Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles
Abstract
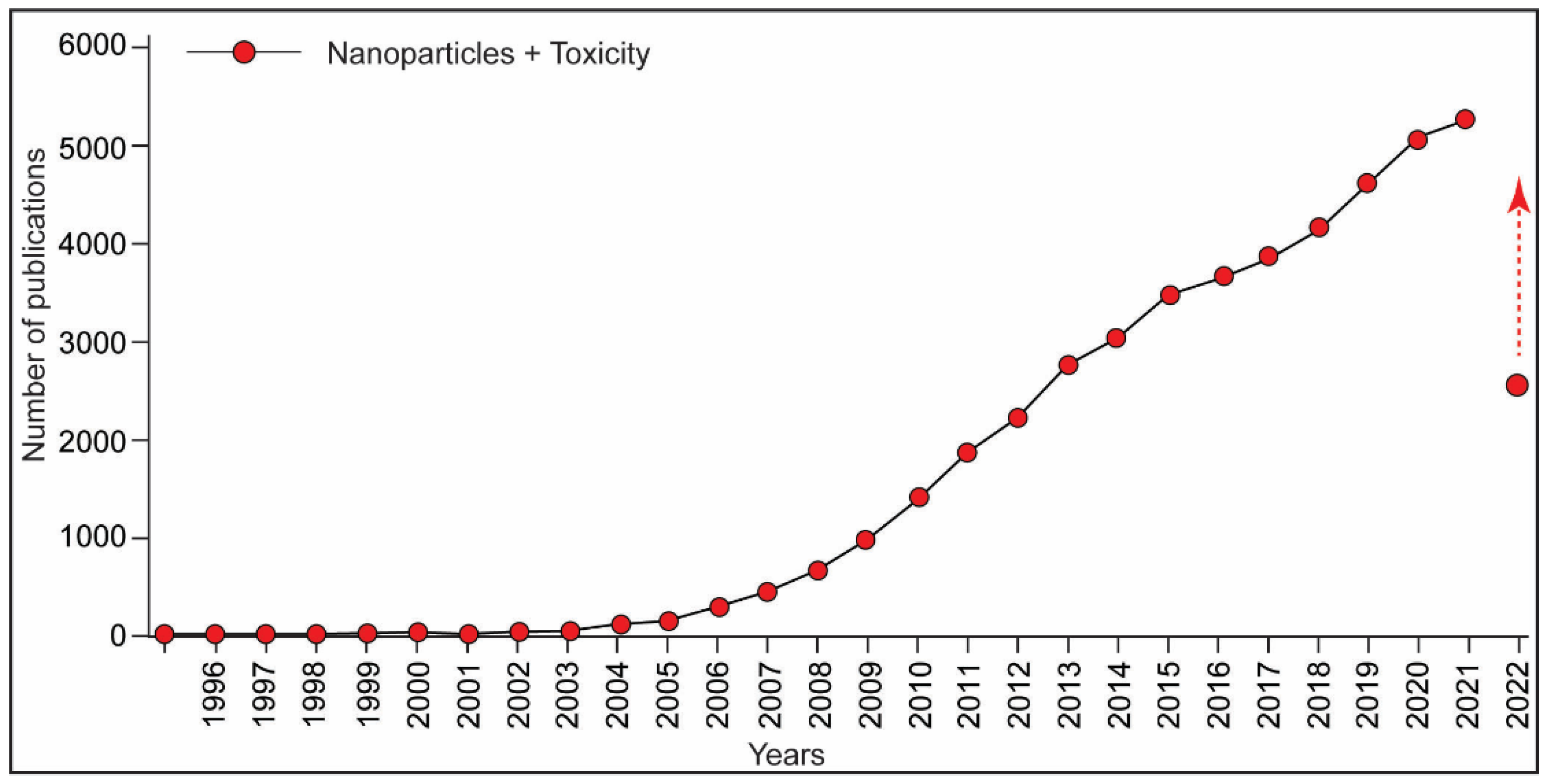
:1. Introduction
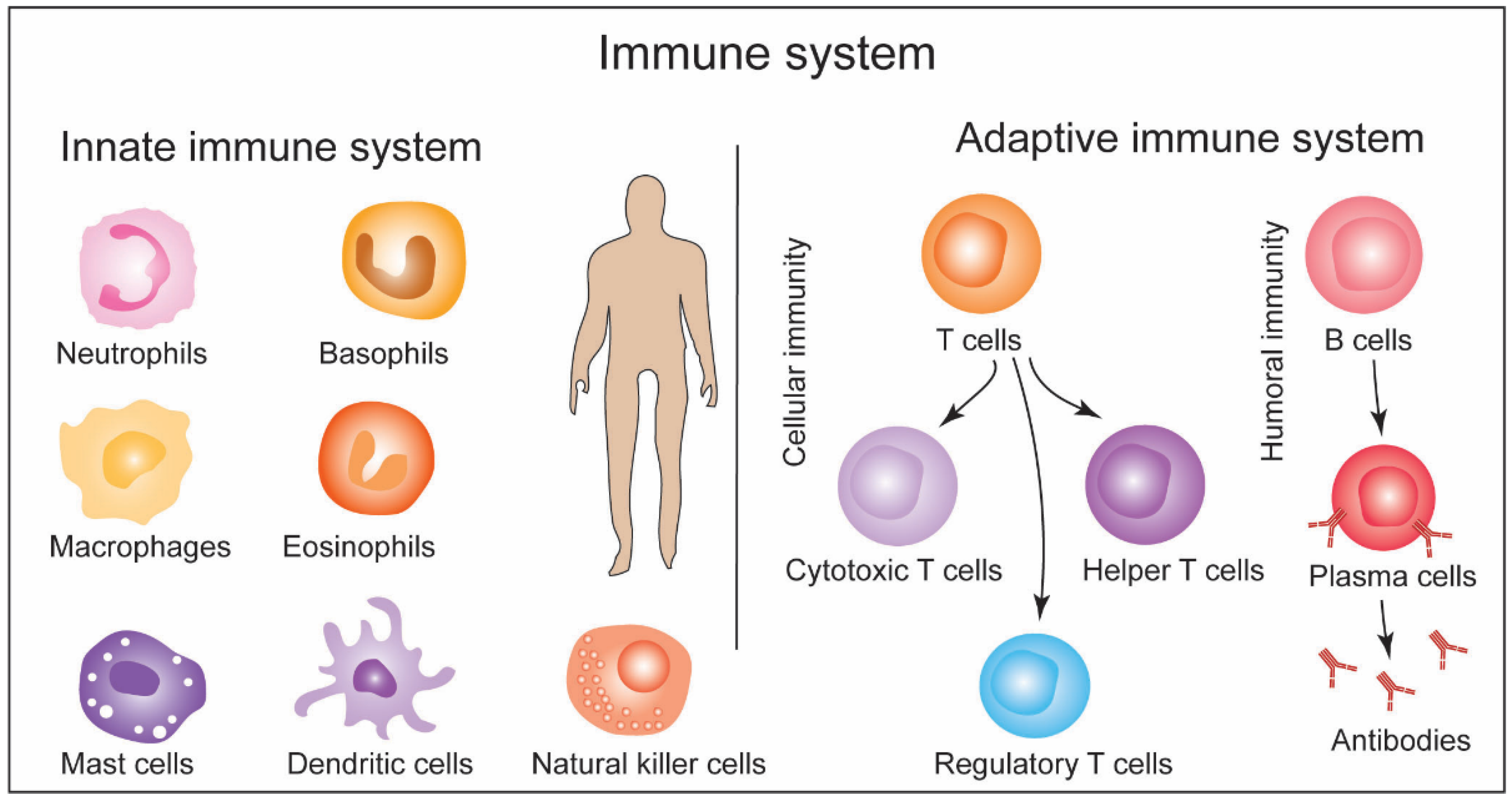
2. General Structure of Immune System and Response Machinery: A Brief Overview and Methods
3. Immunotoxicity of Xenobiotics: Exposure Routes, Signaling Pathways, and Diseases Implications
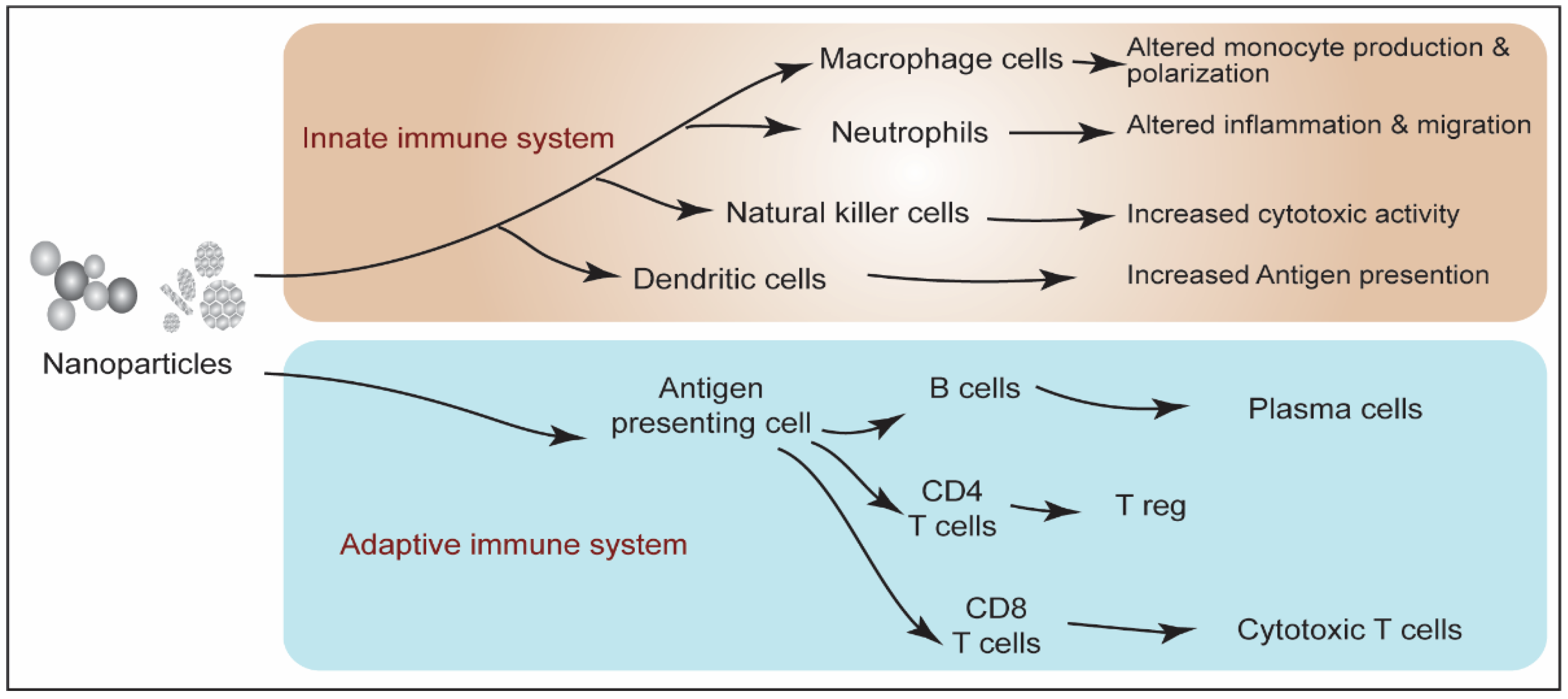
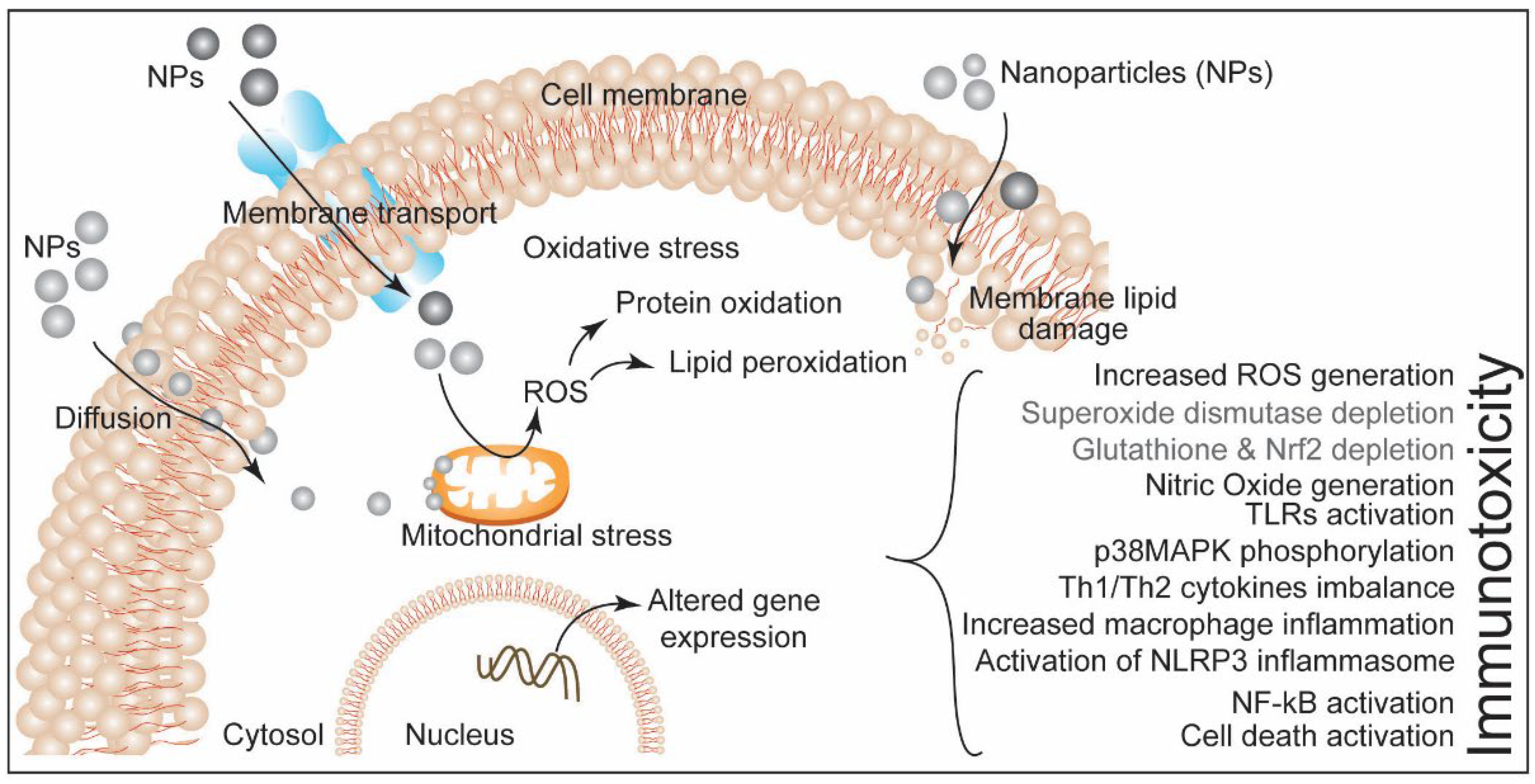
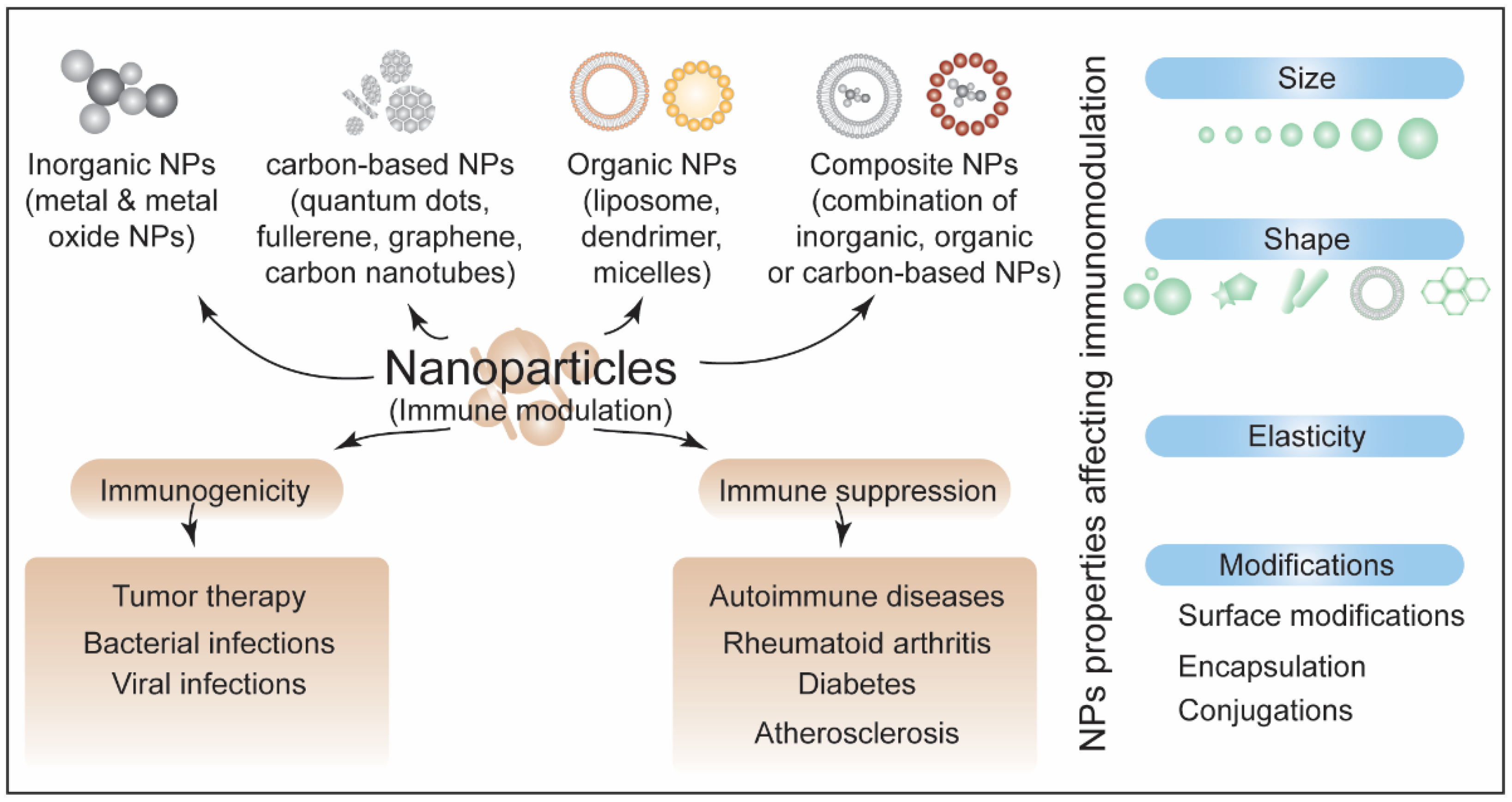
4. Nanoparticles-Induced Immunotoxicity
4.1. Inorganic-Based NPs and Immunomodulation
4.2. Carbon-Based NPs and Immunomodulation
4.3. Organic-Based and Composite NPs and Immunomodulation
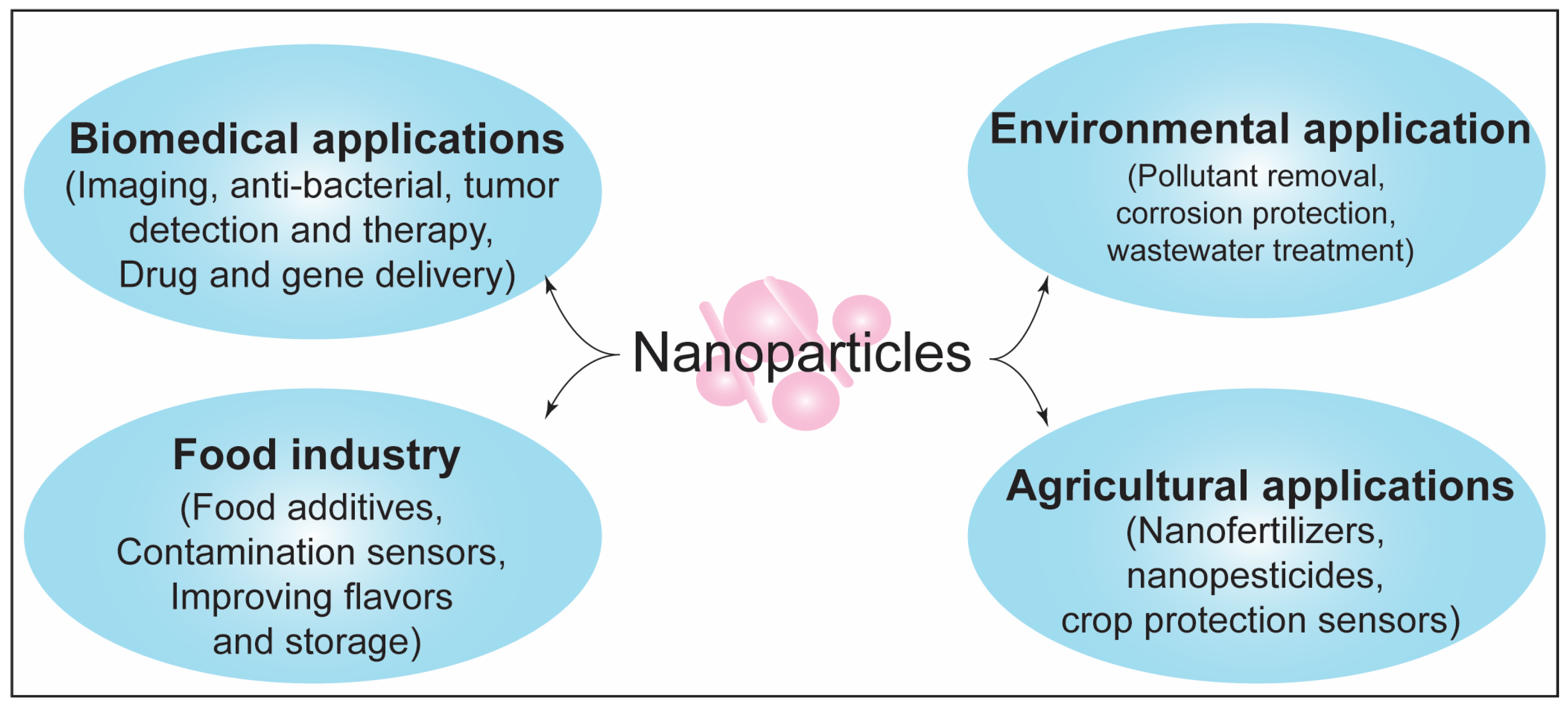
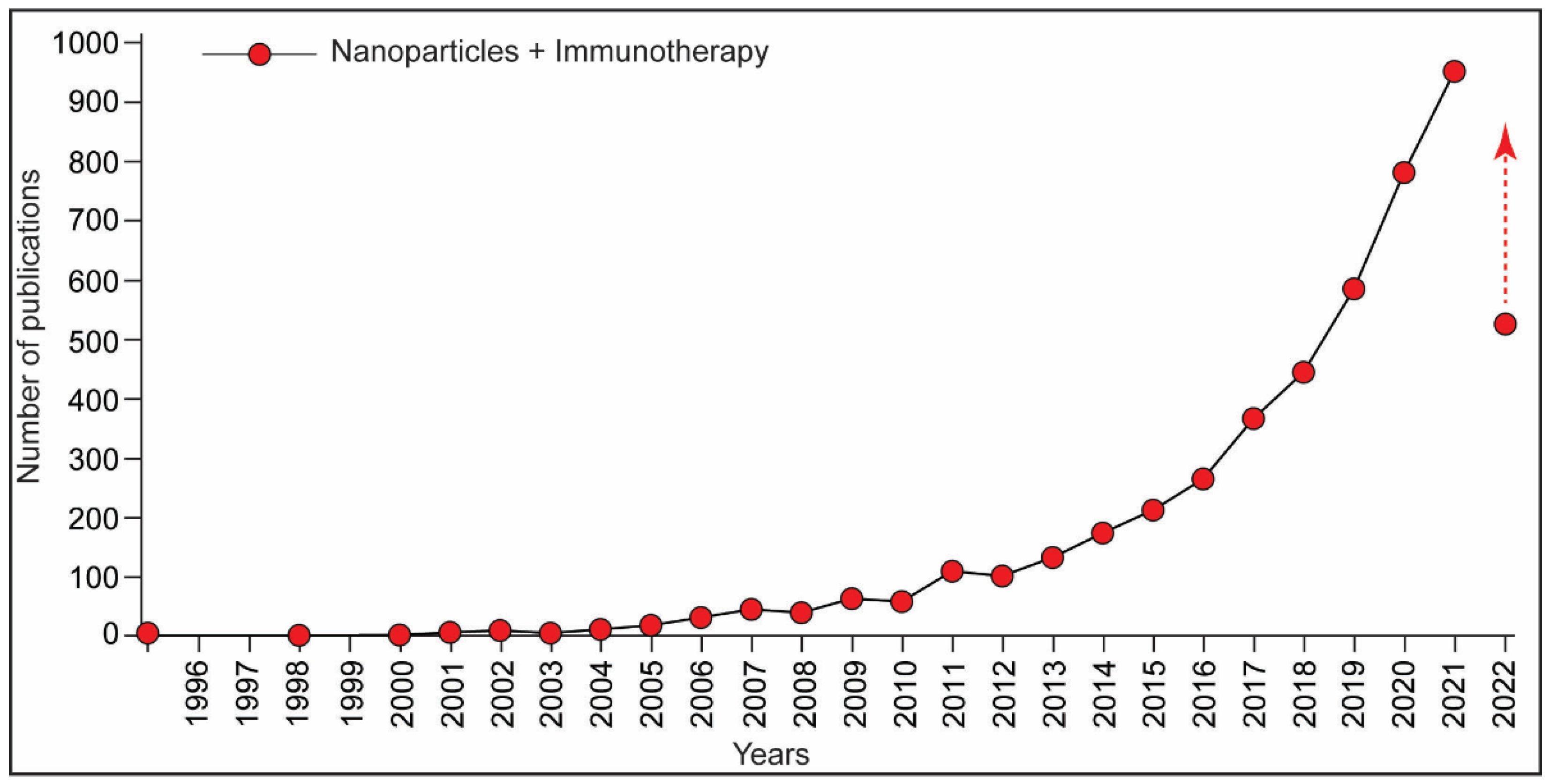
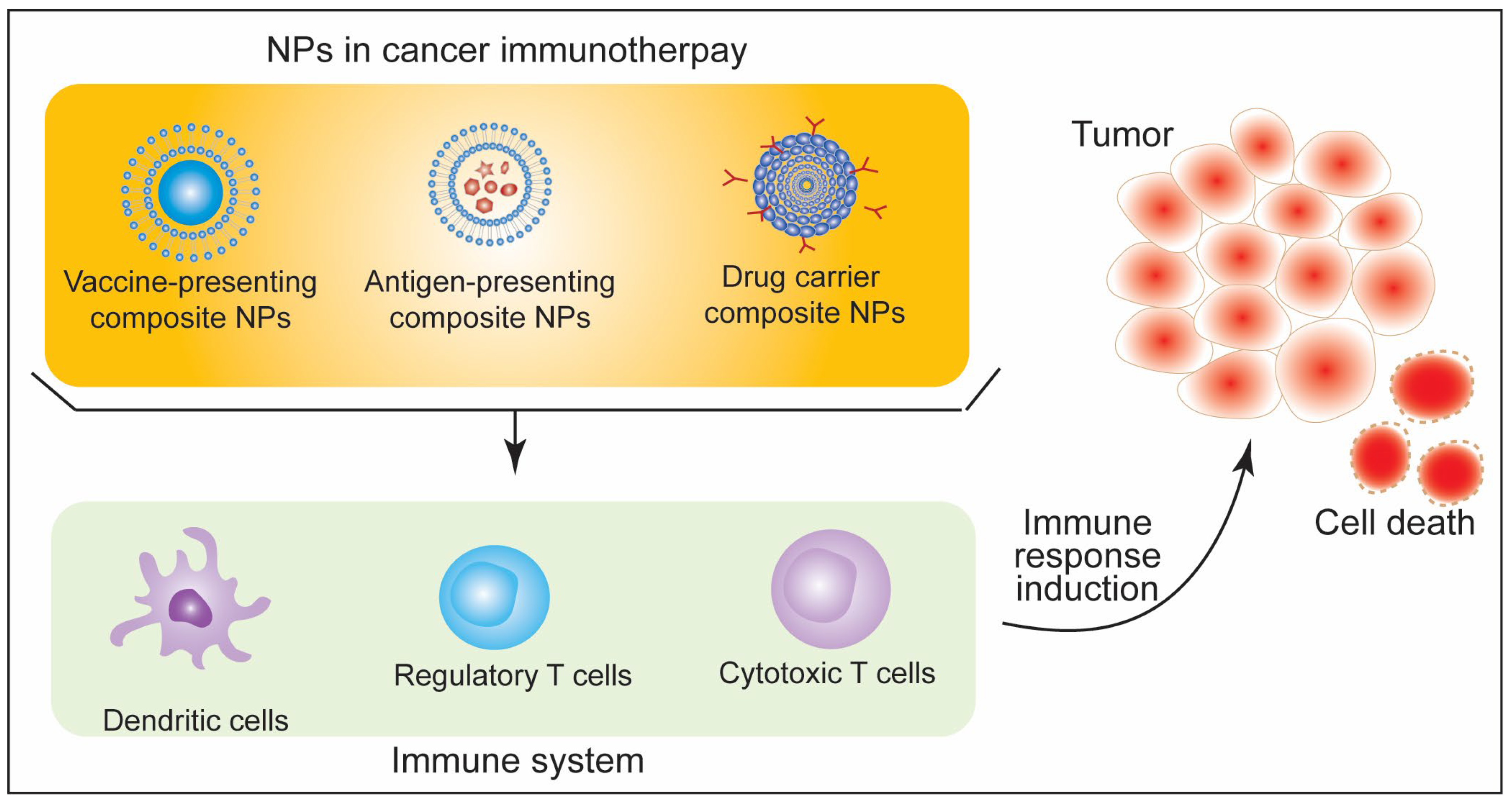
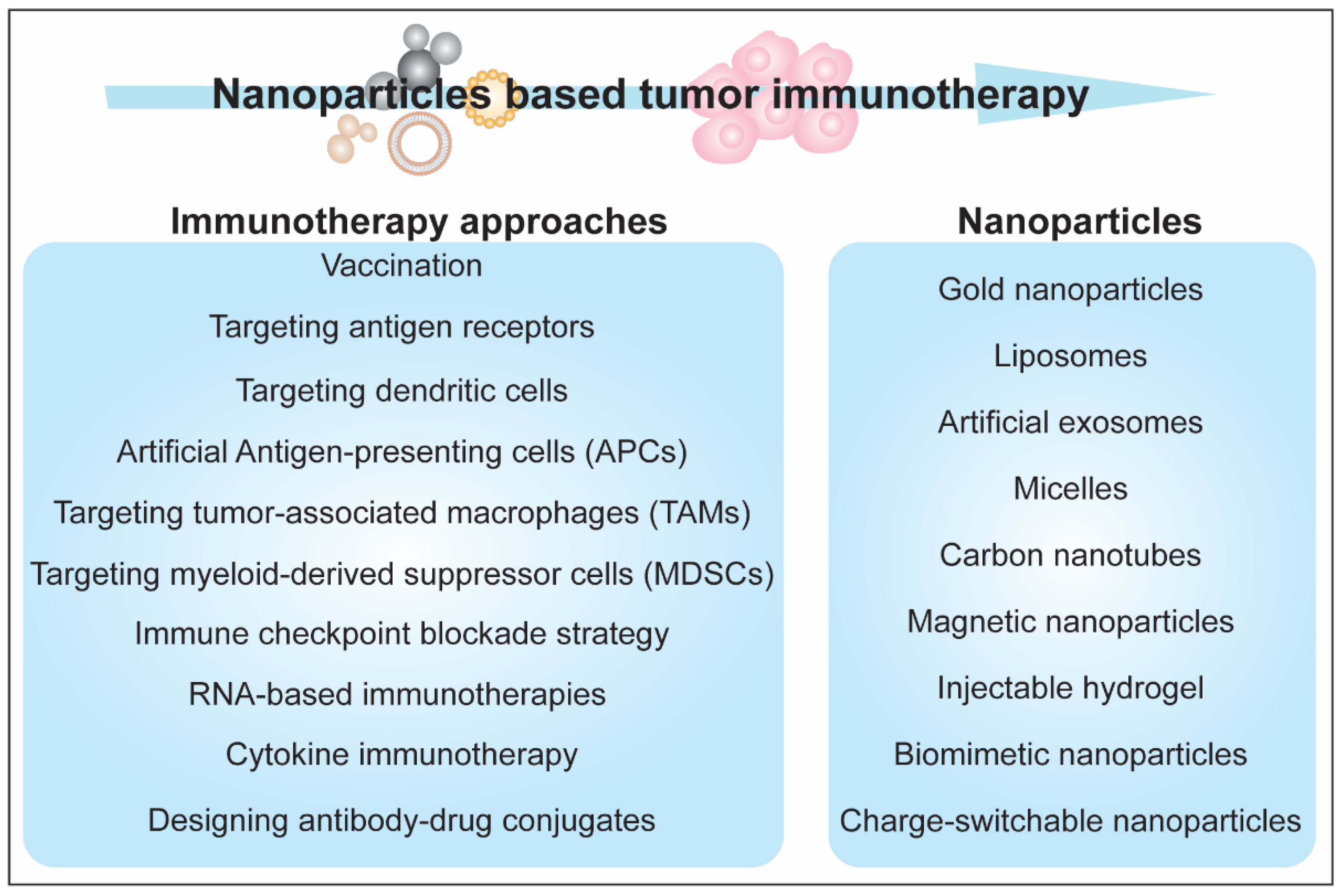
5. Immunotherapeutic Aspects of Nanoparticles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Seaton, A.; Tran, L.; Aitken, R.; Donaldson, K. Nanoparticles, human health hazard and regulation. J. R. Soc. Interface 2010, 7, S119–S129. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.Y.; Nash, A.; Notman, R. The aggregation of striped nanoparticles in mixed phospholipid bilayers. Nanoscale 2020, 12, 4868–4881. [Google Scholar] [CrossRef]
- Pandey, A.; Chandra, S.; Chauhan, L.K.; Narayan, G.; Chowdhuri, D.K. Cellular internalization and stress response of ingested amorphous silica nanoparticles in the midgut of Drosophila melanogaster. Biochim. Biophys. Acta 2013, 1830, 2256–2266. [Google Scholar] [CrossRef]
- Al-Fahdawi, M.Q.; Al-Doghachi, F.A.J.; Abdullah, Q.K.; Hammad, R.T.; Rasedee, A.; Ibrahim, W.N.; Alshwyeh, H.A.; Alosaimi, A.A.; Aldosary, S.K.; Eid, E.E.M.; et al. Oxidative stress cytotoxicity induced by platinum-doped magnesia nanoparticles in cancer cells. Biomed. Pharmacother. 2021, 138, 111483. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Kilic, G.; Costa, C.; Fernandez-Bertolez, N.; Pasaro, E.; Teixeira, J.P.; Laffon, B. Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2015, 56, 125–148. [Google Scholar] [CrossRef]
- Galbiati, V.; Cornaghi, L.; Gianazza, E.; Potenza, M.A.; Donetti, E.; Marinovich, M.; Corsini, E. In vitro assessment of silver nanoparticles immunotoxicity. Food Chem. Toxicol. 2018, 112, 363–374. [Google Scholar] [CrossRef]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2016, 2, 54–88. [Google Scholar] [CrossRef]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology, The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Loose, L.D.; Pittman, K.A.; Benitz, K.F.; Silkworth, J.B.; Mueller, W.; Coulston, F. Environmental chemical-induced immune dysfunction. Ecotoxicol. Environ. Saf. 1978, 2, 173–198. [Google Scholar] [CrossRef]
- Bunderson-Schelvan, M.; Pfau, J.C.; Crouch, R.; Holian, A. Nonpulmonary outcomes of asbestos exposure. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 122–152. [Google Scholar] [CrossRef]
- Uber, C.L.; McReynolds, R.A. Immunotoxicology of silica. Crit. Rev. Toxicol. 1982, 10, 303–319. [Google Scholar] [CrossRef]
- Attiq, A.; Yao, L.J.; Afzal, S.; Khan, M.A. The triumvirate of NF-kappaB, inflammation and cytokine storm in COVID-19. Int. Immunopharmacol. 2021, 101, 108255. [Google Scholar] [CrossRef]
- Pandey, A.; Mishra, A.K. From Innate Immunity to Inflammation: A Primer on Multiple Facets of NF-κB Signaling in COVID-19. Physiologia 2022, 2, 34–45. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, Y.C.; Yu, Y.H.; Tao, M.H.; Leung, P.S.; Ansari, A.A.; Gershwin, M.E.; Chuang, Y.H. Innate immunity drives xenobiotic-induced murine autoimmune cholangitis. Clin. Exp. Immunol. 2014, 177, 373–380. [Google Scholar] [CrossRef]
- Pollard, K.M.; Hultman, P.; Kono, D.H. Toxicology of autoimmune diseases. Chem. Res. Toxicol. 2010, 23, 455–466. [Google Scholar] [CrossRef]
- Pollard, K.M.; Christy, J.M.; Cauvi, D.M.; Kono, D.H. Environmental Xenobiotic Exposure and Autoimmunity. Curr. Opin. Toxicol. 2018, 10, 15–22. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sachan, N.; Mutsuddi, M.; Mukherjee, A. Kinase active Misshapen regulates Notch signaling in Drosophila melanogaster. Exp. Cell Res. 2015, 339, 51–60. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sharma, V.; Mutsuddi, M.; Mukherjee, A. Signaling cross-talk during development: Context-specific networking of Notch, NF-kappaB and JNK signaling pathways in Drosophila. Cell Signal. 2021, 82, 109937. [Google Scholar] [CrossRef]
- Weaver, L.N.; Drummond-Barbosa, D. The Nuclear Receptor Seven Up Regulates Genes Involved in Immunity and Xenobiotic Response in the Adult Drosophila Female Fat Body. G3 Genes Genomes Genet. 2020, 10, 4625–4635. [Google Scholar] [CrossRef]
- Crowe, W.; Allsopp, P.J.; Watson, G.E.; Magee, P.J.; Strain, J.J.; Armstrong, D.J.; Ball, E.; McSorley, E.M. Mercury as an environmental stimulus in the development of autoimmunity—A systematic review. Autoimmun. Rev. 2017, 16, 72–80. [Google Scholar] [CrossRef]
- Miller, F.W.; Alfredsson, L.; Costenbader, K.H.; Kamen, D.L.; Nelson, L.M.; Norris, J.M.; De Roos, A.J. Epidemiology of environmental exposures and human autoimmune diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J. Autoimmun. 2012, 39, 259–271. [Google Scholar] [CrossRef]
- Pollard, K.M.; Cauvi, D.M.; Toomey, C.B.; Morris, K.V.; Kono, D.H. Interferon-gamma and systemic autoimmunity. Discov. Med. 2013, 16, 123–131. [Google Scholar]
- Schiraldi, M.; Monestier, M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009, 30, 502–509. [Google Scholar] [CrossRef]
- Toomey, C.B.; Cauvi, D.M.; Pollard, K.M. The role of decay accelerating factor in environmentally induced and idiopathic systemic autoimmune disease. Autoimmune Dis. 2014, 2014, 452853. [Google Scholar] [CrossRef]
- Bautista-Olivier, C.D.; Elizondo, G. PXR as the tipping point between innate immune response, microbial infections, and drug metabolism. Biochem. Pharmacol. 2022, 202, 115147. [Google Scholar] [CrossRef]
- Corsiero, E.; Nerviani, A.; Bombardieri, M.; Pitzalis, C. Ectopic Lymphoid Structures: Powerhouse of Autoimmunity. Front. Immunol. 2016, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.W.; Jones, S.A. Ectopic lymphoid follicles: Inducible centres for generating antigen-specific immune responses within tissues. Immunology 2016, 147, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Vu Van, D.; Beier, K.C.; Pietzke, L.J.; Al Baz, M.S.; Feist, R.K.; Gurka, S.; Hamelmann, E.; Kroczek, R.A.; Hutloff, A. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat. Commun. 2016, 7, 10875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, S.; Chen, L.; Ye, L.; Jiang, Y.; Peng, H.; Guo, Z.; Li, M.; Jiang, X.; Guo, P.; et al. Single-cell transcriptomics reveals immune dysregulation mediated by IL-17A in initiation of chronic lung injuries upon real-ambient particulate matter exposure. Part. Fibre Toxicol. 2022, 19, 42. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Huang, E.; Peng, N.; Xiao, F.; Hu, D.; Wang, X.; Lu, L. The Roles of Immune Cells in the Pathogenesis of Fibrosis. Int. J. Mol. Sci. 2020, 21, 5203. [Google Scholar] [CrossRef]
- Chen, Y.; Surinkaew, S.; Naud, P.; Qi, X.Y.; Gillis, M.A.; Shi, Y.F.; Tardif, J.C.; Dobrev, D.; Nattel, S. JAK-STAT signalling and the atrial fibrillation promoting fibrotic substrate. Cardiovasc. Res. 2017, 113, 310–320. [Google Scholar] [CrossRef]
- Lawrence, J.; Nho, R. The Role of the Mammalian Target of Rapamycin (mTOR) in Pulmonary Fibrosis. Int. J. Mol. Sci. 2018, 19, 778. [Google Scholar] [CrossRef]
- Milara, J.; Hernandez, G.; Ballester, B.; Morell, A.; Roger, I.; Montero, P.; Escriva, J.; Lloris, J.M.; Molina-Molina, M.; Morcillo, E.; et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 24. [Google Scholar] [CrossRef]
- Wang, W.; Bhattacharyya, S.; Marangoni, R.G.; Carns, M.; Dennis-Aren, K.; Yeldandi, A.; Wei, J.; Varga, J. The JAK/STAT pathway is activated in systemic sclerosis and is effectively targeted by tofacitinib. J. Scleroderma Relat. Disord. 2020, 5, 40–50. [Google Scholar] [CrossRef]
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine 2018, 14, 977–990. [Google Scholar] [CrossRef]
- Chen, R.J.; Huang, C.C.; Pranata, R.; Lee, Y.H.; Chen, Y.Y.; Wu, Y.H.; Wang, Y.J. Modulation of Innate Immune Toxicity by Silver Nanoparticle Exposure and the Preventive Effects of Pterostilbene. Int. J. Mol. Sci. 2021, 22, 2536. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef]
- Frohlich, E. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. Int. J. Nanomed. 2015, 10, 3761–3778. [Google Scholar] [CrossRef] [Green Version]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Lee, A.R.; Lee, S.J.; Lee, M.; Nam, M.; Lee, S.; Choi, J.; Lee, H.J.; Kim, D.U.; Hoe, K.L. Editor’s Highlight: A Genome-wide Screening of Target Genes Against Silver Nanoparticles in Fission Yeast. Toxicol. Sci. 2018, 161, 171–185. [Google Scholar] [CrossRef]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Gonzalez-Fernandez, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Liz, R.; Simard, J.C.; Leonardi, L.B.; Girard, D. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int. Immunopharmacol. 2015, 28, 616–625. [Google Scholar] [CrossRef]
- Alsaleh, N.B.; Minarchick, V.C.; Mendoza, R.P.; Sharma, B.; Podila, R.; Brown, J.M. Silver nanoparticle immunomodulatory potential in absence of direct cytotoxicity in RAW 264.7 macrophages and MPRO 2.1 neutrophils. J. Immunotoxicol. 2019, 16, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Khalaf, A.A.; Tohamy, A.F.; Mohammed, E.R.; Farroh, K.Y. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int. J. Nanomed. 2019, 14, 4723–4739. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Fernandez, M.J.F.; Furst, P.; Gundert-Remy, U.; Gurtler, R.; Hysoy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- Auttachoat, W.; McLoughlin, C.E.; White, K.L., Jr.; Smith, M.J. Route-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nanoparticles. J. Immunotoxicol. 2014, 11, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Di Giampaolo, L.; Zaccariello, G.; Benedetti, A.; Vecchiotti, G.; Caposano, F.; Sabbioni, E.; Groppi, F.; Manenti, S.; Niu, Q.; Poma, A.M.G.; et al. Genotoxicity and Immunotoxicity of Titanium Dioxide-Embedded Mesoporous Silica Nanoparticles (TiO2@MSN) in Primary Peripheral Human Blood Mononuclear Cells (PBMC). Nanomaterials 2021, 11, 270. [Google Scholar] [CrossRef]
- Dhupal, M.; Oh, J.M.; Tripathy, D.R.; Kim, S.K.; Koh, S.B.; Park, K.S. Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation. Int. J. Nanomed. 2018, 13, 6735–6750. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Zhou, Y.; Wang, L. Immunotoxic effects of thymus in mice following exposure to nanoparticulate TiO2. Environ. Toxicol. 2017, 32, 2234–2243. [Google Scholar] [CrossRef]
- Chang, X.; Fu, Y.; Zhang, Y.; Tang, M.; Wang, B. Effects of Th1 and Th2 cells balance in pulmonary injury induced by nano titanium dioxide. Environ. Toxicol. Pharmacol. 2014, 37, 275–283. [Google Scholar] [CrossRef]
- Sang, X.; Fei, M.; Sheng, L.; Zhao, X.; Yu, X.; Hong, J.; Ze, Y.; Gui, S.; Sun, Q.; Ze, X.; et al. Immunomodulatory effects in the spleen-injured mice following exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 3562–3572. [Google Scholar] [CrossRef]
- Keerthana, S.; Kumar, A. Potential risks and benefits of zinc oxide nanoparticles: A systematic review. Crit. Rev. Toxicol. 2020, 50, 47–71. [Google Scholar] [CrossRef]
- Li, S.W.; Huang, C.W.; Liao, V.H. Early-life long-term exposure to ZnO nanoparticles suppresses innate immunity regulated by SKN-1/Nrf and the p38 MAPK signaling pathway in Caenorhabditis elegans. Environ. Pollut. 2020, 256, 113382. [Google Scholar] [CrossRef]
- Senapati, V.A.; Gupta, G.S.; Pandey, A.K.; Shanker, R.; Dhawan, A.; Kumar, A. Zinc oxide nanoparticle induced age dependent immunotoxicity in BALB/c mice. Toxicol. Res. 2017, 6, 342–352. [Google Scholar] [CrossRef]
- Abass, M.A.; Selim, S.A.; Selim, A.O.; El-Shal, A.S.; Gouda, Z.A. Effect of orally administered zinc oxide nanoparticles on albino rat thymus and spleen. IUBMB Life 2017, 69, 528–539. [Google Scholar] [CrossRef]
- Essa, S.S.; El-Saied, E.M.; El-Tawil, O.S.; Gamal, I.M.; El-Rahman, S.S.A. Nanoparticles of zinc oxide defeat chlorpyrifos-induced immunotoxic effects and histopathological alterations. Vet. World 2019, 12, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Cytotoxicity and cellular mechanisms involved in the toxicity of CdS quantum dots in hemocytes and gill cells of the mussel Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 39–52. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Yong, K.T.; Zhu, X.; Lin, M.C.; Jiang, W.; Li, J.; Huang, Q.; Lin, G. Immunotoxicity assessment of CdSe/ZnS quantum dots in macrophages, lymphocytes and BALB/c mice. J. Nanobiotechnol. 2016, 14, 10. [Google Scholar] [CrossRef]
- Wu, T.; Liang, X.; He, K.; Liu, X.; Li, Y.; Wang, Y.; Kong, L.; Tang, M. The NLRP3-Mediated Neuroinflammatory Responses to CdTe Quantum Dots and the Protection of ZnS Shell. Int. J. Nanomed. 2020, 15, 3217–3233. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. Biomed. Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Deng, Y.; Liao, X.; Zhang, J.; Cheng, B.; Cao, Z.; Lu, H. Graphene oxide nanoparticles induce hepatic dysfunction through the regulation of innate immune signaling in zebrafish (Danio rerio). Nanotoxicology 2020, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Kim, J.H. Differential Immunomodulatory Effect of Graphene Oxide and Vanillin-Functionalized Graphene Oxide Nanoparticles in Human Acute Monocytic Leukemia Cell Line (THP-1). Int. J. Mol. Sci. 2019, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.; Lysaght, J.; Liptrott, N.J.; Prina-Mello, A. Immunotoxicity Considerations for Next Generation Cancer Nanomedicines. Adv. Sci. 2019, 6, 1900133. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Tzemach, D.; Mak, L.; Bronstein, M.; Horowitz, A.T. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J. Drug Target 2002, 10, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Shaunak, S.; Thomas, S.; Gianasi, E.; Godwin, A.; Jones, E.; Teo, I.; Mireskandari, K.; Luthert, P.; Duncan, R.; Patterson, S.; et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat. Biotechnol. 2004, 22, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Kendall, M.; Lynch, I. Long-term monitoring for nanomedicine implants and drugs. Nat. Nanotechnol. 2016, 11, 206–210. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; Haanen, J.B.; Punt, C.J. Cancer immunotherapy—Revisited. Nat. Rev. Drug Discov. 2011, 10, 591–600. [Google Scholar] [CrossRef]
- Adams, J.L.; Smothers, J.; Srinivasan, R.; Hoos, A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 2015, 14, 603–622. [Google Scholar] [CrossRef]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Toy, R.; Hayden, E.; Shoup, C.; Baskaran, H.; Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011, 22, 115101. [Google Scholar] [CrossRef]
- Toy, R.; Hayden, E.; Camann, A.; Berman, Z.; Vicente, P.; Tran, E.; Meyers, J.; Pansky, J.; Peiris, P.M.; Wu, H.; et al. Multimodal in vivo imaging exposes the voyage of nanoparticles in tumor microcirculation. ACS Nano 2013, 7, 3118–3129. [Google Scholar] [CrossRef]
- Muro, S.; Garnacho, C.; Champion, J.A.; Leferovich, J.; Gajewski, C.; Schuchman, E.H.; Mitragotri, S.; Muzykantov, V.R. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008, 16, 1450–1458. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of shape, size, and aspect ratio on nanoparticle penetration and distribution inside solid tissues using 3D spheroid models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control Release 2015, 220, 141–148. [Google Scholar] [CrossRef]
- Fromen, C.A.; Robbins, G.R.; Shen, T.W.; Kai, M.P.; Ting, J.P.; DeSimone, J.M. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc. Natl. Acad. Sci. USA 2015, 112, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Smyth, N.R.; Muskens, O.L.; Nitti, S.; Heuer-Jungemann, A.; Ardern-Jones, M.R.; Kanaras, A.G. Interactions of skin with gold nanoparticles of different surface charge, shape, and functionality. Small 2015, 11, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Huang, L. Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials 2014, 35, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Ahmad, A.; Tarique, M.; Suhail, M.; Zughaibi, T.A.; Tabrez, S.; Khan, R. Advancement of Cancer Immunotherapy Using Nanoparticles-Based Nanomedicine. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Fadel, T.R.; Steenblock, E.R.; Stern, E.; Li, N.; Wang, X.; Haller, G.L.; Pfefferle, L.D.; Fahmy, T.M. Enhanced cellular activation with single walled carbon nanotube bundles presenting antibody stimuli. Nano Lett. 2008, 8, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, M.; Martinson, H.; Mann, K.; Anchordoquy, T.J. Complement C3 mediated targeting of liposomes to granulocytic myeloid derived suppressor cells. Nanomedicine 2015, 11, 1355–1363. [Google Scholar] [CrossRef]
- Li, K.; Chang, S.; Wang, Z.; Zhao, X.; Chen, D. A novel micro-emulsion and micelle assembling method to prepare DEC205 monoclonal antibody coupled cationic nanoliposomes for simulating exosomes to target dendritic cells. Int. J. Pharm. 2015, 491, 105–112. [Google Scholar] [CrossRef]
- Thomas, S.N.; Vokali, E.; Lund, A.W.; Hubbell, J.A.; Swartz, M.A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 2014, 35, 814–824. [Google Scholar] [CrossRef]
- Yuba, E.; Kanda, Y.; Yoshizaki, Y.; Teranishi, R.; Harada, A.; Sugiura, K.; Izawa, T.; Yamate, J.; Sakaguchi, N.; Koiwai, K.; et al. pH-sensitive polymer-liposome-based antigen delivery systems potentiated with interferon-gamma gene lipoplex for efficient cancer immunotherapy. Biomaterials 2015, 67, 214–224. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Du, J.; Li, Y.; Zhou, Y.; Fu, Q.; Zhang, J.; Wang, X.; Zhan, L. Different-Sized Gold Nanoparticle Activator/Antigen Increases Dendritic Cells Accumulation in Liver-Draining Lymph Nodes and CD8+ T Cell Responses. ACS Nano 2016, 10, 2678–2692. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Chen, X.; Nie, X.; Xue, F.; Xu, W.; Luan, Y. An Injectable Hydrogel to Modulate T Cells for Cancer Immunotherapy. Small 2022, 18, e2202663. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Tan, N. Cancer-cell-biomimetic nanoparticles systemically eliminate hypoxia tumors by synergistic chemotherapy and checkpoint blockade immunotherapy. Acta Pharm. Sin. B 2022, 12, 2103–2119. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Wang, Y.J.; Han, Y.; Zhao, M.; Hu, H.; Qiao, M.; Chen, D. Blockage of the IDO1 pathway by charge-switchable nanoparticles amplifies immunogenic cell death for enhanced cancer immunotherapy. Acta Biomater. 2022, 22, 415–419. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Liu, J.; Xu, M.; Ji, H.; Wu, S.; Chen, D.; Hu, H. Charge-switchable nanoparticles enhance Cancer immunotherapy based on mitochondrial dynamic regulation and immunogenic cell death induction. J. Control. Release 2021, 335, 320–332. [Google Scholar] [CrossRef]
- Raza, A.; Rossi, G.R.; Janjua, T.I.; Souza-Fonseca-Guimaraes, F.; Popat, A. Nanobiomaterials to modulate natural killer cell responses for effective cancer immunotherapy. Trends Biotechnol. 2022, in press. [Google Scholar] [CrossRef]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Gao, J.; Wal, R.V.; Gigliotti, A.; Burchiel, S.W.; McDonald, J.D. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007, 100, 203–214. [Google Scholar] [CrossRef]
- Moraes, A.S.; Paula, R.F.; Pradella, F.; Santos, M.P.; Oliveira, E.C.; von Glehn, F.; Camilo, D.S.; Ceragioli, H.; Peterlevitz, A.; Baranauskas, V.; et al. The suppressive effect of IL-27 on encephalitogenic Th17 cells induced by multiwalled carbon nanotubes reduces the severity of experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2013, 19, 682–687. [Google Scholar] [CrossRef]
- Ryan, J.J.; Bateman, H.R.; Stover, A.; Gomez, G.; Norton, S.K.; Zhao, W.; Schwartz, L.B.; Lenk, R.; Kepley, C.L. Fullerene nanomaterials inhibit the allergic response. J. Immunol. 2007, 179, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartzwelter, B.J.; Barbero, F.; Verde, A.; Mangini, M.; Pirozzi, M.; De Luca, A.C.; Puntes, V.F.; Leite, L.C.C.; Italiani, P.; Boraschi, D. Gold Nanoparticles Modulate BCG-Induced Innate Immune Memory in Human Monocytes by Shifting the Memory Response towards Tolerance. Cells 2020, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.P.; Shen, C.C.; Huang, C.H.; Lin, Y.C.; Jan, T.R. Iron oxide nanoparticles attenuate T helper 17 cell responses in vitro and in vivo. Int. Immunopharmacol. 2018, 58, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef]
- McCarthy, D.P.; Hunter, Z.N.; Chackerian, B.; Shea, L.D.; Miller, S.D. Targeted immunomodulation using antigen-conjugated nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 298–315. [Google Scholar] [CrossRef]
- Serra, P.; Santamaria, P. Nanoparticle-based autoimmune disease therapy. Clin. Immunol. 2015, 160, 3–13. [Google Scholar] [CrossRef]
- Mitarotonda, R.; Giorgi, E.; Eufrasio-da-Silva, T.; Dolatshahi-Pirouz, A.; Mishra, Y.K.; Khademhosseini, A.; Desimone, M.F.; De Marzi, M.; Orive, G. Immunotherapeutic nanoparticles: From autoimmune disease control to the development of vaccines. Biomater. Adv. 2022, 135, 212726. [Google Scholar] [CrossRef]
- Kroll, A.V.; Jiang, Y.; Zhou, J.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanoparticle Vaccines for Cancer Therapy. Adv. Biosyst. 2019, 3, e1800219. [Google Scholar] [CrossRef]
- Li, A.V.; Moon, J.J.; Abraham, W.; Suh, H.; Elkhader, J.; Seidman, M.A.; Yen, M.; Im, E.J.; Foley, M.H.; Barouch, D.H.; et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 2013, 5, 204ra130. [Google Scholar] [CrossRef]
- Stano, A.; Scott, E.A.; Dane, K.Y.; Swartz, M.A.; Hubbell, J.A. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 2013, 34, 4339–4346. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, H.; Zhao, Q.; Wang, S.; Zou, M.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358. [Google Scholar] [CrossRef]
- Thomann-Harwood, L.J.; Kaeuper, P.; Rossi, N.; Milona, P.; Herrmann, B.; McCullough, K.C. Nanogel vaccines targeting dendritic cells: Contributions of the surface decoration and vaccine cargo on cell targeting and activation. J. Control. Release 2013, 166, 95–105. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Mishra, A.K. Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles. BioTech 2022, 11, 42. https://doi.org/10.3390/biotech11030042
Pandey A, Mishra AK. Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles. BioTech. 2022; 11(3):42. https://doi.org/10.3390/biotech11030042
Chicago/Turabian StylePandey, Ashutosh, and Abhinava K. Mishra. 2022. "Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles" BioTech 11, no. 3: 42. https://doi.org/10.3390/biotech11030042
APA StylePandey, A., & Mishra, A. K. (2022). Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles. BioTech, 11(3), 42. https://doi.org/10.3390/biotech11030042






