Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review
Abstract
1. Introduction
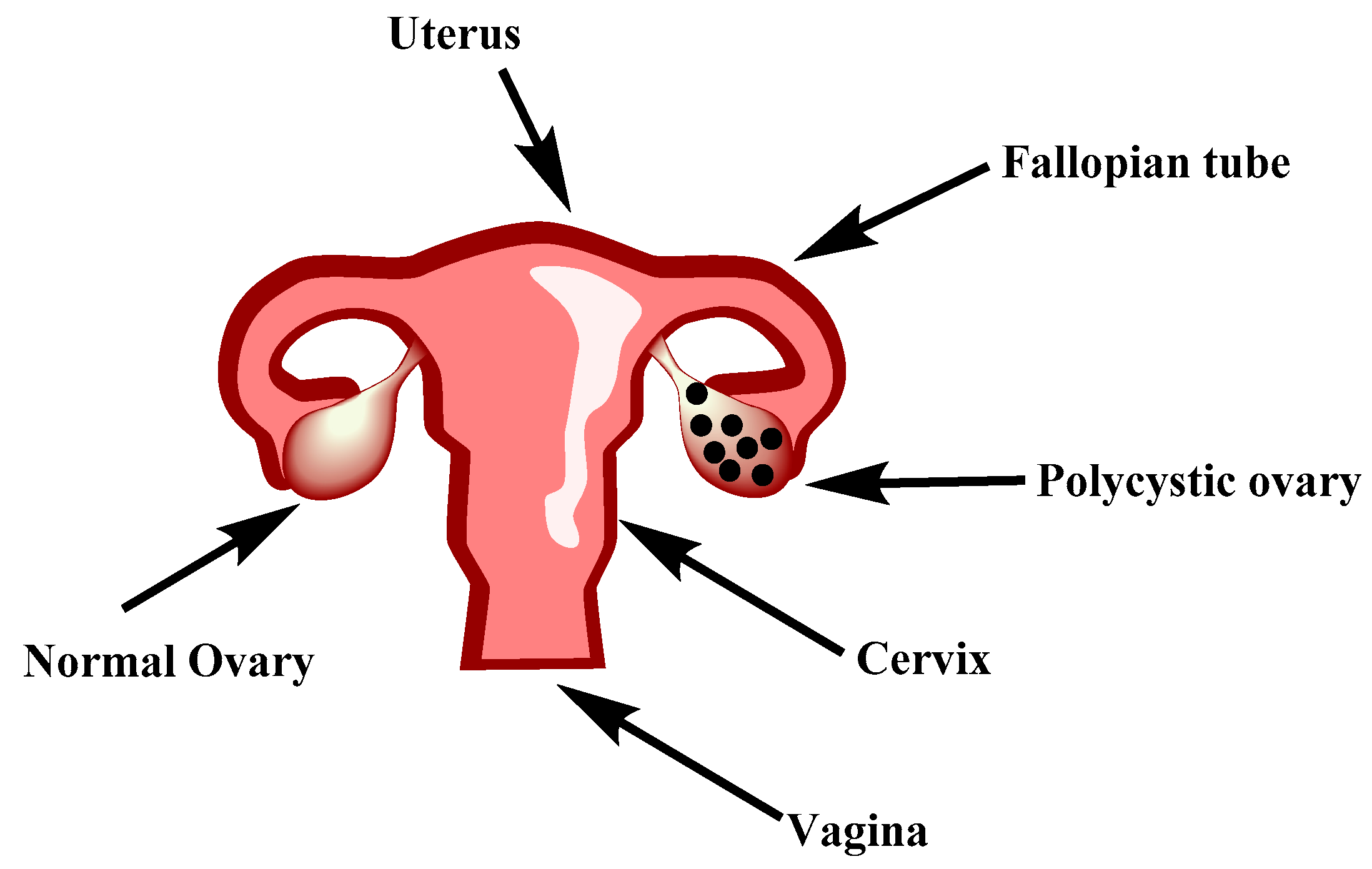
1.1. Etiology
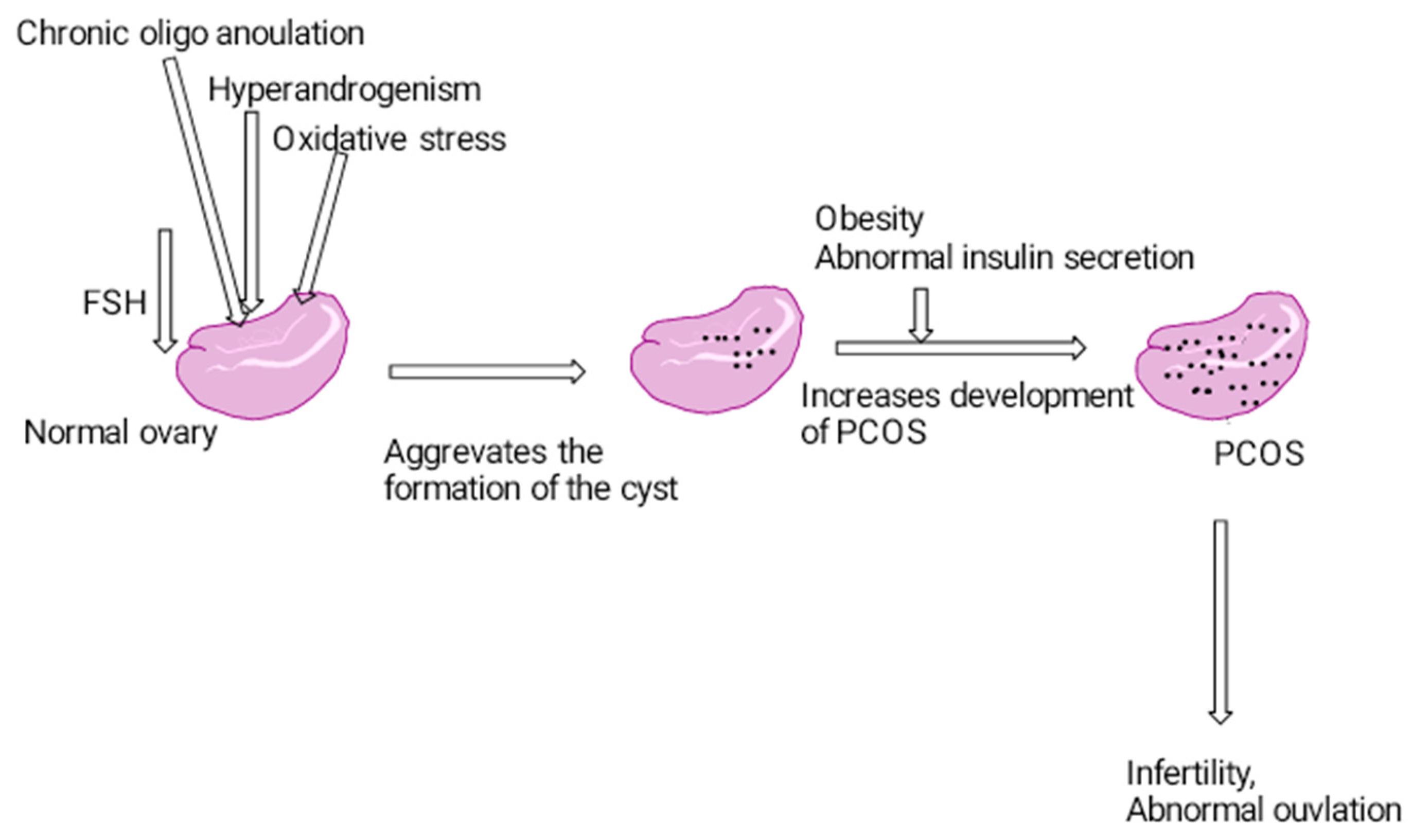
1.2. Pathophysiology
2. Materials and Methods
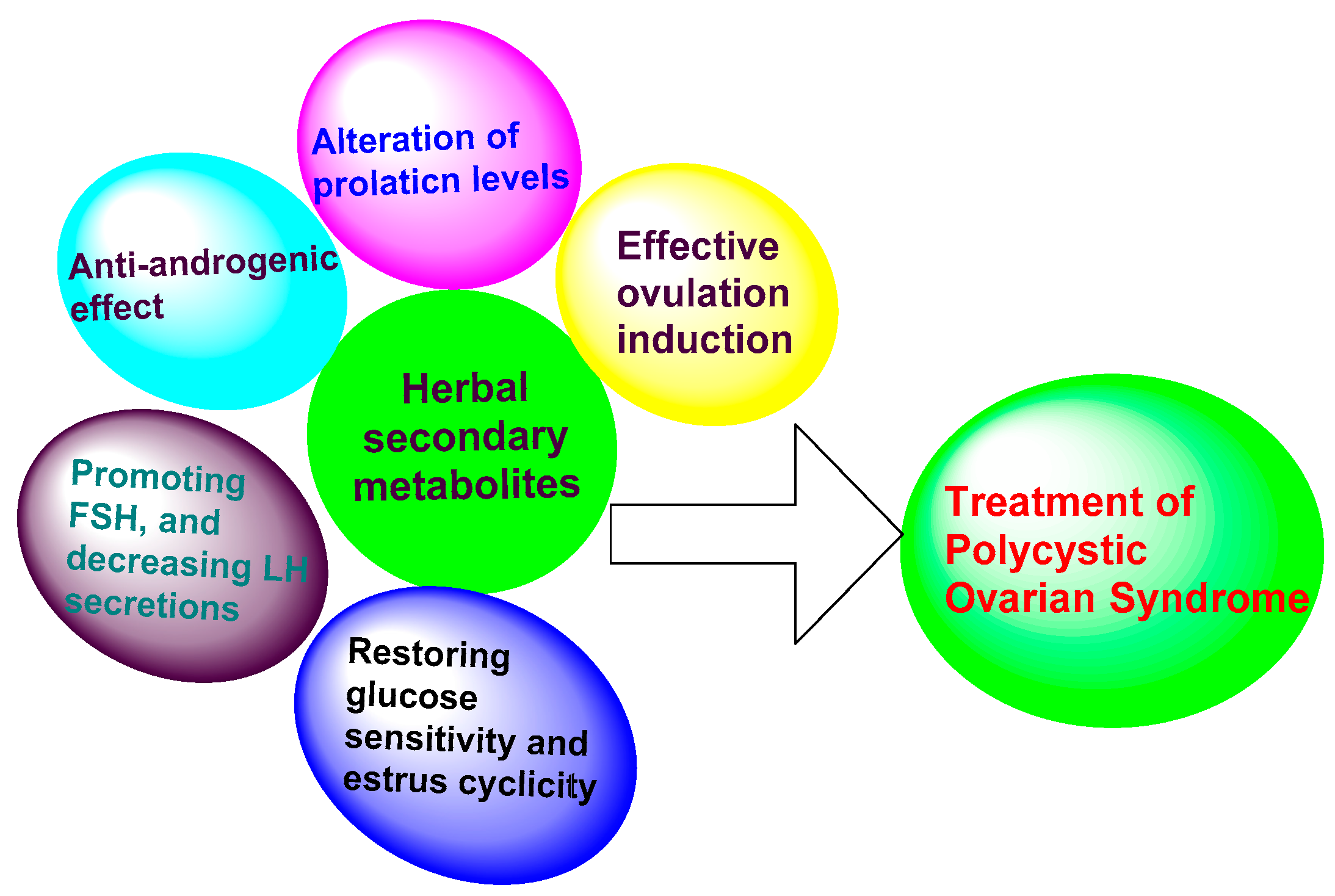
3. Results
3.1. Herbs That Increase Ovulatory Cycles
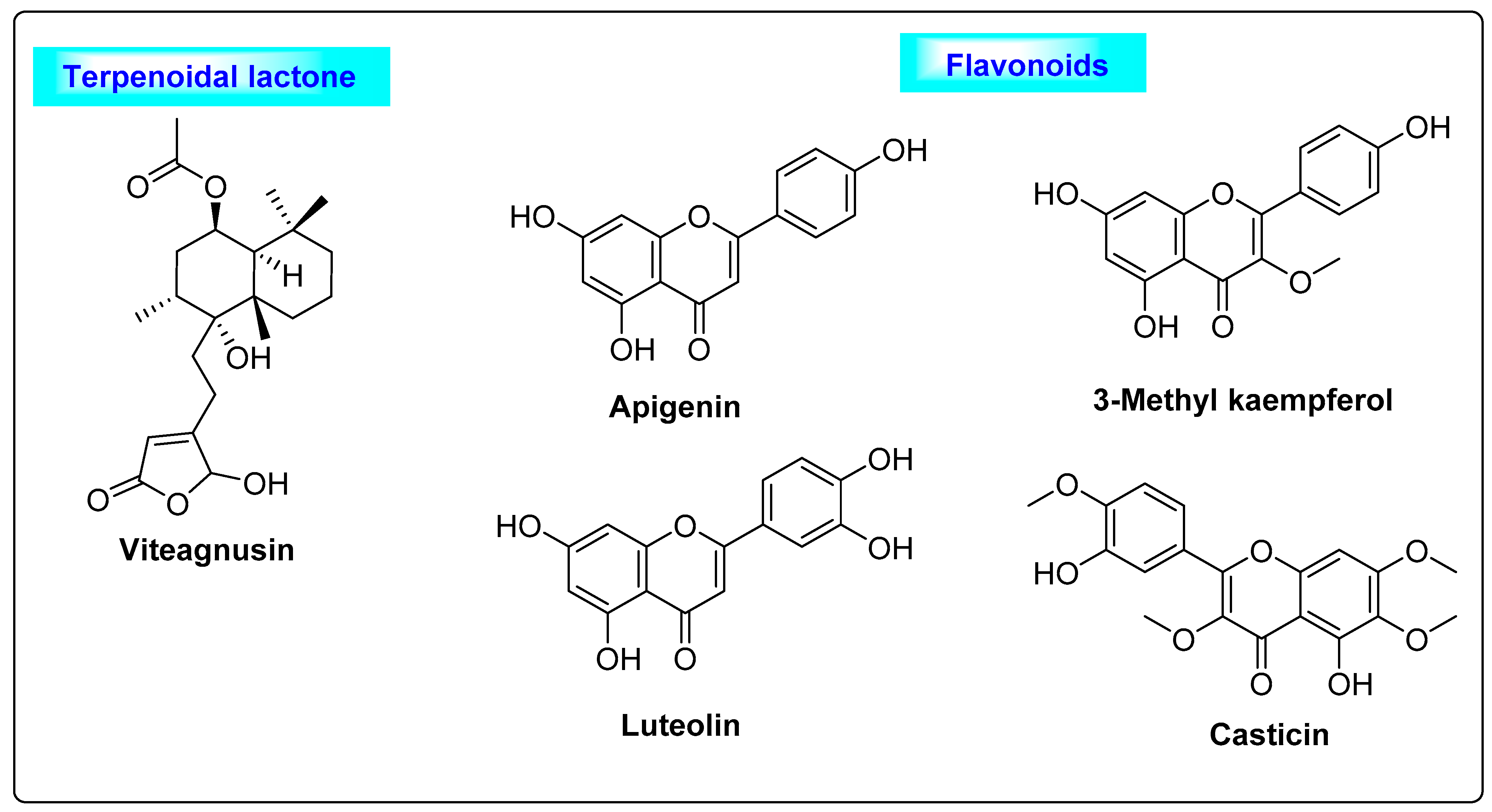
3.1.1. Vitex agnus castus
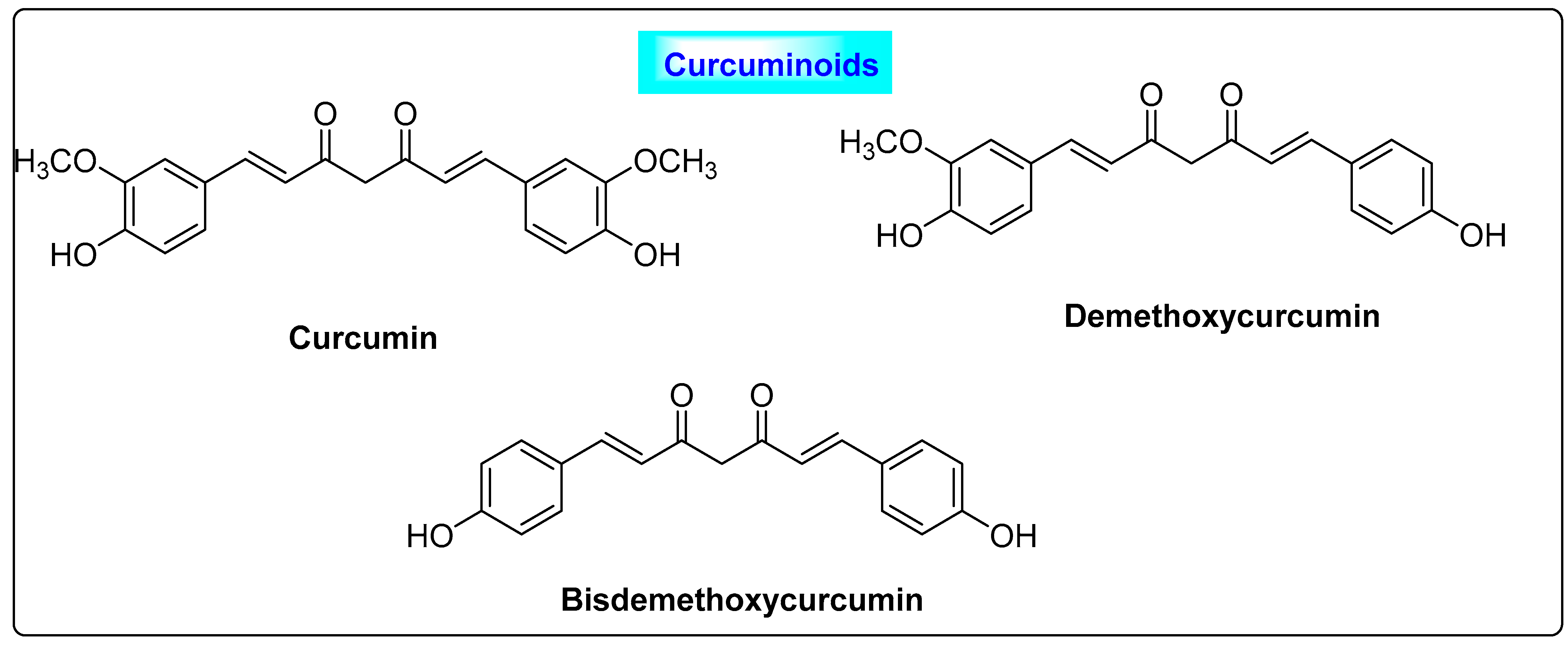
3.1.2. Curcuma longa
3.2. Herbs with Anti-Androgen Properties
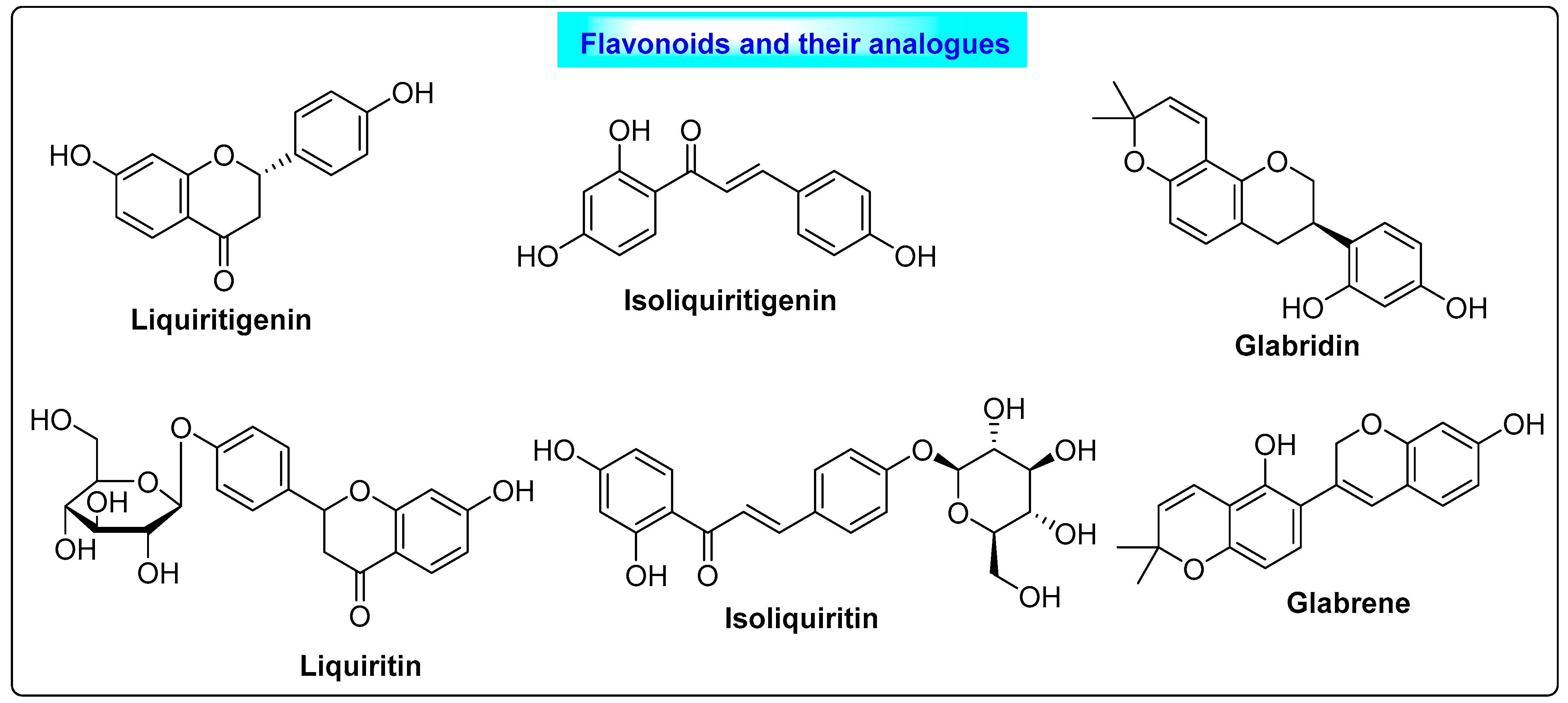
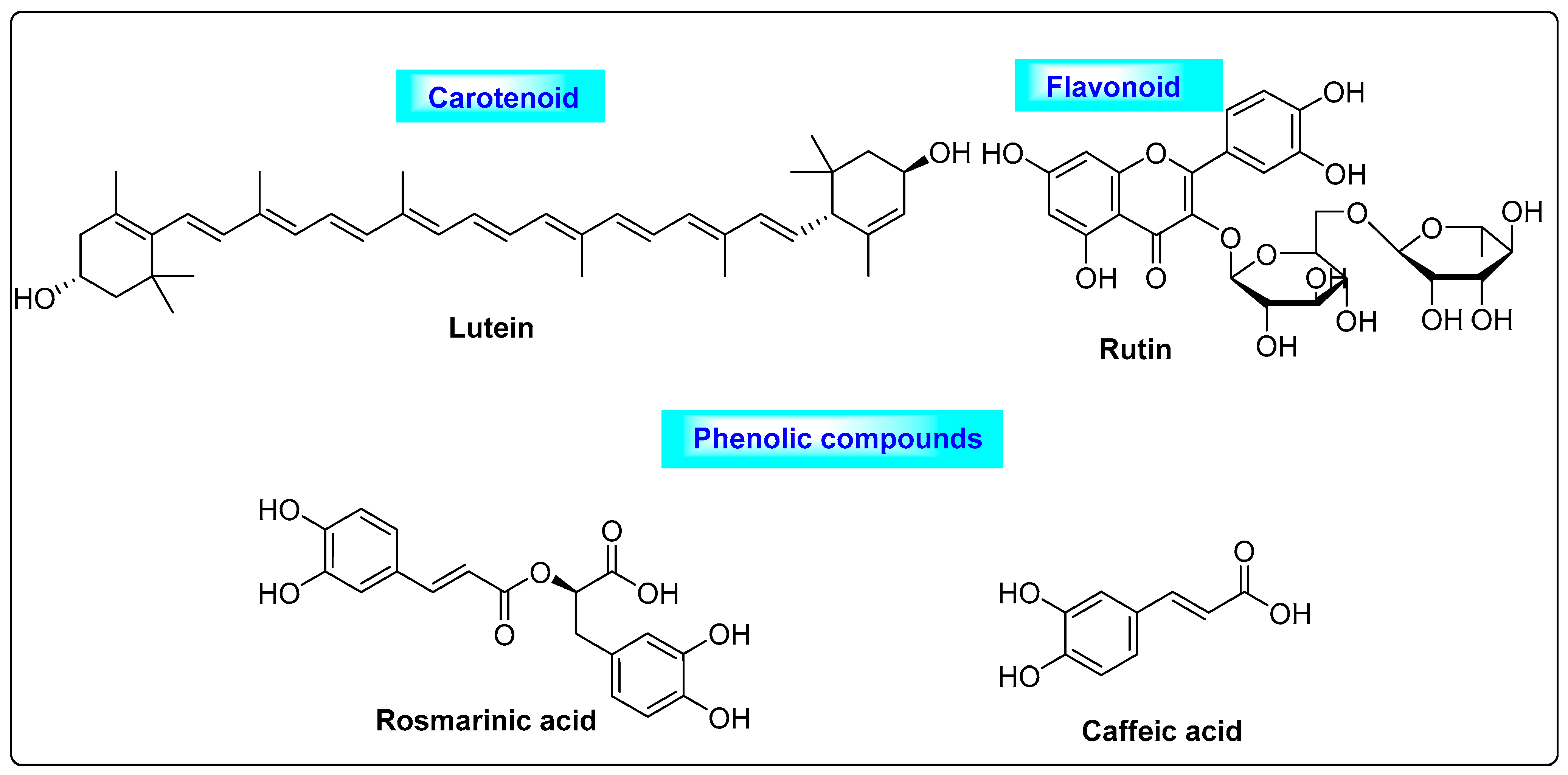
3.2.1. Glycyrrhiza glabra
3.2.2. Linum usitatissimum
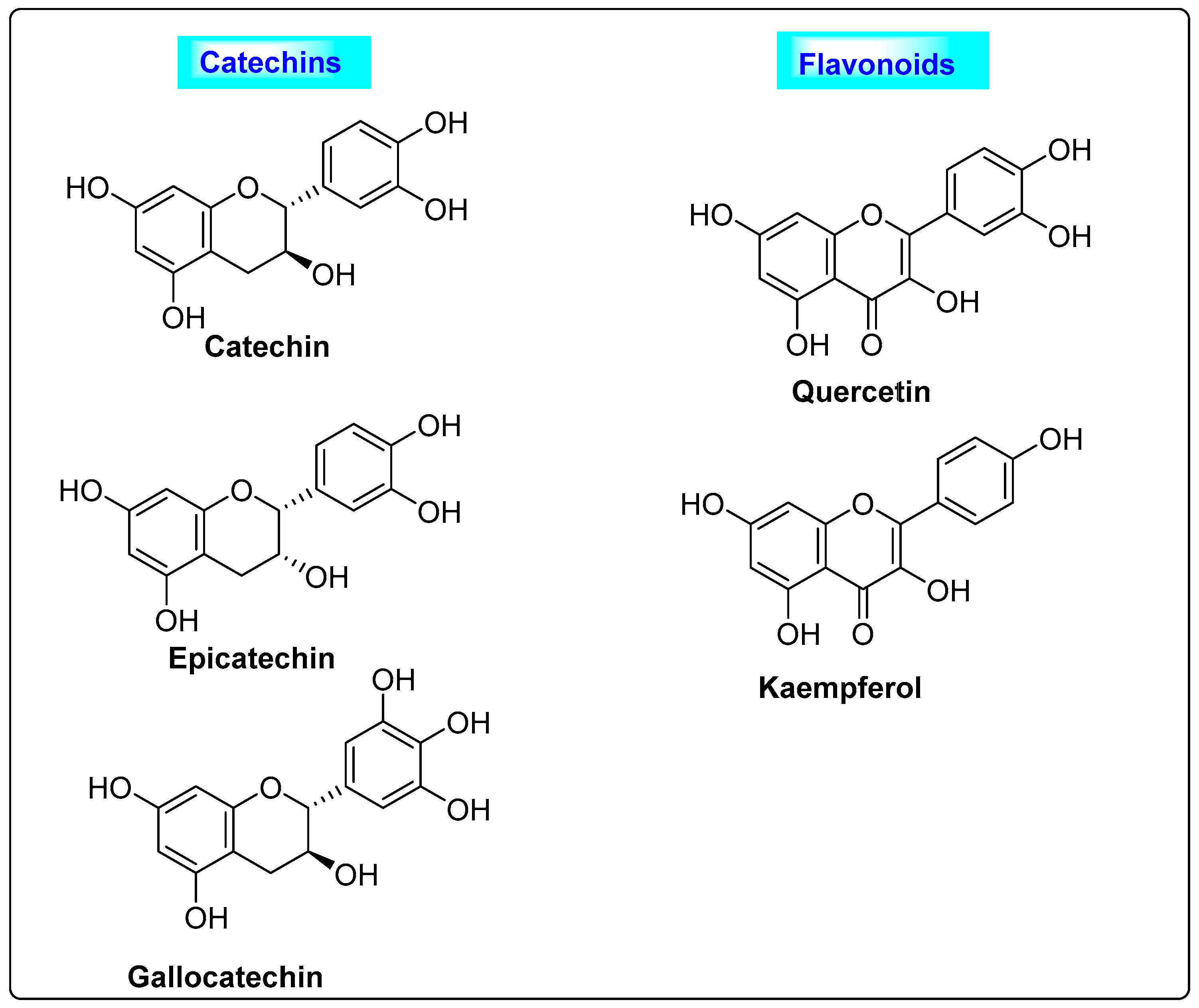
3.2.3. Mentha spicata
3.2.4. Cocos nucifera
3.2.5. Punica granatum
3.3. Herbs That Restore Glucose Sensitivity, Estrus Cyclicity and Enzyme Activity
3.3.1. Cinnamomum cassia
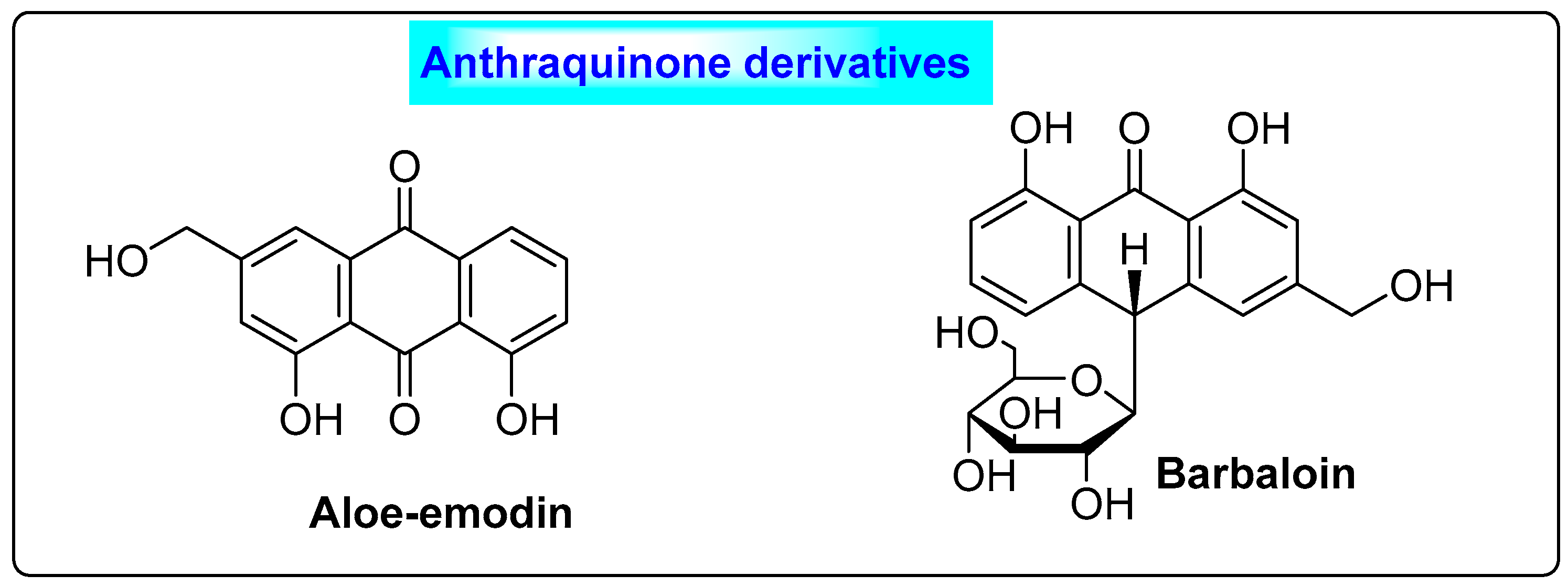
3.3.2. Aloe vera
3.4. Herbs That Promote FSH and Decrease LH Secretions
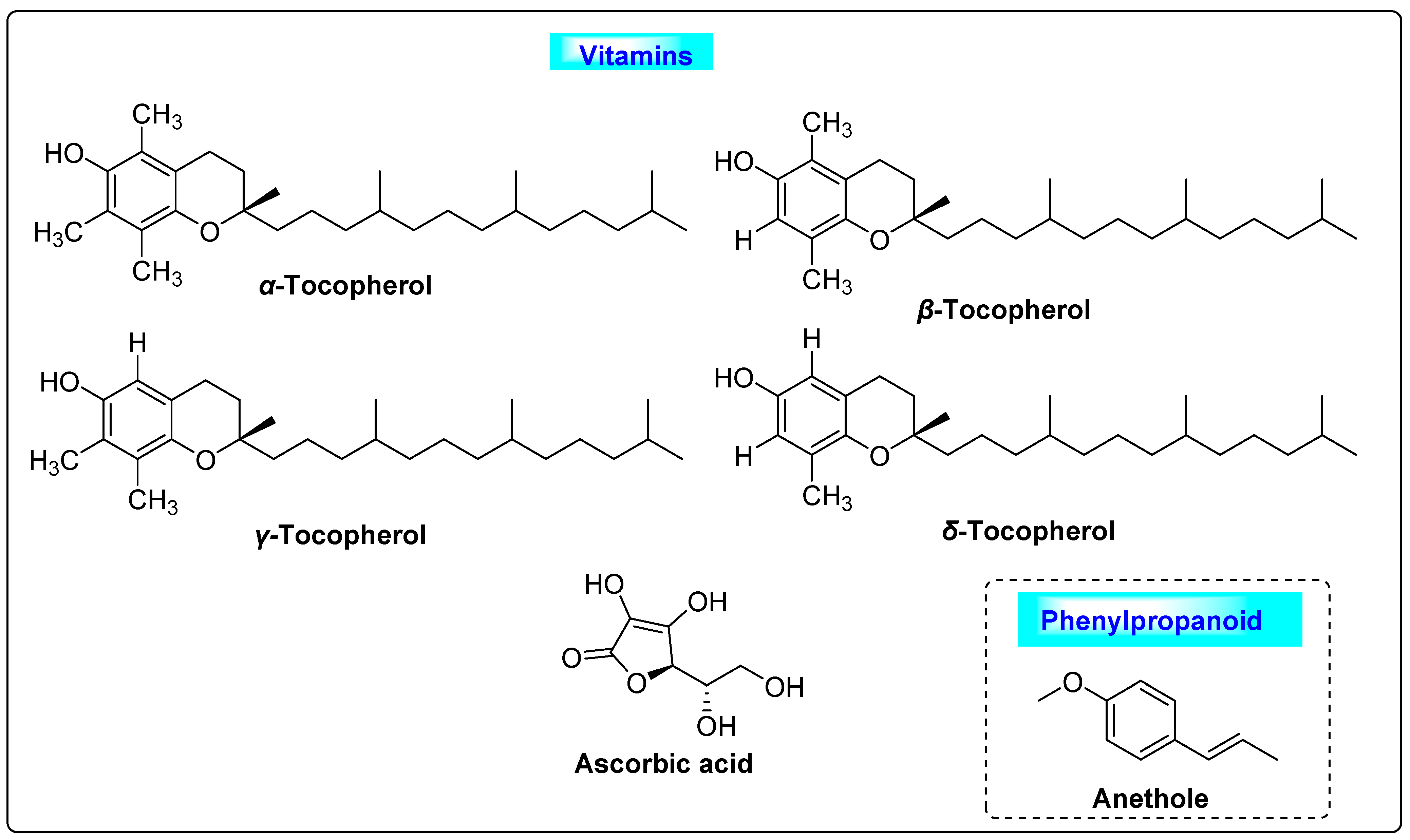
3.4.1. Foeniculum vulgare
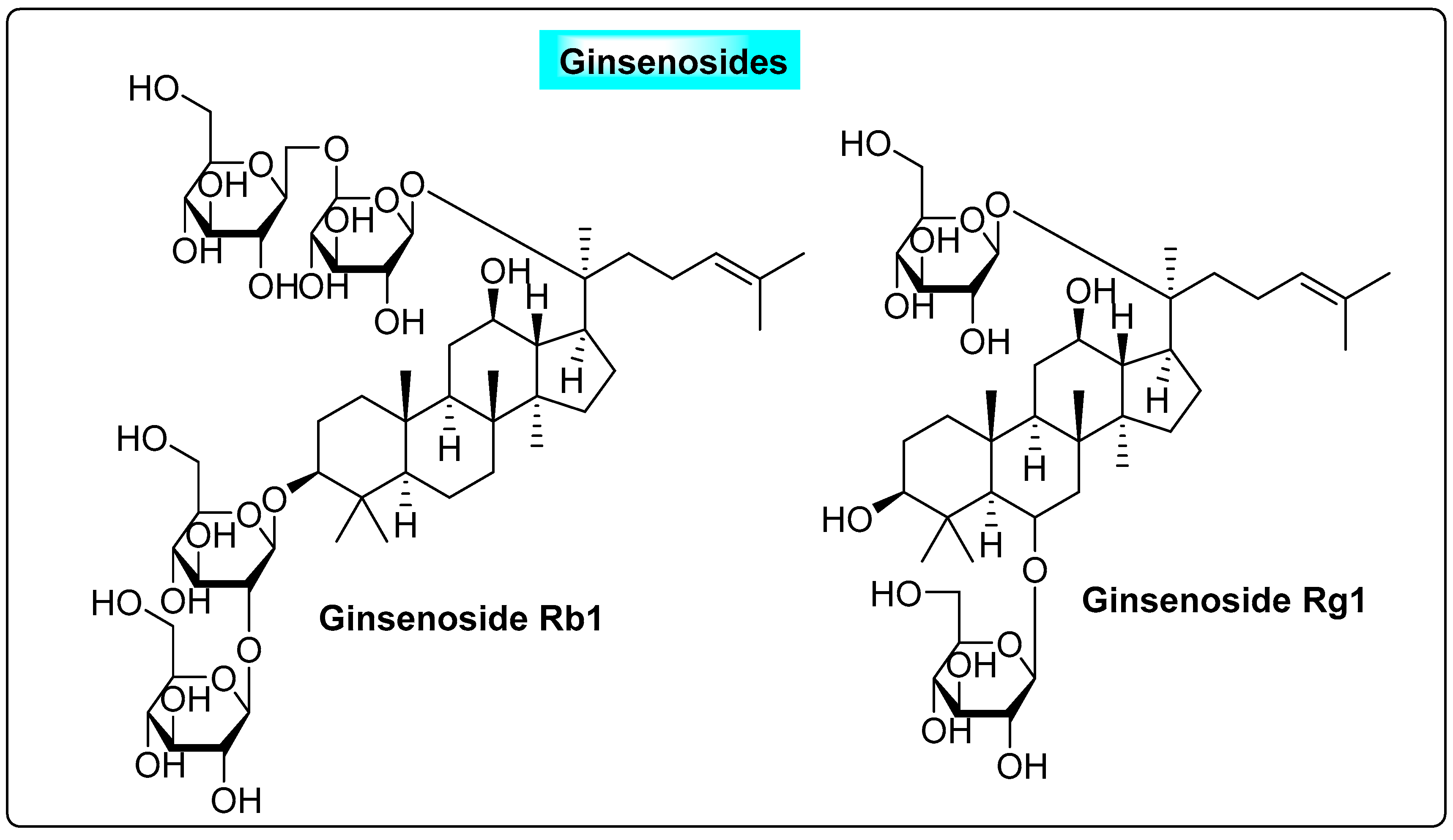
3.4.2. Panax ginseng
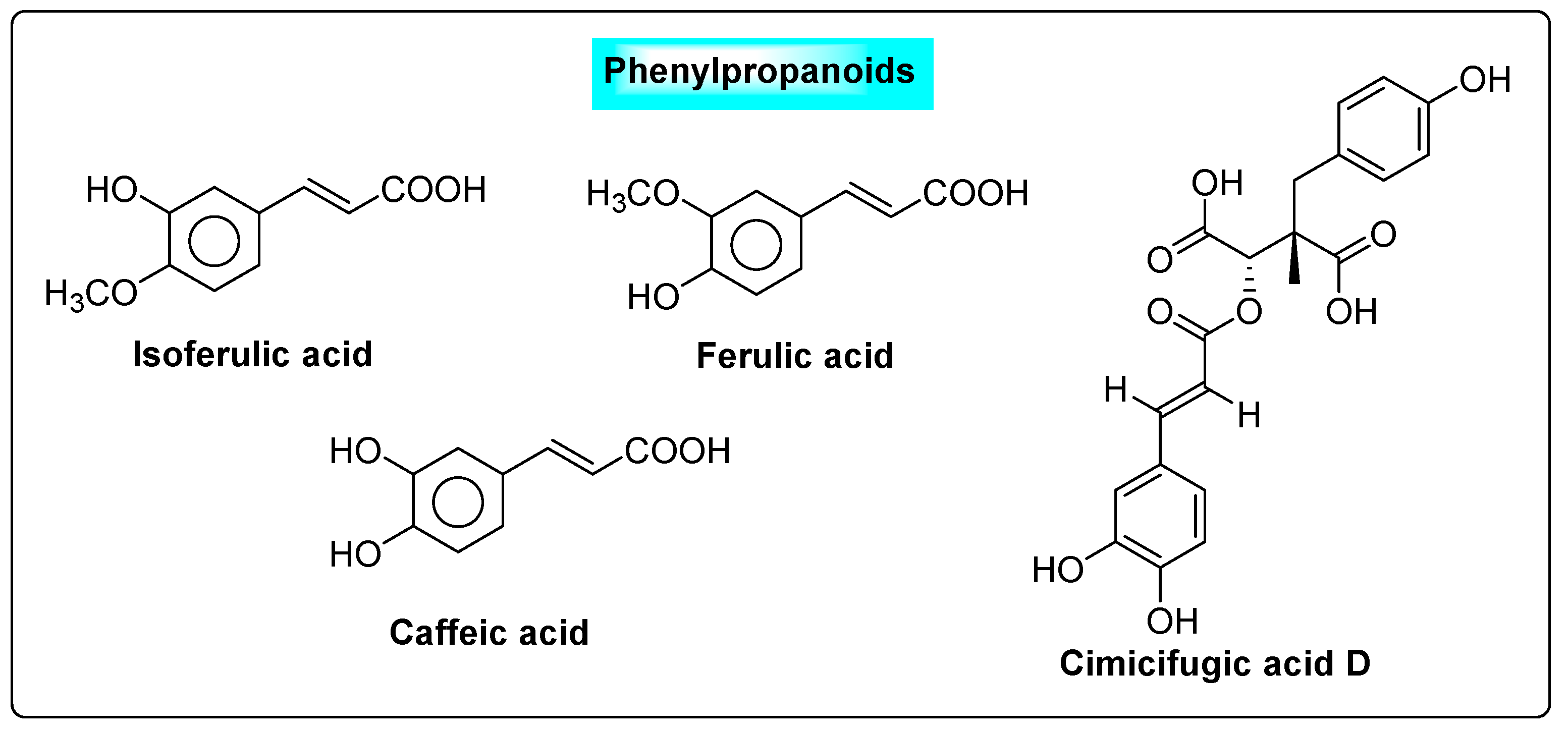
3.4.3. Cimicifuga racemosa
3.4.4. Pimpinella anisum L.
3.4.5. Trigonella foenum-graecum
3.5. Effective Ovulation Induction Agents
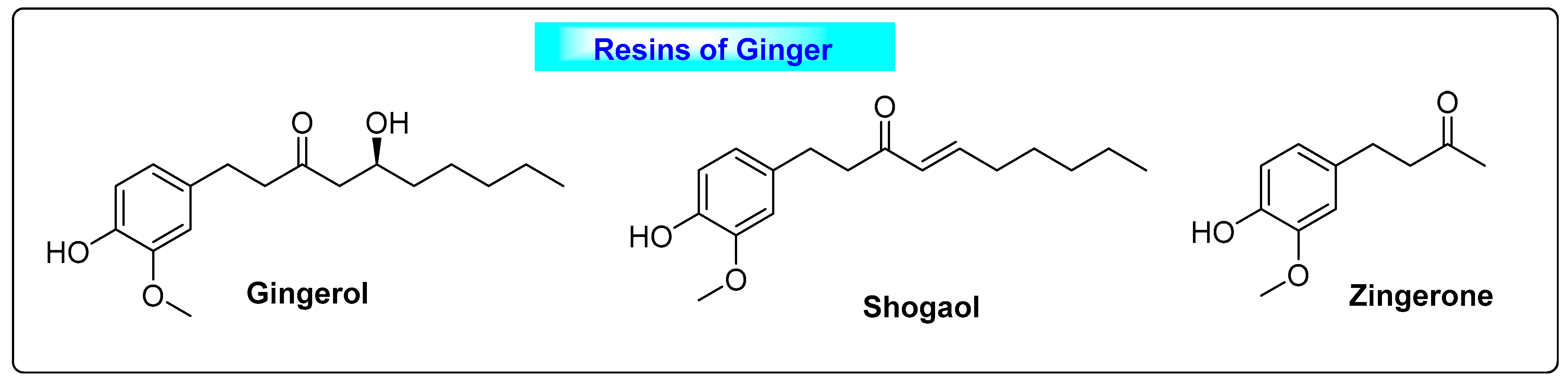
3.5.1. Zingiber officinalis
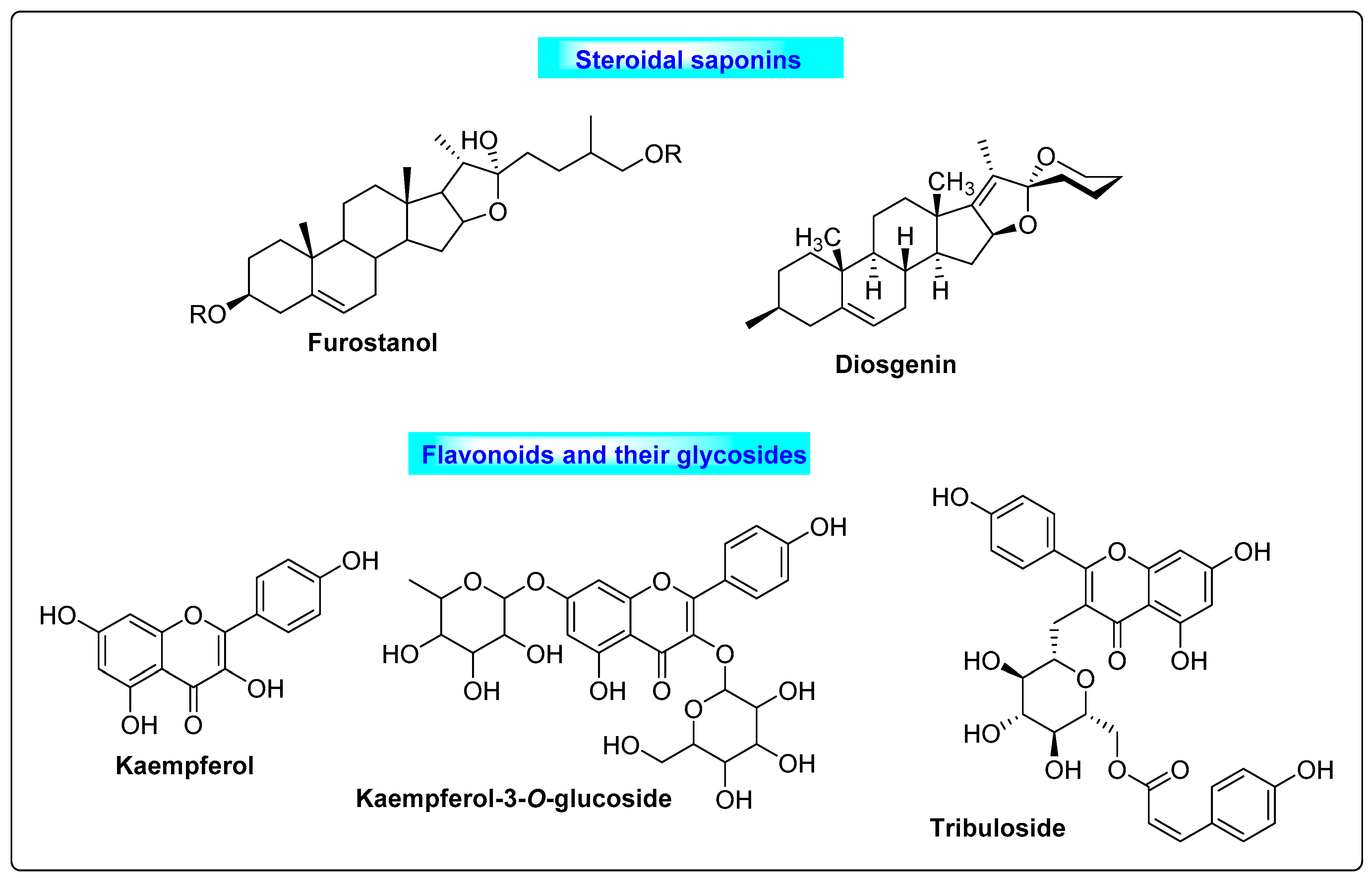
3.5.2. Tribulus terrestris
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ndefo, U.A.; Eaton, A.; Green, M.R. Polycystic ovary syndrome: A review of treatment options with a focus on pharmacological approaches. Pharm. Ther. 2013, 38, 336–355. [Google Scholar]
- Bharathi, R.V.; Swetha, S.; Neerajaa, J.; Madhavica, J.V.; Janani, D.M.; Rekha, S.N.; Ramya, S.; Usha, B. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertil. Soc. J. 2017, 22, 313–316. [Google Scholar] [CrossRef]
- Yadav, K.; Ghadge, P.; Langeh, A.; Kalbhare, S.; Phadtare, P.; Bhoite, R. A Review on Herbal Medicinal Plant for Treatment of Polycystic Ovarian Syndrome (PCOS). Asian J. Pharm. Res. Dev. 2020, 8, 83–87. [Google Scholar]
- Liu, J.; Wu, Q.; Hao, Y.; Jiao, M.; Wang, X.; Jiang, S.; Han, L. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum. Reprod. 2021, 36, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Abasian, Z.; Rostamzadeh, A.; Mohammadi, M.; Hosseini, M.; Rafieian-Kopaei, M. A review on role of medicinal plants in polycystic ovarian syndrome: Pathophysiology, neuroendocrine signaling, therapeutic status and future prospects. Middle East Fertil. Soc. J. 2018, 23, 255–262. [Google Scholar] [CrossRef]
- Ganie, M.A.; Vasudevan, V.; Wani, I.A.; Baba, M.S.; Arif, T.; Rashid, A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J. Med. Res. 2019, 150, 333–344. [Google Scholar]
- Kwon, C.Y.; Cho, I.H.; Park, K.S. Therapeutic Effects and Mechanisms of Herbal Medicines for Treating Polycystic Ovary Syndrome: A Review. Front. Pharmacol. 2020, 11, 1192. [Google Scholar] [CrossRef]
- Goswami, P.K.; Khale, A. Natural Remedies for Polycystic Ovarian Syndrome (PCOS): A Review. Int. J. Pharm. Phytopharm. Res. 2012, 1, 396–402. [Google Scholar]
- Chan, Y.K.; Boram, L.; Kyoung, S.P. Oriental herbal medicine and moxibustion for polycystic ovary syndrome A meta-analysis. Medicine 2018, 43, 97. [Google Scholar]
- Hoberg, E.; Orjala, J.; Meier, B.; Sticher, O. Diterpenoids from the fruits of Vitex agnus-castus. Phytochemistry 1999, 52, 1555–1558. [Google Scholar] [CrossRef]
- Balen, A. The pathophysiology of polycystic ovary syndrome: Trying to understand PCOS and its endocrinology. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 685–706. [Google Scholar] [CrossRef]
- Begum, G.S.; Shariff, A.; Ayman, G.; Mohammad, B.; Housam, R.; Khaled, N. Assessment of Risk Factors for development of polycystic ovarian syndrome. Int. J. Contemp. Med. Res. 2017, 4, 2454–7379. [Google Scholar]
- Pelluri, R.; Srikanth, K.; Paritala, H.; Ravi, V.; Kamma, S.P.; Piduguralla, K.D.; Venkateswarlu, U. The role of high serum uric acid levels in androgenic and non-androgenic polycystic ovarian syndrome patients. Clin. Epidemiol. Glob. Health 2021, 12, 100910. [Google Scholar] [CrossRef]
- O’Reilly, M.; Gathercole, L.; Capper, F.; Arlt, W.; Tomlinson, J. Effect of insulin on AKR1C3 expression in female adipose tissue: In-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet 2015, 385, S16. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Tehranian, N.; Movahedin, M.; Tehrani, F.R.; Ziaee, S. Antioxidants and management of polycystic ovary syndrome in Iran: A systematic review of clinical trials. Iran. J. Reprod. Med. 2015, 13, 1–8. [Google Scholar] [PubMed]
- Susan, A.; Jason, A.A.; Caroline, A.S.; Alan, B. Herbal medicine for the management of polycysticovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogeni sm; a review of the laboratory evidence for effects with corroborative clinical findings. Complement. Altern. Med. 2014, 14, 511. [Google Scholar]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef]
- Khanage, S.G.; Subhash, T.Y.; Bhaiyyasaheb, I.R. Herbal drugs for the treatment of Polycystic ovary syndrome (pcos) and its Complications. Pharm. Res. 2019, 2, 5–13. [Google Scholar]
- Dunne, N.; Slater, W. The Natural Diet Solution for PCOS and Infertility: How to Manage Polycystic Ovary Syndrome Naturally; Natural Solutions for PCOS, Health Solutions Plus: Seattle, WA, USA, 2006. [Google Scholar]
- Elsheikh, M.; Caroline, M. Polycystic Ovary Syndrome; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Legro, R.S. Clomiphene, Metformin, or Both for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2007, 356, 551–566. [Google Scholar] [CrossRef]
- Decio, A. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 61–67. [Google Scholar]
- Felenbam, A. Laparoscopic treatment of polycystic ovaries with insulated needle cautery: A reappraisal. Fertil. Steril. 2000, 73, 266–269. [Google Scholar]
- Miller, L.G.; Murray, W.J. Herbal Medicinals: A Clinician’s Guide; Routledge: London, UK, 1998; p. 326. [Google Scholar]
- Tilburt, J.C.; Kaptchuk, T.J. Bulletin of the World Health Organization, 86th ed.; World Health Organization: Geneva, Switzerland, 2008; pp. 594–599.
- Anonymous. Zanzibar Traditional and Alternative Medicine Policy, Ministry of Health and Social Welfare in Collaboration with: World Health Organization. 2008. Available online: https://www.afro.who.int/publications/zanzibar-traditional-and-alternative-medicine-policy-2008 (accessed on 15 September 2021).
- Benzie, I.F.; Galor, S.W. Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011; Volume 7. [Google Scholar]
- Merz, P.G.; Schrödter, A.; Rietbrock, S.; Gorkow, C.; Löw, D. Prolactin secretion and tolerance during treatment with an Agnus castus extract—Effect on prolactin secretion. Phytopharm. Forsch. Klin. Anwend. 1996, 104, 447–453. [Google Scholar]
- Webster, D.E.; Lu, J.; Chen, S.N.; Farnsworth, N.R.; Wang, Z.J. Activation of the mu-opiate receptor by Vitex agnus-castus methanol extracts: Implication for its use in PMS. J. Ethnopharmacol. 2006, 106, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Burdette, J.E.; Xu, H.; Gu, C.; Van Breemen, R.B.; Bhat, K.P.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H.; et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.M.; Katsiotis, S.T. Variation in essential oil yield and composition of Cretan Vitex agnuscastus L. fruits. J. Essent. Oil Res. 1999, 11, 599–605. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Ozcan, M. Chemical composition and antibacterial activity of essential oil from fruits of Vitex agnus-castus L. (Verbenaceae) growing in Turkey. J. Essent. Oil-Bear. Plants 2003, 6, 185–190. [Google Scholar] [CrossRef]
- Wollenweber, E.; Mann, K. Flavonols from fruits of Vitex agnus-castus. Planta Med. 1983, 48, 126–127. [Google Scholar] [CrossRef]
- Hirobe, C.; Qiao, Z.S.; Takeya, K.; Itokawa, H. Cytotoxic flavonoids from Vitex agnus-castus. Phytochemistry 1997, 46, 521–554. [Google Scholar] [CrossRef]
- Görler, K.; Öhlke, D.; Soicke, H. Iridoid derivatives from Vitex agnus-castus. Planta Med. 1985, 50, 530–531. [Google Scholar] [CrossRef]
- Ono, M.; Yamasaki, T.; Konoshita, M.; Ikeda, T.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Five new diterpenoids, viteagnusins A-E, from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2008, 56, 1621–1624. [Google Scholar] [CrossRef]
- Ono, M.; Nagasawa, Y.; Ikeda, T.; Tsuchihashi, R.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Three new diterpenoids from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2009, 57, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.C.; Brent Friesenb, J.; Donna, W.; Dejan, N.; Richard, B.; Breemena, V. Phytoconstituents from Vitex agnus-castus fruits. Fitoterapia 2011, 82, 528–533. [Google Scholar]
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007, 595, 127–148. [Google Scholar] [PubMed]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017, 8471785. [Google Scholar] [CrossRef]
- Jimenez, A.S.; Monroy, A.; Alavez, S. Curcumin and insulin resistance-molecular targets and clinical evidences. Biofactors 2016, 42, 561–580. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.J.; Pyun, B.-J.; Lee, H.W. Licorice ethanol extract improves symptoms of polycytic ovary syndrome in Letrozole induced female rats. Integr. Med. Res. 2018, 7, 264. [Google Scholar] [CrossRef]
- Baby, B.T.; Rani, S.; Rasheed, S.P.; Bency, B. Polycystic ovarian syndrome: Therapeutic potential of herbal remedies—A review. Int. J. Herb. Med. IJHM 2016, 91, 91–96. [Google Scholar]
- Jelodar, G.; Masoomi, S.; Rahmanifar, F. Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iran. J. Basic Med. Sci. 2018, 21, 645–650. [Google Scholar]
- Aziz, E.; Ameer, K.; Elham, A.; Solmaz, M.D.; Amir, H.; Magali, C. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar]
- Kamel, H.H. Role of phyto-oestrogens in ovulation induction in women with polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Int. 2016, 2016, 5201879. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J. Chemical composition of essential oils of thymus and mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Pendry, B. Phytotherapy for polycystic ovarian syndrome: A review of the literature and evaluation of practitioners’ experiences. J. Herbal Med. 2014, 4, 159–171. [Google Scholar] [CrossRef]
- Wang, J.; Ji, J.; Song, Z.; Zhang, W.; He, X.; Li, F.; Zhang, C.; Guo, C.; Wang, C.; Yuan, C. Hypocholesterolemic effect of emodin by simultaneous determination of in vitro and in vivo bile salts binding. Fitoterapia 2016, 110, 116–122. [Google Scholar] [CrossRef]
- Xueli, C.; Chong, T.; Jingyi, W.; Baochang, H.; Jinbang, X. Meta-analysis of therapeutic efficacy and effects of integrated traditional Chinese and Western medicine on coagulation and fibrinolysis system in patients with threatened abortion and polycystic ovary syndrome. Am. J. Transl. Res. 2022, 14, 2768–2778. [Google Scholar]
- Costa, C.T.; Bevilaqua, C.M.; Morais, S.M.; Camurca, V.A.L.; Maciel, M.V.; Braga, R.R. Anthelmintic activity of Cocos nucifera L. on intestinal nematodes of mice. Res. Vet. Sci. 2010, 88, 101–103. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef]
- Escalante Erosa, F.; Gamboa-León, M.R.; Lecher, J.G.; Arroyo-Serralta, G.A.; Zizumbo-Villareal, D.; Oropeza-Salín, C.; Peña-Rodríguez, L.M. Major components from the epicuticular wax of Cocos nucifera. Rev. Soc. Quím. Mex. 2002, 46, 247–250. [Google Scholar]
- Lima, E.B.C.; Sousa, C.N.S.; Meneses, L.N.; Ximenes, N.C.; Júnior, S.; Vasconcelos, G.S.; Lima, N.B.C.; Patrocínio, M.C.A.; Macedo, D.; Vasconcelos, S.M.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 2015, 48, 953–964. [Google Scholar] [CrossRef]
- Bhandary, M.J.; Chandrashekar, K.R.; Kaveriappa, K.M. Medical ethnobotany of the Siddis of Uttara Kannada district, Karnataka, India. J. Ethnopharmacol. 1995, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Brondegaard, V.J. Contraceptive plant drugs. Planta Med. 1973, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.; von Eichel, J.; Asskali, F. Wound management with coconut oil in Indonesian folk medicine. Chirurg 2002, 73, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, H.H. Botanical remedies of the former Dutch East Indies (Indonesia). Part I: Eumycetes, Pteridophyta, Gymnospermae, Angiospermae (Monocotyledones only). J. Ethnopharmacol. 1983, 7, 123–156. [Google Scholar] [CrossRef]
- Hossein, K.J.; Leila, K.; Ebrahim, T.K.; Nazanin, S.J.; Farzad, P.; Elham, R. The effect of pomegranate juice extract on hormonal changes of female Wistar rats caused by polycystic ovarian syndrome. Biomed. Pharmacol. J. 2015, 8, 971–977. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Chemical composition of volatile oil from Cinnamomum zeylanicum buds. Z. Nat. Sect. C J. Biosci. 2002, 57, 990–993. [Google Scholar] [CrossRef]
- Muchuweti, M.; Kativu, E.; Mupure, H.; Chidewe, C.; Ndhlala, R.A.; Benhura, N.A.M. Phenolic composition and antioxidant properties of some spices. Am. J. Food Technol. 2007, 2, 414–420. [Google Scholar] [CrossRef]
- Evans, P.; Halliwell, B. Micronutrients: Oxidant/antioxidant status. Br. J. Nutr. 2001, 85 (Suppl. 2), 67–74. [Google Scholar] [CrossRef]
- Wang, J.G.; Anderson, R.A.; Graham, G.M.; Chu, M.C.; Sauer, M.V.; Guarnaccia, M.M. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: A pilot study. Fertil. Steril. 2007, 88, 240–243. [Google Scholar] [CrossRef]
- Khodaeifar, F.; Fazljou, S.M.B.; Khaki, A.; Torbati, M.; Ouladsahebmadarek, E.; Khaki, A.A. Investigating the Role of Hydroalcoholic Extract of Apium graveolens and Cinnamon zeylanicum on Metabolically Change and Ovarian Oxidative Injury in a Rat Model of Polycystic Ovary Syndrome. Int. J. Women’s Health Reprod. Sci. 2019, 7, 92–98. [Google Scholar] [CrossRef]
- Ainehchi, N.; Farshbaf-Khalili, A.; Ghasemzadeh, A.; Hamdi, K.; Khaki, A.; Ouladsahebmadarek, E.; Delazar, A.; Bakhtyari, F.; Mazandarani, M. The Effect of Herbal Medicine Supplementation on Clinical and Para-clinical Outcomes in Women with PCOS: A Systematic Review and Meta-analysis. Int. J. Women’s Health Reprod. Sci. 2019, 7, 423–433. [Google Scholar] [CrossRef]
- Desai, B.N.; Maharjan, R.H.; Nampoothiri, L.P. Aloe barbadensis Mill. formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacogn. Res. 2012, 4, 109–115. [Google Scholar]
- Farideh, Z.Z.; Bagher, M.; Ashraf, A.; Akram, A.; Kazem, M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertil. 2010, 11, 169–174. [Google Scholar]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.; Uzunov, D.; Menichini, F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) coutinho seeds. Biol. Pharm. Bull. 2006, 29, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Križman, M.; Baričevič, D.; Prošek, M. Determination of phenolic compounds in fennel by HPLC and HPLC-MS using a monolithic reversed-phase column. J. Pharm. Biomed. Anal. 2007, 43, 481–485. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Sadrefozalayi, S.; Farokhi, F. Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna J. Phytomed. 2014, 4, 110. [Google Scholar]
- Pak, S.C.; Lim, S.C.; Nah, S.Y.; Lee, J.; Hill, J.A.; Bae, C.S. Role of Korean red ginseng total saponins in rat infertility induced by polycystic ovaries. Fertil. Steril. 2005, 84, 1139–1143. [Google Scholar] [CrossRef]
- Wuttke, W.; Seidlova, W.D.; Gorkow, C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: Effects on menopause symptoms and bone markers. Maturitas 2003, 44 (Suppl. 1), 67–77. [Google Scholar] [CrossRef]
- Frei, K.S.; Schaffner, W.; Rahlfs, V.W.; Bodmer, C.; Birkhäuser, M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: A double-blind placebo-controlled clinical trial. Maturitas 2005, 51, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.M.; Herrmann, K. Occurrence of hydroxybenzoic acid and hydroxycinnamic acid in spices. IV. Phenolics of spices. Z. Lebensm.-Unters.-Forsch. 1980, 171, 193–199. [Google Scholar]
- Moghazi, E.L.; Ali, A.M.; Ross, K.; Mottaleb, B.U.T. Flavonoids of Pimpinella anisum L. growing in Egypt. Fitoterapia 1979, 50, 267–268. [Google Scholar]
- Kumaravel, S.; Alagusundaram, K. Antimicrobial activity and phytochemical analysis of selected Indian spices. J. Pure Appl. Microbiol. 2014, 8, 4131–4136. [Google Scholar]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CABI: Oxfordshire, UK, 2008; pp. 405–407. [Google Scholar]
- Girardon, P.; Bessiere, J.M.; Baccou, J.C.; Sauvaire, Y. Volatile constituents of fenugreek seeds. Planta Med. 1985, 6, 533–534. [Google Scholar] [CrossRef]
- Ahmad, A.; Alghamdi, S.S.; Mahmood, K.; Afzal, M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016, 23, 300–310. [Google Scholar] [CrossRef]
- Khanna, A.; John, F.; Das, S.; Thomas, J.R.; Maliakel, B. Efficacy of a novel extract of fenugreek seeds in alleviating vasomotor symptoms and depression in perimenopausal women: A randomized, double-blinded, placebo-controlled study. J. Food Biochem. 2020, 44, 1350–1357. [Google Scholar] [CrossRef]
- Dayani, S.A.; Karunathilaka, L.P.; Kodituwakku, N.D.; Karunarathne, Y.A. Clinical efficacy of Ayurveda treatment regimen on Subfertility with Poly Cystic Ovarian Syndrome (PCOS). Ayu 2010, 31, 24–27. [Google Scholar] [CrossRef]
- Bhingardive, K.B.; Sarvade, D.D.; Bhatted, S. Clinical efficacy of Vamana Karma with Ikshwaaku Beeja Yoga followed by Shatapushpadi Ghanavati in the management of Artava Kshayawsr to polycystic ovarian syndrome. Ayu 2017, 38, 127–132. [Google Scholar] [CrossRef]
- Anand, S.; Amrita, J.; Sushil, K.G.; Manashi, B.; Pawan, K.; Harry, G.; Debasis, B. Efficacy of a Novel Fenugreek Seed Extract (Trigonella foenum-graecum, Furocyst TM) in Polycystic Ovary Syndrome (PCOS). Int. J. Med. Sci. 2015, 12, 825–831. [Google Scholar]
- Charles, D.J. Antioxidant properties of spices, herbs and other sources. Front. Nat. Prod. 2013, 1, 4310–4614. [Google Scholar]
- Cheng, X.L.; Liu, Q.; Peng, Y.B.; Qi, L.W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Atashpour, S.; Kargar, J.H.; Kargar, J.Z.; Maleknasab, M. Comparison of the effects of ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. Int. J. Reprod. Biomed. 2017, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Poojari, P.; Paramanya, A.; Singh, D.; Ali, A. Polycystic ovarian syndrome: Causes and therapies by herbal medicine. In Herbal Medicines: A Boon for Healthy Human Life; Academic Press: Cambridge, MA, USA, 2022; pp. 435–451. [Google Scholar]
- Saiyed, A.; Jahan, N.; Makbul, S.A.A.; Ansari, M.; Bano, H.; Habib, S.H. Effect of combination of Withania somnifera Dunal and Tribulus terrestris Linn on letrozole induced polycystic ovarian syndrome in rats. Integr. Med. Res. 2016, 5, 293–300. [Google Scholar] [CrossRef]
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–54. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Moini Jazani, A.; Nasimi Doost Azgomi, H.; Nasimi Doost Azgomi, A.; Nasimi Doost Azgomi, R. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). DARU J. Pharm. Sci. 2019, 27, 863–877. [Google Scholar] [CrossRef]
- Dhiman, K. Ayurvedic intervention in the management of uterine fibroids: A Case series. Ayu 2014, 35, 303. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshmi, J.N.; Babu, A.N.; Kiran, S.S.M.; Nori, L.P.; Hassan, N.; Ashames, A.; Bhandare, R.R.; Shaik, A.B. Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review. BioTech 2023, 12, 4. https://doi.org/10.3390/biotech12010004
Lakshmi JN, Babu AN, Kiran SSM, Nori LP, Hassan N, Ashames A, Bhandare RR, Shaik AB. Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review. BioTech. 2023; 12(1):4. https://doi.org/10.3390/biotech12010004
Chicago/Turabian StyleLakshmi, Jada Naga, Ankem Narendra Babu, S. S. Mani Kiran, Lakshmi Prasanthi Nori, Nageeb Hassan, Akram Ashames, Richie R. Bhandare, and Afzal B. Shaik. 2023. "Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review" BioTech 12, no. 1: 4. https://doi.org/10.3390/biotech12010004
APA StyleLakshmi, J. N., Babu, A. N., Kiran, S. S. M., Nori, L. P., Hassan, N., Ashames, A., Bhandare, R. R., & Shaik, A. B. (2023). Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review. BioTech, 12(1), 4. https://doi.org/10.3390/biotech12010004






