Effects of Intramuscular Injections of Vitamins AD3E and C in Combination on Fertility, Immunity, and Proteomic and Transcriptomic Analyses of Dairy Cows during Early Gestation
Abstract
1. Introduction
2. Materials and Methods
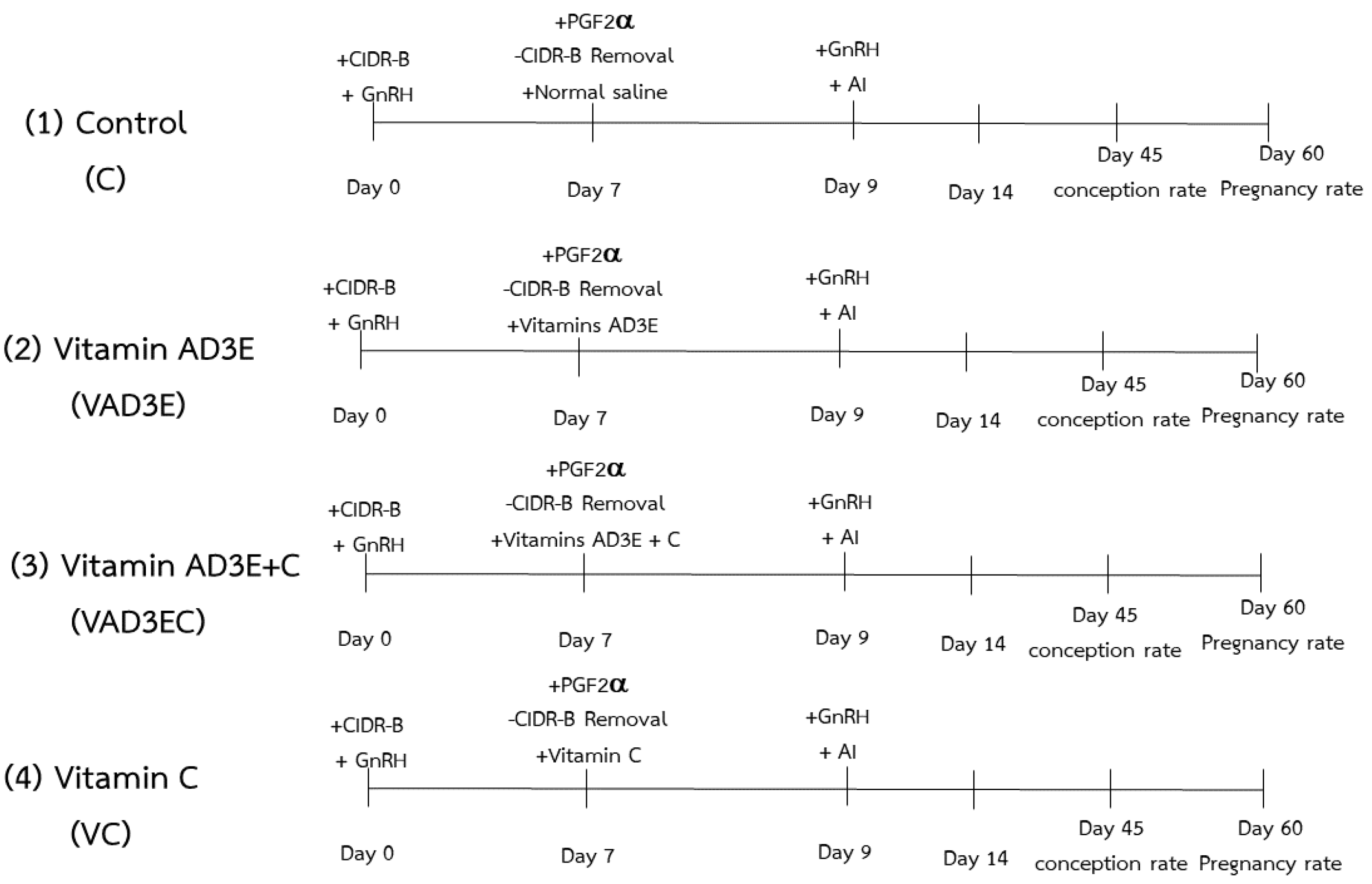
2.1. Cows and Experimental Design
- (1)
- C: the control treatment for which cows were injected with normal saline;
- (2)
- VAD3E: cows were administered a single intramuscular injection (I/M) of vitamin AD3E (Phenix, Brussels, Belgium; vitamin A 300,000 IU, vitamin D3 100,000 IU, vitamin E acetate 50 mg; 1 mL per 50 kg live weight use) and progesterone release device (CIDR) was removed at night on day 7 (cows received the CIDR on day 0);
- (3)
- VAD3EC: cows received vitamins AD3E and C in combination (a total dose of 1500 mg of vitamin C, ascorbic acid, Q.P., Reasol; 500 mg) at night on day 7;
- (4)
- VC: cows were injected with a single dose of vitamin C on the same day as the other treatment.
2.2. Estrus Synchronization
2.3. Blood and Serum Sample Collection
2.4. Serum Assay
2.5. Proteomic Analysis Using LC-MS/MS Technique
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Conception Rate and Pregnancy Rate
3.2. Hematological Parameters
3.3. Biochemical Parameters and Hormone Contents
3.4. Immune and Antioxidation Activity
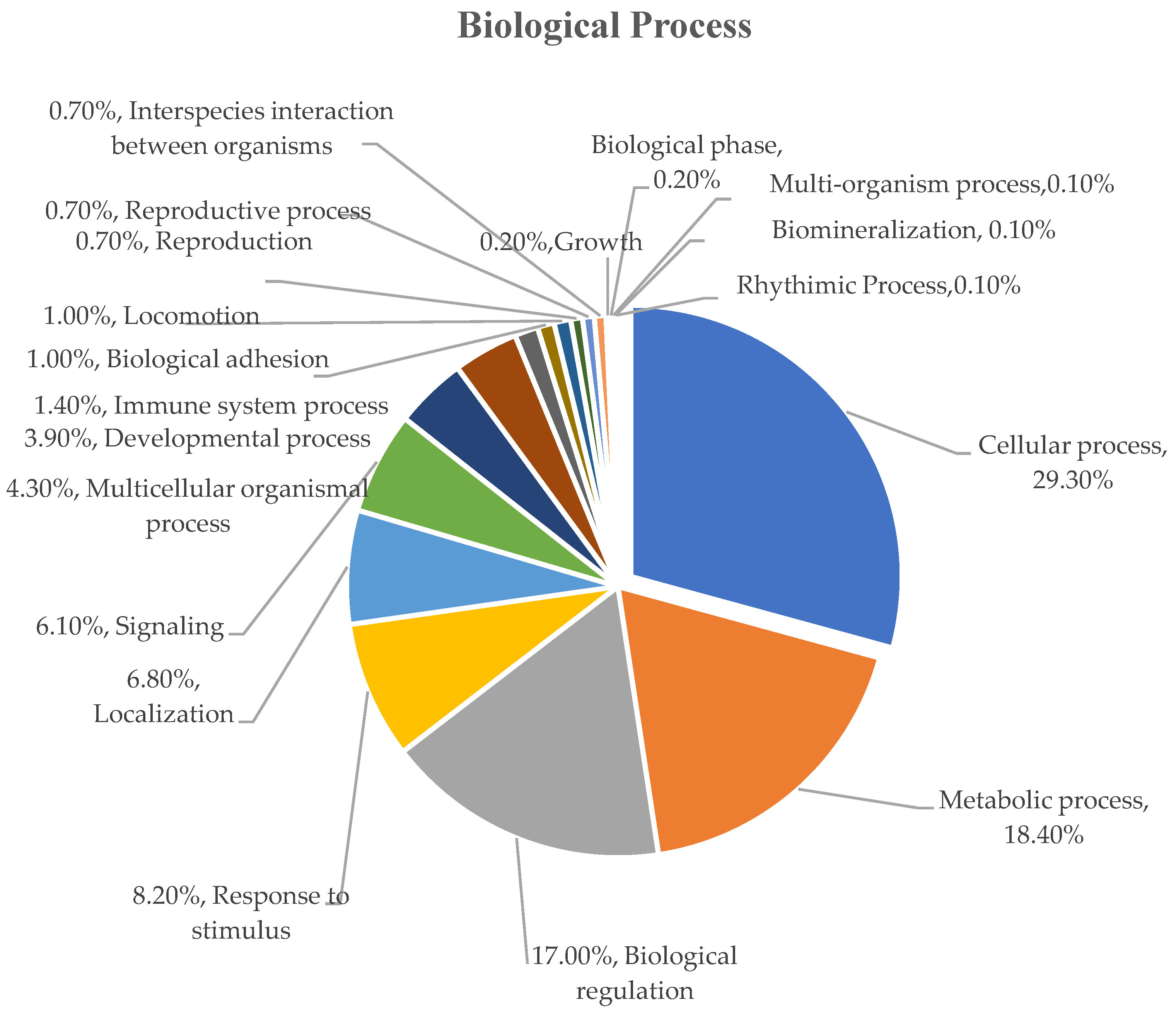
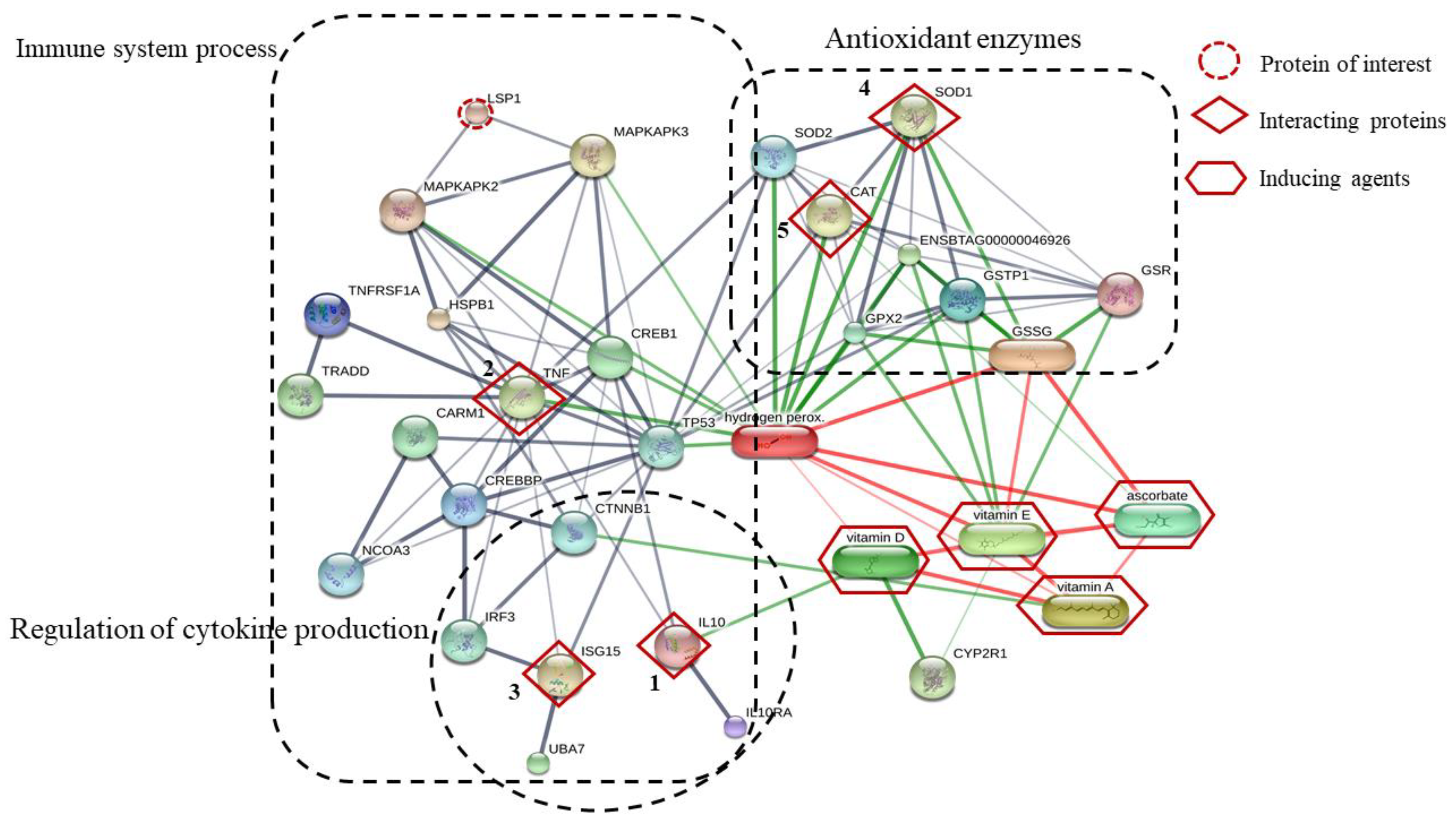
3.5. Functional Classification of Identified Serum Proteins
3.6. LC-MS/MS Identification
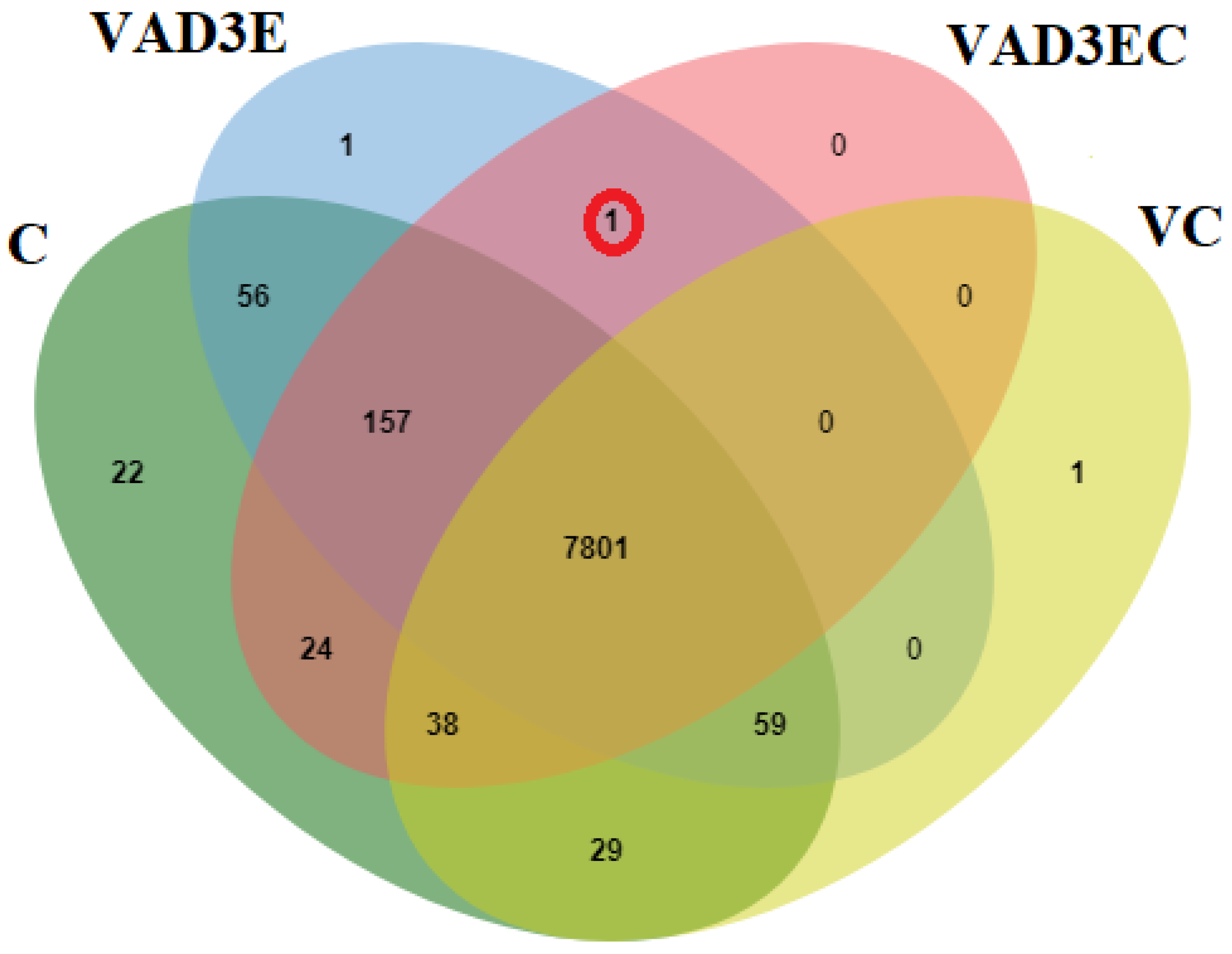
3.7. Differentially Abundant Protein in All Treatments during Induction Times
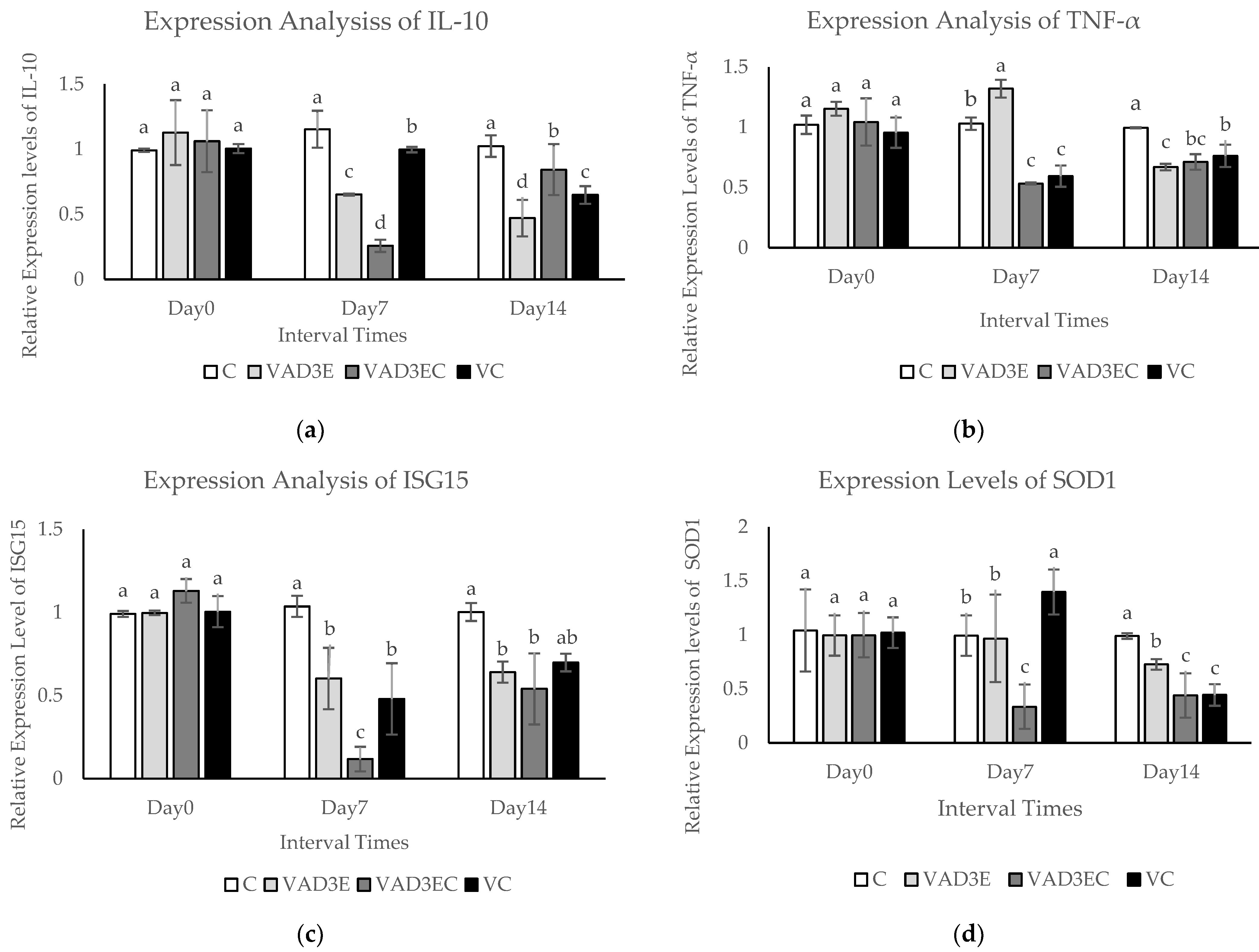
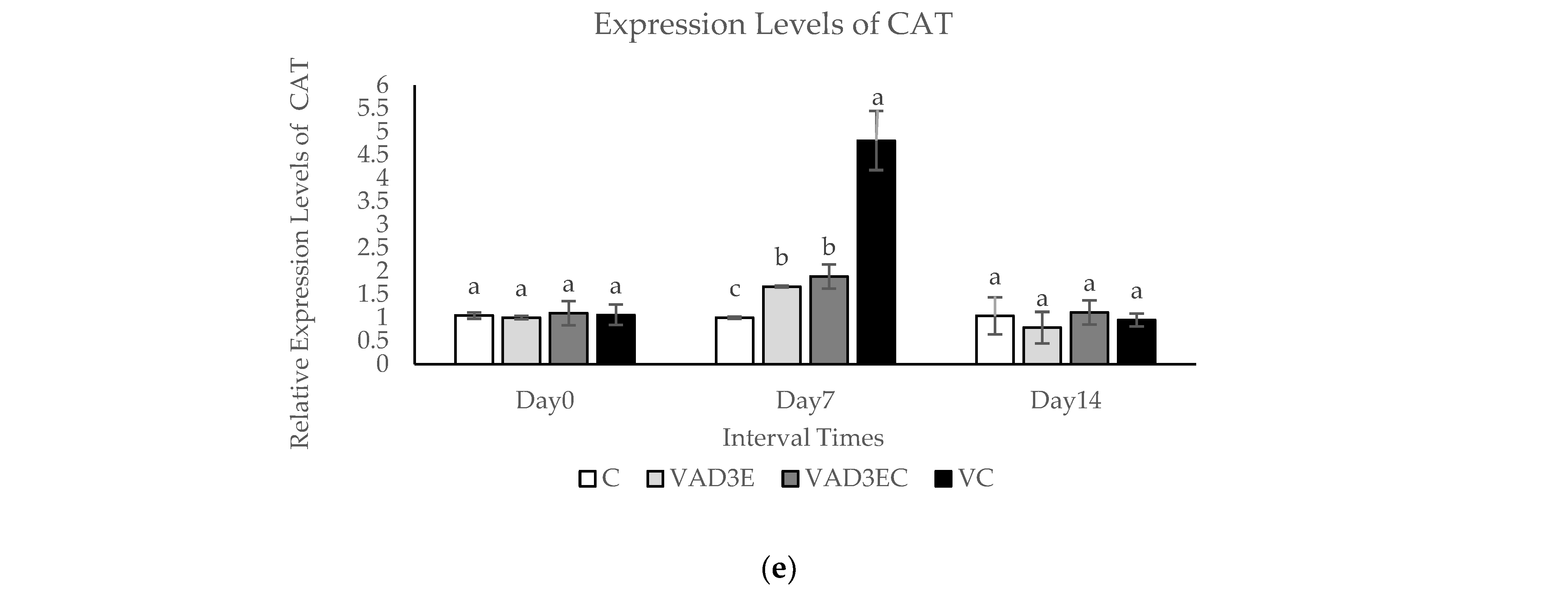
3.8. Quantitative Real-Time Reverse Transcription PCR (qPCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, E. Oxygen free radicals and their clinical implications. Acta Chir. Hung. 1997, 36, 302–305. [Google Scholar] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Dunne, L.D.; Diskin, M.G.; Sreenan, J.M. Embryo and foetal loss in beef heifers between day 14 of gestation and full term. Anim. Reprod. Sci. 2000, 58, 39–44. [Google Scholar] [CrossRef]
- Roche, J.F.; Bolandl, M.P.; McGeady, T.A. Reproductive wastage following artificial insemination of heifers. Vet. Rec. 1981, 109, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Arechiga, C.F.; Ortiz, O.; Hasen, P.J. Effect of prepartum injection of vitamin E and selenium on postpartum reproductive function of dairy cattle. Theriogenology 1994, 41, 1251–1258. [Google Scholar] [CrossRef]
- Habeeb, A.A.M.; AbdelHafez, M.A.M.; ELGohary, E.S.H.; Fathala, M.M.; Salama, O.A. Effect of vitamin AD3E injection on age and weight of weaning and reproductive activity of goats. 1-physiological response and reprductive perfomance of goat bucks during different season in egypt. J. Anim. Prod. 2015, 6, 719–739. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Vitamin A: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 1636S–1680S. [Google Scholar] [CrossRef]
- Azzi, A. Molecular mechanism of α-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Kılınç, F.; Uğuz, A.C.; Çelik, Ö.; Bal, R.; Butterworth, P.J. Oral vitamin C and E combination modulates blood lipid peroxidation and antioxidant vitamin levels in maximal exercising basketball players. Cell Biochem. Funct. 2010, 28, 300–305. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Oliveira, W.D.C.D.; Silva, T.P.D.; Araújo, M.J.D.; Edvan, R.L.; Oliveira, R.L.; Bezerra, L.R. Changes in hematological biomarkers of Nellore cows at different reproductive stages. Acta Sci. Anim. Sci. 2019, 41, e45725. [Google Scholar] [CrossRef]
- Incharoen, T.; Roytrakul, S.; Likittrakulwong, W. Dietary Germinated Paddy Rice and Stocking Density Affect Egg Performance, Serum Biochemical Properties, and Proteomic and Transcriptomic Response of Laying Hens Exposed to Chronic Heat Stress. Proteomes 2021, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Likittrakulwong, W.; Na-Nakorn, U.; Poompuang, S.; Koonawootrittriron, S.; Srisapoome, P. Molecular identification and expression profiling of a novel alpha2-macroglobulin gene in giant freshwater prawn (Macrobrachium rosenbergii, De Man). Agric. Nat. Resour. 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Shirasuna, K.; Matsumoto, H.; Kobayashi, E.; Nitta, A.; Haneda, S.; Matsui, M. Upregulation of interferon-stimulated genes and interleukin-10 in peripheral blood immune cells during early pregnancy in dairy cows. J. Reprod. Dev. 2012, 58, 84–90. [Google Scholar] [CrossRef]
- Côrtes, C.; Palin, M.F.; Gagnon, N.; Benchaar, C.; Lacasse, P.; Petit, H.V. Mammary gene expression and activity of antioxidant enzymes and concentration of the mammalian lignan enterolactone in milk and plasma of dairy cows fed flax lignans and infused with flax oil in the abomasum. Br. J. Nutr. 2012, 108, 1390–1398. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 1262. [Google Scholar] [CrossRef]
- Maldonado, J.G.L.; Santos, R.R.; De Lara, R.R.; Valverde, G.R. Impacts of vitamin C and E injections on ovarian structures and fertility in Holstein cows under heat stress conditions. Turk. J. Vet. Anim. Sci. 2017, 41, 345–350. [Google Scholar] [CrossRef]
- Yildiz, A.; Balikci, E.; Gurdogan, F. Effect of injection of vitamin E and selenium administered immediately before the Ovsynch synchronization on conception rate, antioxidant activity and progesterone levels in dairy cows. Fırat Üniv. Sağlık Bilimleri Vet. Derg. 2015, 29, 183–186. [Google Scholar]
- Sarker, P.K.; Rahman, M.M.; Bhuiyan, M.M.U.; Shamsuddin, M. Effects of GnRH Analogue and Vitamin AD3E on Induction of Cyclicity in Anoestrus Heifers. Bangladesh J. Vet. Med. 2015, 13, 33–40. [Google Scholar] [CrossRef][Green Version]
- Raval, R.; Parmar, K.; Vala, K.; Solanki, G.; Parikh, S.S. Comparative Efficacy of Different Medicaments for Induction of Estrus in True Anestrus Jafarabadi Buffalo Heifers. Indian J. Vet. Sci. Biotechnol. 2017, 12, 30–33. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Toma, F.B.; Kamil, D.Q.; Dabagh, A.S.A.L. Effect of vitamin AD3E utilization at the pregnancy period and parturition on blood parameter for dairy cattle. Int. J. Adv. Res. Biol. Sci. 2016, 3, 227–231. [Google Scholar]
- Müller, U.; Kesser, J.; Koch, C.; Helfrich, H.P.; Rietz, C. Monitoring predictive and informative indicators of the energy status of dairy cows during early lactation in the context of monthly milk recordings using mid-infrared spectroscopy. Livest Sci. 2019, 221, 6–14. [Google Scholar] [CrossRef]
- Habeeb, A.A.M.; El-Gohary, E.S.H.; Saleh, H.M.; Aboelnaga, A.I. Effect of summer heat stress conditions and feeding protein level on blood components in Ossimi ewes and their suckling lambs. Egypt. J. Appl. Sci. 2008, 23, 388–408. [Google Scholar]
- Al-Asadi, F.A.A.; Habib, H.N.; Hassan, A.F. Effect of the injection of vitamins AD3E and the seasons on some blood traits, biochemical components and hormones of Arabi rams. J. Anim. Behav. Biometeorol. 2020, 8, 74–81. [Google Scholar] [CrossRef]
- Duntas, L.H. The evolving role of selenium in the treatment of Graves’ disease and ophthalmopathy. J. Thyroid Res. 2012, 2012, 736161. [Google Scholar] [CrossRef]
- Ali, Z.Y. Neurotoxic effect of lambda-cyhalothrin, a synthetic pyrethroid pesticide: Involvement of oxidative stress and protective role of antioxidant mixture. N. Y. Sci. J. 2012, 5, 93–103. [Google Scholar]
- Sen, C.K.; Packer, L. Thiol homeostasis and supplements in physical exercise. Am. J. Clin. Nutr. 2000, 72, 653S–669S. [Google Scholar] [CrossRef]
- Çimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Evans, W.J. Vitamin E, vitamin C, and exercise. Am. J. Clin. Nutr. 2000, 72, 647S–652S. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Aroonluk, S.; Roytrakul, S.; Jantasuriyarat, C. Identification and Characterization of Phosphoproteins in Somatic Embryogenesis Acquisition during Oil Palm Tissue Culture. Plants 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Said, G.; Yılmaz, Ö.; Güray, T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol. Cell. Biochem. 2008, 309, 109–116. [Google Scholar] [CrossRef]
- Jongstra, J.; Ittel, M.E.; Iscove, N.N.; Brady, G. The LSP1 gene is expressed in cultured normal and transformed mouse macrophages. Mol. Immunol. 1994, 31, 1125–1131. [Google Scholar]
- Pulford, K.; Jones, M.; Banham, A.H.; Haralambieva, E.; Mason, D.Y. Lymphocyte-specific protein 1: A specific marker of human leucocytes. Immunology 1999, 96, 262. [Google Scholar] [CrossRef]
| Gene 1 | Sequence (5′-3′) | Accession No. | Ref. |
|---|---|---|---|
| IL-10 | F: TTCTGCCCTGCGAAAACAA | NM_174088 | [16] |
| R: TCTCTTGGAGCTCACTGAAGACTCT | |||
| TNFα | F: TGACGGGCTTTACCTCATCT | AF_348421 | [16] |
| R: TGATGGCAGACAGGATGTTG | |||
| ISG15 | F: GGTATCCGAGCTGAAGCAGTT | NM_174366 | [16] |
| R: ACCTCCCTGCTGTCAAGGT | |||
| SOD1 | F: TGTTGCCATCGTGGATATTGTAG | NM_174615 | [17] |
| R: CCCAAGTCATCTGGTTTTTCATG | |||
| CAT | F: GCTCCAAATTACTACCCCAATAGC | NM_001035386 | [17] |
| R: GCACTGTTGAAGCGCTGTACA | |||
| Beta-actin | F: GCGTGGCTACAGCTTCACC | AY141970 | [17] |
| R: TTGATGTCACGGACGATTTC |
| Items | C | VAD3E | VAD3EC | VC | p-Value |
|---|---|---|---|---|---|
| Estrus detection AI% | 100.0 | 90.00 | 100.00 | 100.00 | 0.380 |
| Detections/all cows | 10/10 | 9/10 | 10/10 | 10/10 | |
| Conception Rate AI% | 10.0 | 30.0 | 20.0 | 40.0 | 0.446 |
| Detections/all cows | 1/10 | 3/10 | 2/10 | 4/10 | |
| Pregnancy Rate% | 10.0 | 30.0 | 20.0 | 40.0 | 0.446 |
| Detections/all cows | 1/10 | 3/10 | 2/10 | 4/10 |
| Items | C | VAD3E | VAD3EC | VC | p-Value |
|---|---|---|---|---|---|
| RBC (×106 cells/mm3) | 6.44 ± 0.34 | 4.96 ± 0.55 | 5.78 ± 0.49 | 5.22 ± 0.40 | 0.138 |
| Ht (%) | 30.20 ± 1.53 | 23.20 ± 2.76 | 28.40 ± 3.37 | 24.40 ± 2.99 | 0.270 |
| Hgb (g/dL) | 9.52 ± 0.41 | 7.46 ± 0.81 | 8.48 ± 0.88 | 7.94 ± 0.87 | 0.299 |
| MCV (fL) | 43.38 ± 1.27 | 47.92 ± 2.85 | 49.04 ± 1.82 | 46.82 ± 2.68 | 0.910 |
| MCHC (g/dL) | 31.28 ± 0.33 | 31.26 ± 0.33 | 28.74 ± 0.87 | 32.20 ± 1.48 | 0.075 |
| WBC (cells/mm3) | 16,640 ± 7124.79 b | 16,620 ± 5956.37 b | 46,580 ± 7362.50 a | 8460 ± 1148.74 b | 0.002 |
| Neu (%) | 51.80 ± 12.46 a | 24.80 ± 8.35 b | 14.80 ± 3.11 b | 19.40 ± 4.83 b | 0.015 |
| Eos (%) | 2.80 ± 0.86 | 1.40 ± 0.24 | 1.40 ± 0.40 | 2.00 ± 0.55 | 0.280 |
| Lin (%) | 43.80 ± 13.07 b | 72.40 ± 8.29 a | 81.40 ± 1.40 a | 77.40 ± 2.23 a | 0.015 |
| Mon (%) | 1.80 ± 0.20 | 1.80 ± 0.37 | 2.20 ± 0.49 | 1.60 ± 0.40 | 0.730 |
| PLT (cells/mm3) | 201,600 ± 29,512.03 b | 208,400 ± 8518.22 b | 231,200 ± 35,319.12 b | 376,400 ± 36,912.87 a | 0.002 |
| Items | C | VAD3E | VAD3EC | VC | p-Value |
|---|---|---|---|---|---|
| BUN, mg/dL | 4.60 ± 0.51 c | 28.60 ± 1.12 a | 15.00 ± 1.52 b | 8.00 ± 2.07 c | ≤0.01 |
| GLU, mg/dL | 4.50 ± 0.22 | 4.60 ± 1.29 | 2.00 ± 0.32 | 2.96 ± 0.50 | 0.057 |
| ALB, g/dL | 2.48 ± 0.12 b | 3.14 ± 0.18 a | 3.32 ± 0.17 a | 3.16 ± 0.06 a | 0.003 |
| TG, mg/dL | 8.20 ± 0.20 | 9.20 ± 0.37 | 10.60 ± 1.36 | 8.40 ± 1.25 | 0.303 |
| CHO, mg/dL | 82.40 ± 2.98 c | 168.40 ± 3.97 a | 122.60 ± 8.73 b | 126.40 ± 7.34 b | ≤0.01 |
| Ca, mg/dL | 8.36 ± 0.27 | 8.36 ± 0.42 | 8.56 ± 0.25 | 9.24 ± 0.09 | 0.132 |
| P, mg/dL | 9.54 ± 0.74 | 8.30 ± 0.18 | 9.40 ± 0.33 | 8.94 ± 0.88 | 0.486 |
| P4, ng/mL | 0.80 ± 0.25 | 0.82 ± 0.34 | 1.02 ± 0.10 | 1.09 ± 0.39 | 0.856 |
| E, pg/mL | 21.20 ± 10.80 | 11.60 ± 1.03 | 40.16 ± 16.83 | 11.60 ± 1.03 | 0.168 |
| Cortisol, µg/dL | 1.19 ± 0.46 | 0.43 ± 0.24 | 0.71 ± 0.55 | 0.33 ± 0.18 | 0.190 |
| Items | C | VAD3E | VAD3EC | VC | p-Value |
|---|---|---|---|---|---|
| ACH50, U/mL | 294.11 ± 6.24 c | 394.95 ± 20.04 a | 365.05 ± 22.94 ab | 342.14 ± 10.04 bc | 0.004 |
| Total Ig, mg/mL | 1.34 ± 0.12 b | 2.61 ± 0.13 a | 2.53 ± 0.20 a | 2.59 ± 0.14 a | ≤0.01 |
| LZY, U/mL | 335.10 ± 13.26 b | 499.00 ± 33.60 a | 478.83 ± 30.80 a | 328.53 ± 24.05 b | <0.01 |
| SOD, inhibition rate, % | 85.22 ± 0.73 c | 89.26 ± 1.72 b | 94.48 ± 0.72 a | 91.42 ± 0.49 ab | ≤0.01 |
| CAT, mU/mL | 0.20 ± 0.00 b | 0.01 ± 0.00 d | 0.23 ± 0.01 a | 0.07 ± 0.01 c | ≤0.01 |
| GPx, mU/mL | 0.09 ± 0.01 c | 0.09 ± 0.00 bc | 0.10 ± 0.00 ab | 0.11 ± 0.01 a | 0.003 |
| GR, U/mL | 0.16 ± 0.01 c | 0.23 ± 0.02 a | 0.26 ± 0.01 a | 0.20 ± 0.01 b | ≤0.01 |
| Log2 Abundance of Protein 1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |||||||||||||||
| Uniprot Accession Number | Protein Name | Unique Peptide Sequence | ID Scores | MH+(Da) | C | VAD3E | VAD3EC | VC | C | VAD3E | VAD3EC | VC | C | VAD3E | VAD3EC | VC | |
| 1. | A0A3Q1M894 | Lymphocyte-specific protein 1 | LEQYTQAVEIAGR | 7.23 | 1477.08 | 0 | 0 | 0 | 0 | 0 | 0 | 17.51 | 0 | 0 | 17.43 | 0 | 0 |
| 2. | A0A3Q1MVU8 | Cytokine synthesis inhibitory factor (interleukin-10) | CHRFLPCENK | 9.90 | 1359.95 | 15.81 | 15.43 | 15.79 | 14.74 | 16.20 | 14.08 | 14.90 | 15.30 | 14.78 | 17.09 | 14.97 | 13.20 |
| 3. | E1BLB2 | TNF-alpha-induced protein 1 | DVIGDEICCWSFYGQGRK | 2.61 | 2190.44 | 15.67 | 14.40 | 16.02 | 17.99 | 15.37 | 14.21 | 13.39 | 14.81 | 14.55 | 14.76 | 13.90 | 14.52 |
| 4. | F6Q4D3 | ISG15 protein conjugation | KAEVAAEATR | 14.75 | 1045.19 | 16.60 | 15.79 | 16.06 | 15.29 | 16.18 | 15.48 | 15.16 | 16.57 | 16.87 | 16.58 | 15.34 | 15.71 |
| 5. | E1BHL1 | Superoxide dismutase (Mn), mitochondrial (EC 1.15.1.1) | AGGGAAAVVSLR | 5.20 | 1029.35 | 16.47 | 16.50 | 16.36 | 16.41 | 16.59 | 16.24 | 16.28 | 16.30 | 15.41 | 16.05 | 15.54 | 16.04 |
| 6. | A0A3Q1LKF1 | Catalase (EC 1.11.1.6) | GIPDGHRHMNGYGSHTFK | 12.03 | 2009.90 | 14.91 | 16.18 | 15.46 | 16.14 | 15.120 | 13.40 | 15.39 | 14.87 | 15.57 | 20.47 | 14.83 | 15.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likittrakulwong, W.; Poolprasert, P.; Hanthongkul, W.; Roytrakul, S. Effects of Intramuscular Injections of Vitamins AD3E and C in Combination on Fertility, Immunity, and Proteomic and Transcriptomic Analyses of Dairy Cows during Early Gestation. BioTech 2022, 11, 20. https://doi.org/10.3390/biotech11020020
Likittrakulwong W, Poolprasert P, Hanthongkul W, Roytrakul S. Effects of Intramuscular Injections of Vitamins AD3E and C in Combination on Fertility, Immunity, and Proteomic and Transcriptomic Analyses of Dairy Cows during Early Gestation. BioTech. 2022; 11(2):20. https://doi.org/10.3390/biotech11020020
Chicago/Turabian StyleLikittrakulwong, Wirot, Pisit Poolprasert, Worawatt Hanthongkul, and Sittiruk Roytrakul. 2022. "Effects of Intramuscular Injections of Vitamins AD3E and C in Combination on Fertility, Immunity, and Proteomic and Transcriptomic Analyses of Dairy Cows during Early Gestation" BioTech 11, no. 2: 20. https://doi.org/10.3390/biotech11020020
APA StyleLikittrakulwong, W., Poolprasert, P., Hanthongkul, W., & Roytrakul, S. (2022). Effects of Intramuscular Injections of Vitamins AD3E and C in Combination on Fertility, Immunity, and Proteomic and Transcriptomic Analyses of Dairy Cows during Early Gestation. BioTech, 11(2), 20. https://doi.org/10.3390/biotech11020020







