Abstract
Poly(butylene adipate-co-terephthalate) (PBAT) possesses mechanical properties and processing advantages comparable to low-density polyethylene (LDPE). However, its poor water vapor barrier properties (~2 orders of magnitude lower than LDPE) limit its applications in agricultural films and packaging. In this study, dodecenyl succinic anhydride (DDSA) was employed as a functional comonomer to synthesize DDSA-modified PBAT-based copolyesters (PBADT) with varying compositions via co-esterification and melt polycondensation, and the effects of the hydrophobic alkylene side chain on surface hydrophobicity, water vapor barrier property, and other physical and mechanical properties of PBADT were systematically investigated. Results indicate that the introduction of DDSA significantly enhanced the surface hydrophobicity and water vapor barrier properties of PBAT. As the DDSA content increased from 0 to 55 mol%, the water contact angle increased from 79° to 101°, and the water vapor barrier performance improved by nearly three times. Crucially, due to the chemical bonding of hydrophobic side chains to the main chains, the PBADT films exhibited excellent stability in its water vapor barrier performance under external mechanical friction. Furthermore, DDSA introduction markedly reduced haze and increased light transmittance, demonstrating improved optical clarity. On the other hand, the existence of the long alkylene side chain of DDSA also significantly inhibited the crystallization and mechanical properties of the copolyesters.
1. Introduction
Traditional polymer materials are non-biodegradable in natural environments and are often discarded after a single use, leading to massive waste accumulation and the growing problem of “white pollution.” To tackle this issue, developing and applying biodegradable polymers as sustainable alternatives to conventional plastics has become a key research focus. Poly(butylene adipate-co-terephthalate) (PBAT) is a commercially available biodegradable polymer with ductility, mechanical properties, thermal stability, and processability comparable to low-density polyethylene (LDPE), making it a promising alternative to polyethylene-based films [1,2,3,4]. However, its poor gas (O2 and CO2) barrier properties and water vapor (WV) barrier property—approximately 80 times higher than LDPE [4,5], severely restrict the widespread application of PBAT in scenarios requiring high barrier properties, such as food packaging and agricultural mulching. Therefore, it is particularly necessary to modify PBAT to improve its barrier performance.
Modification methods for PBAT’s water vapor barrier properties can be categorized into physical and chemical modifications. Physical modification methods include melt blending, nanocomposites, multilayer composites, etc. [5,6,7]. Melt blending involves mixing PBAT with high-gas-barrier biodegradable polymers such as poly(glycolic acid) (PGA) [8,9,10], poly(propylene carbonate) (PPC) [11,12], and poly(ethylene oxalate) (PEO) [13] in a molten state to form a “sea-island” structure to optimize performance. Since the barrier polymers exist as microspheres with a low aspect ratio in the matrix polymer, the improvement in the water vapor barrier is usually no more than five times. Nanocomposites enhance gas barrier properties by introducing gas-impermeable layered nanofillers (e.g., montmorillonite [14,15], mica [16], hydrotalcite [17], and graphene [18,19]) into the polymer matrix to prolong the diffusion path of gas molecules. However, the bottleneck of this method is that to fully utilize the barrier effect of nanofillers, complete exfoliation, uniform dispersion, and high orientation must be achieved at high filler loading. Recently, Wang et al. [20] achieved excellent water vapor barrier performance (15 times that of pure PBAT) by compounding hydrophobically modified lignin micro-nanosheets into the PBAT matrix. This was achieved through the nanosheets’ barrier effect, improved interfacial compatibility, and uniform dispersion. Multilayer films involve compounding high-gas-barrier layers with the polymer substrate through co-extrusion, co-blowing, hot rolling, melt lamination, dip coating, etc. [21]. Since the high-gas-barrier polymer exists as a continuous phase, the function of the barrier layer can be fully exploited. There have been several reports on the co-extrusion of biodegradable barrier-modified materials such as Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) [22] and PPC [23] with PBAT.
Compared to the aforementioned physical modification methods, chemical modification of PBAT by copolymerization remains relatively underexplored. PBAT possesses inherent limitations, including low crystallinity (typically <15%) and absence of strong polar or hydrophobic groups within its polymer chains. These characteristics collectively contribute to its generally poor gas and water vapor barrier properties. The introduction of polar units, for example, can enhance intermolecular interactions, thereby reducing free volume and improving gas barrier properties. For instance, the water vapor barrier properties of aliphatic–aromatic polyesters, such as poly(butylene oxalate-co-terephthalate) (PBOT) and poly(butylene carbonate-co-terephthalate) (PBCT), which have similar compositions to PBAT, are six times [24] and eight times [25] higher than PBAT, respectively. Nonetheless, excessively strong polarity often leads to excessively high hydrophilicity of the material. Our previous research found that although sulfonated PBAT exhibits improved O2 and CO2 barrier properties due to the strong polarity of the sodium 5-sulfonate groups, its strong hydrophilicity causes the material to easily absorb water and swell, which in turn significantly reduces its water vapor barrier performance [26]. To mitigate this trade-off, researchers have incorporated moderately polar comonomers (e.g., diglycolic acid [27] and lactic acid [28]) into the PBAT molecular chain, which can balance intermolecular interactions and hydrophilic effects. Yet, the resulting water vapor barrier improvements remain limited, typically not exceeding a two-fold enhancement.
From a mechanistic perspective, the water vapor permeability coefficient of a polymer is governed by both the diffusion coefficient (D) and the solubility coefficient (S). While existing research has predominantly focused on reducing D, strategies that effectively lower S or simultaneously reduce D and S remain relatively scarce. Intriguingly, although the O2 and CO2 barrier properties of PE are generally moderate, its methylene segments impart extremely strong hydrophobicity, resulting in a very low water vapor solubility (SWV). This unique characteristic compensates for its high free volume and chain mobility, which would otherwise lead to high water vapor diffusion coefficient (DWV), thus exhibiting excellent water vapor barrier properties. Inspired by this mechanism, Yuan et al. [29] introduced glycerol monostearate, a hydrophobic small molecule, reducing SWV and improving the barrier property by three-fold. Similarly, Gao et al. [30] hydrophobically modified SiO2 nanoparticles with vinyltrimethoxysilane (VTMO) and grafted them onto PBAT. At 2 wt% SiO2 loading, the contact angle reached 146°, doubling the water vapor barrier performance. Our prior work also found that blending PBAT with strongly hydrophobic, easily crystallizable long-chain saturated fatty acids (SFAs) to form films can form a hydrophobic surface layer and interior moisture barrier crystal plates, thereby simultaneously reducing water vapor adsorption/dissolution and diffusion, leading to a ten-fold improvement in water vapor barrier properties [31]. However, the interfacial interaction between hydrophobic SFA additives and the PBAT matrix needs to be enhanced both in the interior and on the surface of the PBAT/SFA films.
To effectively address the aforementioned limitations and achieve a more stable, intrinsic modification, this study explores the feasibility of enhancing PBAT’s water vapor barrier properties by copolymerizing it with a diacid comonomer containing a hydrophobic side chain. Specifically, dodecenyl succinic anhydride (DDSA) was chosen as the functional comonomer due to its bifunctional nature, featuring a reactive anhydride group and a long alkylene side chain. In fact, the anhydride group’s high reactivity with active moieties (e.g., epoxy, hydroxyl, and amine groups) makes DDSA widely applicable as a curing agent, polymer additive, and modifier for biopolymers to enhance their dispersion and functionality in hydrophobic environments [32,33,34]. The DDSA-modified PBAT copolyesters (PBADT) with various compositions were synthesized by copolycondensation, the chain structure was characterized with NMR, and the water vapor barrier property, thermal transition, stability, and mechanical and optical properties were measured and assessed. This research proposes a new way for designing high-performance PBAT-based biodegradable films with enhanced and stable moisture barrier performance and improved clarity.
2. Materials and Methods
2.1. Materials
Adipic acid (AA, industrial grade) was purchased from PetroChina Liaoyang Petrochemical Company (Liaoyang, China). Terephthalic acid (TPA, industrial grade) and 1,4-butanediol (BDO, industrial grade) were obtained from Zhejiang Hengyi Petrochemical Co., Ltd. (Hangzhou, China). Dodecenyl succinic anhydride (DDSA, 99%) was supplied by Hangzhou Chenyu Biotechnology Co., Ltd. (Hangzhou, China). Tetrabutyl titanate (TBT) was obtained from TCI Chemicals Co., Ltd. (Shanghai, China). Deuterated chloroform (CDCl3) was purchased from Merck KGaA, Darmstadt, Germany. Chloroform (AR grade) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Anhydrous calcium chloride (CaCl2, 99%) was purchased from Ruichen Chemical (Jinan, China). All raw materials were used directly, without further purification or pretreatment.
2.2. Synthesis
A series of DDSA-modified PBAT copolyesters, denoted as PBADT, were synthesized via esterification–melt polycondensation (Scheme 1). In the first step, a 250 mL four-necked flask equipped with a mechanical stirrer, N2 inlet, and reflux condenser was charged with metered amounts of TPA, AA, DDSA, and BDO. The molar ratio (AA+DDSA)/TPA was fixed at 55/45. Tetrabutyl titanate (TBT, 0.1 mol% of the total diacid content) was added as a catalyst to the monomer mixture. The esterification reaction was carried out at 180–210 °C for 3–4 h until no more water distilled out. In the second step, a pre-polycondensation reaction was performed at 230 °C under approximately 200 Pa for 1 h. Subsequently, the temperature was raised to 240 °C, and then the pressure was controlled below 20 Pa for 3–3.5 h for melt polycondensation. The obtained copolyesters were named PBA55-xDxT, where x represents the molar percentage of DDSA in the total diacid feed.
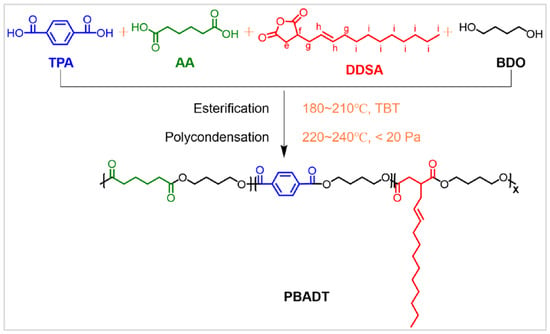
Scheme 1.
Synthesis of DDSA-hydrophobically modified PBAT copolyester (PBADT). (Note: The actual DDSA structure is more complex, and the depicted structure is only a representative one.)
2.3. Characterization
The intrinsic viscosity (IV) of the copolyesters was measured at 25 °C in chloroform at a concentration of 5 g/dL using a IVS300 semi-automatic viscometer tester (Hangzhou Zhongwang Co., Hangzhou, China) equipped with an Ubbelohde viscometer.
1H NMR spectra of the copolyesters were recorded using a Bruker AC-80 spectroscopy (400 M, Bruker BioSpin Co., Karlsruhe, Germany). Deuterated chloroform (CDCl3) was used as the solvent, and tetramethylsilane was used as the internal reference for 1H NMR spectroscopy.
The thermal transition of the copolyesters was recorded by differential scanning calorimetry (DSC, Q200, TA Instrument, New Castle, DE, USA) using a conventional heating–cooling–heating cycle. Samples weighing 6–8 mg were used. The heating/cooling rate was 10 °C/min, the isothermal time was 5 min, and the temperature range was −70 to 180 °C.
Thermogravimetric analysis (TGA) of the copolyesters was performed using a TA Q500 (TA Instrument, New Castle, DE, USA) with 3–5 mg samples. All samples were heated at 10 °C/min under a nitrogen atmosphere with a N2 flow rate of 50 mL/min in the temperature range of 50–600 °C.
Polymer films with a thickness of approximately 200–300 μm were prepared by hot pressing at 160 °C. These films were subsequently used for tensile tests, X-ray diffraction, dynamic mechanical thermal analysis, optical properties tests, water contact angle, and water vapor permeation measurements. For tensile tests, the films were cut into dumbbell-shaped specimens with a length of 35 mm and a neck width of 2 mm using a standard cutter. Prior to testing, all specimens were preconditioned at room temperature for 48 h to eliminate internal stress. Tensile tests of the copolyesters were conducted at 25 °C using a Zwick Roell Z020 (ZwickCo, Ulm, Germany) universal testing machine at a crosshead speed of 50 mm/min. For each sample, at least five specimens were tested.
Wide-angle X-ray diffraction (WAXD) patterns of the copolyesters were recorded on a SmartLab SE (Rigaku Co., Tokyo, Japan) The sample was scanned from 2θ = 10° to 2θ = 40° with a scanning speed of 5°/min.
Dynamic mechanical analysis (DMA) was performed using a TA instrument model Q800 (TA Instrument, New Castle, DE, USA). The oscillation frequency was 1.0 Hz, the amplitude was 10 μm, and the sample size was 30 mm × 6 mm × 0.2 mm. Tensile mode testing was performed. The test temperature range was −80 to 150 °C, and the heating rate was 5 °C·min−1. The peak value of the loss factor within this temperature range was taken as the glass transition temperature Tg.
The transmittance, haze and CIELAB color values (L*, a*, b*) of the polymer films were determined using a CS-821 N desktop spectrophotometric colorimeter (Hangzhou CHNSpec Technology Co., Ltd., Hangzhou, China) used in transmittance mode. L* represents lightness, a* represents the green (−)/red (+) extent, and b* represents the blue (−)/yellow (+) extent.
The water contact angles of the copolyester films were measured using an OCA 20 video optical contact angle measuring device (Dataphysics Co., Filderstadt, Germany). Deionized water (0.35 μL) was dropped at 5–6 positions on the flat surface of the film placed on the sample stage to measure the water contact angle.
The water vapor transmission rate (WVTR) was measured using a moisture permeability cup according to ASTM E96-16 [35] and GB 1037-2021 [36]. The testing cup was put in a chamber controlled at a constant temperature (38 °C) and humidity (90 RH%). The detailed experimental process can be found in our previous study [26,31,37]. The water vapor transmission rate (WVTR) and water vapor permeability coefficient (WVP) were calculated using Equations (1) and (2), respectively. Here, ∆m is the mass of water permeated through the film, ∆t is the time required for water to permeate through the film, S is the test area of the polyester film, which is 1/353.67 m2, d is the thickness of the film, and p is the partial pressure of water vapor, which is 5967 Pa at 38 °C and 90% humidity.
3. Results and Discussion
3.1. Synthesis and Structure Characterization
PBADT copolyesters were synthesized via esterification–melt polycondensation using AA, TPA, DDSA, and BDO as monomers. The molar percentage of TPA in the total diacids was fixed at 45%, while the molar percentage of DDSA in the total diacids varied from 15% to 55%. Detailed synthesis conditions and results are summarized in Table 1.

Table 1.
Synthesis and structural characterization of PBAT and PBADT copolyesters a.
As the DDSA content increased, the intrinsic viscosity (IV) of the copolyesters showed a decreasing trend. The IV of PBA55T was 0.85 dL/g, while that of PBD55T was only 0.45 dL/g. A possible explanation is that the esterification reaction may have been inhibited by both impurities in the DDSA monomer and the steric hindrance effect of its long alkylene side chains.
The copolymer composition and average sequence length of PBADT copolyesters were characterized by 1H-NMR spectroscopy. As shown in Figure 1, the single peak at δ = 8.1 ppm corresponds to the four hydrogen atoms on the benzene ring (T) in the butylene terephthalate (BT) repeat unit, confirming its presence of the BT unit. The peak at δ = 2.3 ppm corresponds to the four methylene hydrogens (a) connected to the ester group in the AA structural unit, and the intensity of this peak gradually decreased with decreasing AA content. When AA was completely replaced by DDSA, this peak disappeared in PBD55T. For the butylene dodecenyl succinate (BD) unit, δ = 5.3–5.5 ppm corresponds to the protons on the double bond of the dodecenyl side chain in the DDSA structural unit (h), δ = 2.1–2.9 ppm corresponds to the methylene hydrogens (e), methine hydrogens (f) connected to the ester group on the DDSA backbone, and methylene hydrogens (g) connected to the double bond in the side chain. The peaks at δ = 0.5–1.5 ppm correspond to the hydrogens on the alkyl side chain (i). The 1H-NMR signals of the BD unit seem to be more complex than expected, possibly because the DDSA monomer is not a pure monomer but a mixture of isomerides with various double-bond positions in the side chain or homologs with various side-chain lengths. The complex 1H NMR signals of the DDSA monomer itself support this point. Nonetheless, the intensity of these three sets of peaks (h, e–g, i) gradually increased with increasing DDSA content, indicating that DDSA was successfully copolymerized into the polyester chains.
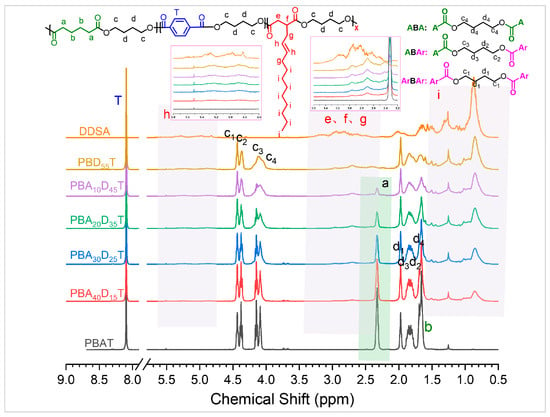
Figure 1.
1H-NMR spectra of PBADT and attribution of the chemical shifts.
The molar percentages of the three repeat units in the copolyesters were determined by calculating the areas of their characteristic peaks in the 1H-NMR spectra. For the above-mentioned reason, precise quantitative analysis of the BD unit is nearly impossible. Therefore, in this study, a subtraction method was employed to determine the molar fraction of the BD repeating units. Based on the peak areas at chemical shifts of δ = 8.1 ppm (T), δ = 2.3 ppm (a), and δ = 4.0–4.5 ppm (c) in the 1H-NMR spectrum, the composition of each unit in the copolyester was calculated according to Equations (3)–(5), where Ia, Ic, and IT represent integrated peak areas corresponding to specific proton signals, ϕBT, ϕBA, and ϕBD: molar fractions of BT, BA, and BD repeating units, respectively.
The results are presented in Table 1. The content of ϕBD increased from 0 to 55.4%, the content of ϕBA gradually decreased from 52.8% to 0, and the value of ϕBT ranged from 47.2% to 44.8%, which is basically consistent with the feed ratio. Therefore, the copolymer composition can be adjusted and controlled by the feed ratio of each diacid monomer.
Furthermore, the splitting of signals (c) and (d) in PBADT is analogous to that in PBAT, implying that the aliphatic BA and BD units can be considered chemically equivalent for the splitting of (c) and (d). In this case, the ternary PBADT copolymers could be regarded as binary copolymers like PBAT, and the randomness degree of PBADT can also be calculated from Equations (6)–(8), in which LBal and LBAr represent number-average sequence lengths of aliphatic (BA and BD) and aromatic (BT) repeating units, respectively. R represents the randomness degree of the copolyester.
As shown in Table 1, the average sequence length of the copolyesters was approximately 2, and the randomness degree was greater than 0.90 and close to 1.0, indicating the successful synthesis of random copolyesters.
3.2. Surface Hydrophilicity/Hydrophobicity and Water Vapor Barrier Property
Table 2 presents the water vapor permeability (WVP) coefficients measured at 38 °C and 90% RH and water contact angle (WCA) data measured at room temperature for PBADT copolyester samples with varying ϕBD content.

Table 2.
WVP and WCA data of PBAT and PBADT.
From the data in Table 2, the WVP value of the newly synthesized PBA55T in the laboratory was 366 g·mm·m−2·d−1·atm−1, which is close to that of commercial PBAT from BASF (406 g·mm·m−2·d−1·atm−1) and agrees well with many literature reports [26,27,37,38]. For PBADT copolyesters, the water vapor permeability coefficient gradually decreased with increasing ϕBD content. When the ϕBD content reached 35%, the water vapor barrier performance improved 1.89 times. Furthermore, when the ϕBD content reached 55%, namely, AA was completely replaced by DDSA, the WVP of PBD55T was 139 g·mm·m−2·d−1·atm−1, indicating a nearly three-fold improvement in water vapor barrier performance compared to pure PBAT. This enhancement is notable when compared to other chemical modification strategies for PBAT. For instance, incorporating moderate polar comonomers such as diglycolic acid [27] or lactic acid [28] typically yields a barrier improvement factor (BIF) of 2.21 and 1.62, respectively. Our strategy, by chemically incorporating the hydrophobic DDSA comonomer, achieves a comparable or even superior water vapor barrier improvement (BIF of 2.71 for PBD55T).
Regarding surface hydrophilicity/hydrophobicity, the WCA of pure PBAT ranged from 76° to 79°, indicating its hydrophilicity. In contrast, the WCA of PBA40D15T was 85°, showing an increase compared to pure PBAT. Furthermore, the water contact angles of PBA30D25T, PBA20D35T, PBA10D45T, and PBD55T exceeded 90°, suggesting that the introduction of the BD unit into the PBAT macromolecular chain can transform the PBAT surface from hydrophilic to hydrophobic. Moreover, as the ϕBD content increased, the water contact angle showed an increasing trend, reaching 100.9° when AA was completely replaced by DDSA.
It is known that lower crystallinity typically leads to lower gas barrier properties [5,39]. In this study, despite the observed decrease in the crystallinity of PBADT copolyesters with increasing DDSA content (as discussed in Section 3.4), their water vapor barrier performance was still clearly improved. This strongly suggests that the primary mechanism for enhanced barrier properties is the reduction in the water vapor solubility coefficient (Swv), driven by the highly hydrophobic long alkyl side chains of the BD units. These bulky, non-polar side chains create a less hospitable environment for water molecules to condense and dissolve into the polymer matrix. Therefore, the dominant effect of reduced water vapor solubility effectively counteracts the impact of potentially higher water vapor diffusion due to lower crystallinity, resulting in an overall superior water vapor barrier performance.
3.3. Water Vapor Barrier Stability
In our previous work on PBAT/SFA films [31], we observed that while the synergistic effect of a hydrophobic rough surface layer and interior moisture barrier crystal plates significantly enhanced WV barrier properties (by tens of times), the hydrophobic surface layer was susceptible to mechanical abrasion. For instance, wiping the film with external friction led to a substantial deterioration in WV barrier performance (e.g., from 98 to 198 g·mm·m−2·d−1·atm−1). To overcome this limitation and investigate the role of copolymerized hydrophobic monomers in stabilizing WV barrier performance, two PBD55T film samples were prepared by hot pressing. One sample (PBD55T-B) was repeatedly wiped with a paper towel, while the other (PBD55T-A) remained unwiped. Macroscopic examination (Figure 2) and thickness measurements revealed no observable changes in the film’s appearance or dimensions before and after wiping. Subsequently, the surface morphologies of these two films were characterized using scanning electron microscopy, and the results are presented in Figure 2. It can be observed that the surfaces of both PBD55T-A and PBD55T-B films appeared relatively smooth and uniform, with no significant changes in surface morphology after wiping. As shown in Table 2, the WVP coefficients for both films were measured to be 128 g·mm·m−2·d−1·atm−1. Additionally, water contact angles were assessed. PBD55T-A exhibited a WCA of 100.9°, while PBD55T-B showed a slightly lower WCA of 96.1°. This minor reduction in WCA for PBD55T-B could potentially be attributed to residual substances from the tissue paper on the film surface. Despite the slight change in WCA, the consistent WVP value, coupled with the unchanged surface morphology, demonstrates that due to the chemical copolymerization effect, PBD55T film exhibits excellent stability in its water vapor barrier performance under external mechanical friction.
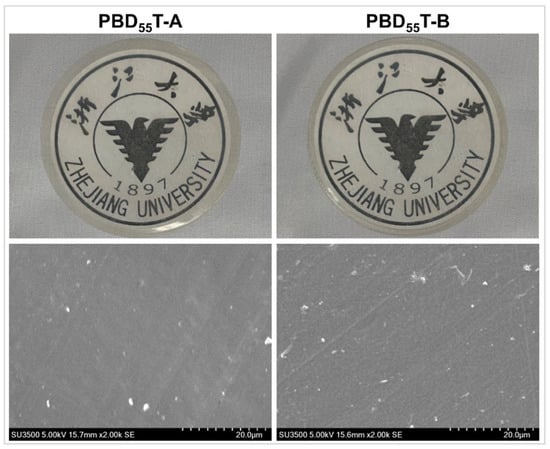
Figure 2.
Macroscopic appearance (upper) and SEM images (lower) of PBD55T film before and after wiping.
3.4. Thermal Transition Behavior and Crystal Structure
Differential scanning calorimetry (DSC) was used to investigate the thermal transition behavior of the PBADT copolyesters. Figure 3 shows the DSC curves of the PBADT copolyesters, including the first heating, cooling, and second heating cycles. The corresponding thermal transition parameters are listed in Table 3.
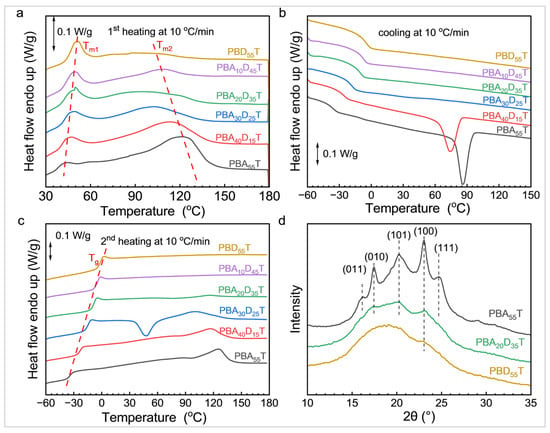
Figure 3.
DSC (a–c) and XRD (d) curves of PBAT and PBADT copolyesters.

Table 3.
DSC and TGA data for PBAT and PBADT copolyesters.
During the first heating cycle (Figure 3a), all samples exhibited two melting peaks (Tm1 and Tm2). Tm2 corresponds to the melting of PBT crystals, while Tm1 likely originates from the slow formation of fringe-micelle PBA or PBD crystals during room-temperature storage [40]. It is important to note that PBD or PBA sequences are inherently challenging to crystallize into sufficiently large structures for significant X-ray diffraction, particularly since their crystallization occurs within the constrained spaces remaining after PBT sequence crystallization [40]. As a result, no characteristic diffraction peaks corresponding to PBA or PBD were observed in Figure 3d. When the ϕBD content increased, the melting peak Tm1 gradually shifted towards higher temperatures, and its intensity showed a trend of gradual increase. Simultaneously, the melting peak Tm2 shifted towards lower temperatures, and its intensity gradually decreased. This indicates that the random incorporation of the BD sequences into the PBAT main chain disrupts the regularity of the PBAT backbone, thereby inhibiting the formation of large-sized, highly perfect crystals of the BT crystals. Consequently, this structural perturbation creates more available space for the crystallization of PBD/PBA sequences, which is manifested by the observed shift of Tm1 to higher temperatures and an increase in its melting enthalpy.
During the cooling process (Figure 3b), pure PBAT only exhibited a distinct BT crystallization peak (Tc = 86 °C, ΔHc = 12.1 J/g), indicating good crystallization ability of the BT sequence. However, upon the introduction of BD segments, for PBA40D15T, the BT crystallization peak temperature Tc decreased to 74 °C, and ΔHc also dropped to 10.3 J/g. More importantly, starting from PBA30D25T, no distinct BT crystallization peak was observed during the cooling process. This indicates that these copolyesters with high BD unit content did not undergo significant crystallization at the tested cooling rate, or their crystallization rate was so slow that it could not be detected during the DSC cooling process. This phenomenon further confirms the strong inhibitory effect of the DDSA component on PBAT’s crystallization ability, leading to a significant reduction in the crystallinity of the copolyester, even transforming it into an amorphous material or one with only a small number of crystalline regions.
The second heating curve eliminates the influence of thermal history and can more accurately reflect the intrinsic thermal transition behavior of the material. As shown in Figure 3c, only one melting peak was observed in the second heating, unlike the two melting peaks in the first heating, verifying that the formation of Tm1 is related to the cold crystallization of BA or BD sequences during room-temperature storage [27]. As the ϕBD content increased, the intensity of the melting peak gradually weakened, and no melting peak could be observed in PBA10D45T and PBD55T. This further confirms that with increasing ϕBD content, the crystallization ability of the copolyester decreased drastically, gradually transforming it into an amorphous polymer. Notably, a distinct exothermic peak (cold crystallization peak) appeared in the PBA30D25T curve. This suggests that PBA30D25T still maintains cold crystallizability though it cannot crystallize from melt. As the ϕBD content increased, the Tg of the copolyester showed a clear increasing trend, from −34 °C for pure PBAT to −1 °C for PBD55T. This indicates that replacing AA with DDSA may have increased the local rigidity of the polymer segments or restricted their movement, thereby increasing the Tg.
Figure 3d shows the X-ray diffraction patterns of three representative samples, PBA55T, PBA20D35T, and PBD55T, which were analyzed to elucidate their crystalline structures. PBA55T exhibits clear and sharp diffraction peaks corresponding to the crystallization of its BT sequence. These peaks are observed at 2θ values of 16.5° (011), 18.5° (010), 20.0° (101), 22.0° (100), and 23.5° (111). For PBA20D35T, the diffraction peak intensities are noticeably reduced, and the peaks appear broader. However, the positions of the crystalline diffraction peaks remain similar to those of PBA55T, suggesting a decrease in crystallinity while still maintaining an unchanged crystalline structure. In contrast, PBD55T displays a broad amorphous scattering peak, characterized by a wide hump around 20°, but lacks sharp diffraction peaks. This pattern indicates that PBD55T is predominantly amorphous or possesses extremely low crystallinity. Overall, as the ϕBD content increased, the BT crystalline diffraction peaks in the XRD patterns gradually diminished and eventually disappeared. This trend signifies a progressive reduction in the crystallization ability of the BT sequence, which is consistent with the observed gradual decrease in the intensity of the Tm2 peak in the DSC heating curves.
3.5. Thermal Stability
Thermal stability is of significant importance in polymer synthesis, processing, and applications. The thermogravimetric analysis (TGA) results of PBADT copolyesters are shown in Figure 4, and the 5% weight loss temperature (Td,5) and maximum weight loss rate temperature (Td,max) are listed in Table 3. The TGA curves (Figure 4a) show that all samples exhibited excellent thermal stability below 300 °C, with almost no mass loss. Significant mass loss occurred only when the temperature increased to 350–450 °C. The Td,5 of pure PBAT was 352 °C. In comparison, the Td,5 values of the PBADT samples (346–334 °C) decreased to varying degrees, but all were above 330 °C, which is 200 °C higher than the melting temperature, sufficient to meet the temperature requirement of the processing. The DTG curves (Figure 4b) show that all samples exhibited a single decomposition peak, indicating that they primarily underwent a one-step thermal decomposition process within the tested temperature range. The Td,max values of the PBADT series samples (388–391 °C) were very close to that of pure PBAT (391 °C), indicating that the introduction of the BD unit did not alter the thermal decomposition mechanism of the material.
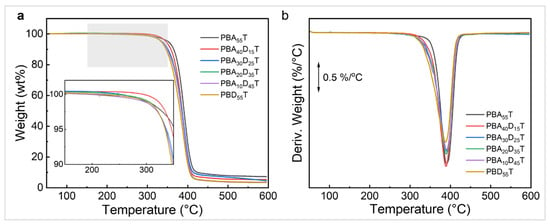
Figure 4.
TGA (a) and DTG (b) curves of PBAT and PBADT copolyesters.
3.6. Mechanical Properties
The tensile mechanical properties of PBAT and PBADT copolyesters were also investigated. The stress–strain curves obtained during the tensile test are shown in Figure 5, and the relevant tensile properties, including Young’s modulus (E), tensile strength at break (σb), and elongation at break (εb), are summarized in Table 4.
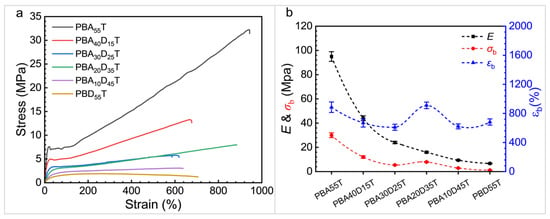
Figure 5.
Stress–strain curves (a) and changes in tensile properties (b) of PBADT copolyesters.

Table 4.
Mechanical properties data of PBADT copolyesters.
The tensile mechanical properties of PBADT copolyesters exhibit a significant dependence on the DDSA content, and their deformation mechanism changes with composition. As shown in Figure 5a, PBA55T displayed typical deformation behavior of a semi-crystalline flexible polyester, including clear elastic deformation, yielding, strain softening, forced highly elastic deformation (cold drawing) in a long plateau region, and subsequent strain hardening, ultimately reaching an elongation at break of up to 885% with a tensile strength of 30 MPa and a Young’s modulus of 95 MPa. This is attributed to its relatively high intrinsic viscosity (IV = 0.85 dL/g) and semi-crystalline structure.
However, the mechanical properties and deformation mode of the copolyesters changed significantly with the introduction of DDSA. At a low DDSA content (PBA40D15T), although Young’s modulus (44 MPa) and tensile strength (12 MPa) decreased, relatively clear elastic deformation, yielding, forced highly elastic deformation, and strain hardening stages were still observed, indicating that its semi-crystalline nature remained dominant. This is consistent with the DSC results, which showed reduced crystallinity, explaining the drop in strength and modulus.
When the DDSA content further increased to PBA30D25T and PBA20D35T, the tensile behavior of the copolyesters changed significantly. The typical yield point and cold drawing plateau in the stress–strain curves became blurred or disappeared, indicating that the material lost its distinct crystalline region yielding and plastic flow capability. This aligns with the sharp decrease in crystallinity observed in the DSC results. Nevertheless, the stress and elongation at break of PBA20D35T were clearly higher than the samples with lower or higher BD content. This phenomenon can be attributed to its relatively high intrinsic viscosity.
At high DDSA content (PBA10D45T and PBD55T), the mechanical properties of the copolyesters continued to deteriorate, with Young’s modulus and tensile strength dropping to their lowest points (PBD55T had a Young’s modulus of only 6.7 MPa and a tensile strength of 1.2 MPa). The stress–strain curves became very flat, completely losing the yield point and strain hardening stages, resembling an amorphous elastomer or a very soft plastic [41]. DSC results confirmed that these samples were almost completely amorphous, lacking sufficient crystalline regions to provide strength support. Furthermore, their intrinsic viscosities (0.53 and 0.45 dL/g) also dropped to the lowest values, indicating a significant decrease in molecular weight and insufficient molecular chain entanglement, which further weakened the mechanical load-bearing capacity of the material.
3.7. Dynamic Mechanical Analysis
Dynamic mechanical analysis (DMA) was conducted to thoroughly investigate the viscoelastic behavior and molecular transitions of the PBADT copolyesters. Figure 6 presents the temperature dependence of the storage modulus (E′, Figure 6a), loss modulus (E″, Figure 6b), and loss tangent (tan δ, Figure 6c) for all synthesized samples.
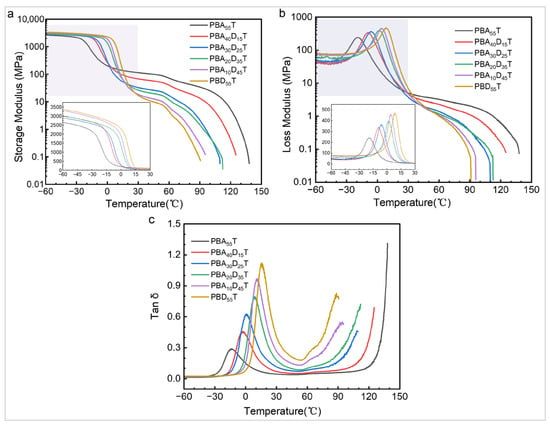
Figure 6.
(a) Storage modulus, (b) loss modulus, and (c) tan δ of PBADT copolyesters.
In the glassy region (temperatures below approximately −20 °C), all copolyesters exhibit a high storage modulus, indicating their rigid and brittle nature. As observed from Figure 6a, the storage modulus in the glassy state ranges from approximately 2500 MPa for PBA55T to over 3000 MPa for PBD55T. Notably, as the DDSA content increases, the storage modulus in the glassy region shows a slight increase, suggesting that the incorporation of the bulky BD units contributes to a stiffer polymer at low temperatures.
As the temperature rises, all samples undergo a prominent glass transition (Tg), characterized by a sharp drop in the storage modulus and distinct peaks in both the loss modulus and tan δ curves. The peak maximum of the tan δ curve is typically defined as the glass transition temperature (summarized in Table 3). A clear and consistent trend of increasing Tg with increasing DDSA content is observed. Specifically, the Tg shifts from −14 °C for PBA55T to 15 °C for PBD55T. This upward shift in Tg is consistent with the DSC results, indicating that the BD units incorporated into the PBAT backbone effectively restrict the segmental mobility of the polymer chains, thereby requiring more thermal energy for cooperative motion. The difference between glass transitions determined from DSC and DMA is normal and attributable to several factors. Firstly, the Tg determined by DMA is sensitive to the frequency of mechanical stimulation and the relaxation times of the polymer chains, while DSC identifies Tg as a step change in heat capacity [42]. Secondly, the heating rates employed in this study are different (10 °C/min for DSC vs. 5 °C/min for DMA). The intensity and breadth of the tan δ peaks also vary with composition; higher BD content samples generally show broader peaks, which might indicate a wider distribution of relaxation times or increased heterogeneity in the amorphous phase due to the varied local environments around the bulky side chains.
Beyond the glass transition, in the rubbery plateau region, the storage modulus continues to decrease, but at a much slower rate. Interestingly, while the glassy modulus increases with DDSA content, the modulus in the rubbery plateau region generally shows a decreasing trend for samples with higher BD content (e.g., PBD55T exhibits a lower rubbery modulus compared to PBA55T). This indicates a reduction in the overall stiffness of the materials in their rubbery state, which aligns well with the observed decrease in Young’s modulus and the more elastomer-like behavior seen in the tensile tests for samples with high BD content. This phenomenon can be attributed to the amorphous nature and reduced crystallinity of the high BD content copolyesters, as confirmed by DSC and XRD results.
At even higher temperatures, a further sharp drop in modulus is observed, indicating the onset of the flow region or melting. Overall, the DMA results provide critical insights into how the chemical modification with DDSA profoundly influences the molecular dynamics and viscoelastic properties of the PBADT copolyesters, corroborating the findings from DSC (Tg and crystallinity changes) and mechanical testing (changes in Young’s modulus and ductility).
3.8. Optical Properties
Optical properties, including transparency, haze, and color, are critical considerations for polymer films, particularly in packaging applications. Figure 7 presents macroscopic photographs of the prepared PBADT copolyester films, clearly illustrating their visual appearance and transparency against an inscription. As the DDSA content increases, the films generally appear to become more transparent and less opaque.
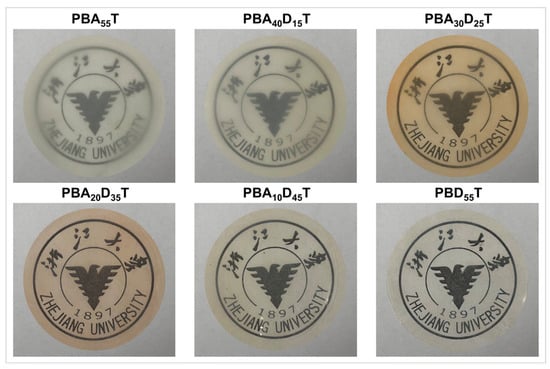
Figure 7.
Macroscopic photographs of PBADT copolyesters.
To quantify these observations, the haze and light transmittance of the films were measured, and the results are summarized in Table 5 and Figure 8. The PBA55T film exhibits a relatively high haze of 98.0% and a relatively low transmittance of 66.5%. With increasing incorporation of DDSA, the haze values significantly decrease, while the light transmittance values increase. For instance, PBA20D35T shows a haze of 36.9% and a transmittance of 80.5%, and the PBD55T film, with the highest DDSA content, demonstrates the lowest haze (33.5%) and the highest transmittance (87.7%). This substantial improvement in transparency is primarily attributed to the inhibitory effect of the bulky DDSA units on the crystallization of PBAT, as evidenced by DSC and XRD results. The reduction in crystallinity leads to a decrease in light scattering within the film, thereby enhancing its clarity.

Table 5.
Color, haze, and light transmittance of PBADT copolyester films.
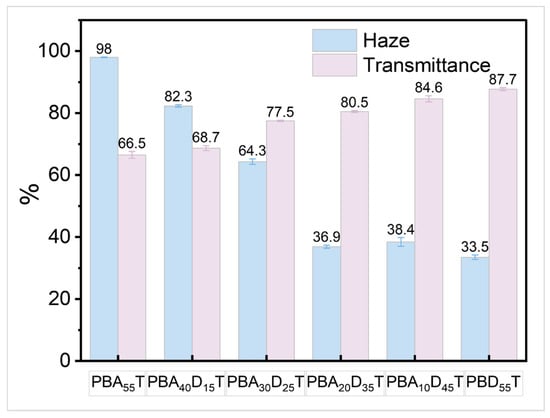
Figure 8.
Haze and light transmittance of PBADT copolyesters.
Furthermore, the color characteristics of the PBADT films were evaluated using the CIELAB color values (Table 5). The L* value, representing lightness, consistently increases from 85.3 for PBA55T to 95.0 for PBD55T, indicating that the films become brighter with higher BD unit content. The a* values (red–green axis) remain close to zero or slightly negative, suggesting a minimal green tint. The b* values (yellow–blue axis) exhibit a non-monotonic trend of first increasing and then decreasing with rising BD unit content. PBA30D25T possessed the highest b* value (11.8), while PBD55T showed the lowest b* value (2.4). This complex change in color, particularly the shift in yellowness, can be attributed to the inherent color of the DDSA monomer, its impact on the thermal stability of the polymer system, and potential discoloration pathways that may occur during high-temperature polymerization as the BD unit content changes.
In summary, the incorporation of DDSA not only enhances the water vapor barrier properties but also significantly improves the optical clarity of the PBADT copolyesters by reducing haze and increasing light transmittance. This dual benefit makes these materials attractive for transparent packaging applications where both barrier performance and visual appeal are crucial.
4. Conclusions
In this study, a series of novel poly(butylene adipate-co-terephthalate) (PBAT) copolyesters, modified with dodecenyl succinic anhydride (DDSA) and denoted as PBADT, were successfully synthesized. The impact of DDSA content on the structure, surface property, water vapor barrier performance, optical characteristics, and thermal and mechanical behaviors was systematically investigated. Replacing AA with DDSA significantly enhanced PBADT’s surface hydrophobicity and improved water vapor barrier performance by nearly three times, confirming the effectiveness of DDSA’s long alkylene side chain. Importantly, due to the chemical bonding of the long hydrophobic side chain, the water vapor barrier performance can remain stable or unchanged even under strong external mechanical abrasion, which is a critical advantage for practical applications. Furthermore, DDSA incorporation markedly improved the optical clarity of the PBADT films by reducing haze and increasing light transmittance, with PBD55T exhibiting the lowest haze and highest transmittance. The results demonstrated a new strategy to improve the water vapor barrier and transparency. On the other hand, the presence of DDSA inhibited PBADT crystallization, leading to a decrease in crystallinity and a corresponding reduction in mechanical properties, particularly at high DDSA content. Compositions with low-to-moderate DDSA content (e.g., PBA40D15T, PBA30D25T) provide a balance of improved barriers and acceptable mechanical strength for general packaging. High DDSA content compositions (e.g., PBA20D35T and PBD55T) are suitable for demanding applications requiring a superior barrier, high transparency, and barrier stability, albeit with reduced mechanical robustness. Future work will be performed to further improve or balance water vapor barrier performance, optical clarity, and mechanical properties to broaden their applicability.
Author Contributions
Conceptualization, L.W. (Lilan Wang) and L.W. (Linbo Wu); data curation, L.W. (Lilan Wang); formal analysis, L.W. (Lilan Wang); funding acquisition, L.W. (Linbo Wu); investigation, L.W. (Lilan Wang); methodology, L.W. (Lilan Wang); project administration, L.W. (Linbo Wu); writing—original draft, L.W. (Lilan Wang); writing—review and editing, L.W. (Linbo Wu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China under Grant No. Z24E030002 and the National Natural Science Foundation of China (NSFC) (52173107).
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. Z24E030002 and the National Natural Science Foundation of China (NSFC) (52173107). The authors also thank Li Xu, Qun Pu, Sudan Shen and Jijiang Hu, Bin Zhang, Yiqing Lu, Wei Liu, Eryuan Fang for their assistance in specimen preparation and instrument measurement at State Key Laboratory of Chemical Engineering (Zhejiang University).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, M.; Chang, H.C.; Zheng, L.; Yan, Q.; Pfleger, B.; Klier, J.; Nelson, K.; Majumder, E.; Huber, G. A Review of Biodegradable Plastics: Chemistry, Applications, Properties, and Future Research Needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zeng, X.B.; Huang, X.B. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Mahataa, D.; Karthikeyana, S.; Godsea, R.; Gupta, V.K. Poly(butylene adipate-co-terephthalate) Polyester Synthesis Process and Product Development. Polym. Sci. Ser. C 2021, 63, 102–111. [Google Scholar] [CrossRef]
- Siegenthaler, K.O.; Künkel, A.; Skupin, G.; Yamamoto, M. Ecoflex® and Ecovio®: Biodegradable, performance-enabling plastics. Adv. Ind. Eng. Polym. Res. 2012, 245, 91–136. [Google Scholar]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Yue, S.S.; Zhang, T.W.; Wang, S.J.; Han, D.M.; Huang, S.; Xiao, M.; Meng, Y.Z. Recent progress of biodegradable polymer package materials: Nanotechnology improving both oxygen and water vapor barrier performance. Nanomaterials 2024, 14, 338. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Zhong, G.J.; Olah, A.; Li, Z.M.; Baer, E.; Zhu, L. Promising strategies and new opportunities for high barrier polymer packaging films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Wei, C.; Guo, P.; Lyu, M.F.; Wang, B.; Li, C.; Sang, L.; Wei, Z.Y. High barrier poly(glycolic acid) modified poly(butylene adipate-co-terephthalate) blown films and accelerated ultraviolet degradability evaluation. ACS Appl. Polym. Mater. 2023, 5, 3457–3467. [Google Scholar] [CrossRef]
- Pan, H.W.; Wang, Y.; Jia, S.L.; Zhao, Y.; Bian, J.J.; Yang, H.L.; Hao, Y.P.; Han, L.J.; Zhang, H.L. Biodegradable poly(butylene adipate-co-terephthalate)/poly(glycolic acid) films: Effect of poly(glycolic acid) crystal on mechanical and barrier properties. Chin. J. Polym. Sci. 2023, 41, 1123–1132. [Google Scholar] [CrossRef]
- Shen, J.N.; Wang, K.; Ma, Z.; Xu, N.; Pang, S.J.; Pan, L.S. Biodegradable blends of poly(butylene adipate-co-terephthalate) and polyglycolic acid with enhanced mechanical, rheological and barrier performances. J. Appl. Polym. Sci. 2021, 138, 51285. [Google Scholar] [CrossRef]
- Guo, B.H.; Shi, K.H.; Bian, H.L.; Xu, J. PBAT/PPC Properties and natural weathering behavior of PBAT/PPC biodegradable Films. Tonji Univ. Nat. Sci. 2023, 51, 1673–1683. [Google Scholar]
- Pan, H.W.; Hao, Y.P.; Zhao, Y.; Lang, X.Z.; Zhang, Y.; Wang, Z.; Zhang, H.L.; Dong, L.S. Improved mechanical properties, barrier properties and degradation behavior of poly(butylenes adipate-co-terephthalate)/poly(propylene carbonate) films. Korean J. Chem. Eng. 2017, 34, 1294–1304. [Google Scholar] [CrossRef]
- Tu, Z.; Wang, B.; Lu, Y.; Wang, L.Z.; Li, Y.; Sang, L.; Zhang, Y.; Wei, Z.Y. Incorporation of Large-Scale Prepared Poly(ethylene oxalate) into biodegradable poly(butylene adipate-co-terephthalate) blown films with enhanced mechanical and barrier performance. ACS Sustain. Chem. Eng. 2023, 11, 9833–9845. [Google Scholar] [CrossRef]
- Chivrac, F.; Kadlecová, Z.; Pollet, E.; Avérous, L. Aromatic copolyester-based nano-biocomposites: Elaboration, structural characterization and properties. J. Polym. Environ. 2006, 14, 393–401. [Google Scholar] [CrossRef]
- Li, J.X.; Lai, L.; Wu, L.B.; Severtson, S.J.; Wang, W.J. Enhancement of water vapor barrier properties of biodegradable poly(butylene adipate-co-terephthalate) films with highly oriented organomontmorillonite. ACS Sustain. Chem. Eng. 2018, 6, 6654–6662. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Zhen, Z.C.; Liu, J.L.; Qiao, R.M.; He, W.Q. Effects of mica modification with ethylene-vinyl acetate wax on the water vapor barrier and mechanical properties of poly(butylene adipate-co-terephthalate) nanocomposite films. J. Appl. Polym. Sci. 2021, 138, 50610. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Li, B.; Li, S.D.; Dou, Y.B.; Han, J.B. Layered double hydroxide/PBAT hybrid films with simultaneously high-barrier and degradable properties. Chem. Eng. Sci. 2023, 280, 119016. [Google Scholar] [CrossRef]
- Ren, P.G.; Liu, X.H.; Ren, F.; Zhong, G.J.; Ji, X.; Xu, L. Biodegradable graphene oxide nanosheets/poly(butylene adipate-co-terephthalate) nanocomposite film with enhanced gas and water vapor barrier properties. Polym. Test. 2017, 58, 173–180. [Google Scholar] [CrossRef]
- Tsou, C.H.; Ge, F.F.; Lin, L.; Yuan, S.; De Guzman, M.R.; Potiyaraj, P. Barrier and biodegradable properties of poly(butylene adipate-co-terephthalate) reinforced with ZnO-decorated graphene rendering it antibacterial. ACS Appl. Polym. Mater. 2023, 5, 1681–1695. [Google Scholar] [CrossRef]
- Wang, H.K.; Liu, J.F.; Jiang, Q.Q.; Liu, X.R.; Wu, M.; Huang, Y. Sustainable clean production and application of hydrophobic straw lignocellulose nanosheets filled biodegradable films. Ind. Crops Prod. 2025, 226, 120602. [Google Scholar] [CrossRef]
- Mckeen, L.W. Permeability Properties of Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 3; pp. 41–60. [Google Scholar]
- Cunha, M.; Fernandes, B.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Film blowing of PHBV blends and PHBV-based multilayers for the production of biodegradable packages. J. Appl. Polym. Sci. 2016, 133, 42165. [Google Scholar] [CrossRef]
- Ran, L.B.; Hong, W.Y.R.; Yu, G.Y.; Du, Q.J.; Guo, S.Y.; Li, C.H. Preparation and improving mechanism of PBAT/PPC-based micro-layer biodegradable mulch film with excellent water resistance and mechanical properties. Polymer 2024, 291, 126614. [Google Scholar] [CrossRef]
- Wang, L.Z.; Tu, Z.; Liang, J.M.; Wei, Z.Y. Poly(butylene oxalate-co-terephthalate): A PBAT-like but rapid hydrolytic degradation plastic. J. Hazard. Mater. 2024, 471, 134349. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Wang, B.; Li, C.; Wei, C.; Wei, Z.Y. Development of high barrier biodegradable poly(butylene carbonate-co-terephthalate)-based blends with balanced mechanical properties by self-reactive compatibility and co-crystallization. Polymer 2023, 281, 126130. [Google Scholar] [CrossRef]
- Huang, F.F.; Wu, L.B.; Li, B.G. Sulfonated biodegradable PBAT copolyesters with improved gas barrier properties and excellent water dispersibility: From synthesis to structure-property. Polym. Degrad. Stab. 2020, 182, 109391. [Google Scholar] [CrossRef]
- Hu, H.; Tian, Y.; Wang, J.G.; Zhang, R.Y.; Zhu, J. Enhanced degradation and gas barrier of PBAT through composition design of aliphatic units. Polym. Degrad. Stab. 2022, 195, 109795. [Google Scholar] [CrossRef]
- Liu, C.Q.; Liu, F.; Yang, T.; Chen, C.H.; Lin, Y.Y.; Wang, J.G.; Zhu, J. Incorporation of lactyl unit to PBAT for enhanced gas barrier property and biodegradability by direct polycondensation via alcoholysis of cyclic anhydride with lactic acid. Polymer 2024, 313, 127758. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, X.P.; Xu, J.; Ding, J.P.; Li, W.L.; Guo, B.H. Effect of glycerol stearates on the thermal and barrier properties of biodegradable poly(butylene adipate-co-terephthalate). Materials 2024, 17, 5732. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; Guo, H.H.; Liang, Z.Y.; Liu, F.Y.; Xiao, K.Y.; Li, W.; Yan, H.; Guo, R.J. Hydrophobic PBAT/SiO2 composite films for food packaging. Mater. Today Commun. 2025, 46, 112696. [Google Scholar] [CrossRef]
- Wu, L.B.; Liu, Z.W.; Wang, L.L. Fully biodegradable superhigh water vapor barrier PBAT films with diffusion-impeded interior crystal plates and a superhydrophobic rough surface. Macromolecules 2025, 58, 6238–6248. [Google Scholar] [CrossRef]
- Shah, N.N.; Soni, N.N.; Singhal, R.S. Modification of proteins and polysaccharides using dodecenyl succinic anhydride: Synthesis, properties and applications—A review. Int. J. Biol. Macromol. 2018, 107, 2224–2233. [Google Scholar] [CrossRef]
- Li, G.T.; Xu, X.; Zhu, F. Physicochemical properties of dodecenyl succinic anhydride (DDSA) modified quinoa starch. Food Chem. 2019, 300, 125201. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Khalid, N.; Nakajima, M. Fabrication and characterization of dodecenyl succinic anhydride modified kudzu starch. Starch-Stärke 2022, 74, 2100188. [Google Scholar] [CrossRef]
- ASTM E96-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016.
- GB 1037-2021; Test Method for Water Vapor Transmission of Plastic Film and Sheeting—Desiccant Method and Water Method. Standards Press of China: Beijing, China, 2021.
- Debeli, D.K.; Huang, F.F.; Wu, L.B. Sulfonated Poly(butylene Adipate-co-terephthalate)/Sodium Montmorillonite Nanocomposite Films with an Ultra-High Oxygen Barrier. Ind. Eng. Chem. Res. 2022, 61, 13283–13293. [Google Scholar] [CrossRef]
- Qin, P.K.; Wu, L.B.; Li, B.G.; Li, N.X.; Pan, X.H.; Dai, J.M. Superior gas barrier properties of biodegradable PBST vs. PBAT copolyesters: A comparative study. Polymers 2021, 13, 3449. [Google Scholar] [CrossRef] [PubMed]
- Lagaron, J.M.; Catalá, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2004, 20, 1–7. [Google Scholar] [CrossRef]
- Zhen, C.; Zhu, G.X.; Shi, Y.; Liu, L.Z.; Ren, M.Q.; Zhang, W.; Han, L. Crystallization, structures and properties of biodegradable poly(butylene succinate-co-butylene terephthalate) with a symmetric composition. Mater. Chem. Phys. 2021, 260, 124183. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Zhang, Q.N.; Wang, Z.; Zhang, L.Q. Synthesis of novel thermoplastic polyester elastomers with biobased amorphous polyester as the soft segment. Polym. Test. 2023, 124, 108088. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Galeski, A.; Pawlak, A. PBAT Green Composites: Effects of Kraft Lignin Particles on the Morphological, Thermal, Crystalline, Macro and Micromechanical Properties. Polymer 2020, 203, 122748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).