Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects
Abstract
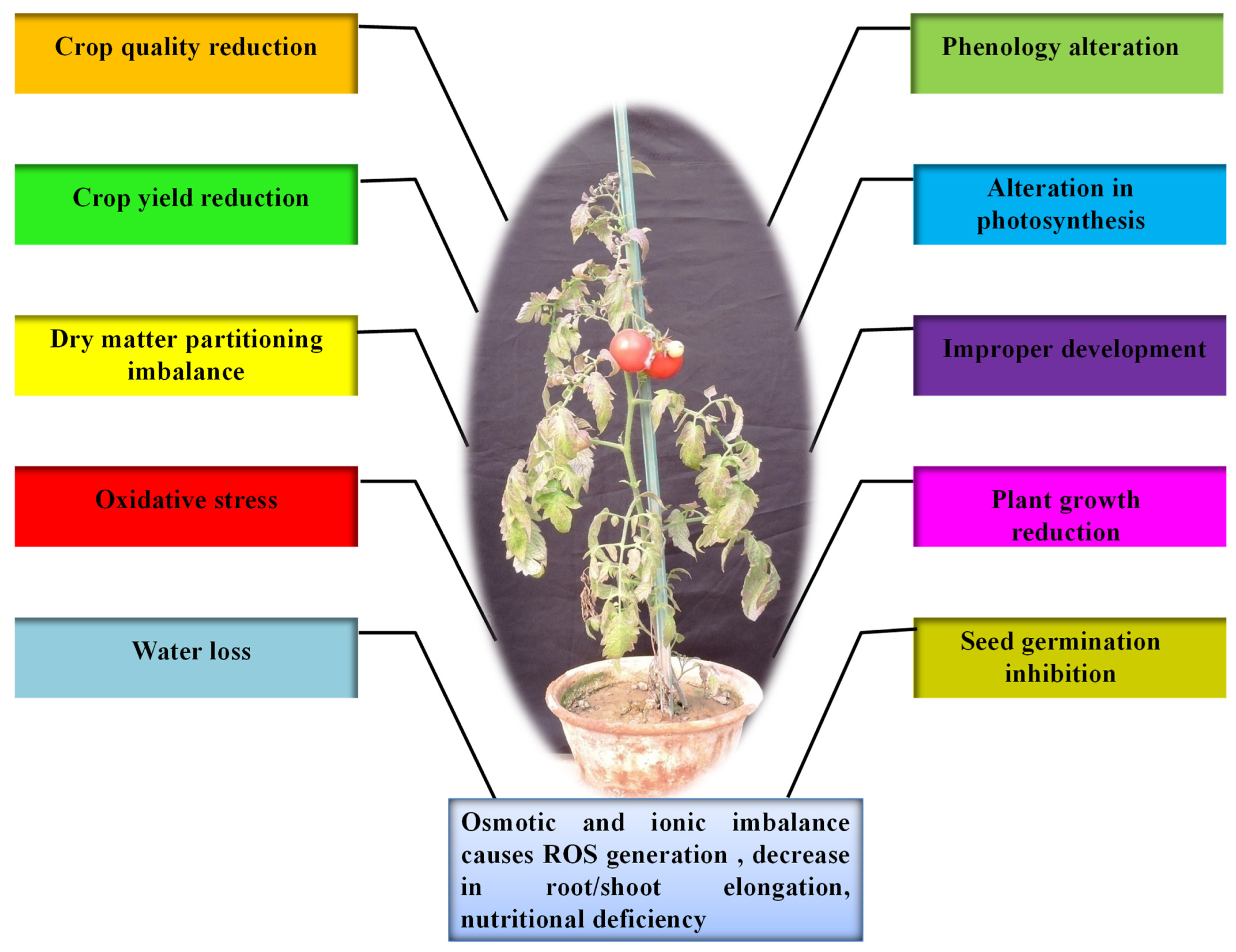
1. Introduction
2. Drought Stress
2.1. Drought-Responsive Transcription Factor Genes in Tomato
2.2. Genetic Engineering of Tomato for Improved Drought Stress Tolerance
2.2.1. DREB1A/CBF3 Gene in Stress Tolerance
2.2.2. ZFP (ZAT) Gene in Stress Tolerance
2.2.3. Multigenic Transgenic Approach for Abiotic Stress Management
2.2.4. Genome Editing in Tomato for Drought Stress Tolerance
3. Yield Potential of Transgenic Tomato under Drought Stress
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linnaeus, C. Species Planatarium, 1st ed.; Holmiae: Stockholm, Sweden, 1753. [Google Scholar]
- Bauchet, G.; Causse, M. Genetic Diversity in Tomato (Solanum lycopersicum) and Its Wild Relatives. Genet. Divers. Plants 2012, 133, 162. [Google Scholar]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tissue Organ Cult. 2015, 120, 881–902. [Google Scholar] [CrossRef]
- Causse, M.; Zhao, J.; Diouf, I.; Wang, J.; Lefebvre, V.; Caromel, B.; Génard, M.; Bertin, N. Genomic designing for climate-smart tomato. In Genomic Designing of Climate-Smart Vegetable Crops; Springer: Cham, Switzerland, 2020; pp. 47–159. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database; FAO: Rome, Italy, 2018. [Google Scholar]
- National Horticultura Board. 2018. Available online: https://nhb.gov.in/statistics/Publication/Horticulture%20Statistics%20at%20a%20Glance-2018.pdf (accessed on 13 October 2022).
- Foolad, M.R. Genome Mapping and Molecular Breeding of Tomato. Int. J. Plant Genom. 2007, 2007, 64358. [Google Scholar] [CrossRef]
- Rai, G.K. Biochemical and Molecular Analysis of AtDREB1A/CBF3 Transcription Factor in Transgenic Tomato under Drought Stress. Ph.D. Thesis, Banaras Hindu University, Varanasi, India, 2012. [Google Scholar]
- Rai, A.C.; Singh, M.; Shah, K. Engineering drought tolerant tomato plants over-expressing BcZAT12 gene encoding a C2H2 zinc finger transcription factor. Phytochemistry 2013, 85, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Devi, M.J.; Reddy, D.S.; Lavanya, M.; Vadez, V.; Serraj, R.; Yamaguchi-Shinozaki, K.; Sharma, K.K. Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Rep. 2007, 26, 2071–2082. [Google Scholar] [CrossRef]
- Atherton, J.; Rudich, J. (Eds.) The Tomato Crop: A Scientific Basis for Improvement; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sestito, R.; Palozza, P. Lycopene and Down-regulation of Cyclin D1, pAKT and pBad. In Lycopene: Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2019; p. 133. [Google Scholar]
- Krishna, R.; Karkute, S.G.; Ansari, W.A.; Jaiswal, D.K.; Verma, J.P.; Singh, M. Transgenic tomatoes for abiotic stress tolerance: Status and way ahead. 3 Biotech 2019, 9, 143. [Google Scholar] [CrossRef]
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Singh, A.K.; Verma, J.P.; Singh, M. Co-overexpression of AtDREB1A and BcZAT12 increases drought tolerance and fruit production in double transgenic tomato (Solanum lycopersicum) plants. Environ. Exp. Bot. 2021, 184, 104396. [Google Scholar] [CrossRef]
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. TOMATOMA: A Novel Tomato Mutant Database Distributing Micro-Tom Mutant Collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef]
- Van der Hoeven, R.; Ronning, C.; Giovannoni, J.; Martin, G.; Tanksley, S. Deductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing. Plant Cell 2002, 14, 1441–1456. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Andaluz, S.; Rodríguez-Celma, J.; Wohlgemuth, G.; Zocchi, G.; Álvarez-Fernández, A.; Fiehn, O.; López-Millán, A.F.; Abadía, J. Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol. 2010, 10, 120. [Google Scholar] [CrossRef]
- Singh, S.; Rathore, M.; Goyary, D.; Singh, R.; Anandhan, S.; Sharma, D.K.; Ahmed, Z. Induced ectopic expression of At-CBF1 in marker-free transgenic tomatoes confers enhanced chilling tolerance. Plant Cell Rep. 2011, 30, 1019–1028. [Google Scholar] [CrossRef]
- Rai, G.K.; Rai, N.P.; Kumar, S.; Yadav, A.; Rathaur, S.; Singh, M. Effects of explant age, germination medium, pre-culture parameters, inoculation medium, pH, washing medium, and selection regime on Agrobacterium-mediated transformation of tomato. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 565–578. [Google Scholar] [CrossRef]
- Rai, G.K.; Rai, N.P.; Rathaur, S.; Kumar, S.; Singh, M. Expression of rd29A: AtDREB1A/CBF3 in tomato alleviates drought-induced oxidative stress by regulating key enzymatic and non-enzymatic antioxidants. Plant Physiol. Biochem. 2013, 69, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Karkute, S.; Krishna, R.; Ansari, W.; Singh, B.; Singh, P.; Singh, M.; Singh, A. Heterologous expression of the AtDREB1A gene in tomato confers tolerance to chilling stress. Biol. Plant. 2019, 63, 268–277. [Google Scholar] [CrossRef]
- Bruening, G.; Lyons, J. The case of the FLAVR SAVR tomato. Calif. Agric. 2000, 54, 6–7. [Google Scholar] [CrossRef]
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Singh, A.K.; Prasad, R.; Verma, J.P.; Singh, M. Overexpression of AtDREB1 and BcZAT12 genes confers drought tolerance by reducing oxidative stress in double transgenic tomato (Solanum lycopersicum L.). Plant Cell Rep. 2021, 40, 2173–2190. [Google Scholar] [CrossRef] [PubMed]
- Behera, T.K.; Krishna, R.; Ansari, W.A.; Aamir, M.; Kumar, P.; Kashyap, S.P.; Pandey, S.; Kole, C. Approaches involved in the vegetable crops salt stress tolerance improvement: Present status and way ahead. Front. Plant Sci. 2021, 12, 787292. [Google Scholar] [CrossRef] [PubMed]
- Ansari, W.A.; Atri, N.; Ahmad, J.; Qureshi, M.I.; Singh, B.; Kumar, R.; Rai, V.; Pandey, S. Drought mediated physiological and molecular changes in muskmelon (Cucumis melo L.). PLoS ONE 2019, 14, e0222647. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Rai, A.C.; Singh, M.; Shah, K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol. Biochem. 2012, 61, 108–114. [Google Scholar]
- Shah, K.; Singh, M.; Rai, A.C. Effect of heat-shock induced oxidative stress is suppressed in BcZAT12 expressing drought tolerant tomato. Phytochemistry 2013, 95, 109–117. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Hussain, S.S.; Kayani, M.A.; Amjad, M. Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol. Prog. 2011, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Harrak, H.; Azelmat, S.; Baker, E.N.; Tabaeizadeh, Z. Isolation and characterization of a gene encoding a drought-induced cysteine protease in tomato (Lycopersicon esculentum). Genome 2001, 44, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Thorstenson, Y.R. Stable carbon isotope composition (δ13C), water use efficiency, and biomass productivity of Lycopersicon esculentum, Lycopersicon pennellii, and the F1 hybrid. Plant Physiol. 1988, 88, 213–217. [Google Scholar] [CrossRef]
- Kahn, T.L.; Fender, S.E.; Bray, E.A.; O’Connell, M.A. Characterization of expression of drought-and abscisic acid-regulated tomato genes in the drought-resistant species Lycopersicon pennellii. Plant Physiol. 1993, 103, 597–605. [Google Scholar] [CrossRef]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Li, C.-W.; Su, R.-C.; Cheng, C.-P.; Tsai, Y.-C.; Chan, M.-T. A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 2010, 231, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.; Cáceres, S.; Orellana, S.; Bastías, A.; Verdugo, I.; Ruiz-Lara, S.; Casaretto, J.A. An abiotic stress-responsive bZIP transcription factor from wild and cultivated tomatoes regulates stress-related genes. Plant Cell Rep. 2009, 28, 1497–1507. [Google Scholar] [CrossRef]
- Orellana, S.; Yanez, M.; Espinoza, A.; Verdugo, I.; Gonzalez, E.; Ruiz-Lara, S.; Casaretto, J.A. The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant Cell Environ. 2010, 33, 2191–2208. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, M.-H. Expression of dehydration responsive element-binding protein-3 (DREB3) under different abiotic stresses in tomato. BMB Rep. 2009, 42, 611–616. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Li, B.; Zhang, J.-T.; Li, Y.; Zhang, H. Generation of selectable marker-free transgenic tomato resistant to drought, cold and oxidative stress using the Cre/loxP DNA excision system. Transgenic Res. 2009, 18, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Creelman, R.A.; Zhu, J.-K. From Laboratory to Field. Using Information from Arabidopsis to Engineer Salt, Cold, and Drought Tolerance in Crops. Plant Physiol. 2004, 135, 615–621. [Google Scholar] [CrossRef]
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, M.-H. Expression profiling of the DREB2 type gene from tomato (Solanum lycopersicum L.) under various abiotic stresses. Hortic. Environ. Biotechnol. 2011, 52, 105–111. [Google Scholar] [CrossRef]
- Li, J.; Sima, W.; Ouyang, B.; Wang, T.; Ziaf, K.; Luo, Z.; Liu, L.; Li, H.; Chen, M.; Huang, Y.; et al. Tomato SlDREB gene restricts leaf expansion and internode elongation by downregulating key genes for gibberellin biosynthesis. J. Exp. Bot. 2012, 63, 6407–6420. [Google Scholar] [CrossRef] [PubMed]
- Labate, J.A.; Grandillo, S.; Fulton, T.; Munos, S. Tomato/Genome mapping and molecular breeding in plants. In Vegetables; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5. [Google Scholar]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Bansal, K.C. Overexpression of osmotin gene confers tolerance to salt and drought stresses in transgenic tomato (Solanum lycopersicum L.). Protoplasma 2010, 245, 133–141. [Google Scholar] [CrossRef]
- Roy, R.; Purty, R.S.; Agrawal, V.; Gupta, S.C. Transformation of tomato cultivar ‘Pusa Ruby’ with bspA gene from Populus tremula for drought tolerance. Plant Cell Tissue Organ Cult. 2006, 84, 56. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Quan, R.; Wang, X.-C.; Huang, R. Transcriptional Regulation of the Ethylene Response Factor LeERF2 in the Expression of Ethylene Biosynthesis Genes Controls Ethylene Production in Tomato and Tobacco. Plant Physiol. 2009, 150, 365–377. [Google Scholar] [CrossRef]
- Khare, N.; Goyary, D.; Singh, N.K.; Shah, P.; Rathore, M.; Anandhan, S.; Sharma, D.; Arif, M.; Ahmed, Z. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2010, 103, 267–277. [Google Scholar] [CrossRef]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Murata, N.; Bansal, K.C. Transformation of tomato with a bacterial codA gene enhances tolerance to salt and water stresses. J. Plant Physiol. 2011, 168, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtílek, M.; Cellini, F.; Nedbal, L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012, 182, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, Y.; Wang, C.; Liu, H.; Niu, D.; Wang, Y.; Guo, J. Enhancement of tomato (Lycopersicon esculentum) tolerance to drought stress by plant-growth-promoting rhizobacterium (PGPR) Bacillus cereus AR156. J. Agric. Biotechnol. 2012, 20, 1097–1105. [Google Scholar]
- Hsieh, T.H.; Lee, J.T.; Yang, P.T.; Chiu, L.H.; Charng, Y.Y.; Wang, Y.C.; Chan, M.T. Heterology expression of the Arabidopsis C-repeat/dehydration response element binding Factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2002, 129, 1086–1094. [Google Scholar] [CrossRef]
- Lee, J.-T.; Prasad, V.; Yang, P.-T.; Wu, J.-F.; Ho, T.-H.D.; Charng, Y.-Y.; Chan, M.-T. Expression of Arabidopsis CBF1 regulated by an ABA/stress inducible promoter in transgenic tomato confers stress tolerance without affecting yield. Plant Cell Environ. 2003, 26, 1181–1190. [Google Scholar] [CrossRef]
- Scippa, G.S.; Griffiths, A.; Chiatante, D.; Bray, E.A. The H1 histone variant of tomato, H1-S, is targeted to the nucleus and accumulates in chromatin in response to water-deficit stress. Planta 2000, 211, 173–181. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Lee, J.-T.; Charng, Y.-Y.; Chan, M.-T. Tomato Plants Ectopically Expressing Arabidopsis CBF1 Show Enhanced Resistance to Water Deficit Stress. Plant Physiol. 2002, 130, 618–626. [Google Scholar] [CrossRef]
- Park, S.; Gilmour, S.J.; Grumet, R.; Thomashow, M.F. CBF-dependent and CBF-independent regulatory pathways contribute to the differences in freezing tolerance and cold-regulated gene expression of two Arabidopsis ecotypes locally adapted to sites in Sweden and Italy. PLoS ONE 2018, 13, e0207723. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Ren, H.; Zhang, Z.; Zhang, H.; Wang, J.; Wang, X.C.; Huang, R. ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta 2007, 226, 815–825. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.-P.; Chen, C.-L.; Wang, Y.; Fu, X.-Z.; Liu, J.-H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.D.O.; Moshelion, M.; Weiss, D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Env. 2014, 37, 113–123. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A New Tomato NAC (NAM/ATAF1/2/CUC2) Transcription Factor, SlNAC4, Functions as a Positive Regulator of Fruit Ripening and Carotenoid Accumulation. Plant Cell Physiol. 2014, 55, 119–135. [Google Scholar] [CrossRef]
- Liao, X.; Guo, X.; Wang, Q.; Wang, Y.; Zhao, D.; Yao, L.; Wang, S.; Liu, G.; Li, T. Overexpression of Ms DREB 6.2 results in cytokinin-deficient developmental phenotypes and enhances drought tolerance in transgenic apple plants. Plant J. 2017, 89, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm drought inducible DREB1 induced expression of DRE/CRT- and non-DRE/CRT-containing genes in lowland transgenic tomato under cold and PEG treatments. Plant Physiol. Biochem. 2017, 112, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, L.; Zhu, J.; Wang, A.; Liu, H. Christolea crassifolia HARDY gene enhances drought stress tolerance in transgenic tomato plants. Plant Cell Tissue Organ Cult. 2017, 129, 469–481. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, R.; Li, R.; Yu, W.; Yang, M.; Sheng, J.; Shen, L. Enhanced drought tolerance in tomato plants by overexpression of SlMAPK1. Plant Cell Tissue Organ Cult. 2018, 133, 27–38. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Wang, X.; Zhang, M.; Xi, Y.; Wang, A.; Zhu, J. Overexpression of Saussurea involucrata dehydrin gene SiDHN promotes cold and drought tolerance in transgenic tomato plants. PLoS ONE 2019, 14, e0225090. [Google Scholar] [CrossRef]
- Lu, J.; Sun, M.-H.; Ma, Q.-J.; Kang, H.; Liu, Y.-J.; Hao, Y.-J.; You, C.-X. MdSWEET17, a sugar transporter in apple, enhances drought tolerance in tomato. J. Integr. Agric. 2019, 18, 2041–2051. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Z.; Gong, B.; Shi, Q. S-adenosylmethionine synthetase 1 confers drought and salt tolerance in transgenic tomato. Environ. Exp. Bot. 2020, 179, 104226. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, T.; Pei, T.; Wang, Z.; Yang, H.; Jiang, J.; Zhang, H.; Chen, X.; Li, J.; Xu, X. Overexpression of SlGATA17 promotes drought tolerance in transgenic tomato plants by enhancing activation of the phenylpropanoid biosynthetic pathway. Front. Plant Sci. 2021, 12, 634888. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, W.; Guo, X.; Yang, L.; Hu, W.; Kuang, L.; Huang, Y.; Xie, J.; Liu, Y. Ectopic Overexpression of CsECR from Navel Orange Increases Cuticular Wax Accumulation in Tomato and Enhances Its Tolerance to Drought Stress. Front. Plant Sci. 2022, 13, 924552. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E.; Repetti, P.P.; Adams, T.R.; Creelman, R.A.; Wu, J.; Warner, D.C.; Anstrom, D.C.; Bensen, R.J.; Castiglioni, P.P.; Donnarummo, M.G.; et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA 2007, 104, 16450–16455. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, M.; Yao, J.; Xiao, B.; Li, X.; Zhang, Q.; Xiong, L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef] [PubMed]
- Gosal, S.S.; Wani, S.H.; Kang, M.S. Biotechnology and Drought Tolerance. J. Crop Improv. 2009, 23, 19–54. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef]
- Haake, V.; Cook, D.; Riechmann, J.; Pineda, O.; Thomashow, M.F.; Zhang, J.Z. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol. 2002, 130, 639–648. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.-H.; Hong, X.; Agarwal, M.; Zhu, J.-K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, X.; Li, M.; Jiang, Y.; Xu, J.; Jin, J.; Li, K. Ectopic expression of Limonium bicolor (Bag.) Kuntze DREB (LbDREB) results in enhanced salt stress tolerance of transgenic Populus ussuriensis Kom. Plant Cell Tissue Organ Cult. 2018, 132, 123–136. [Google Scholar] [CrossRef]
- Sarkar, T.; Thankappan, R.; Mishra, G.P.; Nawade, B.D. Advances in the development and use of DREB for improved abiotic stress tolerance in transgenic crop plants. Physiol. Mol. Biol. Plants 2019, 25, 1323–1334. [Google Scholar] [CrossRef]
- Lehti-Shiu, M.D.; Panchy, N.; Wang, P.; Uygun, S.; Shiu, S.-H. Diversity, expansion, and evolutionary novelty of plant DNA-binding transcription factor families. Biochim. Biophys. Acta 2017, 1860, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.; Mathieu, J.; Dinh, T.T.; Ott, F.; Lanz, C.; Wollmann, H.; Schmid, M. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 2010, 22, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Catalá, R.; Salinas, J. The CBFs: Three arabidopsis transcription factors to cold acclimate. Plant Sci. 2011, 180, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, X.D.; Chi, X.J.; Wu, C.A.; Li, Y.Z.; Song, L.L.; Liu, X.M.; Wang, Y.F.; Wang, F.W.; Zhang, C.; et al. Dwarf apple MbDREB1 enhances plant tolerance to low temperature, drought, and salt stress via both ABA-dependent and ABA-independent pathways. Planta 2011, 233, 219–229. [Google Scholar] [CrossRef]
- Jaglo, K.R.; Kleff, S.; Amundsen, K.L.; Zhang, X.; Haake, V.; Zhang, J.Z.; Deits, T.; Thomashow, M.F. Components of the Arabidopsis C-Repeat/Dehydration-Responsive Element Binding Factor Cold-Response Pathway Are Conserved in Brassica napus and Other Plant Species. Plant Physiol. 2001, 127, 910–917. [Google Scholar] [CrossRef]
- Owens, C.L.; Thomashow, M.F.; Hancock, J.F.; Iezzoni, A.F. CBF1 Orthologs in Sour Cherry and Strawberry and the Heterologous Expression of CBF1 in Strawberry. J. Am. Soc. Hortic. Sci. 2002, 127, 489–494. [Google Scholar] [CrossRef]
- Francia, E.; Rizza, F.; Cattivelli, L.; Stanca, A.M.; Galiba, G.; Toth, B.; Pecchioni, N. Two loci on chromosome 5H determine low-temperature tolerance in a ‘Nure’(winter)בTremois’(spring) barley map. Theor. Appl. Genet. 2004, 108, 670–680. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L.; encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.G.; Zhang, W.K.; He, S.J.; Zhang, J.S.; Liu, Q.; Chen, S.Y. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003, 106, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-G.; Zhang, W.-K.; Yan, D.-Q.; Du, B.-X.; Zhang, J.-S.; Liu, Q.; Chen, S.-Y. Characterization of a DRE-binding transcription factor from a halophyte Atriplex hortensis. Theor. Appl. Genet. 2003, 107, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sakuma, Y.; Li, J.; Liu, Q.; Li, Y.Q.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 2004, 45, 1042–1052. [Google Scholar] [CrossRef]
- Kitashiba, H.; Ishizaka, T.; Isuzugawa, K.; Nishimura, K.; Suzuki, T. Expression of a sweet cherry DREB1/CBF ortholog in Arabidopsis confers salt and freezing tolerance. J. Plant Physiol. 2004, 161, 1171–1176. [Google Scholar] [CrossRef]
- Xiong, Y.; Fei, S.-Z. Functional and phylogenetic analysis of a DREB/CBF-like gene in perennial ryegrass (Lolium perenne L.). Planta 2006, 224, 878–888. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Zhu, K.; Liu, L.; Chen, F.; Yu, D. Identification and characterization of two chrysanthemum (Dendronthema× moriforlium) DREB genes, belonging to the AP2/EREBP family. Mol. Biol. Rep. 2009, 36, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Gilpin, M.; Fuller, M.P. Identification and expression analysis of CBF/DREB1 and COR15 genes in mutants of Brassica oleracea var. botrytis with enhanced proline production and frost resistance. Plant Physiol. Biochem. 2011, 49, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Hong, B.; Tong, Z.; Ma, N.; Li, J.; Kasuga, M.; Yamaguchi-Shinozaki, K.; Gao, J. Heterologous expression of the AtDREB1A gene in chrysanthemum increases drought and salt stress tolerance. Sci. China Ser. C Life Sci. 2006, 49, 436–445. [Google Scholar] [CrossRef]
- Oraby, H.; Ahmad, R. Physiological and biochemical changes of CBF3 transgenic oat in response to salinity stress. Plant Sci. 2012, 185, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kidokoro, S.; Yoshida, T.; Mizoi, J.; Todaka, D.; Fernie, A.R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants. Plant Biotechnol. J. 2017, 15, 458–471. [Google Scholar] [CrossRef]
- Meissner, R.; Michael, A.J. Isolation and characterisation of a diverse family of Arabidopsis two and three-fingered C2H2 zinc finger protein genes and cDNAs. Plant Mol. Biol. 1997, 33, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Bellefroid, E.; Lecocq, P.; Benhida, A.; Poncelet, D.; Belayew, A.; Martial, J. The Human Genome Contains Hundreds of Genes Coding for Finger Proteins of the Krüppel Type. DNA 1989, 8, 377–387. [Google Scholar] [CrossRef]
- Tadepally, H.D.; Burger, G.; Aubry, M. Evolution of C2H2-zinc finger genes and subfamilies in mammals: Species-specific duplication and loss of clusters, genes and effector domains. BMC Evol. Biol. 2008, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, J.; Gong, D.; Kong, Y. The Roles of Arabidopsis C1-2i Subclass of C2H2-type Zinc-Finger Transcription Factors. Genes 2019, 10, 653. [Google Scholar] [CrossRef]
- Takatsuji, H.; Nakamura, N.; Katsumoto, Y. A new family of zinc finger proteins in petunia: Structure, DNA sequence recognition, and floral organ-specific expression. Plant Cell 1994, 6, 947–958. [Google Scholar]
- Yanagisawa, S. A novel DNA-binding domain that may form a single zinc finger motif. Nucleic Acids Res. 1995, 23, 3403–3410. [Google Scholar] [CrossRef]
- Schauser, L.; Christensen, L.; Borg, S.; Poulsen, C. PZF, a cDNA Isolated from Lotus japonicus and Soybean Root Nodule Libraries, Encodes a New Plant Member of the RING-Finger Family of Zinc-Binding Proteins. Plant Physiol. 1995, 107, 1457–1458. [Google Scholar] [CrossRef]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The Zinc-Finger Protein Zat12 Plays a Central Role in Reactive Oxygen and Abiotic Stress Signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Guo, S.Q.; Yang, X.; Bao, Y.M.; Tang, H.J.; Sun, H.; Huang, J.; Zhang, H.S. Functional analysis of a novel Cys2/His2-type zinc finger protein involved in salt tolerance in rice. J. Exp. Bot. 2010, 61, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Z.; Xu, X.; Zhang, H.; Li, C. Genome-Wide Analysis of C2H2 Zinc-Finger Family Transcription Factors and Their Responses to Abiotic Stresses in Poplar (Populus trichocarpa). PLoS ONE 2015, 10, e0134753. [Google Scholar] [CrossRef]
- Wang, K.; Ding, Y.; Cai, C.; Chen, Z.; Zhu, C. The role of C2H2 zinc finger proteins in plant responses to abiotic stresses. Physiol. Plant. 2019, 165, 690–700. [Google Scholar] [CrossRef]
- Rizhsky, L.; Davletova, S.; Liang, H.; Mittler, R. The Zinc Finger Protein Zat12 Is Required for Cytosolic Ascorbate Peroxidase 1 Expression during Oxidative Stress in Arabidopsis. J. Biol. Chem. 2004, 279, 11736–11743. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Schnell, J.; Miki, B. Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3. BMC Genom. 2010, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Zao, J.K.; Hu, J.S.; Chiou, J.C.; Huang, Y.L.; Li, S.C.; Kuo, Z.Y.; Chuang, M.C.; Hsu, S.H.; Tseng, Y.C.; Liu, J.W.; et al. October. Kannon: Ubiquitous Sensor/Actuator Technologies for Elderly Living and Care: A Multidisciplinary Effort in National Chiao Tung University, Taiwan. In Proceedings of the 2006 IEEE International Conference on Systems, Man and Cybernetics, Taipei, Taiwan, 8–11 October 2006; Volume 5, pp. 4297–4303. [Google Scholar]
- Bhaskaran, S.; Savithramma, D.L. Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J. Exp. Bot. 2011, 62, 5561–5570. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.F.; Inostroza-Blancheteau, C.; Timmermann, T.; González, M.; Arce-Johnson, P. Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol. Biol. Rep. 2013, 40, 3281–3290. [Google Scholar] [CrossRef]
- Bao, A.K.; Wang, Y.W.; Xi, J.J.; Liu, C.; Zhang, J.L.; Wang, S.M. Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation. Funct. Plant Biol. 2014, 41, 203–214. [Google Scholar] [CrossRef]
- Shen, G.; Wei, J.; Qiu, X.; Hu, R.; Kuppu, S.; Auld, D.; Blumwald, E.; Gaxiola, R.; Payton, P.; Zhang, H. Co-overexpression of AVP1 and AtNHX1 in Cotton Further Improves Drought and Salt Tolerance in Transgenic Cotton Plants. Plant Mol. Biol. Rep. 2015, 33, 167–177. [Google Scholar] [CrossRef]
- Wang, D.; Luo, W.; Khurshid, M.; Gao, L.; Sun, Z.; Zhou, M.; Wu, Y. Co-expression of PeDREB2a and KcERF Improves Drought and Salt Tolerance in Transgenic Lotus corniculatus. J. Plant Growth Regul. 2018, 37, 550–559. [Google Scholar] [CrossRef]
- Kim, R.J.; Kim, H.U.; Suh, M.C. Development of camelina enhanced with drought stress resistance and seed oil production by co-overexpression of MYB96A and DGAT1C. Ind. Crop. Prod. 2019, 138, 1114755. [Google Scholar] [CrossRef]
- Zhao, F.-Y.; Zhang, X.-J.; Li, P.-H.; Zhao, Y.-X.; Zhang, H. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol. Breed. 2006, 17, 341–353. [Google Scholar] [CrossRef]
- Chang, Y.; Nguyen, B.H.; Xie, Y.; Xiao, B.; Tang, N.; Zhu, W.; Mou, T.; Xiong, L. Co-overexpression of the Constitutively Active Form of OsbZIP46 and ABA-Activated Protein Kinase SAPK6 Improves Drought and Temperature Stress Resistance in Rice. Front. Plant Sci. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Vu, H.T.; Nguyen, T.T.; Nguyen, L.-A.T.; Nguyen, M.-C.D.; Hoang, K.L.; Nguyen, K.T.; Quach, T.N. Co-expression of Arabidopsis AtAVP1 and AtNHX1 to Improve Salt Tolerance in Soybean. Crop Sci. 2019, 59, 1133–1143. [Google Scholar] [CrossRef]
- Gouiaa, S.; Khoudi, H.; Leidi, E.O.; Pardo, J.M.; Masmoudi, K. Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Mol. Biol. 2012, 79, 137–155. [Google Scholar] [CrossRef]
- Augustine, S.M.; Narayan, J.A.; Syamaladevi, D.P.; Appunu, C.; Chakravarthi, M.; Ravichandran, V.; Tuteja, N.; Subramonian, N. Overexpression of EaDREB2 and pyramiding of EaDREB2 with the pea DNA helicase gene (PDH45) enhance drought and salinity tolerance in sugarcane (Saccharum spp. hybrid). Plant Cell Rep. 2015, 34, 247–263. [Google Scholar] [CrossRef]
- Baghour, M.; Gálvez, F.J.; Sánchez, M.E.; Aranda, M.N.; Venema, K.; Rodríguez-Rosales, M.P. Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant Physiol. Biochem. 2019, 135, 77–86. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Fuhrmann-Aoyagi, M.B.; Yuan, S.; Iwama, T.; Kobayashi, M.; Miura, K. CRISPR/Cas9 Technique for Temperature, Drought, and Salinity Stress Responses. Curr. Issues Mol. Biol. 2022, 44, 182. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Xu, J.; Li, Y.; Guo, L.; Wang, Z.; Zhang, X.; Zhao, B.; Guo, Y.-D.; Zhang, N. CRISPR/Cas9 targeted mutagenesis of SlLBD40, a lateral organ boundaries domain transcription factor, enhances drought tolerance in tomato. Plant Sci. 2020, 301, 110683. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Zhao, R.; Wang, L.; Chen, L.; Yu, W.; Zhang, S.; Sheng, J.; Shen, L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 2019, 19, 38. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Liu, X.; Wu, C.; Yu, C.; Hu, G.; Chen, L.; Chen, R.; Bouzayen, M.; Zouine, M.; et al. Knockout of Auxin Response Factor SlARF4 Improves Tomato Resistance to Water Deficit. Int. J. Mol. Sci. 2021, 22, 3347. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Ratcliffe, O.J.; Riechmann, J.L. Arabidopsis transcription factors and the regulation of flowering time: A genomic perspective. Curr. Issues Mol. Biol. 2002, 4, 77–92. [Google Scholar] [PubMed]
- Wang, L.; Qing-Tian, L.; Qiong, L.; Chao, F.; Xiaodong, Z.; Fangfang, Z.; Lingzi, L.; Xuan, L.; Zhi, W.; Jin, K. Ectopically expressing MdPIP1; 3, an aquaporin gene, increased fruit size and enhanced drought tolerance of transgenic tomatoes. BMC Plant Biol. 2017, 17, 246. [Google Scholar] [CrossRef]
- Kelly, G.; Lugassi, N.; Belausov, E.; Wolf, D.; Khamaisi, B.; Brandsma, D.; Kottapalli, J.; Fidel, L.; Ben-Zvi, B.; Egbaria, A.; et al. The Solanum tuberosum KST1 partial promoter as a tool for guard cell expression in multiple plant species. J. Exp. Bot. 2017, 68, 2885–2897. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Gene | Function | Mechanism of Action | Reference |
|---|---|---|---|---|
| 1 | CBF1 | Stress-inducible transcription factors | Expression of stress-responsive gene | [55] |
| 2 | H1-S, drought induced linker histone | DNA packaging and organisation of chromosomes in the nucleus | Modulation of mechanisms related to the stomatal function | [56] |
| 3 | AtCBF-1 | Stress-inducible transcription factors | Expression of stress-responsive gene | [57] |
| 4 | H+-pyrophosphatase | Facilitate auxin fluxes | Enhance pyrophosphate-driven cation transport into root vacuolar fractions | [58] |
| 5 | bspA | Protein protection | Enhance desiccation tolerance by protecting proteins in membranes and cytosol | [48] |
| 6 | coda | Accumulation of glycine betaine | Osmolyte accumulation to protect against oxidative damage. | [59] |
| 7 | LeNCED1 | Increase in abscisic acid (ABA) accumulation | Stomatal closure and increased water-use efficiency (WUE) | [59] |
| 8 | Osmotin | Stress-responsive multifunctional protein | Osmotin provides protection via different mechanisms related with programmed cell death | [47] |
| 9 | PtADC | Induce the stress-responsive gene | Improves dehydration and drought tolerance | [60] |
| 10 | DREBs/ CBFs; ABF3 | Stress-induced transcription factors | Enhanced expression of downstream stress-related genes confers drought tolerance. | [19] |
| 11 | ZAT12 | Stress-induced transcription factors | Enhanced expression of downstream stress-related genes confers drought | [27] |
| 12 | AtGAMT1 | Suppress gibberellin | GAMT1 overexpression inhibited the expansion of leaf epidermal cells. | [61] |
| 13 | SlNAC4 | Stress-responsive transcription factor | Modulation of ABA-independent signaling networks | [62] |
| 14 | GalUR | GalUR encodes Lgalactono- 1,4-lactone as a precursor of ascorbic acid | Ascorbic acid detoxifies superoxide anion radical and hydroxyl radical and also plays a crucial role in scavenging ROS | [63] |
| 15 | EgDREB1 | Enhances the expression of DRE/CRT and DRE/CRT-containing genes | Elevated level of antioxidative enzymes scavenge the superoxide radical and accumulation of nonenzymatic antioxidants maintains the cell basic structure under drought and cold stress | [64] |
| 16 | CcHRD | AP2/ERF-like tanscritpion factor | Regulate many pathways involved in stress tolerance | [65] |
| 17 | SlMAPK1 | Encodes for mitogen-activated protein kinases | SlMAPK1 improves drought stress tolerance by activating antioxidant enzymes, reducing oxidative damage, and modulating transcription of stress-related genes. | [66] |
| 18 | SiDHN | Encodes for Dehydrins (DHNs) commonly hydrophilin LEA proteins | Increased chlorophyll a and b, carotenoid and relative water, proline and soluble sugar content and improve photosynthetic efficiency and suppress the formation of malondialdehyde H2O2 and O2 | [67] |
| 19 | MdSWEET17 | Sugar transporters | Enhances accumulation of sugars, such as glucose and fructose, which act as osmoprotestants and carbon source under drought stress | [68] |
| 20 | SlSAMS1 | S-adenosylmethionine synthetase (SAMS) | SlSAMS1 modulates the production of polyamines and H2O2 and maintains the cellular homeostatasis | [69] |
| 21 | AtDREB1A and BcZAT12 | Encodes for transcription factors | Independent expression of AtDREB1A and BcZAT12 gene enhances drought tolerance in tomato | [14,23] |
| 22 | SlGATA17 | Improves phenylpropanoid biosynthesis pathway activity | DNA-binding domain of GATA TFs regulates many pathways in plants and enhances drought stress tolerance | [70] |
| 23 | CsECR | Encodes for enoyl-CoA reductase (ECR) enzyme, which is involved in biosynthesis of cuticular waxes and catalyses the last step of very long-chain fatty acids (VLCFAs) elongation | Ectopic overexpression of CsECR increased the contents of total waxes and aliphatic wax leaves and fruits of the transgenic tomato and improves drought tolerance | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishna, R.; Ansari, W.A.; Soumia, P.S.; Yadav, A.; Jaiswal, D.K.; Kumar, S.; Singh, A.K.; Singh, M.; Verma, J.P. Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects. BioTech 2022, 11, 48. https://doi.org/10.3390/biotech11040048
Krishna R, Ansari WA, Soumia PS, Yadav A, Jaiswal DK, Kumar S, Singh AK, Singh M, Verma JP. Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects. BioTech. 2022; 11(4):48. https://doi.org/10.3390/biotech11040048
Chicago/Turabian StyleKrishna, Ram, Waquar Akhter Ansari, P. S. Soumia, Akhilesh Yadav, Durgesh Kumar Jaiswal, Sudhir Kumar, Achuit Kumar Singh, Major Singh, and Jay Prakash Verma. 2022. "Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects" BioTech 11, no. 4: 48. https://doi.org/10.3390/biotech11040048
APA StyleKrishna, R., Ansari, W. A., Soumia, P. S., Yadav, A., Jaiswal, D. K., Kumar, S., Singh, A. K., Singh, M., & Verma, J. P. (2022). Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects. BioTech, 11(4), 48. https://doi.org/10.3390/biotech11040048









