Biotechnological and Medical Aspects of Lactic Acid Bacteria Used for Plant Protection: A Comprehensive Review
Abstract
:1. Introduction
2. Current Status of Pesticides
3. Transition to Biopesticides: A Human Health-Based Perspective
Negative Effects of Fungicides Compared to Biofungicides
4. LAB Species and Their Antifungal Compounds
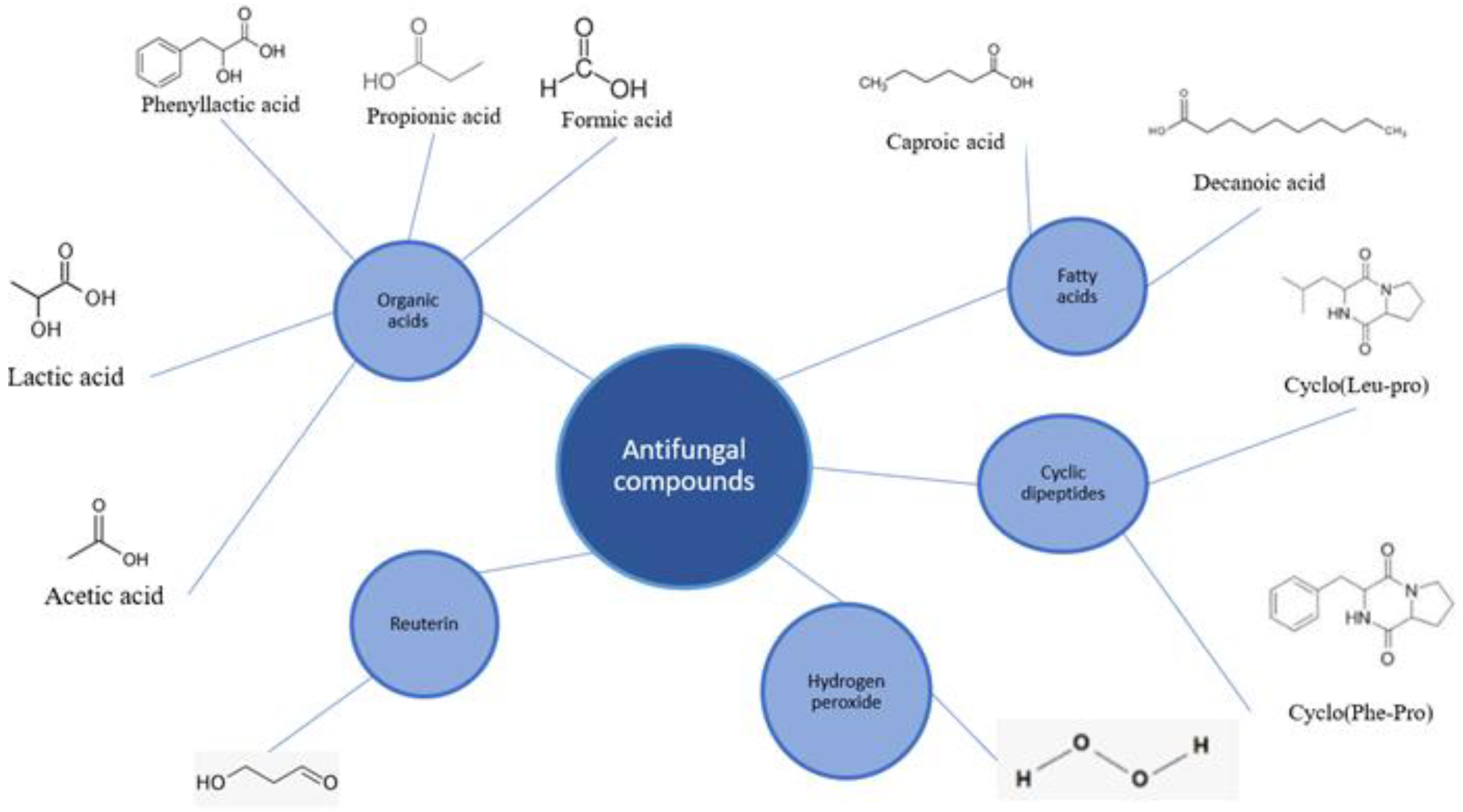
4.1. Antifungal Compounds Produced by LABs
4.1.1. Organic Acids
4.1.2. Reuterin
4.1.3. Fatty Acids
4.1.4. Cyclic Dipeptides
4.1.5. Hydrogen Peroxide
4.1.6. Proteinaceous Compounds
5. Mycotoxins
5.1. Mycotoxin Degradation
5.2. Mycotoxin Adsorption
5.3. Effect Fusarium Mycotoxins on Human and Animal Health
6. Symbiotic Relations between Plants and LABs
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bakker, M.G.; Brown, D.W.; Kelly, A.C.; Kim, H.S.; Kurtzman, C.P.; Mccormick, S.P.; O’Donnell, K.L.; Proctor, R.H.; Vaughan, M.M.; Ward, T.J. Fusarium mycotoxins: A trans-disciplinary overview. Can. J. Plant Pathol. 2018, 40, 161–171. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Proctor, R.H. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Zhang, Y.; Teng, Y.; Xu, Z. Effects of fungicide iprodione and nitrification inhibitor 3, 4-dimethylpyrazole phosphate on soil enzyme and bacterial properties. Sci. Total Environ. 2017, 599, 254–263. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a traditional Mexican alcoholic fermented beverage: Historical, microbiological, and technical aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef]
- Liu, A.; Li, X.; Pu, B.; Ao, X.; Zhou, K.; He, L.; Liu, S. Use of psychrotolerant lactic acid bacteria (Lactobacillus spp. and Leuconostoc spp.) Isolated from Chinese Traditional Paocai for the Quality Improvement of Paocai Products. J. Agric. Food Chem. 2017, 65, 2580–2587. [Google Scholar] [CrossRef]
- Schmidt, M.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Fundamental study on the improvement of the antifungal activity of Lactobacillus reuteri R29 through increased production of phenyllactic acid and reuterin. Food Control 2018, 88, 139–148. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- López-Seijas, J.; García-Fraga, B.; da Silva, A.F.; Sieiro, C. Wine Lactic Acid Bacteria with Antimicrobial Activity as Potential Biocontrol Agents against Fusarium oxysporum f. sp. lycopersici. Agronomy 2020, 10, 31. [Google Scholar] [CrossRef]
- Paul, D.; Mandal, S.M. Microbial Adaptation and Resistance to Pesticides. In Bacterial Adaptation to Co-Resistance; Springer: Singapore, 2019; pp. 233–249. [Google Scholar]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- FAO. Pesticide Use. 2020. Available online: http://www.fao.org/faostat/en/#data/RP/visualize (accessed on 1 November 2020).
- Xie, Y.; Li, J.; Guo, X.; Zhao, J.; Yang, B.; Xiao, W.; Yang, H. Health status among greenhouse workers exposed to different levels of pesticides: A genetic matching analysis. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Langer, P. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front. Neuroendocrinol. 2010, 31, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Malalgoda, M.; Simsek, S. Pesticide residue in grain-based food: Effects on health, grain quality, and chemical properties of biomacromolecules. Cereal Chem. 2020, 98, 8–16. [Google Scholar] [CrossRef]
- Hamadamin, A.Y.; Hassan, K.I. Gas chromatography–mass spectrometry based sensitive analytical approach to detect and quantify non-polar pesticides accumulated in the fat tissues of domestic animals. Saudi J. Biol. Sci. 2020, 27, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Baibakova, E.V.; Nefedjeva, E.E.; Suska-Malawska, M.; Wilk, M.; Sevriukova, G.A.; Zheltobriukhov, V.F. Modern Fungicides: Mechanisms of Action, Fungal Resistance and Phytotoxic Effects. Annu. Res. Rev. Biol. 2019, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, G.; Gao, B.; Hu, K.; Kaziem, A.E.; Li, L.; He, Z.; Shi, H.; Wang, M. Stereoselective endocrine-disrupting effects of the chiral triazole fungicide prothioconazole and its chiral metabolite. Environ. Pollut. 2019, 251, 30–36. [Google Scholar] [CrossRef]
- Shrestha, S.; Parks, C.G.; Goldner, W.S.; Kamel, F.; Umbach, D.M.; Ward, M.H.; Sandler, D.P. Incident thyroid disease in female spouses of private pesticide applicators. Environ. Int. 2018, 118, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Pathan, S.I. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Hirt, H. Healthy soils for healthy plants for healthy humans: How beneficial microbes in the soil, food and gut are interconnected and how agriculture can contribute to human health. EMBO Rep. 2020, 21, e51069. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Ludwicki, J.K. Biopesticides–towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present status and the future prospects. J Fertil Pestic 2015, 6, 100–129. [Google Scholar] [CrossRef]
- Lee, J.J.; Choi, Y.J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.R.; Lee, M.A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef]
- Mandal, V.; Sen, S.K.; Mandal, N.C. Detection, isolation and partial characterization of antifungal compound (s) produced by Pediococcus acidilactici LAB 5. Nat. Prod. Commun. 2007, 2, 671–674. [Google Scholar] [CrossRef]
- Gerez, C.L.; Carbajo, M.S.; Rollán, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef]
- Sellamani, M.; Kalagatur, N.K.; Siddaiah, C.; Mudili, V.; Krishna, K.; Natarajan, G.; Rao Putcha, V.L. Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 2016, 7, 890. [Google Scholar] [CrossRef]
- Bulgasem, B.Y.; Lani, M.N.; Hassan, Z.; Yusoff, W.M.W.; Fnaish, S.G. Antifungal activity of lactic acid bacteria strains isolated from natural honey against pathogenic Candida species. Mycobiology 2016, 44, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilavenil, S.; Vijayakumar, M.; Kim, D.H.; Valan Arasu, M.; Park, H.S.; Ravikumar, S.; Choi, K.C. Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J. Sci. Food Agric. 2016, 96, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Bustos, A.Y.; de Valdez, G.F.; Gerez, C.L. Optimization of phenyllactic acid production by Pediococcus acidilactici CRL 1753. Application of the formulated bio-preserver culture in bread. Biol. Control. 2018, 123, 137–143. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.S. Antifungal activities against Candida albicans, of cell-free supernatants obtained from probiotic Pediococcus acidilactici HW01. Arch. Oral Biol. 2019, 99, 113–119. [Google Scholar] [CrossRef]
- Valerio, F.; Di Biase, M.; Lattanzio, V.M.; Lavermicocca, P. Improvement of the antifungal activity of lactic acid bacteria by addition to the growth medium of phenylpyruvic acid, a precursor of phenyllactic acid. Int. J. Food Microbiol. 2016, 222, 1–7. [Google Scholar] [CrossRef]
- Voulgari, K.; Hatzikamari, M.; Delepoglou, A.; Georgakopoulos, P.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Antifungal activity of non-starter lactic acid bacteria isolates from dairy products. Food Control 2010, 21, 136–142. [Google Scholar] [CrossRef]
- Le Lay, C.; Mounier, J.; Vasseur, V.; Weill, A.; Le Blay, G.; Barbier, G.; Coton, E. In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 2016, 60, 247–255. [Google Scholar] [CrossRef]
- Yépez, A.; Luz, C.; Meca, G.; Vignolo, G.; Mañes, J.; Aznar, R. Biopreservation potential of lactic acid bacteria from Andean fermented food of vegetal origin. Food Control 2017, 78, 393–400. [Google Scholar] [CrossRef]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and L actobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing C andida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef]
- Adedokun, E.O.; Rather, I.A.; Bajpai, V.K.; Park, Y.H. Biocontrol efficacy of Lactobacillus fermentum YML014 against food spoilage moulds using the tomato puree model. Front. Life Sci. 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Bian, X.; Muhammad, Z.; Evivie, S.E.; Luo, G.W.; Xu, M.; Huo, G.C. Screening of antifungal potentials of Lactobacillus helveticus KLDS 1.8701 against spoilage microorganism and their effects on physicochemical properties and shelf life of fermented soybean milk during preservation. Food Control 2016, 66, 183–189. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicki, R.; Klewicka, E.; Kołodziejczyk, K.; Sójka, M.; Nowak, A. Antifungal activity of lactobacillus sp. bacteria in the presence of xylitol and galactosyl-xylitol. BioMed Res. Int. 2016, 5897486. [Google Scholar]
- Huh, C.K.; Hwang, T.Y. Identification of antifungal substances of Lactobacillus sakei subsp. ALI033 and antifungal activity against Penicillium brevicompactum strain FI02. Prev. Nutr. Food Sci. 2016, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Garric, G.; Harel-Oger, M.; Chatel, M.; Coton, E. Antifungal activity of lactic acid bacteria combinations in dairy mimicking models and their potential as bioprotective cultures in pilot scale applications. Front. Microbiol. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Romanens, E.; Leischtfeld, S.F.; Volland, A.; Stevens, M.J.; Krähenmann, U.; Isele, D.; Schwenninger, S.M. Screening of lactic acid bacteria and yeast strains to select adapted anti-fungal co-cultures for cocoa bean fermentation. Int. J. Food Microbiol. 2019, 290, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Janashia, I.; Choiset, Y.; Jozefiak, D.; Déniel, F.; Coton, E.; Moosavi-Movahedi, A.A.; Haertlé, T. Beneficial protective role of endogenous lactic acid bacteria against mycotic contamination of honeybee beebread. Probiotics Antimicrob. Proteins 2018, 10, 638–646. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; Beux, M.; Pagnoncelli, M.G.B.; Soccol, V.T.; Rodrigues, C.; Soccol, C.R. Isolation, selection and evaluation of antagonistic yeasts and lactic acid bacteria against ochratoxigenic fungus Aspergillus westerdijkiae on coffee beans. Lett. Appl. Microbiol. 2016, 62, 96–101. [Google Scholar] [CrossRef]
- Fernandez, B.; Vimont, A.; Desfossés-Foucault, É.; Daga, M.; Arora, G.; Fliss, I. Antifungal activity of lactic and propionic acid bacteria and their potential as protective culture in cottage cheese. Food Control 2017, 78, 350–356. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A. In vitro antifungal, probiotic, and antioxidant functional properties of a novel Lactobacillus paraplantarum isolated from fermented dates in Saudi Arabia. J. Sci. Food Agric. 2017, 97, 5287–5295. [Google Scholar] [CrossRef] [PubMed]
- Kharazian, Z.A.; Jouzani, G.S.; Aghdasi, M.; Khorvash, M.; Zamani, M.; Mohammadzadeh, H. Biocontrol potential of Lactobacillus strains isolated from corn silages against some plant pathogenic fungi. Biol. Control 2017, 110, 33–43. [Google Scholar] [CrossRef]
- Lačanin, I.; Mounier, J.; Pawtowski, A.; Dušková, M.; Kameník, J.; Karpíšková, R. Assessment of the antifungal activity of Lactobacillus and Pediococcus spp. for use as bioprotective cultures in dairy products. World J. Microbiol. Biotechnol. 2017, 33, 188. [Google Scholar] [CrossRef]
- Mieszkin, S.; Hymery, N.; Debaets, S.; Coton, E.; Le Blay, G.; Valence, F.; Mounier, J. Action mechanisms involved in the bioprotective effect of Lactobacillus harbinensis K. V9. 3.1. Np against Yarrowia lipolytica in fermented milk. Int. J. Food Microbiol. 2017, 248, 47–55. [Google Scholar] [CrossRef]
- Barman, S.; Ghosh, R.; Sengupta, S.; Mandal, N.C. Longterm storage of post-packaged bread by controlling spoilage pathogens using Lactobacillus fermentum C14 isolated from homemade curd. PloS ONE 2017, 12, e0184020. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018, 89, 99–106. [Google Scholar] [CrossRef]
- Bazukyan, I.; Matevosyan, L.; Toplaghaltsyan, A.; Trchounian, A. Antifungal activity of lactobacilli isolated from Armenian dairy products: An effective strain and its probable nature. AMB Express 2018, 8, 87. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef]
- Peláez, A.L.; Cataño, C.S.; Yepes, E.Q.; Villarroel, R.G.; De Antoni, G.L.; Giannuzzi, L. Inhibitory activity of lactic and acetic acid on Aspergillus flavus growth for food preservation. Food Control 2012, 24, 177–183. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; Rossi, J.; Damiani, P. Antimould activity of sourdough lactic acid bacteria: Identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 1998, 50, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.; Plumridge, A.; Nebe-von-Caron, G.; Archer, D.B. Inhibition of spoilage mould conidia by acetic acid and sorbic acid involves different modes of action, requiring modification of the classical weak-acid theory. Int. J. Food Microbiol. 2009, 136, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ström, K.; Sjögren, J.; Broberg, A.; Schnürer, J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo (L-Phe-L-Pro) and cyclo (L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef] [PubMed]
- Aunsbjerg, S.D.; Honoré, A.H.; Marcussen, J.; Ebrahimi, P.; Vogensen, F.K.; Benfeldt, C.; Skov, T.; Knøchel, S. Contribution of volatiles to the antifungal effect of Lactobacillus paracasei in defined medium and yogurt. Int. J. Food Microbiol. 2015, 194, 46–53. [Google Scholar] [CrossRef]
- Vimont, A.; Fernandez, B.; Ahmed, G.; Fortin, H.P.; Fliss, I. Quantitative antifungal activity of reuterin against food isolates of yeasts and moulds and its potential application in yogurt. Int. J. Food Microbiol. 2019, 289, 182–188. [Google Scholar] [CrossRef]
- Sjörgen, S.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar]
- Honoré, A.H.; Aunsbjerg, S.D.; Ebrahimi, P.; Thorsen, M.; Benfeldt, C.; Knøchel, S.; Skov, T. Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal. Bioanal. Chem. 2016, 408, 83–96. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, Y.S.; Chang, H.C. Purification and characterization of antifungal δ-dodecalactone from Lactobacillus plantarum AF1 isolated from kimchi. J. Food Prot. 2011, 74, 651–657. [Google Scholar] [CrossRef]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-activity-producing lactic acid bacteria as biocontrol agents in plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef]
- Baek, E.; Kim, H.; Choi, H.; Yoon, S.; Kim, J. Antifungal activity of Leuconostoc citreum and Weissella confusa in rice cakes. J. Microbiol. 2012, 50, 842–848. [Google Scholar] [CrossRef]
- Valerio, F.; Favilla, M.; De Bellis, P.; Sisto, A.; de Candia, S.; Lavermicocca, P. Antifungal activity of strains of lactic acid bacteria isolated from a semolina ecosystem against Penicillium roqueforti, Aspergillus niger and Endomyces fibuliger contaminating bakery products. Syst. Appl. Microbiol. 2009, 32, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Coloretti, F.; Carri, S.; Armaforte, E.; Chiavari, C.; Grazia, L.; Zambonelli, C. Antifungal activity of lactobacilli isolated from salami. FEMS Microbiol. Lett. 2007, 271, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.M.; Brosnan, B.; Furey, A.; Coffey, A.; Zannini, E.; Arendt, E.K. Lactic acid bacteria bioprotection applied to the malting process. Part I Strain Charact. Identif. Antifung. Compd. Food Control 2015, 51, 433–443. [Google Scholar]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Avis, T.J.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, Ó.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Black, B.A.; Zannini, E.; Curtis, J.M.; Gï, M.G. Antifungal hydroxy fatty acids produced during sourdough fermentation: Microbial and enzymatic pathways, and antifungal activity in bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Laitila, A.; Mattila-Sandholm, T.; Haikara, A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 1999, 86, 29–35. [Google Scholar] [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Yan, P.S.; Song, Y.; Sakuno, E.; Nakajima, H.; Nakagawa, H.; Yabe, K. Cyclo (L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 2004, 70, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.I.; Zhou, T.; Bullerman, L.B. Sourdough lactic acid bacteria as antifungal and mycotoxin-controlling agents. Food Sci. Technol. Int. 2016, 22, 79–90. [Google Scholar] [CrossRef]
- Ponts, N.; Couedelo, L.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Fusarium response to oxidative stress by H2O2 is trichothecene chemotype-dependent. FEMS Microbiol. Lett. 2009, 293, 255–262. [Google Scholar] [CrossRef]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.M.; Haertlé, T.; Choiset, Y.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hong, Y.; Deng, L.; Yi, L.; Zeng, K. Screening and characterization of lactic acid bacteria with antifungal activity against Penicillium digitatum on citrus. Biol. Control 2019, 138, 104044. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Bowen, Y.; Fengwei, T.; Jianxin, Z.; Hao, Z.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Garvey, G.S.; McCormick, S.P.; Rayment, I. Structural and Functional Characterization of the TRI101 Trichothecene 3-O-Acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum Kinetic insights to combating fusarium head blight. J. Biol. Chem. 2008, 283, 1660–1669. [Google Scholar] [CrossRef]
- Young, J.C.; Zhou, T.; Yu, H.; Zhu, H.; Gong, J. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 2007, 45, 136–143. [Google Scholar] [CrossRef]
- Kimura, M.; Kaneko, I.; Komiyama, M.; Takatsuki, A.; Koshino, H.; Yoneyama, K.; Yamaguchi, I. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins cloning and characterization of Tri101. J. Biol. Chem. 1998, 273, 1654–1661. [Google Scholar] [CrossRef]
- Tokai, T.; Fujimura, M.; Inoue, H.; Aoki, T.; Ohta, K.; Shibata, T.; Kimura, M. Concordant evolution of trichothecene 3-O-acetyltransferase and an rDNA species phylogeny of trichothecene-producing and non-producing fusaria and other ascomycetous fungi. Microbiology 2005, 151, 509–519. [Google Scholar] [CrossRef]
- Manoharan, M.; Dahleen, L.S.; Hohn, T.M.; Neate, S.M.; Yu, X.H.; Alexander, N.J.; Horsley, R.D. Expression of 3-OH trichothecene acetyltransferase in barley (Hordeum vulgare L.) and effects on deoxynivalenol. Plant Sci. 2006, 171, 699–706. [Google Scholar] [CrossRef]
- Chen, S.W.; Hsu, J.T.; Chou, Y.A.; Wang, H.T. The application of digestive tract lactic acid bacteria with high esterase activity for zearalenone detoxification. J. Sci. Food Agric. 2018, 98, 3870–3879. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Schauerhamer, B.; Christensen, C.M.; Niku-Paavola, M.L.; Nummi, M. Incidence of zearalenol (Fusarium mycotoxin) in animal feed. Appl. Environ. Microbiol. 1979, 38, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Verheecke, C.; Liboz, T.; Mathieu, F. Microbial degradation of aflatoxin B1: Current status and future advances. Int. J. Food Microbiol. 2016, 237, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perczak, A.; Goliński, P.; Bryła, M.; Waśkiewicz, A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arch. Ind. Hyg. Toxicol. 2018, 69, 32. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ohta, Y. Binding of mutagens by fractions of the cell wall skeleton of lactic acid bacteria on mutagens. J. Dairy Sci. 1991, 74, 1477–1481. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Fuchs, S.; Sontag, G.; Stidl, R.; Ehrlich, V.; Kundi, M.; Knasmüller, S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008, 46, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Niderkorn, V.; Morgavi, D.P.; Aboab, B.; Lemaire, M.; Boudra, H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J. Appl. Microbiol. 2009, 106, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef]
- Oatley, J.T.; Rarick, M.D.; Ji, G.E.; Linz, J.E. Binding of aflatoxin B1 to bifidobacteria in vitro. J. Food Prot. 2000, 63, 1133–1136. [Google Scholar] [CrossRef]
- Li, A.; Zheng, Y.; Liu, L.; Chen, S.; He, L.; Ao, X.; Liu, S. Decontamination of Aflatoxins by Lactic Acid Bacteria. Curr. Microbiol. 2020, 77, 3821–3830. [Google Scholar] [CrossRef]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef]
- Summerell, B.A. Resolving Fusarium: Current status of the genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Foster, G.D. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Reyes-Velázquez, W.P.; Anguiano-Sevilla, C.N.; Anguiano-Estrella, R.; Rojo, F.G. Association of acute equine leukoencephalomalacia (ELEM) with fumonisins concentrations in corn stover in an outbreak in the state of Jalisco, Mexico. Austral J. Vet. Sci. 2018, 50, 111–113. [Google Scholar] [CrossRef]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 109–143. [Google Scholar]
- Fedorka-Cray, P.J.; Gray, J.T.; Wray, C. Salmonella infections in pigs. Salmonella Domest. Anim. 2000, 191–207. [Google Scholar]
- Birmingham, C.L.; Smith, A.C.; Bakowski, M.A.; Yoshimori, T.; Brumell, J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006, 281, 11374–11383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Verbrugghe, E.; Vandenbroucke, V.; Dhaenens, M.; Shearer, N.; Goossens, J.; De Saeger, S.; Boyen, F. T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs, despite marked effects on Salmonella-host cell interactions. Vet. Res. 2012, 43, 22. [Google Scholar] [CrossRef]
- Shrestha, A.; Kim, B.S.; Park, D.H. Biological control of bacterial spot disease and plant growth-promoting effects of lactic acid bacteria on pepper. Biocontrol Sci. Technol. 2014, 24, 763–779. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Park, J.M.; Lee, S.M.; Lee, I.J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.Q.; Li, H.X. Inoculation of phosphate-solubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 252–259. [Google Scholar]
| Continents | Pesticide Use (tons) | Countries | Pesticide Use (tons) |
|---|---|---|---|
| Africa | 82,851 | China | 1,763,000 |
| Americas | 1,329,563 | USA | 407,776 |
| Asia | 2,161,869 | Brazil | 377,176 |
| Europe | 478,326 | Argentina | 172,928 |
| Oceania | 69,725 | Canada | 90,839 |
| Name of the LAB Species | Source | Activity Spectrum | Antifungal Agents | Initial pH d | Temperature d | References |
|---|---|---|---|---|---|---|
| Pediococcus species | ||||||
| Pd. acidilactici | A. fumigatus, A. parasitius, and F. oxysporum | A phenolic compound | 6.5/6.8 | 20/28/37 °C | [30] | |
| Pd. pentosaceus | Dairy products | P. digitatum and Geotrichum candidum var citri-aurantii | Organic acids | 6 | 30 °C | [31] |
| Pd. pentosaceus | Dairy products | F. graminearum | Phenolic antioxidants | 6–6.5 a | 37 °C | [32] |
| Pd. pentosaceus HM | Honey | C. krusei, C. glabrota, and C. albicans | - | 5.6 b | 35 °C | [33] |
| Pd. pentosaceus KCC-23 | Italian ryegrass | P. chrysogenum, F. oxysporum, P. roqueforti, Botrytis elliptica, and A. fumigatus | - | 6–6.5 a | 30 °C | [34] |
| Pd. acidilactici and P. pentosaceus | CC e | F. culmorum and F. poae | Organic acids | 6–6.5 a | 32/35 °C | [35] |
| Pd. acidilactici CRL 1753 | silage | A. niger, A. japonicus, P. roqueforti, and Metschnikowia pulcherrima | - | 6.5 | 37 °C | [36] |
| Pd. acidilactici | malt | C. albicans | Organic acids | 6–6.5 a | 37 °C | [37] |
| Leuconostoc species | ||||||
| L. citreum | Italian durum wheat semolina and whole durum wheat semolina | A. niger, P. roqueforti, and Endomyces fibuliger | Organic acids | 6–6.5 a | 30 °C | [38] |
| L. mesenteroides | Feta cheese and yoghurt | P. candidum and Debaryomyces hansenii | Bacteriocin | 6–6.5 a | 30 °C | [39] |
| L. spp. | Milk bread rolls and pound cakes | P. corylophilum, A. niger, Wallenia sebi, and Cladosporium sphaerospermum | - | 6–6.5 a | 30 °C | [40] |
| L. mesenteroides | Traditional fermented Andean food | Meyerozyma guillermondii, P. roqueforti, A. oryzae, and A. niger | Phenyllactic and 3,5-Di-O-caffeoylquinic acids | 6–6.5 a | 30 °C | [41] |
| Lactobacillus species | ||||||
| Lacticaseibacillus. rhamnosus GR-1 and Limosilactobacillus. reuteri RC-14 | CC e | C. glabrata | Aggregation abilities | 6–6.5 a | 37 °C | [42] |
| Limosilactobacillus fermentum | Cassava, a Nigerian fermented product | A. niger, A. flavus, and P. expansum | - | 6–6.5 a | 37 °C | [43] |
| L. helveticus | A dairy product | P. sp. | Organic acids | 6–6.5 a | 37 °C | [44] |
| Lacticaseibacillus paracasei LOCK0921 | Culture collection center | Alternari brassicicola | - | 6–6.5 a | 37 °C | [45] |
| Latilactobacillus sakei ALI033 | Kimchi | P. brevicompactum FIO2 | Organic acids | 6–6.5 a | 37 °C | [46] |
| Schleiferilactobacillus. harbinensis L172 | CCe | P. commune, Galactomyces, Y. lipolytica, and Mucor racemosus | - | 4.8–4.97 | 10–12 °C | [47] |
| Limosilactobacillus. fermentum | Cocoa bean | P. citrinum and G.moniliformis | Organic acids | 4–4.5 | 25 °C | [48] |
| Lentilactobacillus. buchneri UTAD104 | Silage | P. nordicum | Organic acids | 6–6.5 a | 30 °C | [49] |
| Apilactobacillus. kunkeei | Honeybee | Z. rouxii | - | 6–6.5 a | 34 °C | [50] |
| Limosilactobacillus. reuteri | Whole wheat sourdough | A. niger | n-Decanoic,3-hydroxydodecanoic acid and 3-hydroxydecanoic acid | 6–6.5 a | 37 °C | [51] |
| furfurilactobacillus Rossiae, the companilactobacillus group, and the Lentilactobacillus bucheri group | Milk bread rolls and pound cakes | P. corylophilum, A. niger, Wallenia sebi, and Cladosporium sphaerospermum | - | 6–6.5 a | 30 °C | [40] |
| the Schleiferilactobacillus perolens group | - | Eurotium repens, Wallenia sebi, and Cladosporium sphaerospermum | - | 6–6.5 a | 30 °C | [40] |
| Levilactobacillus. brevis LPBB03 | coffee fruit | A. Westerdijkiae | - | 6–6.5 a | 30 °C | [52] |
| Limosilactobacillus fermentum | Traditional fermented Andean products (chica and tocosh) | Meyerozyma guillermondii, P. roqueforti, Aspergilus oryzae, and A. niger | Phenyllactic and 3,5-Di-O-caffeoylquinic acids | 6–6.5 a | 30 °C | [41] |
| Lacticaseibacillus paracasei, Lactiplantibacillus pentosus, Lacticaseibacillus rhamnosus, Limosilactobacillus fermentum, and L. helveticus | Cheese | P. chrysogenum, Mucor racemosus, and Cladosporium harbarum | Organic acids | 6–6.5 a | 37 °C | [53] |
| Lactiplantibacillus paraplantarum | Fermented dates | A. fumigates, Curvularia lunata, F. oxysporum, Gibberella moniliformis, and P. chrysogenum | Organic acids | 3 | 37 °C | [54] |
| Lactiplantibacillus plantarum, companilactobacillus paralimentarius, Lactiplantibacillus pentosus, Lentilactobacillus buchneri, and Limosilactobacillus fermentum | Corn silage | F. verticilioides | - | 2.7/3.7/4.7/5.7/6.7/7.7/8.7 | 30 °C | [55] |
| Lacticaseibacillus paracasei SYR90 and Lacticaseibacillus rhamnosus BIOIII28 | Whey and Cheese samples | Y. lipotica, R. mucilaginosa, and P. brevicompactum | - | 6–6.5 a | 30 °C | [56] |
| Limosilactobacillus fermentum, L. sakei, and L. zeae | Cheese and meat | P. brevicompactum | - | 6–6.5 a | 30 °C | [56] |
| Schleiferilactobacillus harbinensis K.V9.3.1 Np | Cow milk | Y. lipotica | Organic acids | 6–6.5 a | 30 °C | [57] |
| Limosilactobacillus fermentum C14 | Homemade curd | P. digitatum, Mucor sp. and Trichophyton rubrum | Organic acids | 6–6.5 a | 28 °C | [58] |
| Lacticaseibacillus rhamnosus A238 | Biena culture collection (st-Hyacinthe, QC, Canada) | P. chrysogenum | Organic acids | 6–6.5 a | 37 °C | [53] |
| Latilactobacillus. sakei | CC e | F. culmorum and F. Poae | Organic acids | 6–6.5 a | 30 °C | [35] |
| Lactiplantibacillus pentosus LAP1 | A fermented fish product | C. tropicalis, C. albicans and C. krusei | - | 3/4/5/6 | 30 °C | [59] |
| Apilactobacillus kunkeei | Honeybee beebread | A. niger, Zygosaccharomyces rouxii, and Candida sp. | - | 3.0–4.0 c | 34 °C | [50] |
| Lacticaseibacillus rhamnosus MDC 9661 | Armenian dairy product | P. aurantioviolaceum and Mucor plumbeus | proteinaceous compounds | 6–6.5 a | 30–42 °C | [60] |
| Compound | MIC (mM) | Activity Spectrum | References |
|---|---|---|---|
| Lactic acid | 274–405 | A. flavus | [62] |
| Acetic acid | 38–41, 8.33, 80 | A. flavus, F. graminearum 623, A. niger | [62,63,64] |
| Butyric acid | 9.08 | F. graminearum 623 | [63] |
| Propionic acid | 8.1 | F. graminearum 623 | [63] |
| Formic acid | 19.5 | F. graminearum 623 | [63] |
| Caprioc acid | 4.3 | F. graminearum 623 | [63] |
| Phenyllactic acid | 45.1 | A. fumigatus and P. roqueforti | [65] |
| cyclo(l-Phe-l-Pro) | 81.9 | A. fumigatus and P. roqueforti | [65] |
| Diacetyl | 0.005 | Penicillium spp. | [66] |
| Reuterin | 0.1–2.0 | A. niger, A. versicolor, P. chrysogenum, P. citrinum, P. commune, P. crustosum, P. roqueforti | [67] |
| decanoic acid | 0.15–0.58 | P. roqueforti, P. commune, A. nidulans, A. fumigatus, P. anomala | [68] |
| 2-hydroxydecanoic acid | 0.027–0.13 | P. roqueforti, P. commune, A. nidulans, A. fumigatus, P. anomala | [68] |
| 3-hydroxyundecanoic acid | 0.049–0.25 | P. roqueforti, P. commune, A. nidulans, A. fumigatus, P. anomala | [68] |
| Indolelactic acid | 24 | P. solitum DCS 302, P. sp. nov. DCS 1541 | [69] |
| 2-hydroxy-(4-methylthio)butanioc acid | 66 | P. solitum DCS 302, P. sp. nov. DCS 1541 | [69] |
| 2-hydroxy-3-methylbutanioc acid | 42 | P. solitum DCS 302, P. sp. nov. DCS 1541 | [69] |
| 2-hydroxy-4-methylthiopentanioc acid | 38 | P. solitum DCS 302, P. sp. nov. DCS 1541 | [69] |
| δ-dodecalactone | 1.8–3.3 | A. flavus, A. fumigatus, A. petrakii, A. ochraceus, A. nidulans, P. roqueforti. | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergsma, S.; Euverink, G.J.W.; Charalampogiannis, N.; Poulios, E.; Janssens, T.K.S.; Achinas, S. Biotechnological and Medical Aspects of Lactic Acid Bacteria Used for Plant Protection: A Comprehensive Review. BioTech 2022, 11, 40. https://doi.org/10.3390/biotech11030040
Bergsma S, Euverink GJW, Charalampogiannis N, Poulios E, Janssens TKS, Achinas S. Biotechnological and Medical Aspects of Lactic Acid Bacteria Used for Plant Protection: A Comprehensive Review. BioTech. 2022; 11(3):40. https://doi.org/10.3390/biotech11030040
Chicago/Turabian StyleBergsma, Simon, Gerrit Jan Willem Euverink, Nikolaos Charalampogiannis, Efthymios Poulios, Thierry K. S. Janssens, and Spyridon Achinas. 2022. "Biotechnological and Medical Aspects of Lactic Acid Bacteria Used for Plant Protection: A Comprehensive Review" BioTech 11, no. 3: 40. https://doi.org/10.3390/biotech11030040
APA StyleBergsma, S., Euverink, G. J. W., Charalampogiannis, N., Poulios, E., Janssens, T. K. S., & Achinas, S. (2022). Biotechnological and Medical Aspects of Lactic Acid Bacteria Used for Plant Protection: A Comprehensive Review. BioTech, 11(3), 40. https://doi.org/10.3390/biotech11030040





