Loss of Cell-Cell Contact Inhibits Cellular Differentiation of α-Catenin Knock Out P19 Embryonal Carcinoma Cells and Their Colonization into the Developing Mouse Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of Cells Lines Using CRISPR/Cas9 Plasmid-Mediated Gene KO
2.3. Heat Map Analysis
2.4. Generation of Embryoid Bodies (EB)s and Retinoic Acid (RA)-Based Induction for Cellular Differentiation
2.5. In Vivo Teratoma Formation Assay
2.6. Formation of Aggregation Chimeras between EC Cells and an 8-Cell Embryo
2.7. Western Blotting
2.8. Immunostaining
2.9. Fluorescence Observation
2.10. Statistical Analysis
3. Results
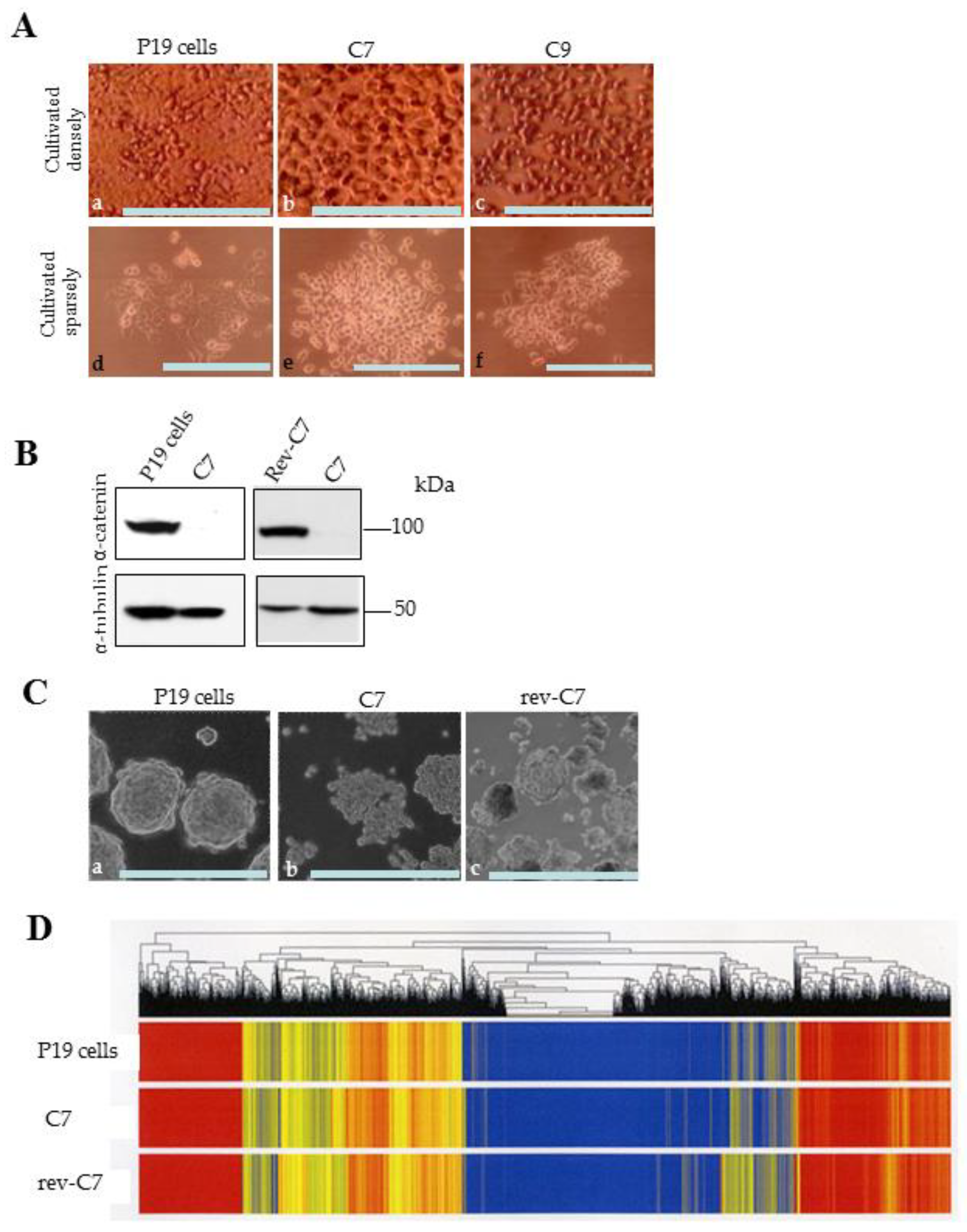
3.1. Generation of α-Cat KO P19 Cell Lines and Their Revertant
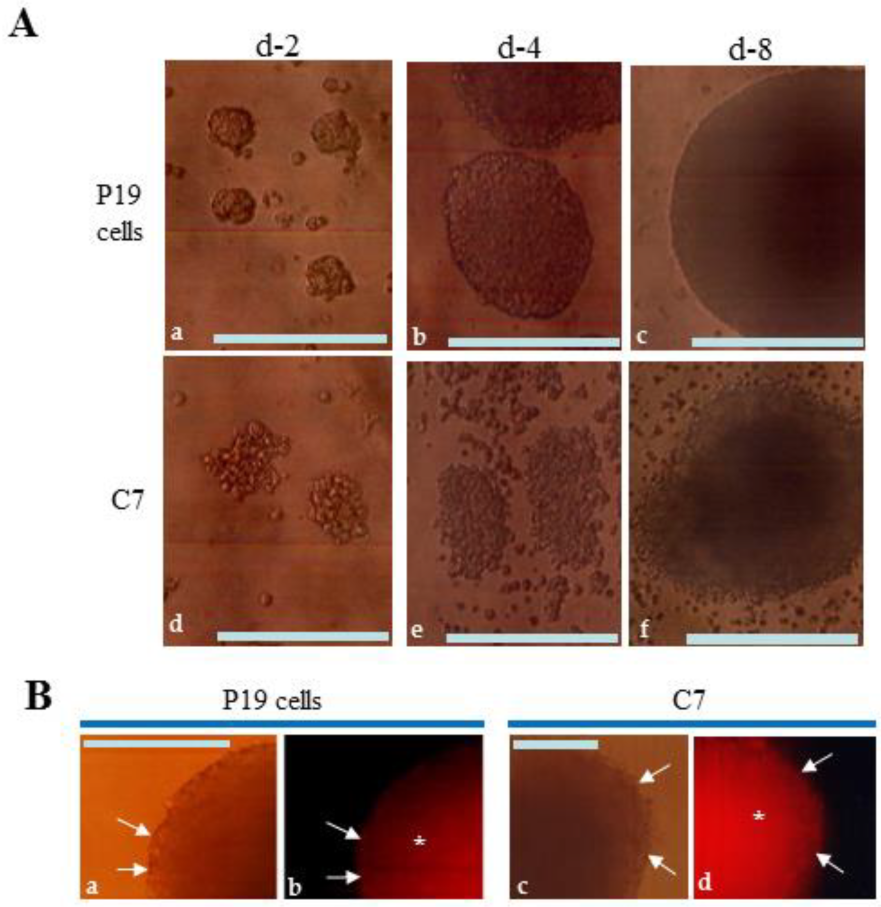
3.2. EBs Generated after Culture on Non-Adhesive Dishes and Their Immunohistochemical Property
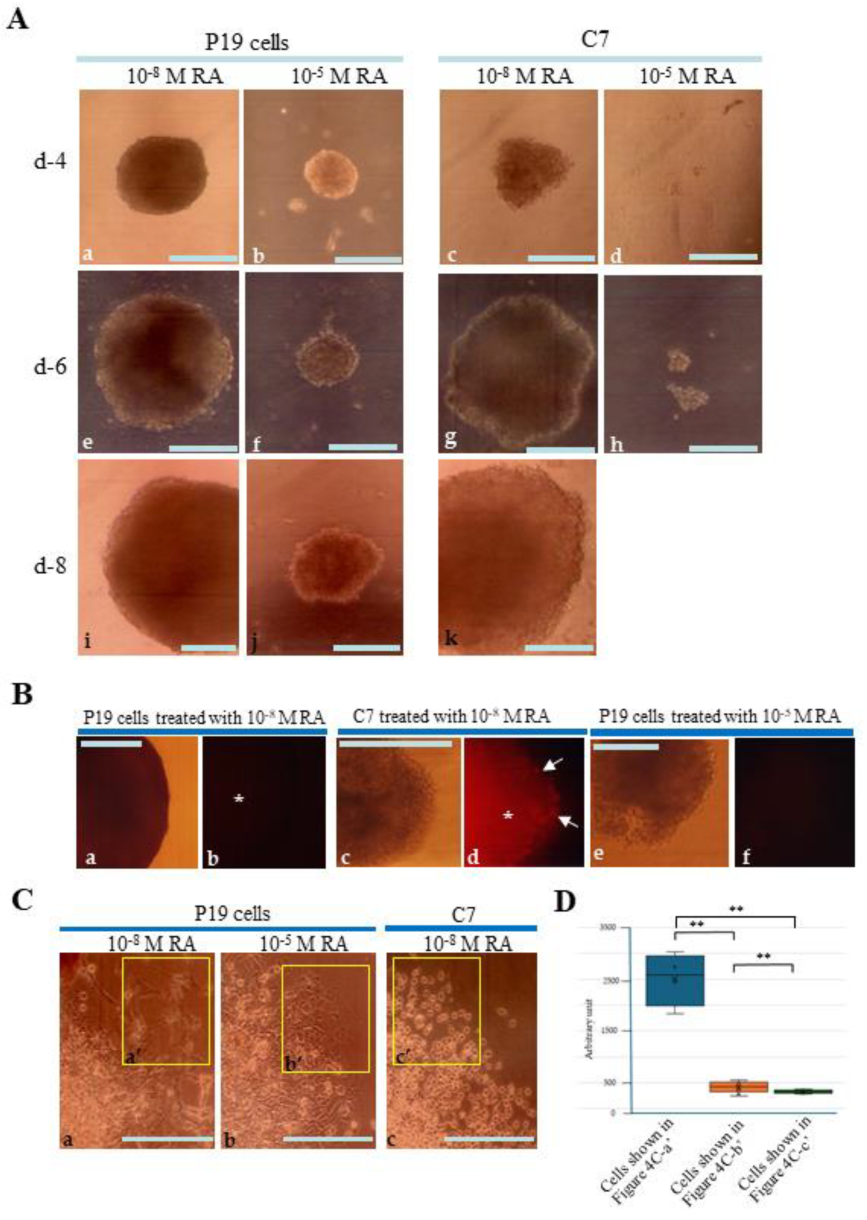
3.3. Forced Induction for Differentiation of EBs by Cultivating in RA-Containing Medium
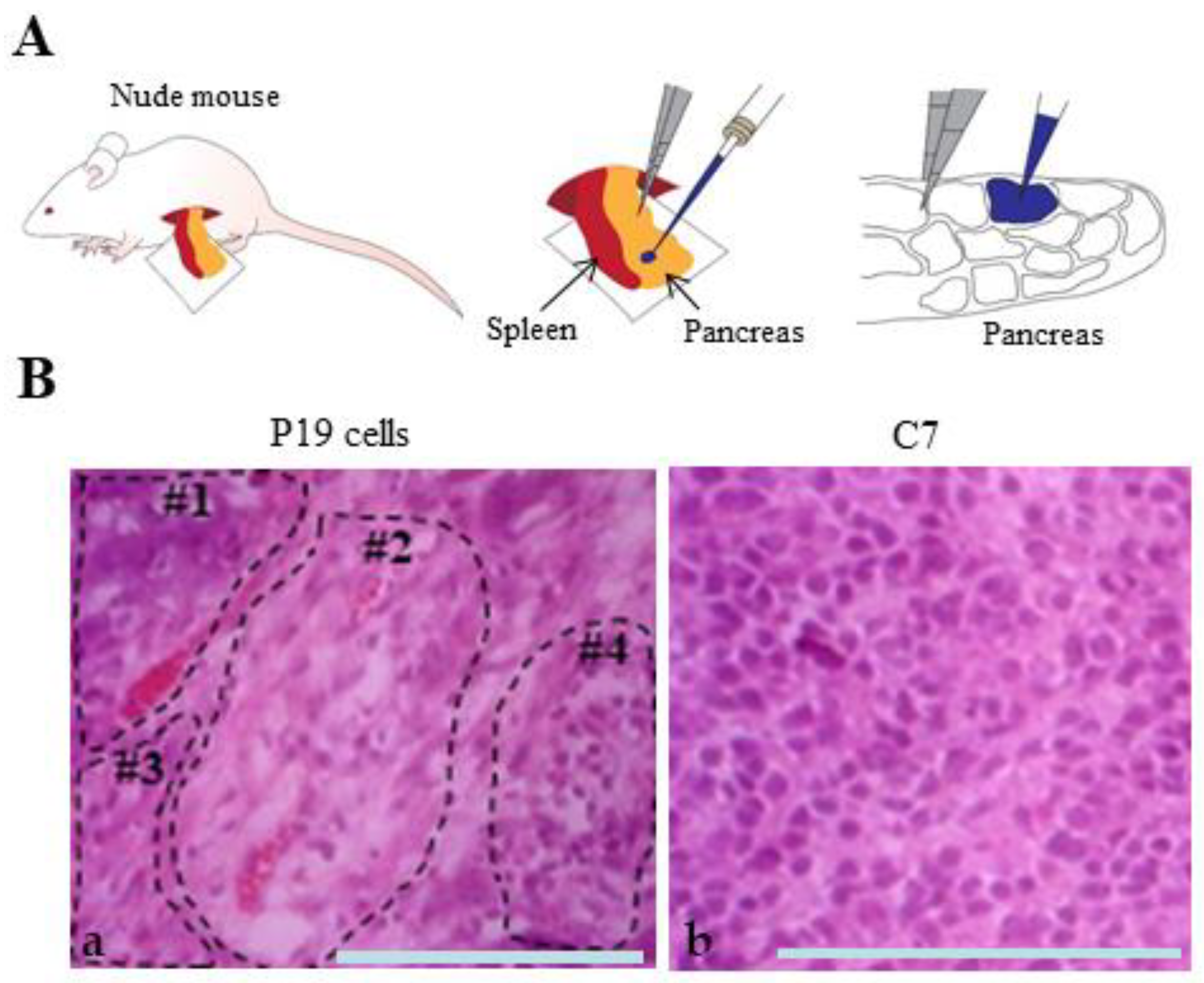
3.4. In Vivo Teratoma Formation Assay through Transplantation of P19 and Its Derivative into Pancreatic Tissues
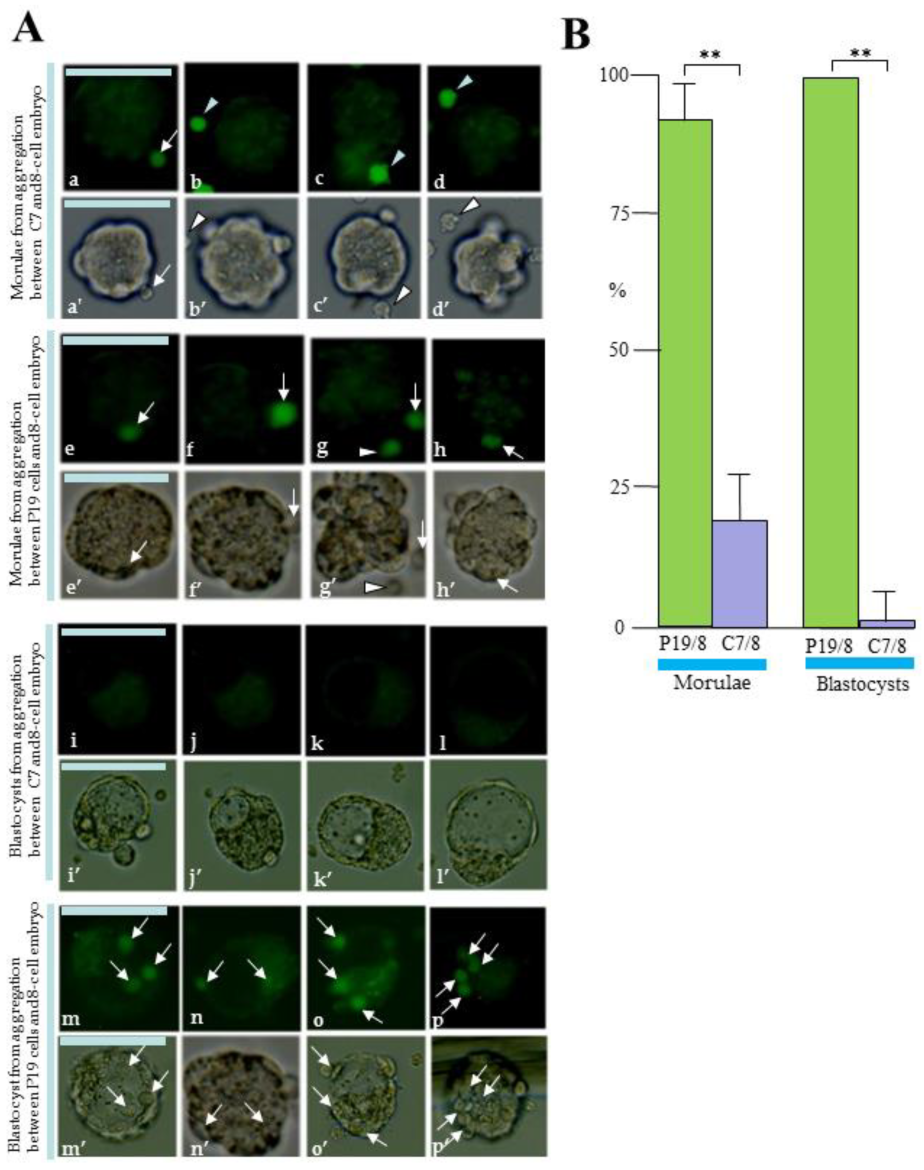
3.5. EC Cells/8-Cell Embryo Aggregation Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ozawa, M.; Baribault, H.; Kemler, R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989, 8, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Nagafuchi, A.; Shirayoshi, Y.; Okazaki, K.; Yasuda, K.; Takeichi, M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 1987, 329, 341–343. [Google Scholar] [CrossRef]
- Rimm, D.L.; Koslov, E.R.; Kebriaei, P.; Cianci, C.D.; Morrow, J.S. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA 1995, 92, 8813–8817. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Nagafuchi, A.; Moroi, S.; Tsukita, S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 1997, 138, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Wada, Y.; Watanabe, T.; Nagafuchi, A.; Shibata, M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 2010, 12, 533–542. [Google Scholar] [CrossRef]
- Takeichi, M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodeling. Nat. Rev. Mol. Cell Biol. 2014, 15, 397–410. [Google Scholar] [CrossRef]
- Larue, L.; Ohsugi, M.; Hirchenhain, J.; Kemler, R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA 1994, 91, 8263–8267. [Google Scholar] [CrossRef]
- Riethmacher, D.; Brinkmann, V.; Birchmeier, C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 1995, 92, 855–859. [Google Scholar] [CrossRef]
- Poh, Y.C.; Chen, J.; Hong, Y.; Yi, H.; Zhang, S.; Chen, J.; Wu, D.C.; Wang, L.; Jia, Q.; Singh, R.; et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 2014, 5, 4000. [Google Scholar] [CrossRef]
- Fujiwara, M.; Fujimura, K.; Obata, S.; Yanagibashi, R.; Sakuma, T.; Yamamoto, T.; Suzuki, S.T. Epithelial DLD-1 Cells with disrupted E-cadherin gene retain the ability to form cell junctions and apico-basal polarity. Cell Struct. Funct. 2015, 40, 79–94. [Google Scholar] [CrossRef]
- Torres, M.; Stoykova, A.; Huber, O.; Chowdhury, K.; Bonaldo, P.; Mansouri, A.; Butz, S.; Kemler, R.; Gruss, P. An α-E-catenin gene trap mutation defines its function in preimplantation development. Proc. Natl. Acad. Sci. USA 1997, 94, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M. E-cadherin cytoplasmic domain inhibits cell surface localization of endogenous cadherins and fusion of C2C12 myoblasts. Biol. Open 2015, 4, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Hehlgans, S.; Rödel, F.; Otto, B.; Cordes, N. Differential effects of α-catenin on the invasion and radiochemosensitivity of human colorectal cancer cells. Int. J. Oncol. 2018, 52, 1117–1128. [Google Scholar] [PubMed]
- McBurney, M.W.; Rogers, B.J. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev. Biol. 1982, 89, 503–508. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W. P19 embryonal carcinoma cells. Int. J. Dev. Biol. 1993, 37, 135–140. [Google Scholar]
- Hogan, B.L.M.; Taylor, A.; Adamson, E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature 1981, 291, 235–237. [Google Scholar] [CrossRef]
- Strickland, S.; Mahdavi, V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell 1978, 15, 393–403. [Google Scholar] [CrossRef]
- Jones-Villeneuve, E.M.V.; Rudnicki, M.A.; Harris, J.F.; McBurney, M.W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol. Cell. Biol. 1983, 3, 2271–2279. [Google Scholar]
- Edwards, M.K.; McBurney, M.W. The concentration of retinoic acid determines the differentiated cell types formed by a teratocarcinoma cell line. Dev. Biol. 1983, 98, 187–191. [Google Scholar] [CrossRef]
- Rossant, J.; McBurney, M.W. The developmental potential of a euploid male teratocarcinoma cell line after blastocyst injection. J. Embryol. Exp. Morphol. 1982, 70, 99–112. [Google Scholar] [CrossRef]
- Ozawa, M. Nonmuscle myosin IIA is involved in recruitment of apical junction components through activation of α-catenin. Biol. Open 2018, 7, bio031369. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Hiver, S.; Yamamoto, T.; Shibata, T.; Upadhyayula, S.; Mimori-Kiyosue, Y.; Takeichi, M. Adherens junction regulates cryptic lamellipodia formation for epithelial cell migration. J. Cell Biol. 2020, 219, e202006196. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Kobayashi, W. Cadherin cytoplasmic domains inhibit the cell surface localization of endogenous E-cadherin, blocking desmosome and tight junction formation and inducing cell dissociation. PLoS ONE 2014, 9, e105313. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Miyazaki, M.; Miyashita, Y.; Arima, T.; Ozawa, M. Identification of regions of alpha-catenin required for desmosome organization in epithelial cells. Int. J. Mol. Med. 2005, 16, 1003–1008. [Google Scholar] [PubMed]
- Sato, M.; Saitoh, I.; Murakami, T.; Kubota, N.; Nakamura, S.; Watanabe, S.; Inada, E. Intrapancreatic parenchymal injection of cells as a useful tool for allowing a small number of proliferative cells to grow in vivo. Int. J. Mol. Sci. 2017, 18, 1678. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Yanagamachi, R.; Yanagamachi, H. Ultrastructural localisation of lectin-binding sites on the zonzae pellucidae and plasma membranes of mammalian eggs. J. Cell Biol. 1975, 66, 263–274. [Google Scholar] [CrossRef]
- Mintz, B.; Gearhart, J.D.; Guymont, A.O. Phytohemagglutinin-mediated blastomere aggregation and development of allophenic mice. Dev. Biol. 1973, 31, 195–199. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kubota, N.; Iwase, Y.; Murakami, T.; Sawami, T.; Yamasaki, Y.; Sato, M. Increased expression of cell surface SSEA-1 is closely associated with naïve-like conversion from human deciduous teeth dental pulp cells-derived iPS cells. Int. J. Mol. Sci. 2019, 20, 1651. [Google Scholar] [CrossRef]
- Solter, D.; Knowles, B.B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569. [Google Scholar] [CrossRef]
- Grover, A.; Oshima, R.G.; Adamson, E.D. Epithelial layer formation in differentiating aggregates of F9 embryonal carcinoma cells. J. Cell Biol. 1983, 96, 1690–1696. [Google Scholar] [CrossRef]
- Wesselschmidt, R.L. The teratoma assay: An in vivo assessment of pluripotency. Methods Mol. Biol. 2011, 767, 231–241. [Google Scholar] [PubMed]
- Nagy, A.; Gócza, E.; Diaz, E.M.; Prideaux, V.R.; Iványi, E.; Markula, M.; Rossant, J. Embryonic stem cells alone are able to support fetal development in the mouse. Development 1990, 110, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Allen, N.D.; Rossant, J.; Auerbach, A.; Nagy, A. Non-injection methods for the production of embryonic stem cell–embryo chimaeras. Nature 1993, 365, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Gertsenstein, M.; Vintersten, K.; Behringer, R. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2003. [Google Scholar]
- Alexandrova, S.; Kalkan, T.; Humphreys, P.; Riddell, A.; Scognamiglio, R.; Trumpp, A.; Nichols, J. Selection and dynamics of embryonic stem cell integration into early mouse embryos. Development 2016, 143, 24–34. [Google Scholar] [CrossRef]
- Mallikarjunan, N.; Marathe, S.; Rajalakshmi, D.; Mahesh, S.; Jamdar, S.; Sharma, A. Effect of ionizing radiation on structural and functional attributes of red kidney bean (Phaseolus vulgaris L.) lectin. LWT Food Sci. Technol. 2014, 59, 300–307. [Google Scholar] [CrossRef]
- Mohamet, L.; Hawkins, K.; Ward, C.M. Loss of function of E-cadherin in embryonic stem cells and the relevance to models of tumorigenesis. J. Oncol. 2011, 2011, 352616. [Google Scholar] [CrossRef]
- Bruner, H.C.; Derksen, P.W.B. Loss of E-Cadherin-dependent cell–cell adhesion and the development and progression of cancer. Cold Spring Harb. Perspect. Biol. 2018, 10, a029330. [Google Scholar] [CrossRef]
- Smith, Q.; Stukalin, E.; Kusuma, S.; Gerecht, S.; Sun, S.X. Stochasticity and spatial interaction govern stem cell differentiation dynamics. Sci. Rep. 2015, 5, 12617. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, X.; An, J.; Zhang, Z.; Chen, X.; Tan, J.; Zhang, P.; Lu, H.; Liu, Y. Cell-cell connection enhances proliferation and neuronal differentiation of rat embryonic neural stem/progenitor cells. Front. Cell Neurosci. 2017, 11, 200. [Google Scholar] [CrossRef]
- Armingol, E.; Officer, A.; Harismendy, O.; Lewis, N.E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet. 2021, 22, 71–88. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kolasa, M.; Podkowa, D.; Kloc, M.; Kubiak, J.Z. Tissue-specific knockout of E-cadherin (Cdh1) in developing mouse gonads causes germ cells loss. Reproduction 2019, 158, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tinkle, C.L.; Lechler, T.; Pasolli, H.A.; Fuchs, E. Conditional targeting of E-cadherin in skin: Insights into hyperproliferative and degenerative responses. Proc. Natl. Acad. Sci. USA 2004, 101, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Vasioukhin, V.; Bauer, C.; Degenstein, L.; Wise, B.; Fuchs, E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 2001, 104, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Bondow, B.J.; Faber, M.L.; Wojta, K.J.; Walker, E.M.; Battle, M.A. E-cadherin is required for intestinal morphogenesis in the mouse. Dev. Biol. 2012, 371, 1–12. [Google Scholar] [CrossRef]
- Zyzynska-Galenska, K.; Piliszek, A.; Modlinski, J.A. From blastomeres to somatic cells: Reflections on cell developmental potential in light of chimaera studies—A review. Anim. Sci. Pap. Rep. 2017, 35, 225–240. [Google Scholar]
- Burnside, A.S.; Collas, P. Induction of Oct-3/4 expression in somatic cells by gap junction-mediated cAMP signaling from blastomeres. Eur. J. Cell Biol. 2002, 81, 585–591. [Google Scholar] [CrossRef]
- Lou, Y.; Preobrazhenska, O.; auf dem Keller, U.; Sutcliffe, M.; Barclay, L.; McDonald, P.C.; Roskelley, C.; Overall, C.M.; Dedhar, S. NIH 3T3 fibroblasts do not express E-cadherin or γ-catenin (Epithelial–mesenchymal transition (EMT) is not sufficient for spontaneous murine breast cancer metastasis. Dev. Dyn. 2008, 237, 2755–2768. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Inada, E.; Kubota, N.; Ozawa, M. Loss of Cell-Cell Contact Inhibits Cellular Differentiation of α-Catenin Knock Out P19 Embryonal Carcinoma Cells and Their Colonization into the Developing Mouse Embryos. BioTech 2024, 13, 41. https://doi.org/10.3390/biotech13040041
Sato M, Inada E, Kubota N, Ozawa M. Loss of Cell-Cell Contact Inhibits Cellular Differentiation of α-Catenin Knock Out P19 Embryonal Carcinoma Cells and Their Colonization into the Developing Mouse Embryos. BioTech. 2024; 13(4):41. https://doi.org/10.3390/biotech13040041
Chicago/Turabian StyleSato, Masahiro, Emi Inada, Naoko Kubota, and Masayuki Ozawa. 2024. "Loss of Cell-Cell Contact Inhibits Cellular Differentiation of α-Catenin Knock Out P19 Embryonal Carcinoma Cells and Their Colonization into the Developing Mouse Embryos" BioTech 13, no. 4: 41. https://doi.org/10.3390/biotech13040041
APA StyleSato, M., Inada, E., Kubota, N., & Ozawa, M. (2024). Loss of Cell-Cell Contact Inhibits Cellular Differentiation of α-Catenin Knock Out P19 Embryonal Carcinoma Cells and Their Colonization into the Developing Mouse Embryos. BioTech, 13(4), 41. https://doi.org/10.3390/biotech13040041







