Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences
Abstract
1. Introduction
2. Origin, Development, and Mechanism of the CRISPR-Cas9 System
3. Ethical Issues in Genome Editing by CRISPR-Cas9 System
4. Applications of CRISPER-Cas9 Technology
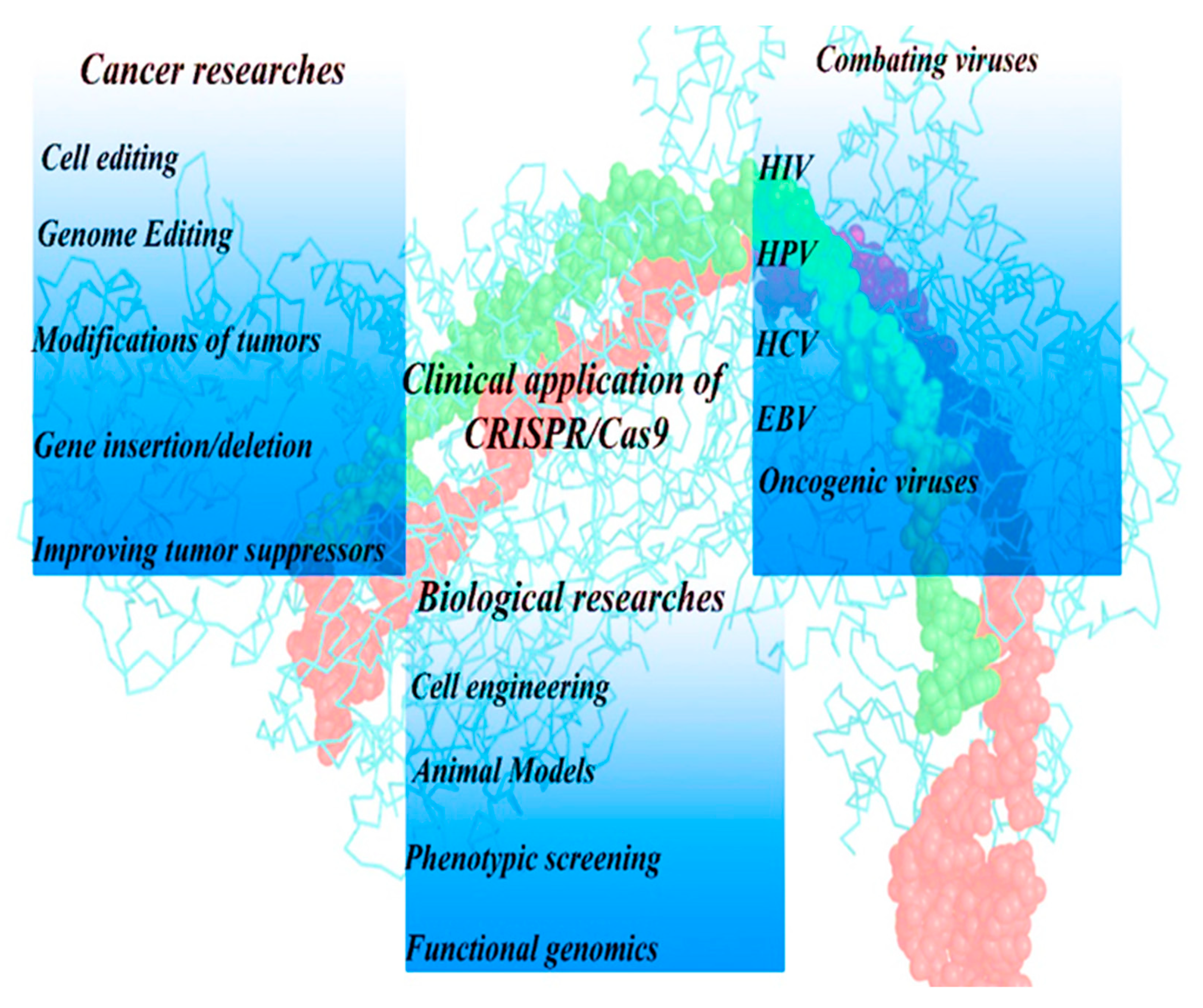
4.1. Human Science
4.1.1. Clinical Trials Using CRISPR-Cas9 Technology
4.1.2. Clinical Trials of the Eye Based on CRISPR-Cas9
4.1.3. Limitations of CRISPR-Cas-Based Gene Therapy
4.2. Plant Science
4.2.1. Plant Disease Resistance
4.2.2. Yield of Crop Plants
4.2.3. Genome Modification for Nutritional Improvement
4.2.4. Medicinal Plants
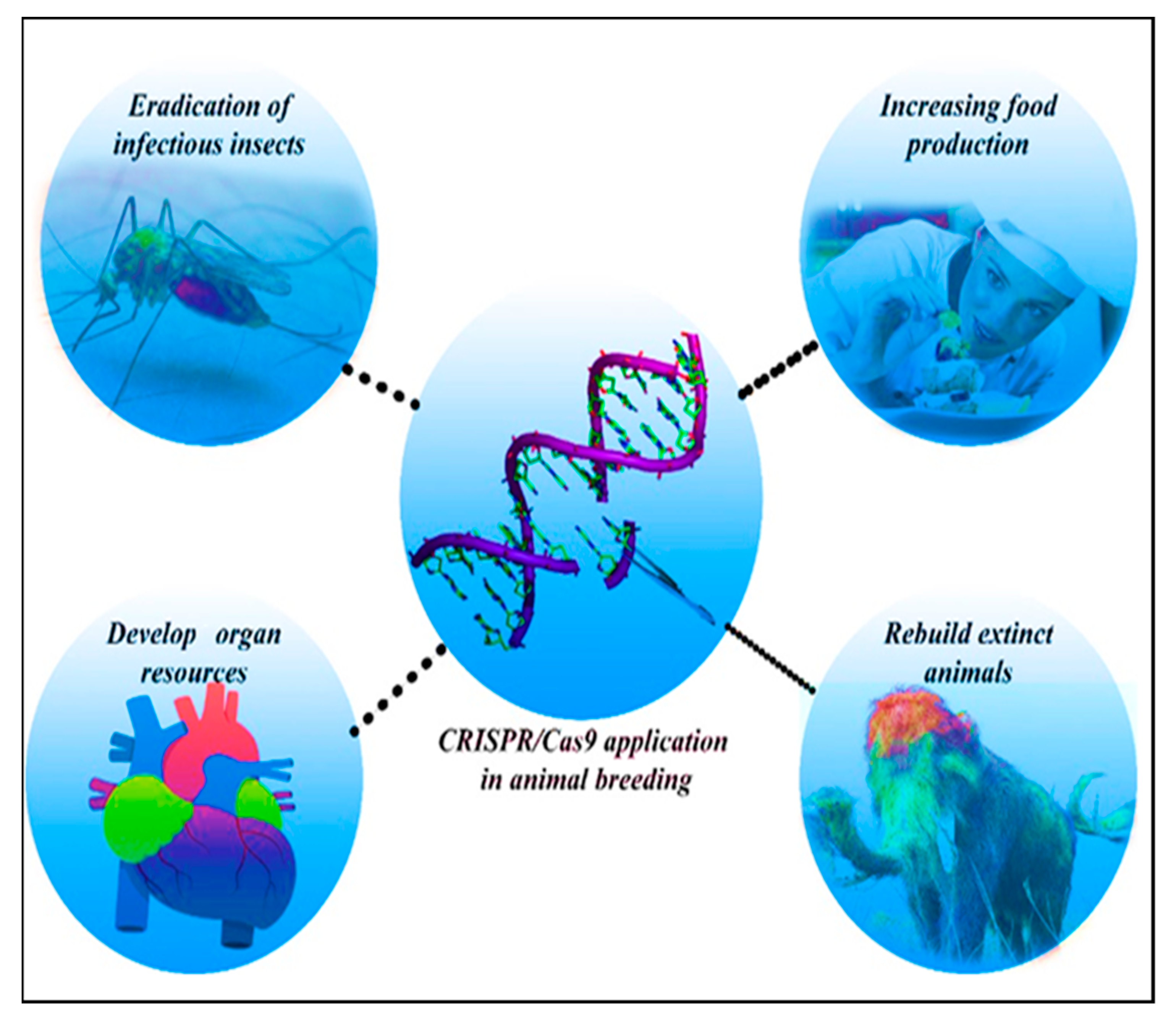
4.3. Animal Breeding
4.3.1. Modification of Pigs for Xenotransplantation Research
4.3.2. Application of CRISPR-Cas9 Technology in Insects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.; Huang, X. Methods and applications of CRISPR/Cas system for genome editing in stem cells. Cell Regen. 2019, 8, 33–41. [Google Scholar] [CrossRef]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas System: Recent Advances and Future Prospects for Genome Editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef]
- Mirza, Z.; Karim, S. Advancements in CRISPR/Cas9 technology—focusing on cancer therapeutics and beyond. Semin. Cell Dev. Biol. 2019, 96, 13–21. [Google Scholar] [CrossRef]
- Mehravar, M.; Shirazi, A.; Nazari, M.; Banan, M. Mosaicism in CRISPR/Cas9-mediated genome editing. Dev. Biol. 2019, 445, 156–162. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Al-Sadi, A.M.; Pour-Aboughadareh, A.; Burritt, D.J.; Tran, L.-S.P. Genome editing using CRISPR/Cas9–targeted mutagenesis: An opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol. Biochem. 2018, 131, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lage, M.; Puig-Serra, P.; Menendez, P.; Torres-Ruiz, R.; Rodriguez-Perales, S. CRISPR/Cas9 for Cancer Therapy: Hopes and Challenges. Biomedicines 2018, 6, 105. [Google Scholar] [CrossRef]
- Raguz, N.; Lukic, B. Potential gain of genome editing for improved animal breeding. In Proceedings of the 55th Croatian & 15th International Symposium on Agriculture, Vodice, Croatia, 16–21 February 2020. [Google Scholar]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.V.; Nunez, J.K.; Doudna, J.A. Biology and applications of CRISPR systems: Harnessing nature’s toolbox for genome engineering. Cell 2016, 164, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-programmed genome editing in human cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Karakikes, I. Translating genomic insights into cardiovascular medicines: Opportunities and challenges of CRISPR-Cas9. Trends Cardiovasc. Med. 2020. [CrossRef]
- Sun, J.-Y.; Hu, H.-B.; Cheng, Y.-X.; Lu, X.-J. CRISPR in medicine: Applications and challenges. Brief. Funct. Genom. 2020, 19, 151–153. [Google Scholar] [CrossRef]
- Ahmad, S.; Wei, X.; Sheng, Z.; Hu, P.; Tang, S. CRISPR/Cas9 for development of disease resistance in plants: Recent progress, limitations and future prospects. Brief. Funct. Genom. 2020, 19, 26–39. [Google Scholar] [CrossRef]
- Tahir, T.; Ali, Q.; Rashid, M.; Malik, A. The journey of CRISPR-Cas9 from bacterial defense mechanism to a gene editing tool in both animals and plants. Biol. Clin. Sci. Res. J. 2020, 2020, e017. [Google Scholar]
- Hundleby, P.A.C.; Harwood, W.A. Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur. 2019, 8, e00161. [Google Scholar] [CrossRef]
- Hirsch, F.; Iphofen, R.; Koporc, Z. Ethics assessment in research proposals adopting CRISPR technology. Biochem. Med. 2019, 29, 020202. [Google Scholar] [CrossRef]
- Cai, L.; Zheng, L.A.; He, L. The forty years of medical genetics in China. J. Genet. Genom. 2018, 45, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Memi, F.; Ntokou, A.; Papangeli, I. CRISPR/Cas9 gene-editing: Research technologies, clinical applications and ethical considerations. Semin. Perinatol. 2018, 42, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E. Ethical issues in genome editing using Crispr/Cas9 system. J. Clin. Res. Bioeth. 2016, 7, 266. [Google Scholar]
- Esvelt, K.M.; Smidler, A.L.; Catteruccia, F.; Church, G.M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 2014, 3, e03401. [Google Scholar] [CrossRef]
- Shinwari, Z.K.; Tanveer, F.; Khalil, A.T. Ethical issues regarding CRISPR-mediated genome editing. Curr. Issues Mol. Biol. 2017, 26, 103–110. [Google Scholar]
- Polcz, S.; Lewis, A. CRISPR-Cas9 and the non-germline non-controversy. J. Law Biosci. 2016, 3, 413–425. [Google Scholar] [CrossRef]
- Rodriguez, E. Ethical issues in genome editing for non-human organisms using CRISPR/Cas9 system. J. Clin. Res. Bioeth. 2017, 8, 10–4172. [Google Scholar] [CrossRef]
- Eriksson, S.; Jonas, E.; Rydhmer, L.; Röcklinsberg, H. Invited review: Breeding and ethical perspectives on genetically modified and genome edited cattle. J. Dairy Sci. 2018, 101, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J. Human-animal chimeras: The moral insignificance of uniquely human capacities. Hastings Cent. Rep. 2019, 49, 23–32. [Google Scholar] [CrossRef]
- Degrazia, D. Human-animal chimeras, “human” cognitive capacities, and moral status. Hastings Cent. Rep. 2019, 49, 33–34. [Google Scholar] [CrossRef]
- de Graeff, N.; Jongsma, K.R.; Johnston, J.; Hartley, S.; Bredenoord, A.L. The ethics of genome editing in non-human animals: A systematic review of reasons reported in the academic literature. Philos. Trans. R. Soc. B 2019, 374, 20180106. [Google Scholar] [CrossRef] [PubMed]
- Otieno, M.O. CRISPR-Cas9 human genome editing: Challenges, ethical concerns and implications. J. Clin. Res. Bioeth. 2015, 6, 253–255. [Google Scholar]
- Duardo-Sanchez, A. CRISPR-Cas in medicinal chemistry: Applications and regulatory concerns. Curr. Top. Med. Chem. 2017, 17, 3308–3315. [Google Scholar] [CrossRef]
- Greene, M.; Master, Z. Ethical issues of using CRISPR technologies for research on military enhancement. J. Bioethical Inq. 2018, 15, 327–335. [Google Scholar] [CrossRef]
- Sherkow, J.S. The CRISPR patent landscape: Past, present, and future. CRISPR J. 2018, 1, 5–9. [Google Scholar] [CrossRef]
- Cathomen, T.; Schüle, S.; Schüßler-Lenz, M.; Abou-El-Enein, M. The human genome editing race: Loosening regulatory standards for commercial advantage? Trends Biotechnol. 2019, 37, 120–123. [Google Scholar] [CrossRef]
- Lyu, C.; Shen, J.; Wang, R.; Gu, H.; Zhang, J.; Xue, F.; Liu, X.; Liu, W.; Fu, R.; Zhang, L.; et al. Targeted genome engineering in human induced pluripotent stem cells from patients with hemophilia B using the CRISPR-Cas9 system. Stem Cell Res. Ther. 2018, 9, 92. [Google Scholar] [CrossRef]
- Shimo, T.; Hosoki, K.; Nakatsuji, Y.; Yokota, T.; Obika, S. A novel human muscle cell model of Duchenne muscular dystrophy created by CRISPR/Cas9 and evaluation of antisense-mediated exon skipping. J. Hum. Genet. 2018, 63, 365–375. [Google Scholar] [CrossRef]
- Egorova, T.V.; Zotova, E.D.; Reshetov, D.A.; Polikarpova, A.V.; Vassilieva, S.G.; Vlodavets, D.V.; Gavrilov, A.A.; Ulianov, S.V.; Buchman, V.L.; Deykin, A.V. CRISPR/Cas9-generated mouse model of Duchenne muscular dystrophy recapitulating a newly identified large 430 kb deletion in the human DMD gene. Dis. Models Mech. 2019, 12. [Google Scholar] [CrossRef]
- Long, C.; Amoasii, L.; Mireault, A.A.; McAnally, J.R.; Li, H.; Sanchez-Ortiz, E.; Bhattacharyya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 2016, 351, 400–403. [Google Scholar] [CrossRef]
- Nelson, C.E.; Hakim, C.H.; Ousterout, D.G.; Thakore, P.I.; Moreb, E.A.; Rivera, R.M.C.; Madhavan, S.; Pan, X.; Ran, F.A.; Yan, W.X.; et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016, 351, 403–407. [Google Scholar] [CrossRef]
- Tabebordbar, M.; Zhu, K.; Cheng, J.K.W.; Chew, W.L.; Widrick, J.J.; Yan, W.X.; Maesner, C.C.; Wu, E.Y.; Xiao, R.; Ran, F.A.; et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016, 351, 407–411. [Google Scholar] [CrossRef]
- Bjursell, M.; Porritt, M.J.; Ericson, E.; Taheri-Ghahfarokhi, A.; Clausen, M.; Magnusson, L.; Admyre, T.; Nitsch, R.; Mayr, L.; Aasehaug, L.; et al. Therapeutic Genome Editing With CRISPR/Cas9 in a Humanized Mouse Model Ameliorates alpha1-antitrypsin Deficiency Phenotype. EBioMedicine 2018, 29, 104–111. [Google Scholar] [CrossRef]
- Song, C.-Q.; Wang, D.; Jiang, T.; O’Connor, K.; Tang, Q.; Cai, L.; Li, X.; Weng, Z.; Yin, H.; Gao, G.; et al. In Vivo Genome Editing Partially Restores Alpha1-Antitrypsin in a Murine Model of AAT Deficiency. Hum. Gene Ther. 2018, 29, 853–860. [Google Scholar] [CrossRef]
- Gao, X.; Tao, Y.; Lamas, V.; Huang, M.; Yeh, W.-H.; Pan, B.; Hu, Y.-J.; Hu, J.H.; Thompson, D.B.; Shu, Y.; et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Naure 2018, 553, 217–221. [Google Scholar] [CrossRef]
- György, B.; Nist-Lund, C.; Pan, B.; Asai, Y.; Karavitaki, K.D.; Kleinstiver, B.P.; Garcia, S.; Zaborowski, M.P.; Solanes, P.; Spataro, S.; et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 2019, 25, 1123–1130. [Google Scholar] [CrossRef]
- Xu, S.; Luk, K.; Yao, Q.; Shen, A.H.; Zeng, J.; Wu, Y.; Luo, H.-Y.; Brendel, C.; Pinello, L.; Chui, D.H.K.; et al. Editing aberrant splice sites efficiently restores β-globin expression in β-thalassemia. Blood 2019, 133, 2255–2262. [Google Scholar] [CrossRef]
- Canver, M.C.; Smith, E.C.; Sher, F.; Pinello, L.; Sanjana, N.; Shalem, O.; Chen, D.D.; Schupp, P.G.; Vinjamur, D.; Garcia, S.; et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 2015, 527, 192–197. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Roscoe, B.P.; Liu, P.; Yao, Q.; Lazzarotto, C.R.; Clement, M.K.; Cole, M.; Luk, K.; Baricordi, C.; et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 2019, 25, 776–783. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.M.; Dever, D.P.; Davis, T.H.; Camarena, J.; Srifa, W.; Zhang, Y.; Paikari, A.; Chang, A.K.; Porteus, M.H.; et al. Highly efficient editing of the beta-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res. 2019, 47, 7955–7972. [Google Scholar] [CrossRef]
- Castaño, J.; Herrero, A.B.; Bursen, A.; González, F.; Marschalek, R.; Gutierrez, N.; Menendez, P. Expression of MLL-AF4 or AF4-MLL fusions does not impact the efficiency of DNA damage repair. Oncotarget 2016, 7, 30440–30452. [Google Scholar] [CrossRef]
- Reimer, J.; Knoess, S.; Labuhn, M.; Charpentier, E.M.; Goehring, G.; Schlegelberger, B.; Klusmann, J.-H.; Heckl, D. CRISPRCas9-induced t(11;19)/MLL-ENL translocations initiate leukemia in human hematopoietic progenitor cells in vivo. Haematologica 2017, 102, 1558–1566. [Google Scholar] [CrossRef]
- Heckl, D.; Kowalczyk, M.S.; Yudovich, D.; Belizaire, R.; Puram, R.V.; McConkey, M.E.; Thielke, A.; Aster, J.C.; Regev, A.; Ebert, B.L. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 2014, 32, 941–946. [Google Scholar] [CrossRef]
- Shi, J.; Wang, E.; Milazzo, J.P.; Wang, Z.; Kinney, J.B.; Vakoc, C.R. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat. Biotechnol. 2015, 33, 661–667. [Google Scholar] [CrossRef]
- Xu, P.; Tong, Y.; Liu, X.-Z.; Wang, T.-T.; Cheng, L.; Wang, B.-Y.; Lv, X.; Huang, Y.; Liu, D.-P. Both TALENs and CRISPR/Cas9 directly target the HBB IVS2–654 (C > T) mutation in β-thalassemia-derived iPSCs. Sci. Rep. 2015, 5, 12065. [Google Scholar] [CrossRef]
- Antony, J.S.; Latifi, N.; Haque, A.K.M.A.; Lamsfus-Calle, A.; Daniel-Moreno, A.; Graeter, S.; Baskaran, P.; Weinmann, P.; Mezger, M.; Handgretinger, R.; et al. Gene correction of HBB mutations in CD34+ hematopoietic stem cells using Cas9 mRNA and ssODN donors. Mol. Cell. Pediatrics 2018, 5, 9. [Google Scholar] [CrossRef]
- Lin, S.-R.; Yang, H.-C.; Kuo, Y.-T.; Liu, C.-J.; Yang, T.-Y.; Sung, K.-C.; Lin, Y.-Y.; Wang, H.-Y.; Wang, C.-C.; Shen, Y.-C.; et al. The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol. Ther. Nucleic Acids. 2014, 3, e186. [Google Scholar] [CrossRef]
- Hazafa, A.; Mumtaz, M.; Farooq, M.F.; Bilal, S.; Chaudhry, S.N.; Firdous, M.; Naeem, H.; Ullah, M.O.; Yameen, M.; Mukhtiar, M.S.; et al. CRISPR/Cas9: A powerful genome editing technique for the treatment of cancer cells with present challenges and future directions. Life Sci. 2020, 263, 118525. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 22 May 2021).
- He, S. The first human trial of CRISPR-based cell therapy clears safety concerns as new treatment for late-stage lung cancer. Signal Transduct. Target. Ther. 2020, 5, 168. [Google Scholar] [CrossRef]
- Hirakawa, M.P.; Krishnakumar, R.; Timlin, J.A.; Carney, J.P.; Butler, K.S. Gene editing and CRISPR in the clinic: Current and future perspectives. Biosci. Rep. 2020, 40, BSR20200127. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef]
- Molla, K.A.; Yang, Y. CRISPR/Cas-Mediated Base Editing: Technical Considerations and Practical Applications. Trends Biotechnol. 2019, 37, 1121–1142. [Google Scholar] [CrossRef]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866. [Google Scholar] [CrossRef] [PubMed]
- Rich, K.; Terry, S.F. CRISPR-Cas9: New Heights, New Hesitations. Genet. Test. Mol. Biomark. 2018, 22, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Cui, Y.; Liao, X.; Peng, S.; Tang, T.; Huang, C.; Yang, C. OffScan: A universal and fast CRISPR off-target sites detection tool. BMC Genom. 2020, 21, 872. [Google Scholar] [CrossRef]
- Jung, C.; Capistrano-Gossmann, G.; Braatz, J.; Sashidhar, N.; Melzer, S. Recent developments in genome editing and applications in plant breeding. Plant Breed. 2018, 137, 1–9. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/Cas9-Targeted Mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Pillay, M. Genome Editing Technologies for Crop Improvement. In Quantitative Genetics, Genomics and Plant Breeding, 2nd ed.; CABI: Boston, MA, USA, 2020; pp. 33–44. [Google Scholar]
- Hillary, V.E.; Ceasar, S.A. Application of CRISPR/Cas9 Genome Editing System in Cereal Crops. Open Biotechnol. J. 2019, 13, 173–179. [Google Scholar] [CrossRef][Green Version]
- Ji, X.; Zhang, H.; Zhang, Y.; Wang, Y.; Gao, C. Establishing a CRISPR–Cas-like immune system conferring DNA virus resistance in plants. Nat. Plants 2015, 1, 15144. [Google Scholar] [CrossRef]
- Baltes, N.J.; Hummel, A.W.; Konecna, E.; Cegan, R.; Bruns, A.N.; Bisaro, D.M.; Voytas, D.F. Conferring resistance to geminiviruses with the CRISPR–Cas prokaryotic immune system. Nat. Plants 2015, 1, 15145. [Google Scholar] [CrossRef]
- Aman, R.; Ali, Z.; Butt, H.; Mahas, A.; Aljedaani, F.; Khan, M.Z.; Ding, S.; Mahfouz, M. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 2018, 19, 1. [Google Scholar] [CrossRef]
- Ali, Z.; Abulfaraj, A.; Idris, A.; Ali, S.; Tashkandi, M.; Mahfouz, M.M. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 2015, 16, 238. [Google Scholar] [CrossRef]
- Mahas, A.; Mahfouz, M. Engineering virus resistance via CRISPR–Cas systems. Curr. Opin. Virol. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Chilcoat, D.; Liu, Z.-B.; Sander, J. Use of CRISPR/Cas9 for Crop Improvement in Maize and Soybean. In Progress in Molecular Biology and Translational Science; Weeks, D.P., Yang, B., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 149, pp. 27–46. [Google Scholar]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.-S.; Huang, S.; Liu, S.; Cruz, C.V.; Frommer, W.; et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015, 82, 632–643. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Liu, W.; Gao, W.; Liu, C.; Song, G.; Li, W.-X.; Mao, L.; Chen, B.; Xu, Y.; et al. An alternative strategy for targeted gene replacement in plants using a dual-sgRNA/Cas9 design. Sci. Rep. 2016, 6, 23890. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Watanabe, T.; Sugano, S.S.; Ueta, R.; Ishihara, R.; Shinozaki, K.; Osakabe, K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci. Rep. 2016, 6, 26685. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Xie, K.; Yang, Y. RNA-guided genome editing in plants using a CRISPR-Cas system. Mol. Plant 2013, 6, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, T.; Chen, C.-H.; Li, W.; Meyer, C.A.; Wu, Q.; Wu, D.; Cong, L.; Zhang, F.; Liu, J.S.; et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res. 2015, 25, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zheng, Y.; Xiao, K.; Wei, Y.; Zhu, Y.; Cai, Q.; Chen, L.; Xie, H.; Zhang, J. OsPRX2 contributes to stomatal closure and improves potassium deficiency tolerance in rice. Biochem. Biophys. Res. Commun. 2018, 495, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hou, Y.; Wang, H.; Ji, R.; Liu, B.; Wen, J.; Niu, L.; Lin, H. Targeted mutagenesis by CRISPR/Cas9 system in the model legume Medicago truncatula. Plant Cell Rep. 2017, 36, 371–374. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodriguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef]
- Du, H.; Zeng, X.; Zhao, M.; Cui, X.; Wang, Q.; Yang, H.; Cheng, H.; Yu, D. Efficient targeted mutagenesis in soybean by TALENs and CRISPR/Cas9. J. Biotechnol. 2016, 217, 90–97. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 2015, 467, 76–82. [Google Scholar] [CrossRef]
- Butler, N.M.; Baltes, N.J.; Voytas, D.F.; Douches, D.S. Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases. Front. Plant Sci. 2016, 7, 1045. [Google Scholar] [CrossRef]
- Odipio, J.; Alicai, T.; Ingelbrecht, I.; Nusinow, D.A.; Bart, R.; Taylor, N.J. Efficient CRISPR/Cas9 genome editing of phytoene desaturase in cassava. Front. Plant Sci. 2017, 8, 1780. [Google Scholar] [CrossRef]
- Funahashi, H. Animal Biotechnology Roles in Livestock Production. IOP Conf. Ser. Earth Environ. Sci. 2020, 465, 012001. [Google Scholar] [CrossRef]
- Proudfoot, C.; Lillico, S.; Tait-Burkard, C. Genome editing for disease resistance in pigs and chickens. Anim. Front. 2019, 9, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Shrock, E.; Guell, M. CRISPR in animals and animal models. Prog. Mol. Biol. Transl. Sci. 2017, 152, 95–114. [Google Scholar]
- Bailey, L.L.; Nehlsen-Cannarella, S.L.; Concepcion, W.; Jolley, W.B. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA 1985, 254, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Dooldeniya, M.D.; Warrens, A.N. Xenotransplantation: Where are we today? J. R. Soc. Med. 2003, 96, 111–117. [Google Scholar] [CrossRef]
- Ryczek, N.; Hryhorowicz, M.; Zeyland, J.; Lipi’nski, D.; Słomski, R. CRISPR/Cas technology in pig-to-human Xenotransplantation RESEARCH. Int. J. Mol. Sci. 2021, 22, 3196. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wei, H.J.; Lin, L.; George, H.; Wang, T.; Lee, I.; Zhao, H.; Kan, Y.; Shrock, E.; Lesha, E.; et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017, 357, 1303–1307. [Google Scholar] [CrossRef]
- Denner, J. Advances in organ transplant from pigs. Science 2017, 357, 1238–1239. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Zeyland, J.; Stomski, R.; Lipinski, D. Genetically modified pigs as organ donors for Xenotransplantation. Mol. Biotechnol. 2017, 59, 435–444. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Myers, E.W.; Sutton, G.G.; Delcher, A.L.; Dew, I.M.; Fasulo, D.P.; Flanigan, M.J.; Kravitz, S.A.; Mobarry, C.M.; Reinart, K.H.; Remington, K.A.; et al. A whole-genome assembly of Drosophila. Sciene 2000, 287, 2196–2204. [Google Scholar] [CrossRef]
- Gratz, S.J.; Rubinstein, C.D.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. CRISPR-Cas9 genome editing in Drosophila. Curr. Protoc. Mol. Biol. 2015, 111, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R.; Tibbit, C.; Ponting, C.P.; Liu, J.L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013, 4, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, H.; Liu, J.; Zhang, H.; Yan, Y.; Zhu, N.; Guo, Y.; Yang, B.; Chang, Y.; Dai, F.; et al. Various applications of TALEN- and CRISPR/Cas9-mediated homologous recombination to modify the Drosophila genome. Biol. Open 2014, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.J.; Ukken, F.P.; Rubinstein, C.D.; Thiede, G.; Donohue, L.K.; Cummings, A.M.; O’Connor-Giles, K.M. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 2014, 196, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Wang, X.; Chang, J.; Gao, J.; Shi, R.; Zhang, J.; Lu, W.; Liu, Y.; Zhao, P.; et al. Highly efficient multiplex targeted mutagenesis and genomic structure variation in Bombyx mori cells using CRISPR/Cas9. Insect Biochem. Mol Biol. 2014, 49, 35–42. [Google Scholar] [CrossRef]
- Dong, S.; Lin, J.; Held, N.L.; Clem, R.J.; Passarelli, A.L.; Franz, A.W. Heritable CRISPR/Cas9-mediated genome editing in the yellow fever mosquito, Aedes aegypti. PLoS ONE 2015, 10, e0122353. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kato, Y.; Matsuura, T.; Watanabe, H. CRISPR/Cas-mediated targeted mutagenesis in Daphnia magna. PLoS ONE 2014, 9, e98363. [Google Scholar] [CrossRef]
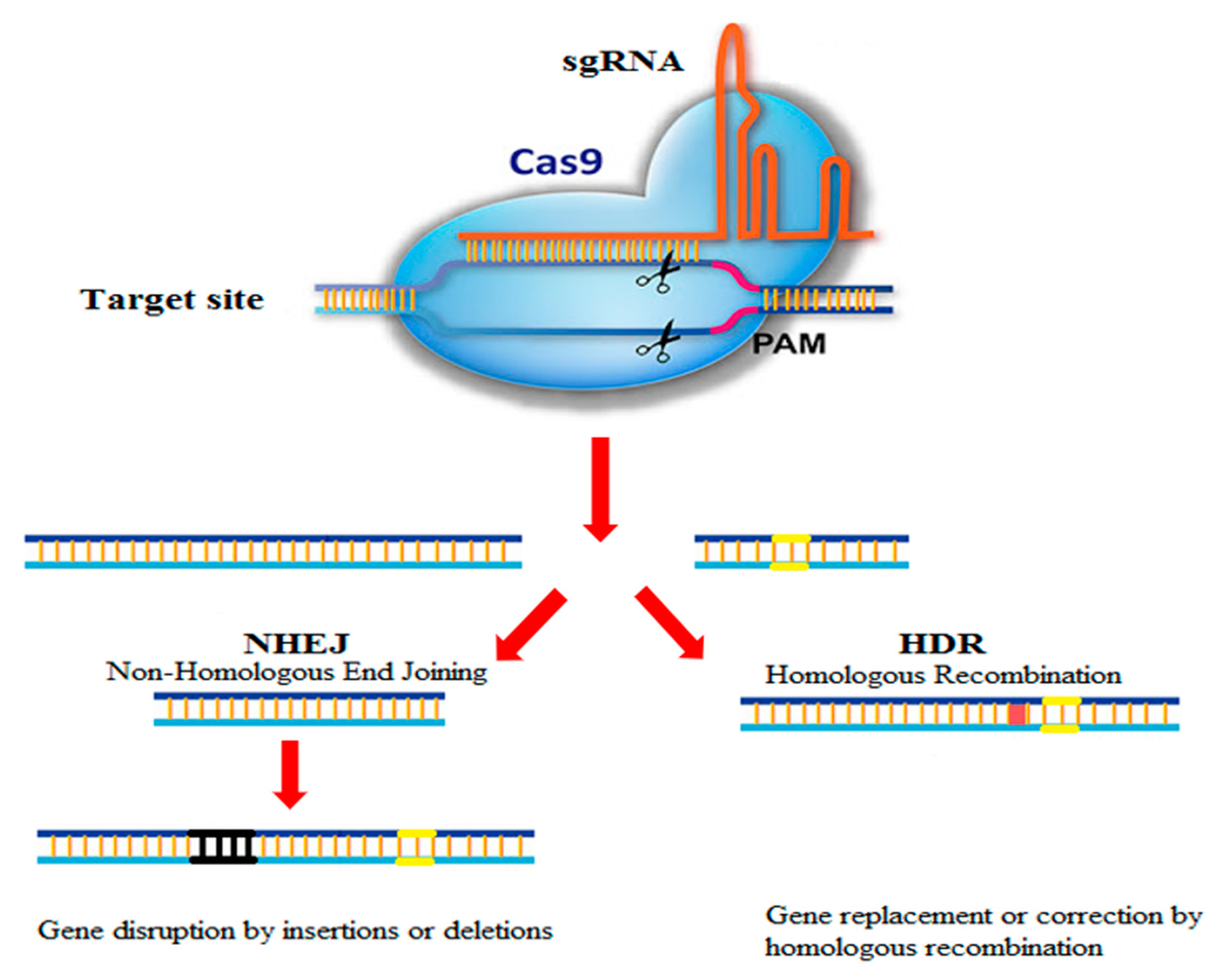
| Feature | CRISPR-Cas | TALEN | ZFN |
|---|---|---|---|
| Cost | Low | High | Low |
| Ease of design | Simple | A little complex | Moderate |
| Specificity | High | Intermediate | Low |
| Pros | Modifies multiple sites in tandem | Highly effective and specific | Highly effective and specific |
| Cons | PAM motif required next to target sequence | Time consuming | Time consuming |
| Multiplex genome editing | High-yield multiplexing | Few models | Few models |
| Organism | Risks | Bioethical Issues | References |
|---|---|---|---|
| Bacteria | Gene mutations/ Gene drifts | Disruption of ecological balance | [20,24,25] |
| Plants | Gene mutations/ Gene drifts | Disruption of ecological balance | [20,26] |
| Animals/ chimeric animals | Gene mutations | Disruption of ecological balance | [24,27,28,29,30,31,32] |
| Humans | Gene mutations Side effects Cost Genetic mosaicism | Eugenics Informed consent Enhancement Accessibility Patenting Safety Incomplete or over legislation | [21,24,26,33,34,35,36,37] |
| Type | Oncogene | Tumor Suppressor Gene | Drug-Resistance Gene |
|---|---|---|---|
| Breast | SHCBP1, MIEN1, miR-27b, mi23b, HER2 exons | CPEB2, ETS1, BRCA1 | HER2, EGFR, ER |
| Prostate | PCAR19, CECAM21, SENP1, miR-302/367, miR-1205 | PGC1a, DEPTOR, p53, RB1 | BLS-211, NANOGP8, NANOG1 |
| Lung | IGFIR, ERBB2, RSF1, FOS, MCM4 | MFN2, MiR-1205, GOT1, TP53 | NRF2, MiR-1205, ER300, RSF1 |
| Liver | Plxnb1, NCAPG, CDK7, IncBRM, Nf1 | BAP1, HELLS, Tp53, Traf3 | NF1, MED12, ERK2 |
| Colorectal | KRAS, HPV16, Fut4, NRAS | LIMCH, PTEN, SOX15 | miR-139-5P, ZEB1 |
| CRISPR-Cas9 function | Knockout | Activate | Promote drug sensitivity |
| Target Gene and Effect | Disease | Intervention |
|---|---|---|
| Cas9-mediated creation of CD19 and CD20 | Leukemia | CAR T cells to CD19 and CD20 or CD19 and CD22 |
| CCR5 knockout | HIV | Modified CD34+ hematopoietic stem cells |
| CD7 knockout in CD7 CAR T cells | T-cell malignancies | CAR T cells to CD7 and knockout of native CD7 to prevent self-targeting |
| Correction of the hemoglobulin subunit β globulin gene | β-thalassemia | Ex vivo modified hematopoietic stem cells |
| Creation of a CD19-directed T cell | Refractory B-cell malignancies | CD19-directed T-cell immunotherapy |
| Cytokine-induced SH2 protein (CISH) knockout | Metastatic gastrointestinal epithelial cancer | Modified tumor-infiltrating lymphocytes |
| disruption of HPK1 | Refractory B cell malignancies | CD19-CAR modified T cells with CAR delivered by lentivirus and Cas9 knockout of HPK1 |
| Disruption of the erythroid enhancer to BCL11A gene | β-thalassemia | Ex vivo modified hematopoietic stem cells |
| Sickle cell anemia | ||
| β-thalassemia and severe sickle cell anemia | Ex vivo- modified hematopoietic stem cells, 15-year follow-up study | |
| E6 and E7 oncogene of HPV16 and HPV18 deletion | HPV-related malignancy | Plasmid in a gel containing a polymer to facilitate delivery |
| Programmed cell death protein 1 (PD-1) knockout | Mesothelin positive solid tumors | CAR T cells to mesothelin with added PD-1 and TCR knockout |
| Hormone refractory prostate cancer | Modified T cells | |
| Esophageal cancer | ||
| Metastatic non-small cell lung cancer | ||
| Stage IV bladder cancer | ||
| Metastatic renal cell carcinoma | ||
| EBV-positive, advanced stage malignancies | Modified T cells selected for those targeting EBV positive cells | |
| Mesothelin positive solid tumors | CAR T cells to mesothelin with PD-1 knockout | |
| Removal of alternative splice site in CEP290 | Leber congenital amaurosis 10 | ZFN-mediated removal of intronic alternative splice site in retinal cells |
| TCRα, TCRβ, PD-1 knockout | Various malignancies | Modified T cells with Cas9-mediated deletions and lentiviral transduction of NY-ESO-1 targeted TCR |
| βTCRα, TCRβ, β-2 microglobin (B2M) knockout | B-cell leukemia | CD19-CAR modified T cells with CAR delivered by lentivirus and Cas9 knockout B2M and TCR to create universal T cells |
| Virus | Type of Nucleic Acid | Involved Protein | Plant under Attack | References |
|---|---|---|---|---|
| Beet severe curly top virus | DNA | Cas9 | Capsicum | [75] |
| Bean yellow dwarf virus | DNA | Cas9 | Oat | [76] |
| Turnip mosaic virus | RNA | Cas13 | Cruciferous plants, Chinese cabbage, turnip, mustard, radish | [77] |
| Tomato yellow leaf curl virus | DNA | Cas9 | Invading a number of seeds, including tomato | [78,79] |
| Yellowing virus | RNA | Cas13 | Cucumber | [80] |
| Zucchini yellow mosaic virus | RNA | Cas13 | Cucumber | [81] |
| Papaya ring spot mosaic virus | RNA | Cas13 | Cucumber | [82] |
| Plant | Targeted Area in Gene | Disease | References |
|---|---|---|---|
| Rice | Mutagenesis of the ERF Transcription Factor Gene OsERF922 | Blast | [72] |
| Duncan grapefruit | Effector-binding element in the promoter of the Lateral Organ Boundaries 1 gene | Citrus bacterial canker (CBC) | [81] |
| Wanjinchen oranges | (CsLOB1G and CsLOB1−) alleles | Citrus bacterial canker (CBC) | [82] |
| Crop | Method | Target Gene | Stress/Trait | References |
|---|---|---|---|---|
| A. thaliana/ N. benthamiana | NHEJ | dsDNA of virus (A7, B7, and C3 regions) | Beet severe curly top virus resistance | [75] |
| N. benthamiana Bean | NHEJ | BeYDV | Yellow dwarf virus (BeYDV) resistance | [76] |
| N. benthamiana | NHEJ | ORFs and the IR sequence sDNA of virus | Tomato yellow leaf curl virus (TYLCV) and Merremia mosaic virus (MeMV) | [77] |
| Rice | NHEJ | OsERF922 (ethylene responsive factor) | Blast Resistance | [78] |
| Cucumber | NHEJ | eIF4E (eukaryotic translation initiation factor 4E) | Cucumber vein yellowing virus (CVYV), Zucchini yellow mosaic virus (ZYMV), and (PRSV-W) | [80] |
| A. thaliana | NHEJ | eIF(iso)4E | Turnip mosaic virus (TuMV) resistance | [84] |
| Rice (IR24) | NHEJ | OsSWEET13 | Bacterial blight disease resistance | [85] |
| Bread wheat | NHEJ | TaMLO-A1, TaMLO-B1, and TaMLOD1 | Powdery mildew resistance | [86] |
| Maize | HDR | ARGOS8 | Increased grain yield under drought stress | [87] |
| Tomato | NHEJ | SlMAPK3 | Drought tolerance | [88] |
| A. thaliana | HDR | MIR169a | Drought tolerance | [89] |
| A. thaliana | NHEJ | OST2 (OPEN STOMATA 2) (AHA1) | Increased stomatal closure in response to abscisic acid (ABA), | [90] |
| Rice | HDR/ NHEJ | OsPDS, OsMPK2, OsBADH2 | Involved in various abiotic stress tolerance | [91] |
| Rice | NHEJ | OsMPK5 | Various abiotic stress tolerance and disease resistance | [92] |
| Rice | NHEJ/ HDR | OsMPK2, OsDEP1 | Yield under stress | [93] |
| Crop | Method | Target Gene | Stress/Trait | References |
|---|---|---|---|---|
| Rice | NHEJ | 2.5604 gRNA for 12,802 genes | Creating genome wide mutant library | [95] |
| Maize | NHEJ | ZmIPK1A ZmIPK and ZmMRP4 | Phytic acid synthesis | [96] |
| Wheat | HDR | TaVIT2 | Fe content | [97] |
| Soybean | NHEJ | GmPDS11 and GmPDS18 | Carotenoid biosynthesis | [98] |
| Tomato | NHEJ | Rin | Fruit ripening | [99] |
| Potato | HDR | ALS1 | Herbicide resistance | [100] |
| Cassava | NHEJ | MePDS | Carotenoid biosynthesis | [101] |
| Rice | NHEJ | 2.5604 gRNA for 12,802 genes | Creating genome wide mutant library | [95] |
| Maize | NHEJ | ZmIPK1A ZmIPK and ZmMRP4 | Phytic acid synthesis | [96] |
| Wheat | HDR | TaVIT2 | Fe content | [97] |
| Soybean | NHEJ | GmPDS11 and GmPDS18 | Carotenoid biosynthesis | [98] |
| Tomato | NHEJ | Rin | Fruit ripening | [99] |
| Potato | HDR | ALS1 | Herbicide resistance | [100] |
| Cassava | NHEJ | MePDS | Carotenoid biosynthesis | [101] |
| Species | Targeted Genes | Strategy | Germline Transmission Rate (%) | G1 Mutation Rate (%) | References |
|---|---|---|---|---|---|
| Drosophila spec. | yellow, whit | mRNA INJ | 0–79 | 0–34.5 | [116] |
| CG4221, CG5961, Chameau | mRNA INJ with donor | 8.1–26.7 | 2.7–10.4 | [117] | |
| yellow | DNA INJ with donor | 5.9–20.7 | 0.25–1.37 | [115] | |
| yellow | Rapid INJ with donor | 8–53 | 15 | [118] | |
| Bombyx mori | BmBLOS2 | mRNA INJ | 95.5 | 35.6 | [119] |
| th, re, fl, yellow-e, kynu, ebony | DNA INJ | 5.7–18.9 | ND | [120] | |
| Aedes aegypti | ECFP | mRNA INJ + DNA INJ | 0 | 5.5 | [121] |
| Daphnia magna | eyeless | mRNA INJ | 18–47 | 8.2 | [122] |
| Tribolium castaneum | eGFP1 | mRNA INJ + DNA INJ with donor | 55–80 | 71–100 | [123] |
| Papilio xuthus, P. machaon | abdominal-B, ebony, frizzled | mRNA INJ | 18.33–90.85 | ND | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, K.; Pour-Aboughadareh, A.; Kianersi, F.; Poczai, P.; Etminan, A.; Shooshtari, L. Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences. BioTech 2021, 10, 14. https://doi.org/10.3390/biotech10030014
Tavakoli K, Pour-Aboughadareh A, Kianersi F, Poczai P, Etminan A, Shooshtari L. Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences. BioTech. 2021; 10(3):14. https://doi.org/10.3390/biotech10030014
Chicago/Turabian StyleTavakoli, Kamand, Alireza Pour-Aboughadareh, Farzad Kianersi, Peter Poczai, Alireza Etminan, and Lia Shooshtari. 2021. "Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences" BioTech 10, no. 3: 14. https://doi.org/10.3390/biotech10030014
APA StyleTavakoli, K., Pour-Aboughadareh, A., Kianersi, F., Poczai, P., Etminan, A., & Shooshtari, L. (2021). Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences. BioTech, 10(3), 14. https://doi.org/10.3390/biotech10030014








