A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application
Abstract
1. Introduction
2. The Selection and Modification of Strains
2.1. The Form of Cell Cultures
2.2. Strain Modification Based on Genetic Engineering
3. The Selection of Substrates for MFC Systems
3.1. Defined Substrates
3.2. Wastewater
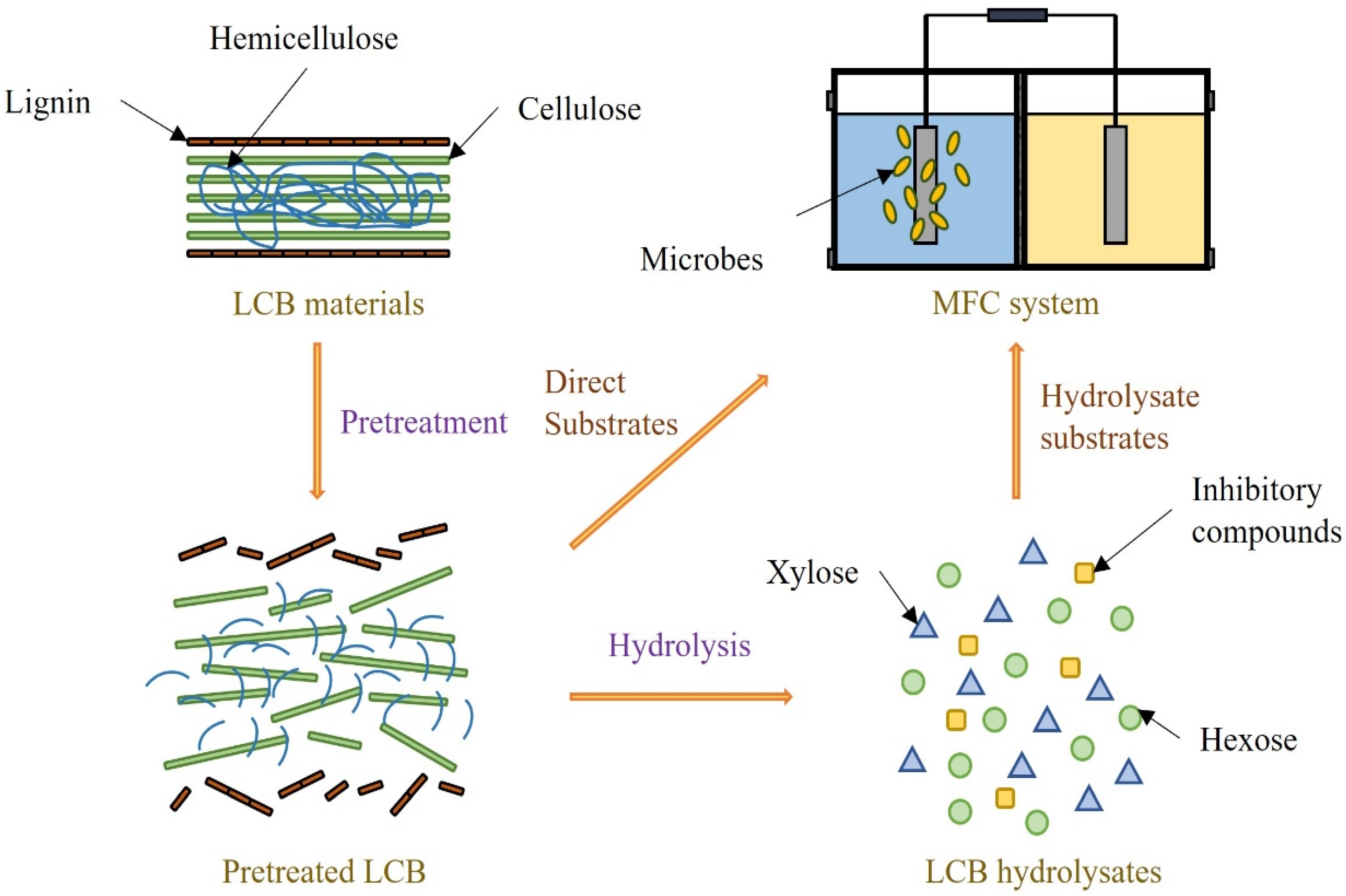
3.3. LCB Substrates
4. The Electrode Modification for MFC Systems
4.1. Anode Modification
4.2. Cathode Catalyst
5. The Operation Environment of MFC Systems
5.1. Electron Transfer Mediators
5.2. Operation Conditions of MFC Systems
6. Recent Progress in the Application of MFC Technology
6.1. Wastewater Treatment
6.2. The Production of Value-Added Products
6.3. The Application of MFC-Based Biosensors
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOD | biochemical oxygen demand |
| CF | carbon felt |
| CNT | carbon nanotubes |
| COD | chemical oxygen demand |
| CW | constructed wetland |
| EET | extracellular electron transfer |
| GO | graphene oxide |
| LCB | lignocellulosic biomass |
| LDH | layered double hydroxide |
| MB | methylene blue |
| MFC | microbial fuel cell |
| NR | neutral red |
| PANI | polyaniline |
| PEM | proton exchange membrane |
| PHB | polyhydroxybutyrate |
| POME | palm oil mill effluent |
| SSCF | simultaneous saccharification and co-fermentation |
| SSF | simultaneous saccharification and fermentation |
| STWW | septic tank wastewater |
| VFAs | volatile fatty acids |
References
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P. Microbial Fuel Cells, A Current Review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Obileke, K.C.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J. Microbial fuel cells as pollutant treatment units: Research updates. Bioresour. Technol. 2016, 217, 121–128. [Google Scholar] [CrossRef]
- Vinayak, V.; Khan, M.J.; Varjani, S.; Saratale, G.D.; Saratale, R.G.; Bhatia, S.K. Microbial fuel cells for remediation of environmental pollutants and value addition: Special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J. Biotechnol. 2021, 338, 5–19. [Google Scholar] [CrossRef]
- Kaur, R.; Marwaha, A.; Chhabra, V.A.; Kim, K.-H.; Tripathi, S.K. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 2020, 119, 109551. [Google Scholar] [CrossRef]
- Gul, H.; Raza, W.; Lee, J.; Azam, M.; Ashraf, M.; Kim, K.-H. Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere 2021, 281, 130828. [Google Scholar] [CrossRef]
- Sivamani, S.; Binnal, P.; Cuento, A.; Al-Shahri, A.; Al-Mahri, M.; Rafeet, M.; Shamas, M.; Al-Awaid, A. A comprehensive review of experimental studies on aerobic digestion of wastewater sludge. In Removal of Toxic Pollutants Through Microbiological and Tertiary Treatment; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Sustainable energy from waste organic matters via efficient microbial processes. Sci. Total Environ. 2020, 722, 137927. [Google Scholar] [CrossRef]
- Peera, S.G.; Maiyalagan, T.; Liu, C.; Ashmath, S.; Lee, T.G.; Jiang, Z.; Mao, S. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int. J. Hydrogen Energy 2021, 46, 3056–3089. [Google Scholar] [CrossRef]
- Islam, M.A.; Karim, A.; Mishra, P.; Dubowski, J.J.; Yousuf, A.; Sarmin, S.; Khan, M.M.R. Microbial synergistic interactions enhanced power generation in co-culture driven microbial fuel cell. Sci. Total Environ. 2020, 738, 140138. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Periyasamy, S.; Zhen, G.; Koók, L.; Bakonyi, P.; Nemestóthy, N.; Kumar, G. Bioelectrochemical systems using microalgae—A concise research update. Chemosphere 2017, 177, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.-H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. New Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Mohanakrishna, G.; Kumar, A.; Lai, C.; Lee, J.-K.; Kalia, V.C. Exploitation of Citrus Peel Extract as a Feedstock for Power Generation in Microbial Fuel Cell (MFC). Indian J. Microbiol. 2019, 59, 476–481. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Song, H.S.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; et al. Engineering of Shewanella marisflavi BBL25 for biomass-based polyhydroxybutyrate production and evaluation of its performance in electricity production. Int. J. Biol. Macromol. 2021, 183, 1669–1675. [Google Scholar] [CrossRef]
- Sani, A.M.; Savla, N.; Pandit, S.; Singh Mathuriya, A.; Gupta, P.K.; Khanna, N.; Pramod Babu, R.; Kumar, S. Recent advances in bioelectricity generation through the simultaneous valorization of lignocellulosic biomass and wastewater treatment in microbial fuel cell. Sustain. Energy Technol. Assess. 2021, 48, 101572. [Google Scholar] [CrossRef]
- Liang, J.; Nabi, M.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y. Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110335. [Google Scholar] [CrossRef]
- Prathiba, S.; Kumar, P.S.; Vo, D.-V.N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 2022, 286, 131856. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Recent trends in upgrading the performance of yeast as electrode biocatalyst in microbial fuel cells. Chemosphere 2021, 284, 131383. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Khilari, S.; Roy, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Reduction of start-up time through bioaugmentation process in microbial fuel cells using an isolate from dark fermentative spent media fed anode. Water Sci. Technol. 2015, 72, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Panda, P.; Bag, B. The Performance Improvement of Microbial Fuel Cells Using Different Waste-Sludge as an Inoculum. Energy Sources Part A: Recover. Util. Environ. Eff. 2013, 35, 1828–1835. [Google Scholar] [CrossRef]
- Schmitz, S.; Rosenbaum, M.A. Boosting mediated electron transfer in bioelectrochemical systems with tailored defined microbial cocultures. Biotechnol. Bioeng. 2018, 115, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Patil, S.A. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol. Adv. 2020, 39, 107468. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fukushima, T.; Prior, A.; Baruch, M.; Zajdel, T.J.; Ajo-Franklin, C.M. Modifying Cytochrome c Maturation Can Increase the Bioelectronic Performance of Engineered Escherichia coli. ACS Synth. Biol. 2020, 9, 115–124. [Google Scholar] [CrossRef]
- Su, L.; Fukushima, T.; Ajo-Franklin, C.M. A hybrid cyt c maturation system enhances the bioelectrical performance of engineered Escherichia coli by improving the rate-limiting step. Biosens. Bioelectron. 2020, 165, 112312. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Xu, A.; Zhang, L.; Liu, H.; Ma, L.Z. Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 1535–1544. [Google Scholar] [CrossRef]
- Lin, T.; Ding, W.; Sun, L.; Wang, L.; Liu, C.-G.; Song, H. Engineered Shewanella oneidensis-reduced graphene oxide biohybrid with enhanced biosynthesis and transport of flavins enabled a highest bioelectricity output in microbial fuel cells. Nano Energy 2018, 50, 639–648. [Google Scholar] [CrossRef]
- Kasai, T.; Tomioka, Y.; Kouzuma, A.; Watanabe, K. Overexpression of the adenylate cyclase gene cyaC facilitates current generation by Shewanella oneidensis in bioelectrochemical systems. Bioelectrochemistry 2019, 129, 100–105. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Xiong, J.R.; Min, D.; Cheng, L.; Liu, D.F.; Li, W.W.; Jin, F.; Yang, M.; Yu, H.Q. Promoting bidirectional extracellular electron transfer of Shewanella oneidensis MR-1 for hexavalent chromium reduction via elevating intracellular cAMP level. Biotechnol. Bioeng. 2020, 117, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Cheng, L.; Zhang, F.; Huang, X.-N.; Li, D.-B.; Liu, D.-F.; Lau, T.-C.; Mu, Y.; Yu, H.-Q. Enhancing Extracellular Electron Transfer of Shewanella oneidensis MR-1 through Coupling Improved Flavin Synthesis and Metal-Reducing Conduit for Pollutant Degradation. Environ. Sci. Technol. 2017, 51, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Y.X.; Cao, Y.X.; Wang, L.; Liu, C.G.; Shi, L.; Song, H. Modular engineering to increase intracellular NAD(H/+) promotes rate of extracellular electron transfer of Shewanella oneidensis. Nat. Commun. 2018, 9, 3637. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, M.J.; Poosarla, V.G.; Kim, K.-Y.; Jasso-Chávez, R.; Logan, B.E.; Wood, T.K. Electricity from methane by reversing methanogenesis. Nat. Commun. 2017, 8, 15419. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Sun, L.; Li, X.; Yin, C.; An, X.; Chen, X.; Tian, Y.; Song, H. Engineering Shewanella oneidensis enables xylose-fed microbial fuel cell. Biotechnol. Biofuels 2017, 10, 196. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Optimization of glucose concentration and glucose/yeast ratio in yeast microbial fuel cell using response surface methodology approach. J. Power Sources 2018, 402, 402–412. [Google Scholar] [CrossRef]
- Haavisto, J.; Dessì, P.; Chatterjee, P.; Honkanen, M.; Noori, M.T.; Kokko, M.; Lakaniemi, A.-M.; Lens, P.N.L.; Puhakka, J.A. Effects of anode materials on electricity production from xylose and treatability of TMP wastewater in an up-flow microbial fuel cell. Chem. Eng. J. 2019, 372, 141–150. [Google Scholar] [CrossRef]
- Li, F.; An, X.; Wu, D.; Xu, J.; Chen, Y.; Li, W.; Cao, Y.; Guo, X.; Lin, X.; Li, C.; et al. Engineering Microbial Consortia for High-Performance Cellulosic Hydrolyzates-Fed Microbial Fuel Cells. Front. Microbiol. 2019, 10, 409. [Google Scholar] [CrossRef]
- Ullah, Z.; Zeshan, S. Effect of substrate type and concentration on the performance of a double chamber microbial fuel cell. Water Sci. Technol. 2020, 81, 1336–1344. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Wu, Y.C.; Li, B.Q.; Zhu, H.D.; Li, Y.P.; Zhuang, M.Z.; Fu, H.Y. Study on the Electricity Generation Characteristics of Microbial Fuel Cell with Different Substrates. IOP Conf. Series: Earth Environ. Sci. 2020, 435, 012036. [Google Scholar] [CrossRef]
- Mateo, S.; Cañizares, P.; Rodrigo, M.A.; Fernandez-Morales, F.J. Driving force behind electrochemical performance of microbial fuel cells fed with different substrates. Chemosphere 2018, 207, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Du, Z.; Li, J.; Cheng, F. Co-metabolism for enhanced phenol degradation and bioelectricity generation in microbial fuel cell. Bioelectrochemistry 2020, 134, 107527. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ndayisenga, F.; Yu, Z.; Zhao, M.; Lay, C.-H.; Zhou, D. Co-substrate strategy for improved power production and chlorophenol degradation in a microbial fuel cell. Int. J. Hydrogen Energy 2019, 44, 20312–20322. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Yan, G.; Phulpoto, I.A.; Li, Q.; Kumar, H.; Fu, L.; Zhou, D. Using easy-to-biodegrade co-substrate to eliminate microcystin toxic on electrochemically active bacteria and enhance bioelectricity generation from cyanobacteria biomass. Sci. Total Environ. 2021, 751, 142292. [Google Scholar] [CrossRef]
- Mancilio, L.B.K.; Ribeiro, G.A.; de Almeida, E.J.R.; de Siqueira, G.M.V.; Rocha, R.S.; Guazzaroni, M.-E.; De Andrade, A.R.; Reginatto, V. Adding value to lignocellulosic byproducts by using acetate and p-coumaric acid as substrate in a microbial fuel cell. Ind. Crop. Prod. 2021, 171, 113844. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Jayabalan, T.; Sethupathi, M.; Kim, W.; Muniyasamy, S.; Sengottuvelan, N.; Nainamohamed, S.; Ponnuchamy, K.; Alagarsamy, A. Bioelectricity generation by natural microflora of septic tank wastewater (STWW) and biodegradation of persistent petrogenic pollutants by basidiomycetes fungi: An integrated microbial fuel cell system. J. Hazard. Mater. 2021, 412, 125228. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Nainamohamed, S.; Samuel, J.O.; Soorangkattan, S.; Muthuramalingam, J.B.; Kulanthaisamy, M.; Balasubramani, R.; Nguyen, D.D.; Chang, S.W.; Bolan, N.; et al. Comparative study on Cronobacter sakazakii and Pseudomonas otitidis isolated from septic tank wastewater in microbial fuel cell for bioelectricity generation. Fuel 2019, 248, 47–55. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Ebenezer, J.O.; Bora, A.; Nagarajan, A.; Pugazhendhi, A.; Jayabalan, T.; Nainamohamed, S.; Doble, M.; Alagarsamy, A. Bioelectricity generation and analysis of anode biofilm metabolites from septic tank wastewater in microbial fuel cells. Int. J. Energy Res. 2021, 45, 17244–17258. [Google Scholar] [CrossRef]
- Ramu, S.M.; Thulasinathan, B.; Hari, D.G.; Bora, A.; Jayabalan, T.; Mohammed, S.N.; Doble, M.; Arivalagan, P.; Alagarsamy, A. Fermentative hydrogen production and bioelectricity generation from food based industrial waste: An integrative approach. Bioresour. Technol. 2020, 310, 123447. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J.; Khanjani, N. Evaluation of dairy industry wastewater treatment and simultaneous bioelectricity generation in a catalyst-less and mediator-less membrane microbial fuel cell. J. Saudi Chem. Soc. 2016, 20, 88–100. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Behera, M. Comparative evaluation of methanogenesis suppression methods in microbial fuel cell during rice mill wastewater treatment. Environ. Technol. Innov. 2020, 17, 100509. [Google Scholar] [CrossRef]
- Ng, F.-L.; Phang, S.-M.; Thong, C.-H.; Periasamy, V.; Pindah, J.; Yunus, K.; Fisher, A.C. Integration of bioelectricity generation from algal biophotovoltaic (BPV) devices with remediation of palm oil mill effluent (POME) as substrate for algal growth. Environ. Technol. Innov. 2020, 21, 101280. [Google Scholar] [CrossRef]
- Islam, M.A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Thiruvenkadam, S.; Prasad, R.; Rahman Khan, M.M. Enhanced Current Generation Using Mutualistic Interaction of Yeast-Bacterial Coculture in Dual Chamber Microbial Fuel Cell. Ind. Eng. Chem. Res. 2018, 57, 813–821. [Google Scholar] [CrossRef]
- Islam, M.A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Rahman Khan, M.M. An Insight of Synergy between Pseudomonas aeruginosa and Klebsiella variicola in a Microbial Fuel Cell. ACS Sustain. Chem. Eng. 2018, 6, 4130–4137. [Google Scholar] [CrossRef]
- Sarmin, S.; Tarek, M.; Roopan, S.M.; Cheng, C.K.; Rahman Khan, M.M. Significant improvement of power generation through effective substrate-inoculum interaction mechanism in microbial fuel cell. J. Power Sources 2020, 484, 229285. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A novel UASB–MFC–BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour. Technol. 2009, 100, 5687–5693. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Thota Karunakaran, R.; Manickam, M. Enhancement of bioelectricity generation from treatment of distillery wastewater using microbial fuel cell. Environ. Prog. Sustain. Energy 2018, 37, 663–668. [Google Scholar] [CrossRef]
- Oyiwona, G.E.; Ogbonna, J.C.; Anyanwu, C.U.; Okabe, S. Electricity generation potential of poultry droppings wastewater in microbial fuel cell using rice husk charcoal electrodes. Bioresour. Bioprocess. 2018, 5, 13. [Google Scholar] [CrossRef]
- Ren, B.; Wang, T.; Zhao, Y. Two-stage hybrid constructed wetland-microbial fuel cells for swine wastewater treatment and bioenergy generation. Chemosphere 2021, 268, 128803. [Google Scholar] [CrossRef]
- Ni, H.; Wang, K.; Lv, S.; Wang, X.; Zhuo, L.; Zhang, J. Effects of Concentration Variations on the Performance and Microbial Community in Microbial Fuel Cell Using Swine Wastewater. Energies 2020, 13, 2231. [Google Scholar] [CrossRef]
- Catal, T.; Liu, H.; Fan, Y.; Bermek, H. A clean technology to convert sucrose and lignocellulose in microbial electrochemical cells into electricity and hydrogen. Bioresour. Technol. Rep. 2019, 5, 331–334. [Google Scholar] [CrossRef]
- Jablonska, M.A.; Rybarczyk, M.K.; Lieder, M. Electricity generation from rapeseed straw hydrolysates using microbial fuel cells. Bioresour. Technol. 2016, 208, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Kim, H.J.; Song, H.S.; Park, S.L.; Lee, S.M.; Lee, H.S.; Kim, S.H.; Yoon, J.J.; et al. Utilization of different lignocellulosic hydrolysates as carbon source for electricity generation using novel Shewanella marisflavi BBL25. J. Clean. Prod. 2020, 277, 124084. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Hassan, S.H.A.; Rahman, M.M.; Oh, S.E. The effect of Nafion membrane fouling on the power generation of a microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 13643–13651. [Google Scholar] [CrossRef]
- Mohd Zaini Makhtar, M.; Tajarudin, H.A. Electricity generation using membrane-less microbial fuel cell powered by sludge supplemented with lignocellulosic waste. Int. J. Energy Res. 2020, 44, 3260–3265. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Nakashima, K.; Kato, M.; Inoue, K.; Okazaki, F.; Soyama, H.; Kawasaki, S. Electricity Generation from Rice Bran by a Microbial Fuel Cell and the Influence of Hydrodynamic Cavitation Pretreatment. ACS Omega 2018, 3, 15267–15271. [Google Scholar] [CrossRef]
- Jenol, M.A.; Ibrahim, M.F.; Bahrin, E.K.; Kim, S.W.; Abd-Aziz, S. Direct Bioelectricity Generation from Sago Hampas by Clostridium beijerinckii SR1 Using Microbial Fuel Cell. Molecules 2019, 24, 2397. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Nosek, D.; Jachimowicz, P.; Cydzik-Kwiatkowska, A. Anode Modification as an Alternative Approach to Improve Electricity Generation in Microbial Fuel Cells. Energies 2020, 13, 6596. [Google Scholar] [CrossRef]
- Xu, H.; Quan, X.; Xiao, Z.; Chen, L. Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem. Eng. J. 2018, 335, 539–547. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Feng, L. Enhancing the performance of soil microbial fuel cells by using a bentonite-Fe and Fe3O4 modified anode. J. Hazard. Mater. 2019, 377, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Veeramani, V.; Rajangam, K.; Nagendran, J. Performance of cobalt oxide/carbon cloth composite electrode in energy generation from dairy wastewater using microbial fuel cells. Sustain. Environ. Res. 2020, 30, 16. [Google Scholar] [CrossRef]
- Li, X.; Hu, M.; Zeng, L.; Xiong, J.; Tang, B.; Hu, Z.; Xing, L.; Huang, Q.; Li, W. Co-modified MoO2 nanoparticles highly dispersed on N-doped carbon nanorods as anode electrocatalyst of microbial fuel cells. Biosens. Bioelectron. 2019, 145, 111727. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; He, H.; Cao, J.; Zhou, S. Adding Zero-Valent Iron to Enhance Electricity Generation during MFC Start-Up. Int. J. Environ. Res. Public Health 2020, 17, 806. [Google Scholar] [CrossRef]
- Paul, D.; Noori, M.T.; Rajesh, P.P.; Ghangrekar, M.M.; Mitra, A. Modification of carbon felt anode with graphene oxide-zeolite composite for enhancing the performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2018, 26, 77–82. [Google Scholar] [CrossRef]
- Fu, L.; Wang, H.; Huang, Q.; Song, T.S.; Xie, J. Modification of carbon felt anode with graphene/Fe2O3 composite for enhancing the performance of microbial fuel cell. Bioprocess Biosyst. Eng. 2020, 43, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhai, H.; Liu, B.; Ji, M.; Li, J. Carbon nanomaterial-modified graphite felt as an anode enhanced the power production and polycyclic aromatic hydrocarbon removal in sediment microbial fuel cells. Sci. Total Environ. 2020, 713, 136483. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Yuan, Y.; Lu, X.; Yang, Z.; Wang, Y.; Sun, J. Long-term effect of carbon nanotubes on electrochemical properties and microbial community of electrochemically active biofilms in microbial fuel cells. Int. J. Hydrogen Energy 2018, 43, 16240–16247. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, X.; Yuan, X.; Li, N.; He, W.; Feng, Y. Enhanced electricity generation and extracellular electron transfer by polydopamine–reduced graphene oxide (PDA–rGO) modification for high-performance anode in microbial fuel cell. Chem. Eng. J. 2020, 387, 123408. [Google Scholar] [CrossRef]
- Kirubaharan, C.J.; Kumar, G.G.; Sha, C.; Zhou, D.; Yang, H.; Nahm, K.S.; Raj, B.S.; Zhang, Y.; Yong, Y.C. Facile fabrication of Au@polyaniline core-shell nanocomposite as efficient anodic catalyst for microbial fuel cells. Electrochim. Acta 2019, 328, 135136. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Mashkour, M.; Soavi, F. Electro-polymerized polyaniline modified conductive bacterial cellulose anode for supercapacitive microbial fuel cells and studying the role of anodic biofilm in the capacitive behavior. J. Power Sources 2020, 478, 228822. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wen, Q.; Zheng, H.; Xu, H.; Qi, L. Electricity generation, energy storage, and microbial-community analysis in microbial fuel cells with multilayer capacitive anodes. Energy 2019, 189, 116342. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, I.; Rajesh, P.P.; Ghangrekar, M.M. Performance Evaluation of Microbial Fuel Cell Operated with Pd or MnO2 as Cathode Catalyst and Chaetoceros Pretreated Anodic Inoculum. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020009. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Ahmad, A.; Das, S.; Ghangrekar, M.M. Metal organic frameworks as emergent oxygen-reducing cathode catalysts for microbial fuel cells: A review. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.; Dong, F.; Lin, Q.; Tong, Y.; Li, Y.; Zhang, P. Electricity generation from banana peels in an alkaline fuel cell with a Cu2O-Cu modified activated carbon cathode. Sci. Total Environ. 2018, 631–632, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.R.; Shahbazi Farahani, F.; Hosseini, M.; Ahadzadeh, I. Low-cost nanowired α-MnO2/C as an ORR catalyst in air-cathode microbial fuel cell. Bioelectrochemistry 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Chiodoni, A.; Salvador, G.P.; Massaglia, G.; Delmondo, L.; Muñoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Margaria, V.; Ahmed, D.; et al. MnxOy- based cathodes for oxygen reduction reaction catalysis in microbial fuel cells. Int. J. Hydrogen Energy 2018, 44, 4432–4441. [Google Scholar] [CrossRef]
- Rout, S.; Nayak, A.K.; Varanasi, J.L.; Pradhan, D.; Das, D. Enhanced energy recovery by manganese oxide/reduced graphene oxide nanocomposite as an air-cathode electrode in the single-chambered microbial fuel cell. J. Electroanal. Chem. 2018, 815, 1–7. [Google Scholar] [CrossRef]
- Mecheri, B.; Ficca, V.C.A.; de Oliveira, M.A.C.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Licoccia, S. Facile synthesis of graphene-phthalocyanine composites as oxygen reduction electrocatalysts in microbial fuel cells. Appl. Catal. B: Environ. 2018, 237, 699–707. [Google Scholar] [CrossRef]
- Lee, C.; Ozden, S.; Tewari, C.S.; Park, O.K.; Vajtai, R.; Chatterjee, K.; Ajayan, P.M. MoS2 —Carbon Nanotube Porous 3 D Network for Enhanced Oxygen Reduction Reaction. ChemSusChem 2018, 11, 2960–2966. [Google Scholar] [CrossRef]
- Li, H.; Ma, H.; Liu, T.; Ni, J.; Wang, Q. An excellent alternative composite modifier for cathode catalysts prepared from bacterial cellulose doped with Cu and P and its utilization in microbial fuel cell. Bioresour. Technol. 2019, 289, 121661. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh, S.; Chhabra, V.A.; Marwaha, A.; Kim, K.-H.; Tripathi, S.K. A sustainable approach towards utilization of plastic waste for an efficient electrode in microbial fuel cell applications. J. Hazard. Mater. 2021, 417, 125992. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, X.; Yu, H.; Wang, X.; Liu, X.; Yang, H.; Li, F.; Zhou, Q. Simultaneous sulfamethoxazole degradation with electricity generation by microbial fuel cells using Ni-MOF-74 as cathode catalysts and quantification of antibiotic resistance genes. Environ. Res. 2021, 197, 111054. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, J.; An, Y.; Han, D.; Chang, S.; Liu, Y.; Yang, R. Enhanced electrochemical performance by nickel-iron layered double hydroxides (LDH) coated on Fe3O4 as a cathode catalyst for single-chamber microbial fuel cells. Sci. Total Environ. 2020, 745, 141163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, J.; Han, D.; Chang, S.; Yang, R.; An, Y.; Liu, Y.; Chen, F. Potential of core-shell NiFe layered double hydroxide@Co3O4 nanostructures as cathode catalysts for oxygen reduction reaction in microbial fuel cells. J. Power Sources 2020, 453, 227877. [Google Scholar] [CrossRef]
- Tajdid Khajeh, R.; Aber, S.; Zarei, M. Comparison of NiCo2O4, CoNiAl-LDH, and CoNiAl-LDH@NiCo2O4 performances as ORR catalysts in MFC cathode. Renew. Energy 2020, 154, 1263–1271. [Google Scholar] [CrossRef]
- Li, J.C.; Wu, X.T.; Chen, L.J.; Li, N.; Liu, Z.Q. Bifunctional MOF-derived Co-N-doped carbon electrocatalysts for high-performance zinc-air batteries and MFCs. Energy 2018, 156, 95–102. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Z.; Hao, L.; Ran, L.; Xu, X.; Dai, Y.; Pan, S.; Jing, B.; Zou, J. Increase of structural defects by N doping in MoS2 cross-linked with N-doped CNTs/carbon for enhancing charge transfer in oxygen reduction. Electrochim. Acta 2018, 283, 448–458. [Google Scholar] [CrossRef]
- Chong, G.W.; Karbelkar, A.A.; El-Naggar, M.Y. Nature’s Conductors: What Can Microbial Multi-Heme Cytochromes Teach Us about Electron Transport and Biological Energy Conversion? Curr. Opin. Chem. Biol. 2018, 47, 7–17. [Google Scholar] [CrossRef]
- Aiyer, K.S. How Does Electron Transfer Occur in Microbial Fuel Cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Pal, M.; Sharma, R.K. Exoelectrogenic response of Pichia fermentans influenced by mediator and reactor design. J. Biosci. Bioeng. 2019, 127, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, F.; Fallah, N.; Nasernejad, B. Biodegradation of synthetic wastewater containing styrene in microbial fuel cell: Effect of adaptation of microbial community. Fuel 2021, 305, 121382. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of methylene blue and methyl red mediators on performance of yeast based microbial fuel cells adopting polyethylenimine coated carbon felt as anode. J. Power Sources 2018, 396, 1–11. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Selvanathan, J.R.; Abdullah, M.O.; Wahab, N.A.; Kanakaraju, D. Effect of Different Mediators on Bio-Energy Generation and Palm Oil Mill Effluent Treatment in an Air-Cathode Microbial Fuel Cell-Adsorption System. Defect Diffus. Forum 2021, 411, 67–78. [Google Scholar] [CrossRef]
- Chauhan, S.; Sharma, V.; Varjani, S.; Sindhu, R.; Chaturvedi Bhargava, P. Mitigation of tannery effluent with simultaneous generation of bioenergy using dual chambered microbial fuel cell. Bioresour. Technol. 2022, 351, 127084. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Y.; Yu, Y.; Ye, J.; Zhang, S.; Chen, J. Exogenous electron transfer mediator enhancing gaseous toluene degradation in a microbial fuel cell: Performance and electron transfer mechanism. Chemosphere 2021, 282, 131028. [Google Scholar] [CrossRef]
- Moreno, L.; Nemati, M.; Predicala, B. Biodegradation of phenol in batch and continuous flow microbial fuel cells with rod and granular graphite electrodes. Environ. Technol. 2018, 39, 144–156. [Google Scholar] [CrossRef]
- Marcílio, R.; Neto, S.A.; Ruvieri, B.M.; Andreote, F.D.; de Andrade, A.R.; Reginatto, V. Enhancing the performance of an acetate-fed microbial fuel cell with methylene green. Braz. J. Chem. Eng. 2021, 38, 471–484. [Google Scholar] [CrossRef]
- Ajunwa, O.M.; Odeniyi, O.A.; Garuba, E.O.; Marsili, E.; Onilude, A.A. Influence of enhanced electrogenicity on anodic biofilm and bioelectricity production by a novel microbial consortium. Process Biochem. 2021, 104, 27–38. [Google Scholar] [CrossRef]
- Larrosa-Guerrero, A.; Scott, K.; Head, I.M.; Mateo, F.; Ginesta, A.; Godinez, C. Effect of temperature on the performance of microbial fuel cells. Fuel 2010, 89, 3985–3994. [Google Scholar] [CrossRef]
- Mohammed, H.; Al-Othman, A.; Nancarrow, P.; Elsayed, Y.; Tawalbeh, M. Enhanced proton conduction in zirconium phosphate/ionic liquids materials for high-temperature fuel cells. Int. J. Hydrogen Energy 2021, 46, 4857–4869. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, C.; Chae, J. Effect of temperature on a miniaturized microbial fuel cell (MFC). Micro Nano Syst. Lett. 2017, 5, 353. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Wade, M.J.; Sloan, W.T.; Quince, C.; Curtis, T.P. Temperature, inocula and substrate: Contrasting electroactive consortia, diversity and performance in microbial fuel cells. Bioelectrochemistry 2018, 119, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martínez, A.; Chengyuan, S.; Rodriguez-Sanchez, A.; Pozo, C.; Gonzalez-Lopez, J.; Vahala, R. Application of microbial fuel cell technology for wastewater treatment and electricity generation under Nordic countries climate conditions: Study of performance and microbial communities. Bioresour. Technol. 2018, 270, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boas, J.V.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Review on microbial fuel cells applications, developments and costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Jin, W.; Shahid, N.; Bhatti, Z.A.; Iqbal, A.; Abbasi, U.; Mahmood, Q.; Ali, A. The performance of microbial fuel cells treating vegetable oil industrial wastewater. Environ. Technol. Innov. 2018, 10, 143–151. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, J.; Shi, Y.; Li, X.; Yang, N.; Wang, X. Anolyte recycling enhanced bioelectricity generation of the buffer-free single-chamber air-cathode microbial fuel cell. Bioresour. Technol. 2017, 244, 1183–1187. [Google Scholar] [CrossRef]
- Wang, C.T.; Huang, Y.S.; Sangeetha, T.; Yan, W.M. Assessment of recirculation batch mode operation in bufferless Bio-cathode microbial Fuel Cells (MFCs). Appl. Energy 2018, 209, 120–126. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Akhter, F.; Mazari, S.A.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Mubarak, N.M.; Baloch, H.; Memon, A.Q.; Ahmed, S. Advanced microbial fuel cell for waste water treatment—A review. Environ. Sci. Pollut. Res. 2021, 28, 5005–5019. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Mirbagheri, S.A. A review of the operating parameters on the microbial fuel cell for wastewater treatment and electricity generation. Water Sci. Technol. 2021, 84, 1309–1323. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.R.; Ethiraj, B.; Cheng, C.K.; Rahman Khan, M.M. Optimization of co-culture inoculated microbial fuel cell performance using response surface methodology. J. Environ. Manag. 2018, 225, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Aljlil, S.A.; Alsubei, M.D.; Ghasemi, M.; Mohammadi, M. Performance optimisation of microbial fuel cell for wastewater treatment and sustainable clean energy generation using response surface methodology. Alex. Eng. J. 2018, 57, 4243–4253. [Google Scholar] [CrossRef]
- Salar-García, M.J.; de Ramón-Fernández, A.; Ortiz-Martínez, V.M.; Ruiz-Fernández, D.; Ieropoulos, I. Towards the optimisation of ceramic-based microbial fuel cells: A three-factor three-level response surface analysis design. Biochem. Eng. J. 2019, 144, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Velayudhaperumal Chellam, P.; Verma, A.; Singh, R.S. A comparative study on the performance of microbial fuel cell for the treatment of reactive orange 16 dye using mixed and pure bacterial species and its optimization using response surface methodology. Sustain. Energy Technol. Assess. 2021, 48, 101667. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Pratiwi, W.Z.; Purwanto, P.; Sudarno, S.; Haryani, K.; Hoang, A.T. Response surface optimization of microalgae microbial fuel cell (MMFC) enhanced by yeast immobilization for bioelectricity production. Chemosphere 2022, 287, 132275. [Google Scholar] [CrossRef]
- Sugumar, M.; Dharmalingam, S. Statistical optimization of process parameters in microbial fuel cell for enhanced power production using Sulphonated Polyhedral Oligomeric Silsesquioxane dispersed Sulphonated Polystyrene Ethylene Butylene Polystyrene nanocomposite membranes. J. Power Sources 2020, 469, 228400. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Thomas, N.; Tamilmani, J.; Boobalan, T.; Matheswaran, M.; Kalaichelvi, P.; Alagarsamy, A.; Pugazhendhi, A. Bioelectricity generation using iron(II) molybdate nanocatalyst coated anode during treatment of sugar wastewater in microbial fuel cell. Fuel 2020, 277, 118119. [Google Scholar] [CrossRef]
- Jayashree, C.; Tamilarasan, K.N.; Rajkumar, M.; Arulazhagan, P.; Yogalakshmi, K.; Srikanth, M.; Banu, J.R. Treatment of seafood processing wastewater using upflow microbial fuel cell for power generation and identification of bacterial community in anodic biofilm. J. Environ. Manag. 2016, 180, 351–358. [Google Scholar] [CrossRef]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sources 2017, 356, 274–287. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Simultaneous pollution treatment and electricity generation of tannery wastewater in air-cathode single chamber MFC. Int. J. Hydrogen Energy 2016, 41, 15632–15637. [Google Scholar] [CrossRef]
- Jamal, M.T.; Pugazhendi, A. Treatment of fish market wastewater and energy production using halophiles in air cathode microbial fuel cell. J. Environ. Manag. 2021, 292, 112752. [Google Scholar] [CrossRef] [PubMed]
- Marassi, R.J.; Queiroz, L.G.; Silva, D.C.V.R.; da Silva, F.T.; Silva, G.C.; de Paiva, T.C.B. Performance and toxicity assessment of an up-flow tubular microbial fuel cell during long-term operation with high-strength dairy wastewater. J. Clean. Prod. 2020, 259, 120882. [Google Scholar] [CrossRef]
- Rathour, R.; Patel, D.; Shaikh, S.; Desai, C. Eco-electrogenic treatment of dyestuff wastewater using constructed wetland-microbial fuel cell system with an evaluation of electrode-enriched microbial community structures. Bioresour. Technol. 2019, 285, 121349. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, R.; Rene, E.R.; Qi, X.; Hao, Q.; Du, Y.; Zhao, C.; Xu, F.; Kong, Q. Characterization of microbial community and resistance gene (CzcA) shifts in up-flow constructed wetlands-microbial fuel cell treating Zn (II) contaminated wastewater. Bioresour. Technol. 2020, 302, 122867. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L. Cathodes of membrane and packed manganese dioxide/titanium dioxide/graphitic carbon nitride/granular activated carbon promoted treatment of coking wastewater in microbial fuel cell. Bioresour. Technol. 2021, 321, 124442. [Google Scholar] [CrossRef]
- Kadivarian, M.; Dadkhah, A.A.; Nasr Esfahany, M. Oily wastewater treatment by a continuous flow microbial fuel cell and packages of cells with serial and parallel flow connections. Bioelectrochemistry 2020, 134, 107535. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, X.; Wang, H.; Zhang, Y.; Gao, Y.; Bian, C.; Wang, X.; Xu, P. Power generation and microbial community analysis in microbial fuel cells: A promising system to treat organic acid fermentation wastewater. Bioresour. Technol. 2019, 284, 72–79. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, Y.; Bo, L.; Zhang, L.; Liu, W.; Zhu, Y. Coupling of electricity generation and denitrification in three-phase single-chamber MFCs in high-salt conditions. Bioelectrochemistry 2020, 133, 107481. [Google Scholar] [CrossRef]
- Srikanth, S.; Kumar, M.; Singh, D.; Singh, M.P.; Das, B.P. Electro-biocatalytic treatment of petroleum refinery wastewater using microbial fuel cell (MFC) in continuous mode operation. Bioresour. Technol. 2016, 221, 70–77. [Google Scholar] [CrossRef]
- Ali, J.; Wang, L.; Waseem, H.; Sharif, H.M.A.; Djellabi, R.; Zhang, C.; Pan, G. Bioelectrochemical recovery of silver from wastewater with sustainable power generation and its reuse for biofouling mitigation. J. Clean. Prod. 2019, 235, 1425–1437. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lai, C.-Y.; Chang, P.-H.; Lin, C.-W.; Chen, Y.-H. Enhancing copper recovery and electricity generation from wastewater using low-cost membrane-less microbial fuel cell with a carbonized clay cup as cathode. J. Clean. Prod. 2020, 247. [Google Scholar] [CrossRef]
- Birjandi, N.; Younesi, H.; Ghoreyshi, A.A.; Rahimnejad, M. Electricity generation, ethanol fermentation and enhanced glucose degradation in a bio-electro-Fenton system driven by a microbial fuel cell. J. Chem. Technol. Biotechnol. 2016, 91, 1868–1876. [Google Scholar] [CrossRef]
- Moradian, J.M.; Xu, Z.A.; Shi, Y.T.; Fang, Z.; Yong, Y.C. Efficient biohydrogen and bioelectricity production from xylose by microbial fuel cell with newly isolated yeast of Cystobasidium slooffiae. Int. J. Energy Res. 2020, 44, 325–333. [Google Scholar] [CrossRef]
- Srikanth, S.; Venkateswar Reddy, M.; Venkata Mohan, S. Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour. Technol. 2012, 125, 291–299. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Bouvier, S.; Feiler, L.; Karacs, J.; Weld, R.J. Geobacter-dominated biofilms used as amperometric BOD sensors. Biochem. Eng. J. 2016, 109, 88–95. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Chung, Y.C. Measurement of biochemical oxygen demand from different wastewater samples using a mediator-less microbial fuel cell biosensor. Environ. Technol. 2014, 35, 2204–2211. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Self-powered, autonomous Biological Oxygen Demand biosensor for online water quality monitoring. Sens. Actuators B: Chem. 2017, 244, 815–822. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Wu, S.; Dong, R.; Angelidaki, I. Innovative operation of microbial fuel cell-based biosensor for selective monitoring of acetate during anaerobic digestion. Sci. Total Environ. 2019, 655, 1439–1447. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Shi, Y.; Qi, Y.; Huang, D.; Tadé, M.; Wang, S.; Liu, S. FePO4 based single chamber air-cathode microbial fuel cell for online monitoring levofloxacin. Biosens. Bioelectron. 2017, 91, 367–373. [Google Scholar] [CrossRef]
- Yang, W.; Wei, X.; Fraiwan, A.; Coogan, C.G.; Lee, H.; Choi, S. Fast and sensitive water quality assessment: A μL-scale microbial fuel cell-based biosensor integrated with an air-bubble trap and electrochemical sensing functionality. Sens. Actuators B: Chem. 2016, 226, 191–195. [Google Scholar] [CrossRef]
- Yu, D.; Bai, L.; Zhai, J.; Wang, Y.; Dong, S. Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta 2017, 168, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, P.; Liu, P.; Yan, X.; Bian, Y.; Huang, X. A cathode-shared microbial fuel cell sensor array for water alert system. Int. J. Hydrogen Energy 2017, 42, 4342–4348. [Google Scholar] [CrossRef]
- Wang, G.H.; Cheng, C.Y.; Liu, M.H.; Chen, T.Y.; Hsieh, M.C.; Chung, Y.C. Utility of Ochrobactrum anthropi YC152 in a Microbial Fuel Cell as an Early Warning Device for Hexavalent Chromium Determination. Sensors 2016, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Tsai, T.H.; Liu, M.H.; Kuo, J.L.; Chang, Y.C.; Chung, Y.C. A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef]
- Nguyen Tran, P.H.; Thi Luong, T.T.; Thi Nguyen, T.T.; Nguyen, H.Q.; van Duong, H.; Kim, B.H.; Pham, H.T. Possibility of using a lithotrophic iron-oxidizing microbial fuel cell as a biosensor for detecting iron and manganese in water samples. Environ. Sci. Process. Impacts 2015, 17, 1806–1815. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, Y.; Zhao, S.; Khan, A.; Ling, Z.; Chen, Y.; Liu, P.; Li, X. A novel biosensor for p-nitrophenol based on an aerobic anode microbial fuel cell. Biosens. Bioelectron. 2016, 85, 860–868. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Liu, H. Hibernations of electroactive bacteria provide insights into the flexible and robust BOD detection using microbial fuel cell-based biosensors. Sci. Total Environ. 2021, 753, 142244. [Google Scholar] [CrossRef]


| MFC Type | Microbial Community | Experimental Design | Optimized Conditions | MFC Performance | Reference |
|---|---|---|---|---|---|
| Dual-chamber MFC | Co-culture of Klebsiella variicola and Pseudomonas aeruginosa | Box–Behnken design | Inoculum composition: 1:1, Initial COD: 26.690 mg/L, pH: 7.21, Time: 15.50 days. | Power density: 12.21 mW/m3 | [123] |
| Dual-chamber MFC | Anaerobic sludge | Central composite design | Degree of sulfonation: 68%, Aeration: 121.62 mL/min, Pt load: 0.42 mg/cm2. | Power density: 58.19 mW/m2 | [124] |
| Cubical ceramic-based MFC | Sludge and human urine in a 1:1 ratio | Box–Behnken design | Membrane thickness: 1.55 mm, External resistance: 895.59 Ω, Anode area: 165.72 cm2. | Maximum absolute power generation: 467.63 μW | [125] |
| Dual-chamber MFC | Acinetobacter pitii | Central composite design | Initial dye: 295 ppm, pH: 7.5, Time: 71.27 h | Current density: 1.06 A/m3 | [126] |
| Microalgae MFC | Immobilized Saccharomyces cerevisiae yeast | Central composite design | Yeast: content: 10.89% w/v Wastewater concentration: 56.94% | Power density: 11.25 mW/m2 | [127] |
| Fabricated tubular MFC | Microorganisms in wastewater | Box–Behnken design | pH: 7, Substrate concentration: 75% Anode material: graphite rod | Maximum power density: 126 mW/m2 | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application. BioTech 2022, 11, 44. https://doi.org/10.3390/biotech11040044
Wang J, Ren K, Zhu Y, Huang J, Liu S. A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application. BioTech. 2022; 11(4):44. https://doi.org/10.3390/biotech11040044
Chicago/Turabian StyleWang, Jianfei, Kexin Ren, Yan Zhu, Jiaqi Huang, and Shijie Liu. 2022. "A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application" BioTech 11, no. 4: 44. https://doi.org/10.3390/biotech11040044
APA StyleWang, J., Ren, K., Zhu, Y., Huang, J., & Liu, S. (2022). A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application. BioTech, 11(4), 44. https://doi.org/10.3390/biotech11040044







