Preparation of PLLA and PLGA Copolymers with Poly(ethylene adipate) Through Reactive Melt Mixing: Structural Characterization, Thermal Properties, and Molecular Mobility Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
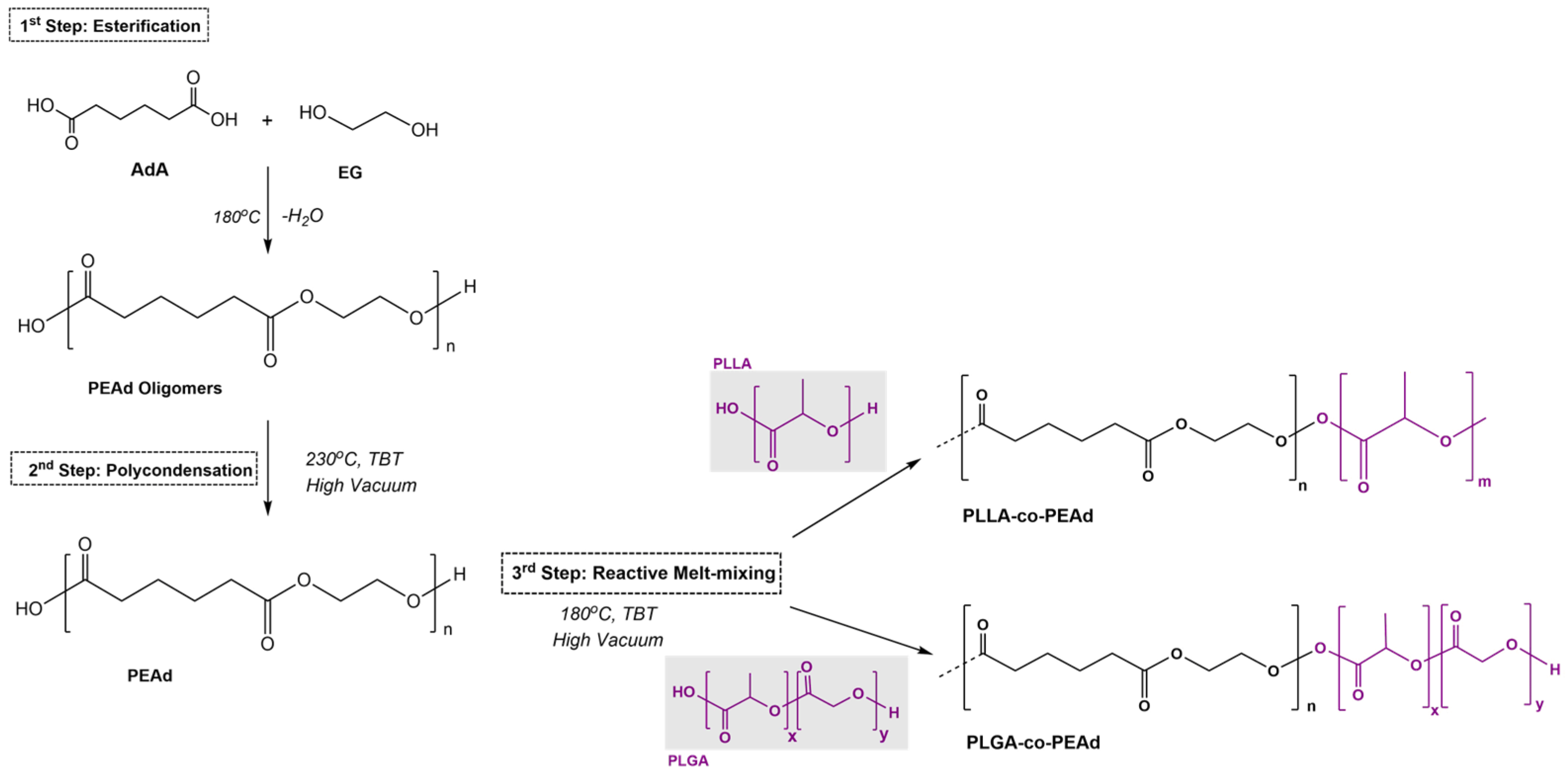
2.2. Synthesis of PLLA/PEAd Block Copolymers
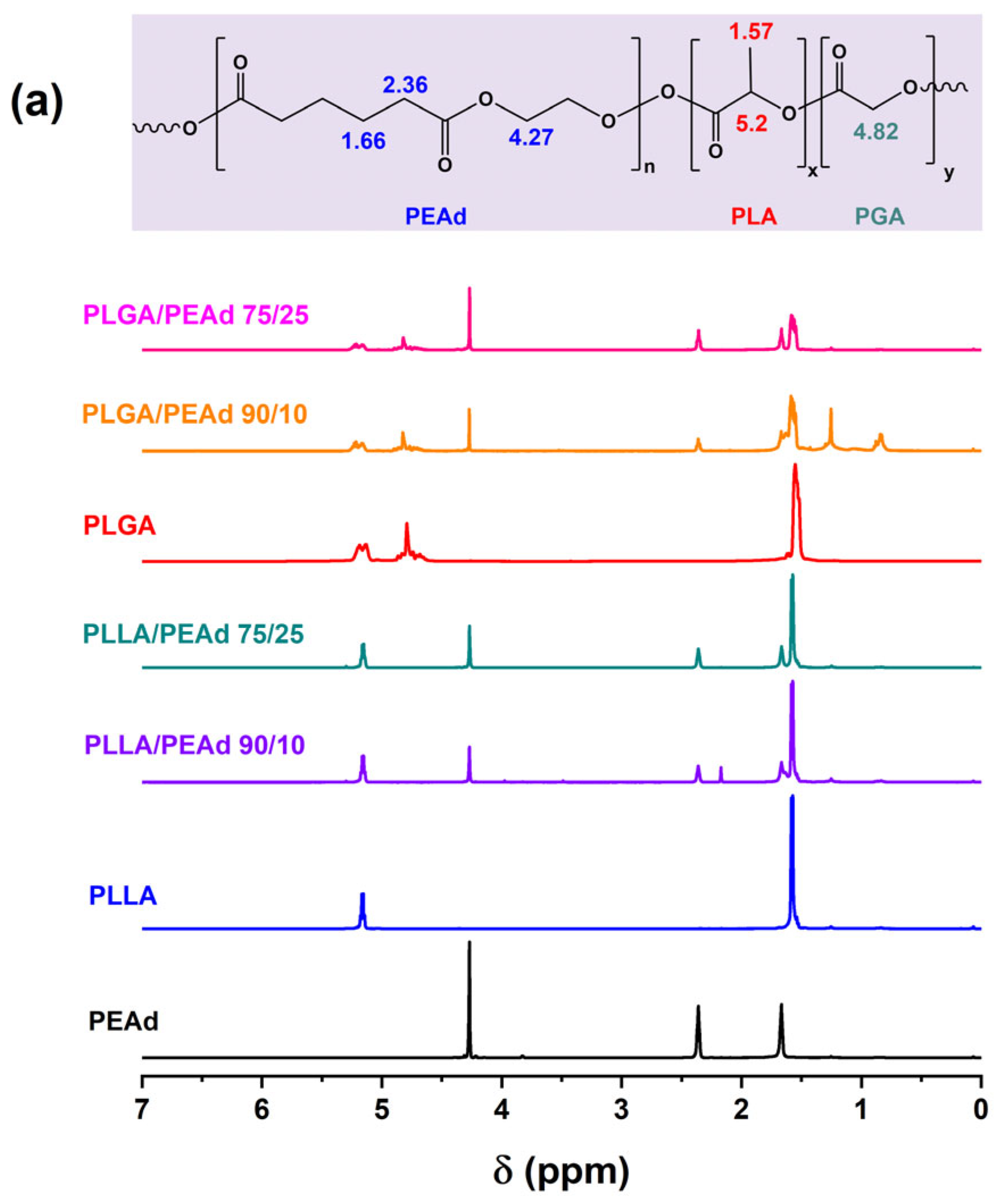
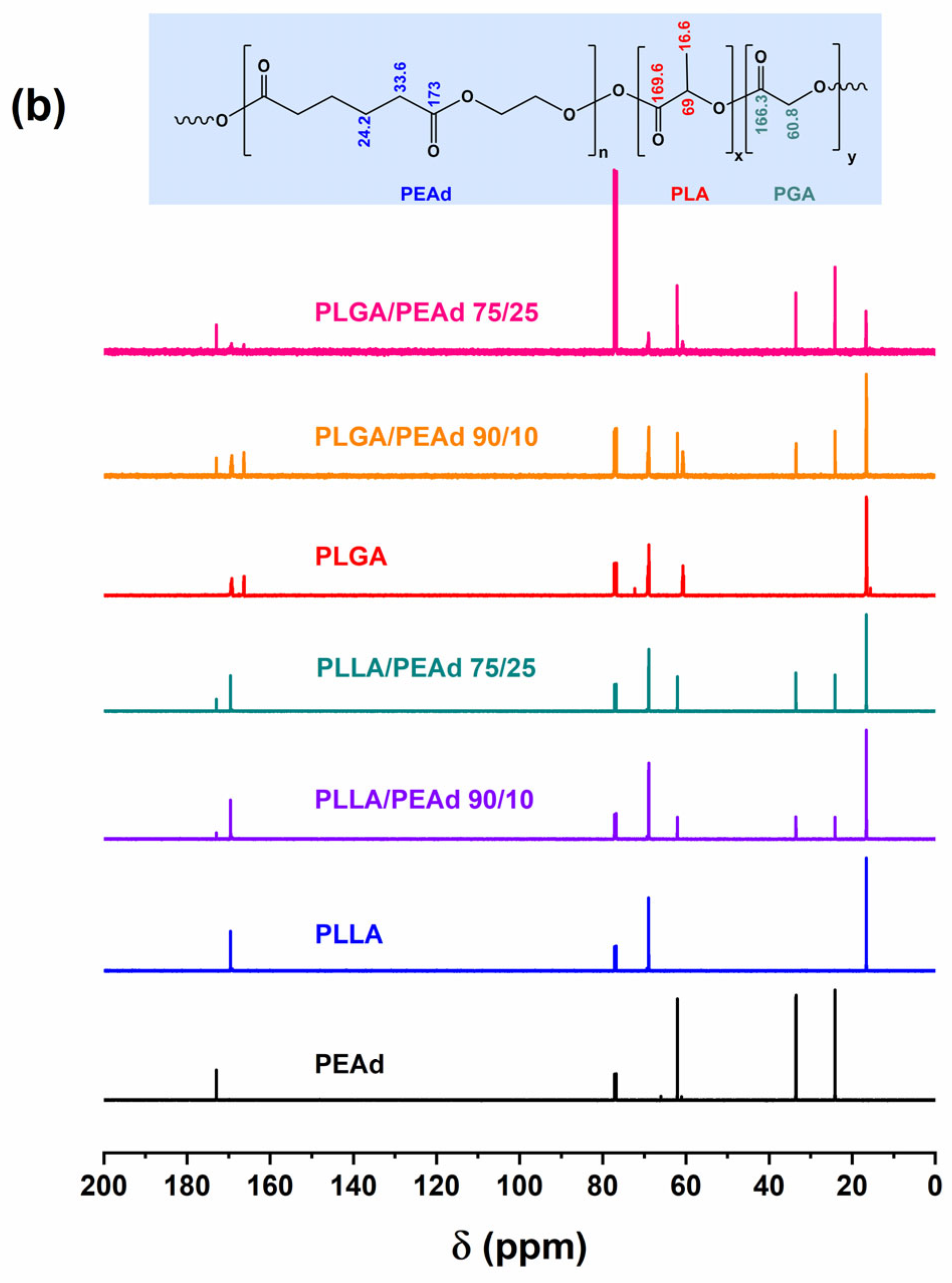
2.3. Experimental Techniques
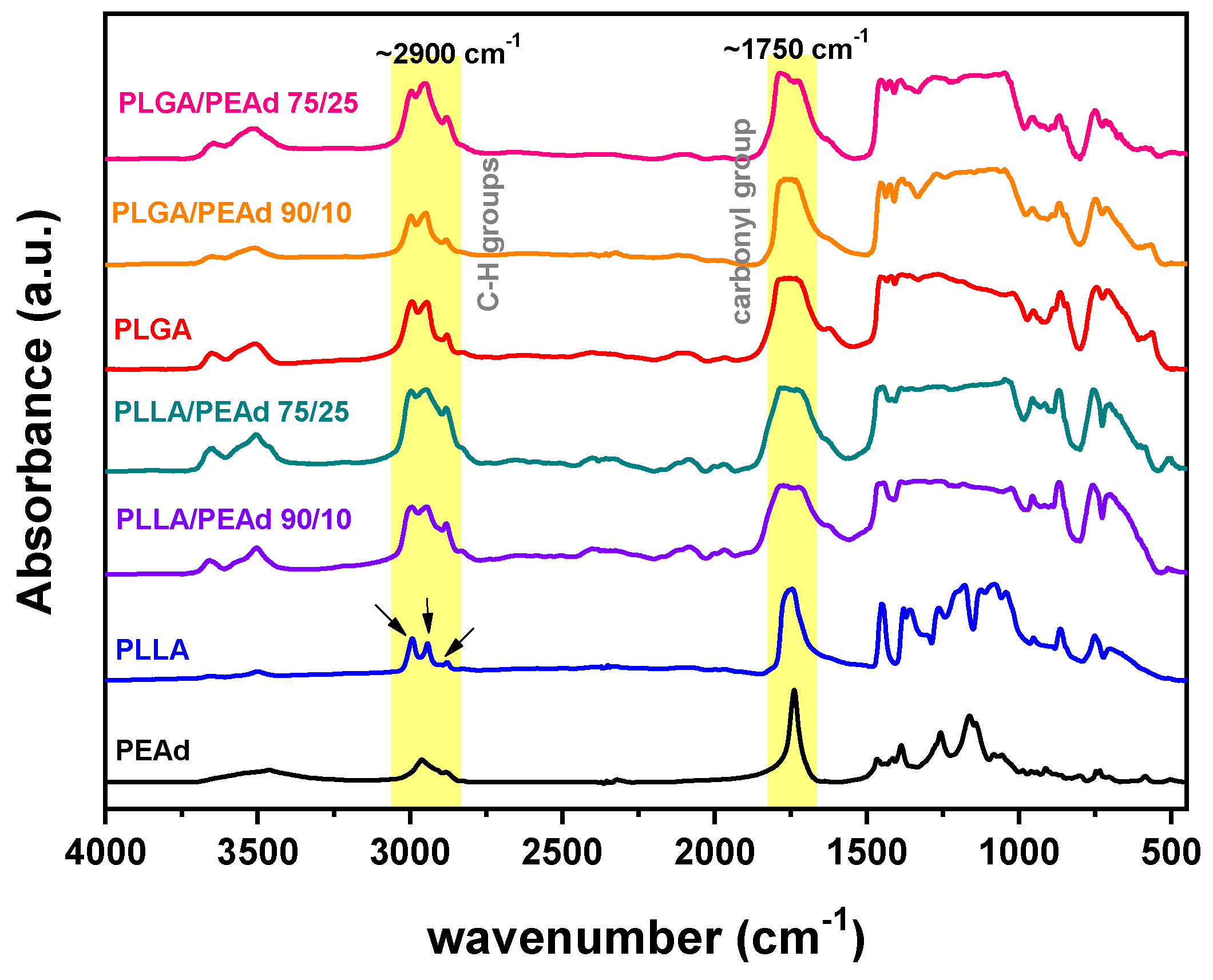
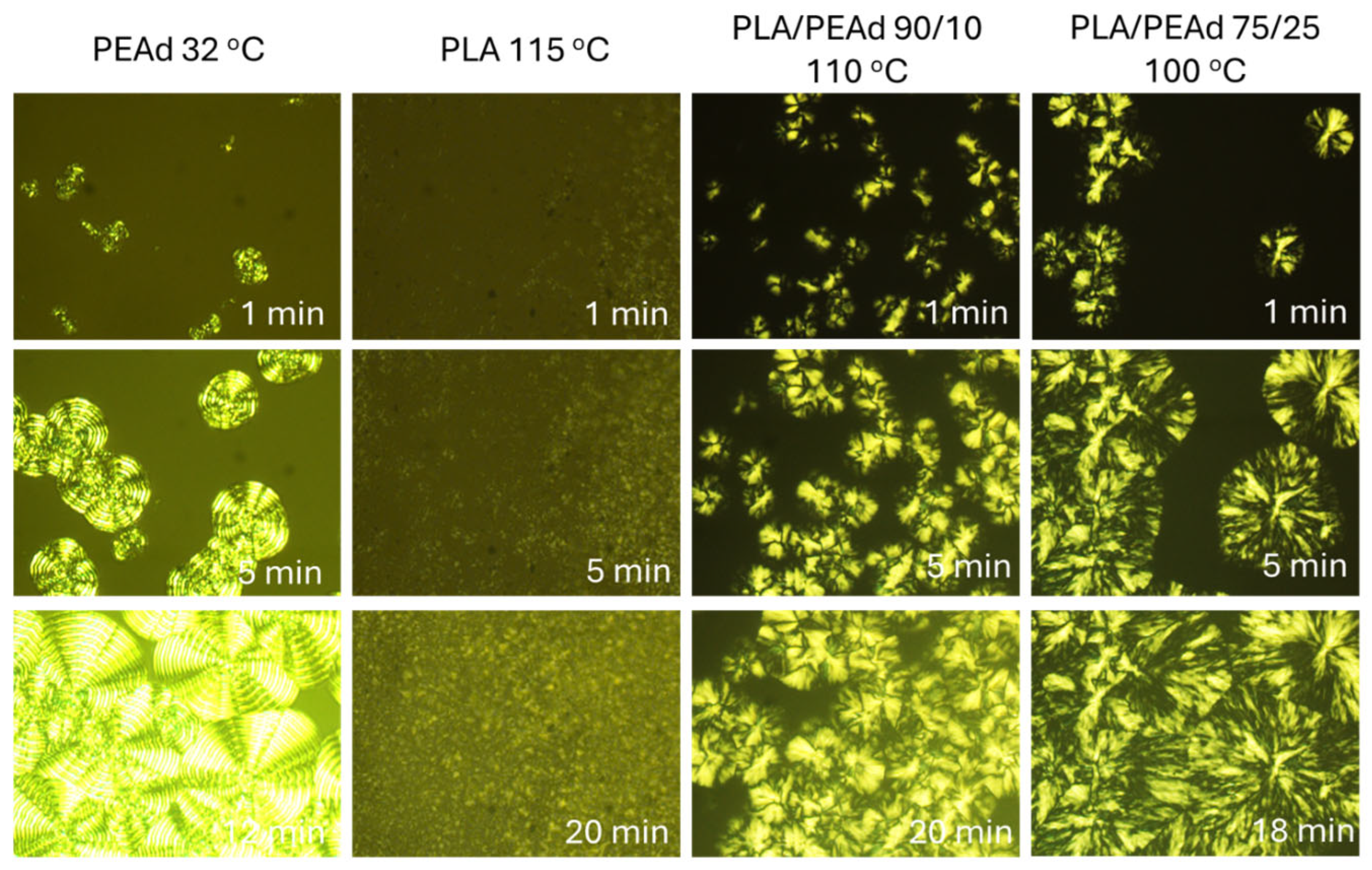
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowan, S.J. 100th Anniversary of Macromolecular Science Viewpoints. ACS Macro Lett. 2021, 10, 466–468. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. The Legacy of Hermann Staudinger: Covalently Linked Macromolecules. Chem 2020, 6, 2855–2861. [Google Scholar] [CrossRef]
- Jafferson, J.M.; Chatterjee, D. A review on polymeric materials in additive manufacturing. Mater. Today Proc. 2021, 46, 1349–1365. [Google Scholar] [CrossRef]
- Blanchard, R.; Mekonnen, T.H. Valorization of plastic waste via chemical activation and carbonization into activated carbon for functional material applications. RSC Appl. Polym. 2024, 2, 557–582. [Google Scholar] [CrossRef]
- Scott, G. ‘Green’ polymers. Polym. Degrad. Stab. 2000, 68, 1–7. [Google Scholar] [CrossRef]
- Chyr, G.; DeSimone, J.M. Review of high-performance sustainable polymers in additive manufacturing. Green Chem. 2023, 25, 453–466. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bin Bakri, M.K.; Bin Julaihi, M.R.M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Sternberg, J.; Sequerth, O.; Pilla, S. Green chemistry design in polymers derived from lignin: Review and perspective. Prog. Polym. Sci. 2021, 113, 101344. [Google Scholar] [CrossRef]
- Meegan, J. Some of the challenges faced by the Composites Industry in its bid to become more sustainable. RSC Sustain. 2023, 1, 1737–1742. [Google Scholar] [CrossRef]
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Norton, M. Green chemistry and the plastic pollution challenge: Towards a circular economy. Green Chem. 2020, 22, 6310–6322. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 483145. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic Acid Based Nanocomposites: Promising Safe and Biodegradable Materials in Biomedical Field. Int. J. Polym. Sci. 2016, 1, 6869154. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K. Polylactides-chemistry, properties and green packaging technology: A review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Degrad. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Rahmanifard, M.; Khademi, S.M.H.; Asheghi-Oskooee, R.; Farizeh, T.; Hemmati, F. Reactive processing-microstructure-mechanical performance correlations in biodegradable poly(lactic acid)/expanded graphite nanocomposites. RSC Adv. 2024, 14, 794–807. [Google Scholar] [CrossRef]
- Sauer, A.; Kapelski, A.; Fliedel, C.; Dagorne, S.; Kol, M.; Okuda, J. Structurally well-defined group 4 metal complexes as initiators for the ring-opening polymerization of lactide monomers. Dalton Trans. 2013, 42, 9007–9023. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.; Gupta, N. Aluminum-Based Catalysts for Cycloaddition Reactions: Moving One Step Ahead in Sustainability. ChemistrySelect 2019, 4, 10452–10465. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization Study and Comparative In Vitro–In Vivo Hydrolysis of PLA Reinforcement Ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef]
- Toda, A.; Androsch, R.; Schick, C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer 2016, 91, 239–263. [Google Scholar] [CrossRef]
- Androsch, R.; Zhang, R.; Schick, C. Melt-recrystallization of poly (l-lactic acid) initially containing α′-crystals. Polymer 2019, 176, 227–235. [Google Scholar] [CrossRef]
- Rizis, G.; Van De Ven, T.G.M.; Eisenberg, A. Crystallinity-driven morphological ripening processes for poly(ethylene oxide)-block-polycaprolactone micelles in water. Soft Matter 2014, 10, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.K.; Zhao, J.; Hadjichristidis, N.; Müller, A.J. How the Complex Interplay between Different Blocks Determines the Isothermal Crystallization Kinetics of Triple-Crystalline PEO-b-PCL-b-PLLA Triblock Terpolymers. Macromolecules 2017, 50, 9683–9695. [Google Scholar] [CrossRef]
- Zhang, R.; Du, F.; Jariyavidyanont, K.; Zhuravlev, E.; Schick, C.; Androsch, R. Glass transition temperature of poly(d,l-lactic acid) of different molar mass. Thermochim. Acta 2022, 718, 179387. [Google Scholar] [CrossRef]
- Wang, Y.; van Putten, R.J.; Tietema, A.; Parsons, J.R.; Gruter, G.J.M. Polyester biodegradability: Importance and potential for optimisation. Green Chem. 2024, 26, 3698–3716. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Tsachouridis, K.; Christodoulou, E.; Zamboulis, A.; Michopoulou, A.; Barmpalexis, P.; Bikiaris, D.N. Evaluation of poly(lactic acid)/and poly(lactic-co-glycolic acid)/poly(ethylene adipate) copolymers for the preparation of paclitaxel loaded drug nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 77, 103918. [Google Scholar] [CrossRef]
- Abdelghafour, M.M.; Orbán, Á.; Deák, Á.; Lamch, Ł.; Frank, É.; Nagy, R.; Ádám, A.; Sipos, P.; Farkas, E.; Bari, F.; et al. The effect of molecular weight on the solubility properties of biocompatible poly(Ethylene succinate) polyester. Polymers 2021, 13, 2725. [Google Scholar] [CrossRef]
- Im, S.S.; Kim, T.J.; Han, S.; Moon, T.J.; Bae, Y.C. Physical Properties of Poly(ethylene adipate)/Low-Density Polyethylene Blends. J. Appl. Polym. Sci. 1997, 65, 1745–1750. [Google Scholar] [CrossRef]
- Klonos, P.A.; Lazaridou, M.; Samiotaki, C.; Kyritsis, A.; Bikiaris, D.N. Dielectric and calorimetric study in renewable polymer blends based on poly(ethylene adipate) and poly(lactic acid) with microphase separation. Polymer 2022, 259, 125329. [Google Scholar] [CrossRef]
- Jiang, J.; He, Z.; Yin, W.; Chen, R.; He, J.; Lang, M. Enhancing the toughness of poly(lactic acid) with a novel, highly flexible and biodegradable polyester: Poly(ethylene adipate-co-terephthalate) terephthalate. J. Polym. Res. 2024, 31, 151. [Google Scholar] [CrossRef]
- Atanase, L.I.; Salhi, S.; Cucoveica, O.; Ponjavic, M.; Nikodinovic-Runic, J.; Delaite, C. Biodegradability Assessment of Polyester Copolymers Based on Poly(ethylene adipate) and Poly(ε-caprolactone). Polymers 2022, 14, 3736. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Kaur, P.; Mehta, R.; Berek, D.; Upadhyay, S.N. Synthesis of polylactide: Effect of dispersion of the initiator. J. Macromol. Sci. Part A 2011, 48, 840–845. [Google Scholar] [CrossRef]
- He, A.; Han, C.C.; Yang, G. Preparation and characterization of PLLA/P(CL-b-LLA) blends by an in situ ring-opening polymerization. Polymer 2004, 45, 8231–8237. [Google Scholar] [CrossRef]
- Sheu, W.S. Molecular weight averages and polydispersity of polymers. J. Chem. Educ. 2001, 78, 554–555. [Google Scholar] [CrossRef]
- Zheng, L.; Kim, M.S.; Xu, S.; Urgun-Demirtas, M.; Huber, G.W.; Klier, J. Biodegradable High-Molecular-Weight Poly(pentylene adipate-co-terephthalate): Synthesis, Thermo-Mechanical Properties, Microstructures, and Biodegradation. ACS Sustain. Chem. Eng. 2023, 11, 13885–13895. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z.; Hussein, M.Z. Effects of Graphene Nanopletelets on Poly(Lactic Acid)/Poly(Ethylene Glycol) Polymer Nanocomposites. Adv. Mater. Res. 2014, 1024, 136–139. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Mahdavi-Shahri, M. Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regen. Biomater. 2017, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Almeida, A.; Tavares, M.I.B.; Oliveira Da Silva, E.; Neto, R.P.C.; Moreira, L.A. Development of hybrid nanocomposites based on PLLA and low-field NMR characterization. Polym. Test. 2012, 31, 267–275. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Zhao, L.; Kong, J.; Tian, X.; Zhang, J.; Qin, S. Isothermal crystallization of poly(L-lactide) and poly(butylene adipate) crystalline/crystalline blends. Polym. J. 2014, 46, 323–329. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Klonos, P.A.; Bikiaris, N.D.; Barmpalexis, P.; Kyritsis, A. Segmental mobility in linear polylactides of various molecular weights. Polymer 2024, 305, 127177. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Klonos, P.A.; Christodoulou, E.; Barmpalexis, P.; Kyritsis, A. Plasticization Effects of PEG of Low Molar Fraction and Molar Mass on the Molecular Dynamics and Crystallization of PLA-b-PEG-b-PLA Triblock Copolymers Envisaged for Medical Applications. J. Phys. Chem. B 2025, 129, 3528. [Google Scholar] [CrossRef]
- Karava, V.; Siamidi, A.; Vlachou, M.; Christodoulou, E.; Bikiaris, N.D.; Zamboulis, A.; Kostoglou, M.; Gounari, E.; Barmpalexis, P. Poly(L-Lactic Acid)-co-poly(Butylene Adipate) New Block Copolymers for the Preparation of Drug-Loaded Long Acting Injectable Microparticles. Pharmaceutics 2021, 13, 930. [Google Scholar] [CrossRef]
- Bouyahya, C.; Bikiaris, N.D.; Zamboulis, A.; Kyritsis, A.; Majdoub, M.; Klonos, P.A. Crystallization and molecular mobility in renewable semicrystalline copolymers based on polycaprolactone and polyisosorbide. Soft Matter 2022, 18, 9216–9230. [Google Scholar] [CrossRef]
- Schönhals, A.; Szymoniak, P. Dynamics of Composite Materials, 1st ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Richert, R.; Agapov, A.; Sokolov, A.P. Appearance of a Debye process at the conductivity relaxation frequency of a viscous liquid. J. Chem. Phys. 2011, 134, 104508. [Google Scholar] [CrossRef]
- Ren, J.; Urakawa, O.; Adachi, K. Dielectric study on dynamics and conformation of poly(d,l-lactic acid) in dilute and semi-dilute solutions. Polymer 2003, 44, 847–855. [Google Scholar] [CrossRef]
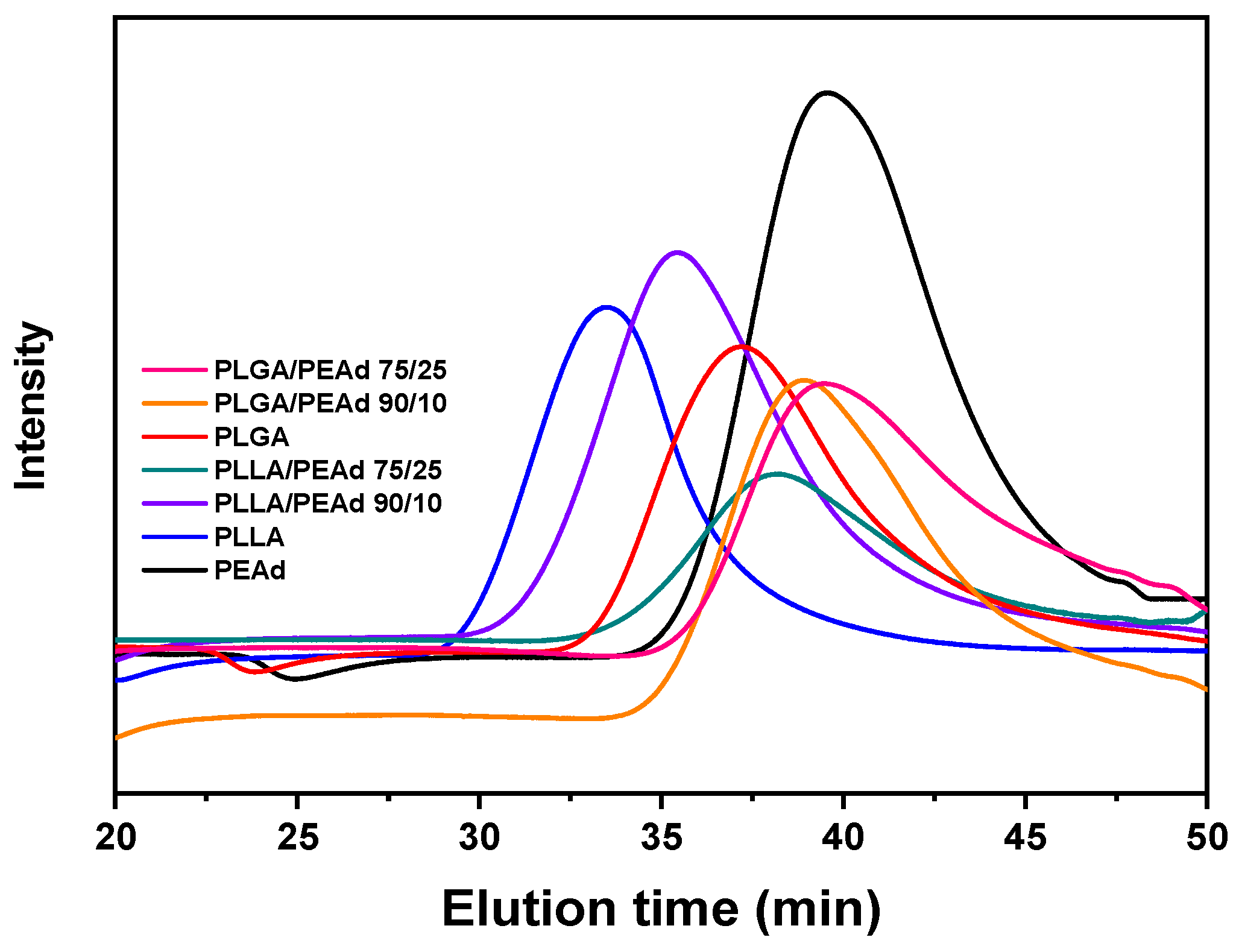



| Sample | Mw (g/mol) | Mn (g/mol) | PDI |
|---|---|---|---|
| PEAd | 14.4k | 6.3k | 2.3 |
| PLLA | 126.3k | 81.7k | 1.5 |
| PLLA/PEAd 90/10 | 59.5k | 34.9k | 1.7 |
| PLLA/PEAd 75/25 | 23.7k | 9.5k | 2.5 |
| PLGA | 33.1k | 21.1k | 1.6 |
| PLGA/PEAd 90/10 | 17.6k | 8.4k | 2.1 |
| PLGA/PEAd 75/25 | 13.2k | 3.5k | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, E.; Samiotaki, C.; Zamboulis, A.; Bikiaris, R.E.; Klonos, P.A.; Kyritsis, A.; Bikiaris, D.N. Preparation of PLLA and PLGA Copolymers with Poly(ethylene adipate) Through Reactive Melt Mixing: Structural Characterization, Thermal Properties, and Molecular Mobility Insights. Macromol 2025, 5, 35. https://doi.org/10.3390/macromol5030035
Christodoulou E, Samiotaki C, Zamboulis A, Bikiaris RE, Klonos PA, Kyritsis A, Bikiaris DN. Preparation of PLLA and PLGA Copolymers with Poly(ethylene adipate) Through Reactive Melt Mixing: Structural Characterization, Thermal Properties, and Molecular Mobility Insights. Macromol. 2025; 5(3):35. https://doi.org/10.3390/macromol5030035
Chicago/Turabian StyleChristodoulou, Evi, Christina Samiotaki, Alexandra Zamboulis, Rizos Evangelos Bikiaris, Panagiotis A. Klonos, Apostolos Kyritsis, and Dimitrios N. Bikiaris. 2025. "Preparation of PLLA and PLGA Copolymers with Poly(ethylene adipate) Through Reactive Melt Mixing: Structural Characterization, Thermal Properties, and Molecular Mobility Insights" Macromol 5, no. 3: 35. https://doi.org/10.3390/macromol5030035
APA StyleChristodoulou, E., Samiotaki, C., Zamboulis, A., Bikiaris, R. E., Klonos, P. A., Kyritsis, A., & Bikiaris, D. N. (2025). Preparation of PLLA and PLGA Copolymers with Poly(ethylene adipate) Through Reactive Melt Mixing: Structural Characterization, Thermal Properties, and Molecular Mobility Insights. Macromol, 5(3), 35. https://doi.org/10.3390/macromol5030035









