Chitin, Chitosan and Its Derivatives: Antimicrobials and/or Mitigators of Water
Abstract
1. Introduction
2. Chitin and Chitin Deacetylases
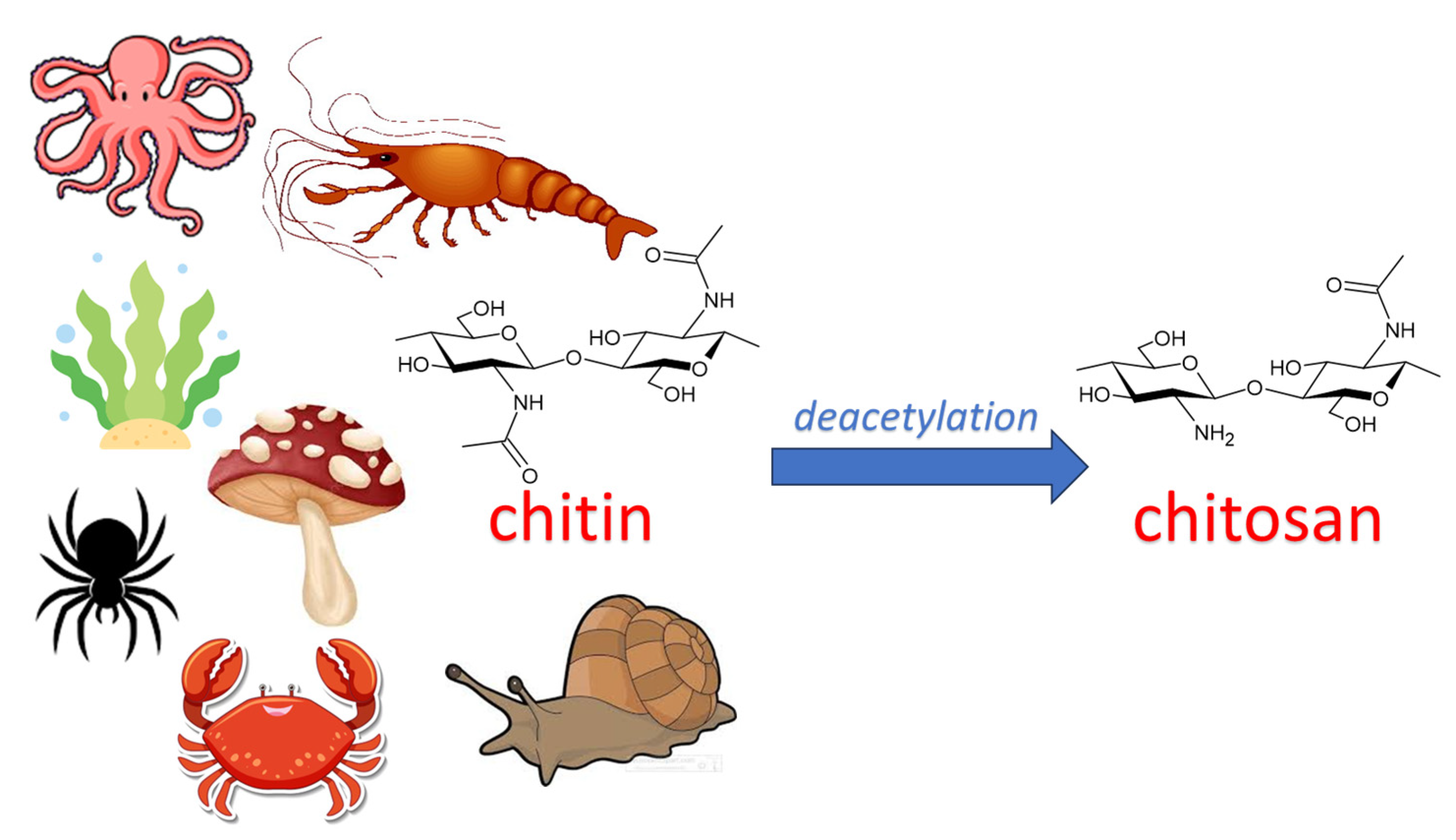
2.1. History of Chitin
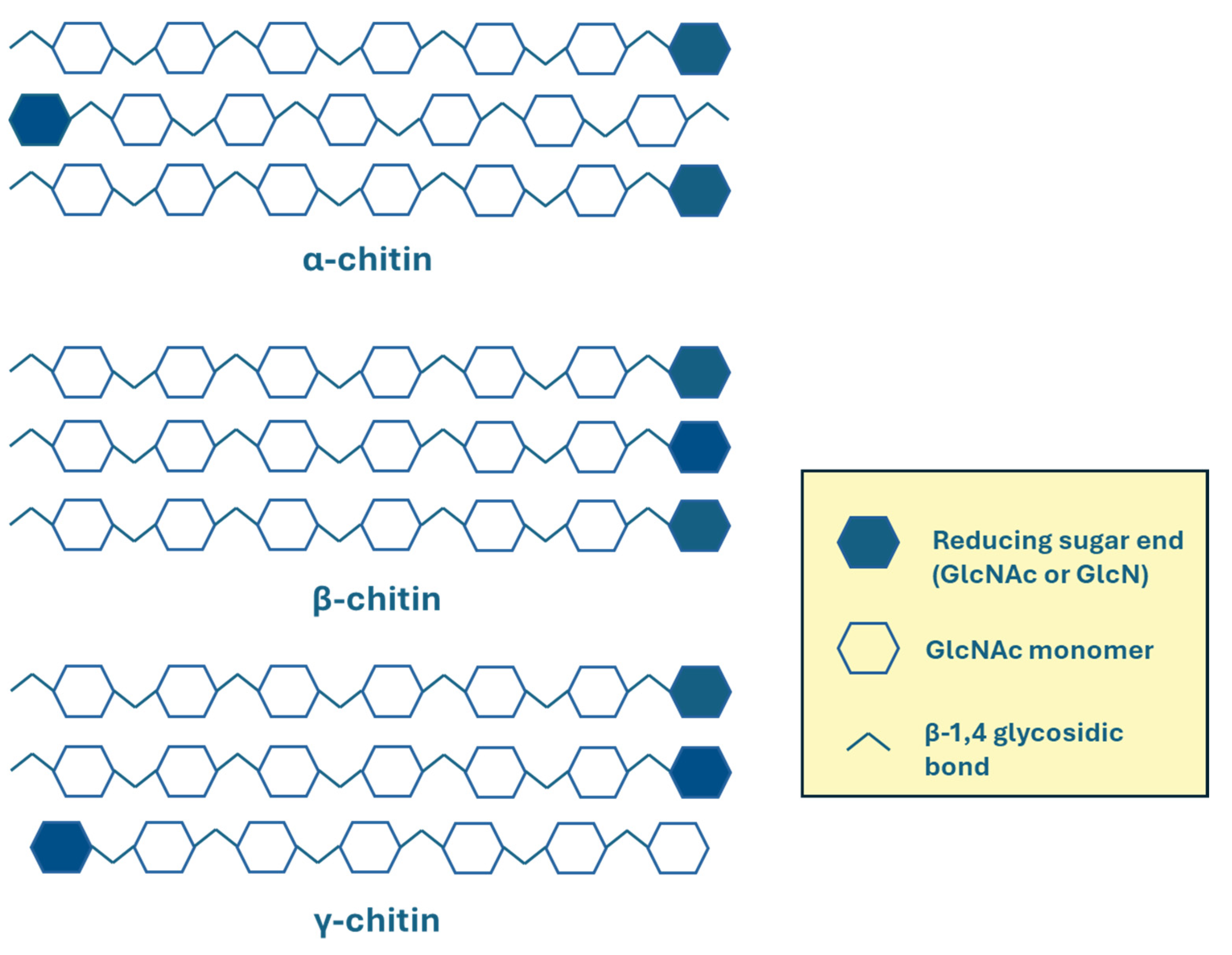
2.2. Chemistry of Chitin
2.3. Chitin Deacetylases
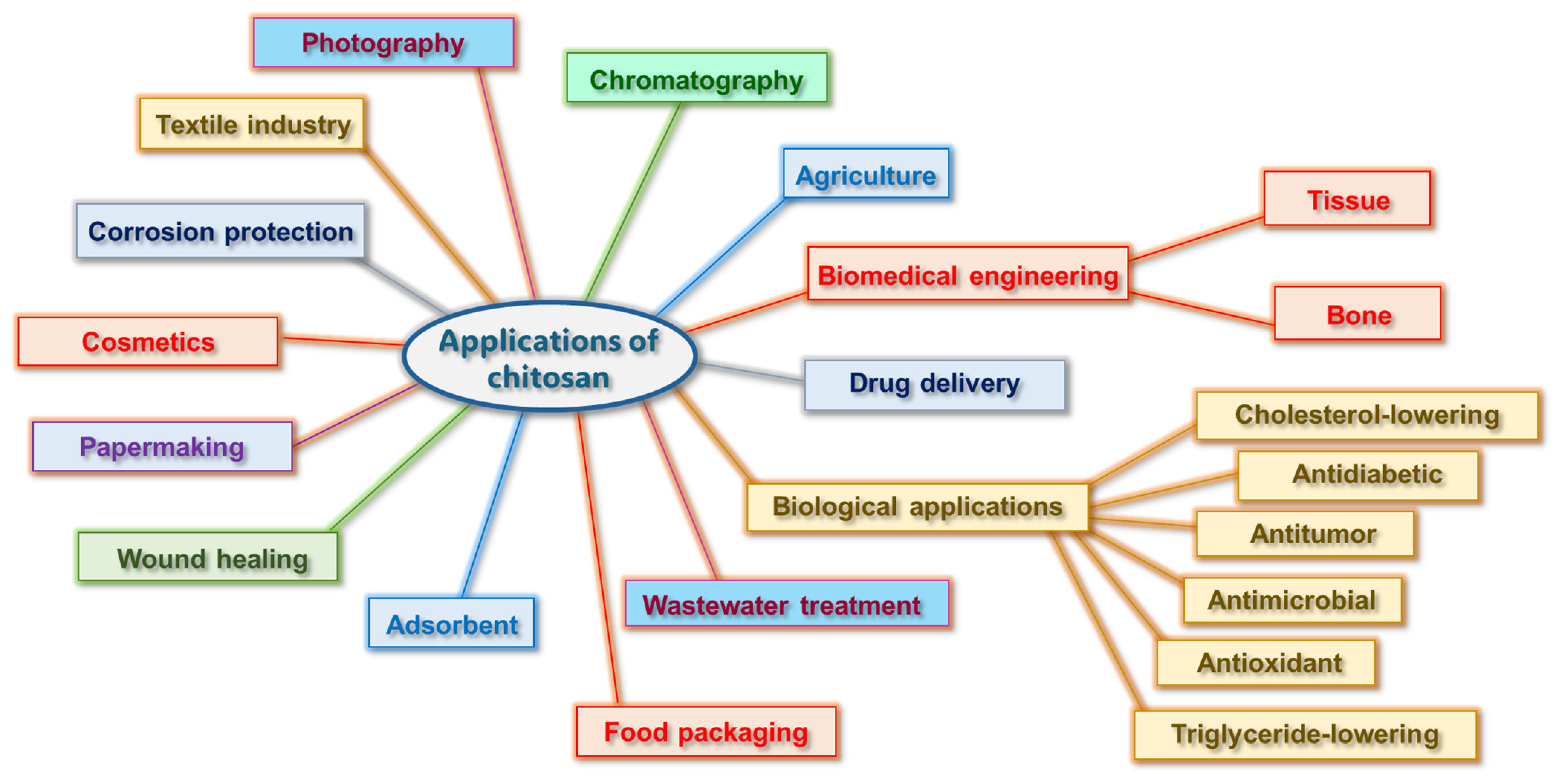
3. Chitosan
4. Recent Studies of Chitosan-Based Materials as Antimicrobials
4.1. Antibacterial Activities of Chitosan-Based Materials for Food Packaging
4.2. Antibacterial Activities of Chitosan-Based Materials in Photodynamic Therapy and/or for Wound Infections
4.3. Antibacterial Activity of Chitosan-Based Materials for Dental Drug Delivery
5. Chitosan for Removing Pharmaceutical Pollutants, Dyes and Heavy Metals from Water Sources
6. Chitosan Market Development
7. Conclusions
8. Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allabi, A.C.; Agbo, A.G.; Boya, B.; Mudenda, S. Antimicrobial Stewardship: Knowledge and Attitudes of Pharmacy Staff on Antibiotic Dispensing Patterns, Use and Resistance in Benin. Pharmacol. Pharm. 2023, 14, 189–214. [Google Scholar] [CrossRef]
- World Health Organization. One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Willyard, C. The Drug-Resistant Bacteria that Pose the Greatest Health Threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Pellegrino, M.; Giuzio, F.; Marra, M.; Rosano, C.; Saturnino, C.; Sinicropi, M.S.; Aquaro, S. Antibiotic-resistant ESKAPE pathogens and COVID-19: The pandemic beyond the pandemic. Viruses 2023, 15, 1843. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibacterial Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Dhlamini, K.S.; Selepe, C.T.; Ramalapa, B.; Tshweu, L.; Ray, S.S. Reimagining Chitosan-Based Antimicrobial Biomaterials to Mitigate Antibiotic Resistance and Alleviate Antibiotic Overuse: A Review. Macromol. Mater. Eng. 2024, 309, 2400018. [Google Scholar] [CrossRef]
- Sampantamit, T.; Ho, L.; Lachat, C.; Sutummawong, N.; Sorgeloos, P.; Goethals, P. Aquaculture Production and Its Environmental Sustainability in Thailand: Challenges and Potential Solutions. Sustainability 2020, 12, 2010. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Elouali, S.; Hamdan, Y.A.; Benali, S.; Lhomme, P.; Gosselin, M.; Raquez, J.M.; Rhazi, M. Extraction of Chitin and Chitosan from Hermetia illucens Breeding Waste: A Greener Approach for Industrial Application. Int. J. Biol. Macromol. 2025, 285, 138302. [Google Scholar] [CrossRef]
- González-Lara, H.; Parra-Pacheco, B.; Rico-García, E.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A.; García-Trejo, J.F. Black Soldier Fly Culture as a Source of Chitin and Chitosan for Its Potential Use in Concrete: An Overview. Polymers 2025, 17, 717. [Google Scholar] [CrossRef]
- Lertjindaporn, M.; Geng, J.T.; Keratimanoch, S.; Lee, G.Y.; Ryo, K.; Osako, K. Chitin and Chitosan from North Pacific krill (Euphausia Pacifica): Comparative Study of Conventional and Microwave-Assisted Extraction Methods and the Potential Use in Chitosan Film Production. Int. J. Biol. Macromol. 2025, 296, 139692. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Ramprasath, R.; Jalaludeen, A.M.; Jayakumar, R.; Jolius, G.; Balu, R.; Mohamed, S.B.; Sridhar, T.M.; Gunasekaran, S.S.; Davoodbasha, M.; et al. Electrospun Nanofibers of Collagen and Chitosan for Tissue Engineering and Drug Delivery Applications: A Review. Int. J. Biol. Macromol. 2025, 296, 139663. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. New Insights into Sources, Bioavailability, Health-Promoting Effects, and Applications of Chitin and Chitosan. J. Agricul. Food Chem. 2024, 72, 17138–17152. [Google Scholar] [CrossRef]
- Rani, Z.; Ridwanto, R.; Nasution, H.M.; Kaban, V.E.; Nasri, N.; Karo, N.B. Antibacterial Activity of Freshwater Lobster (Cherax quadricarinatus) Shell Chitosan Gel Preparation against Escherichia coli and Staphylococcus aureus. J. Appl. Pharm. Sci. 2022, 13, 146–153. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Xin, M.; Li, M.; Shi, L.; Mao, Y. Evaluating the antibacterial and antibiofilm activities of chitosan derivatives containing six-membered heterocyclics against E. coli and S. aureus. Colloids Surf. B Biointerfaces 2024, 242, 114084. [Google Scholar] [CrossRef]
- Yang, Y.; Aghbashlo, M.; Gupta, V.K.; Amiri, H.; Pan, J.; Tabatabaei, M.; Rajaei, A. Chitosan Nanocarriers Containing Essential Oils as a Green Strategy to Improve the Functional Properties of Chitosan: A Review. Int. J. Biol. Macromol. 2023, 236, 123954. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Zhang, H.; Luo, J.; Gao, X. Green Fabrication of Chitin/Chitosan Composite Hydrogels and Their Potential Applications. Macromol. Biosci. 2021, 21, 2000389. [Google Scholar] [CrossRef]
- Boudouaia, N.; Benine, M.L.; Fettal, N.; Abbouni, B.; Bengharez, Z. Antibacterial Action of Chitosan Produced from Shrimp Waste Against the Growth of Escherichia coli, Staphylococcus epidermidis, Proteus mirabilis and Pseudomonas aeruginosa. Waste Biomass Valorization 2024, 15, 1267–1279. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadieh-Yazdi, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestani, M. Factors Influencing the Antimicrobial Mechanism of Chitosan Action and Its Derivatives: A Review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, Z.; Zhao, Z. Preparation, Characterization and Antimicrobial Activities of Cyclic Substituted Chitosan Derivatives. Int. J. Biol. Macromol. 2021, 193, 474–480. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.W. Chitosan-Based Nitric Oxide-Releasing Dressing for Anti-Biofilm and In Vivo Healing Activities in MRSA Biofilm-Infected Wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Cakici, F.; Cakici, E.B. Antimicrobial efficacy of chitosan versus sodium hypochlorite: A systematic review and meta-analysis. Oral Dis. 2024, 30, 5445–5460. [Google Scholar] [CrossRef] [PubMed]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial Activity of Chitin, Chitosan and Its Oligomers Prepared from Shrimp Shell Waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent Developments in Antibacterial and Antifungal Chitosan and Its Derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, M.I.G.; Souza, P.F.N.; Monteiro Júnior, J.E.; Grangeiro, T.B. Chitosan and Chitooligosaccharides: Antifungal Potential and Structural Insights. Chem. Biodiver. 2024, 21, e202400044. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current Trends in Fungal Biosynthesis of Chitin and Chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Gao, M.; Tang, H.; Zhu, H. Advances in Extraction, Utilization, and Development of Chitin/Chitosan and its Derivatives from Shrimp Shell Waste. Comprehens. Rev. Food Sci. Food Saf. 2024, 23, e70008. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of Chitin and Chitosan Based Biomaterials for the Adsorptive Removal of Textile Dyes from Water—A Comprehensive Review. Carbohyd. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef]
- Vieira, H.; Lestre, G.M.; Solstad, R.G.; Cabral, A.E.; Botelho, A.; Helbig, C.; Coppola, D.; de Pascale, D.; Robbens, J.; Raes, K.; et al. Current and Expected Trends for the Marine Chitin/Chitosan and Collagen Value Chains. Mar. Drugs 2023, 21, 605. [Google Scholar] [CrossRef]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish Gelatin: The Novel Potential Applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffery, J.; Manning, L.; Basri, S.M.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and Chitosan Derived from Crustacean Waste Valorization Streams Can Support Food Systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.; Saleh, N.; Kamarudin, N.; Sulong, A. Review of Chitosan Composite as a Heavy Metal Adsorbent: Material Preparation and Properties. Carbohydr. Polym. 2022, 259, 117613. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Y.; Li, J.; Chen, S. Progress and Prospects in Chitosan Derivatives: Modification Strategies and Medical Applications. J. Mater. Sci. Technol. 2021, 89, 209–224. [Google Scholar] [CrossRef]
- Amitaye, A.N.; Elemike, E.E.; Akpeji, H.B.; Amitaye, E.; Hossain, I.; Mbonu, J.I.; Aziza, A.E. Chitosan: A Sustainable Biobased Material For Diverse Applications. J. Environ. Chem. Eng. 2024, 12, 113208. [Google Scholar] [CrossRef]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, Quantification, Characterization, and Application in Food Packaging of Chitin and Chitosan from Mushrooms: A Review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef]
- Wagner, G.P.; Lo, J.; Laine, R.; Almeder, M. Chitin in the Epidermal Cuticle of a Vertebrate (Paralipophrys trigloides, Blenniidae, Teleostei). Cell. Mol. Life Sci 1993, 49, 317–319. [Google Scholar] [CrossRef]
- Nurfikari, A.; de Boer, W. Chitin Determination in Residual Streams Derived from Insect Production by LC-ECD and LC-MS/MS Methods. Front. Sustain. Food Syst. 2021, 5, 795694. [Google Scholar] [CrossRef]
- Kato, K.; Okamura, K.; Hiki, K.; Kintsu, H.; Nohara, K.; Yamagishi, T.; Nakajima, N.; Watanabe, H.; Yamamoto, H. Potential Differences in Chitin Synthesis Ability Cause Different Sensitivities to Diflubenzuron among Three Strains of Daphnia magna. Aquat. Toxicol. 2022, 243, 106071. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.Y.; Hsieh, S.C.; Wong, Y.C.; Hu, S.Y.; Hsieh, J.M.; Chiu, S.T.; Yeh, S.P.; Liu, C.H. Chitin Derived from Daphnia similis and Its Derivate, Chitosan, Promote Growth Performance of Penaeus vannamei. Aquaculture 2021, 531, 735919. [Google Scholar] [CrossRef]
- Campalani, C.; Bertuol, I.; Bersani, C.; Calmanti, R.; Filonenko, S.; Rodríguez-Padrón, D.; Selva, M.; Perosa, A. Green Extraction of Chitin from Hard Spider Crab Shells. Carbohydr. Polym. 2024, 345, 122565. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan Production with Larval Exoskeletons Derived from the Insect Protein Production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baublys, V.; Can, E.; Šatkauskienė, I.; Bitim, B.; Tubelytė, V.; Baran, T. Comparison of Physicochemical Properties of Chitins Isolated from an Insect (Melolontha melolontha) and a Crustacean Species (Oniscus asellus). Zoomorphology 2014, 133, 285–293. [Google Scholar] [CrossRef]
- Kaya, M.; Baublys, V.; Satkauskiene, I.; Akyuz, B.; Bulut, E.; Tubelytė, V. First Chitin Extraction from Plumatella repens (Bryozoa) with Comparison to Chitins of Insect and Fungal Origin. Int. J. Biol. Macromol. 2015, 79, 126–132. [Google Scholar] [CrossRef]
- Elouali, S.; Ait Ali Ouydir, H.; Ait Hamdan, Y.; Eladlani, N.; Rhazi, M. Chitosan from Periplaneta americana L.: A Sustainable Solution for Heavy Metals Removal. Euro-Mediterr. J. Environ. Integr. 2025, 10, 25–36. [Google Scholar] [CrossRef]
- Kaya, M.; Lelešius, E.; Nagrockaitė, R.; Sargin, I.; Arslan, G.; Mol, A.; Bitim, B. Differentiations of Chitin Content and Surface Morphologies of Chitins Extracted from Male and Female Grasshopper Species. PLoS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef]
- Araki, Y.; Ito, E. A Pathway of Chitosan Formation in Mucor rouxii: Enzymatic Deacetylation of Chitin. Eur. J. Biochem. 1975, 55, 71–78. [Google Scholar] [CrossRef]
- Rinaudo, M.; Perez, S. From Chitin to Chitosan. Available online: https://www.glycopedia.eu/IMG/pdf/from_chitin_to_chitosan.pdf (accessed on 16 January 2025).
- Muzzarelli, R.A.A.; Muzzarelli, C.; Phillips, G.O.; Williams, P.A. (Eds.) Chitin and chitosan hydrogels. In Handbook of Hydrocolloids; Woodhead Publishing Ltd.: Cambridge, UK, 2009; pp. 849–888. [Google Scholar]
- Jeuniaux, C.; Domard, A.; Jeuniaux, C.; Muzzarelli, R.A.A.; Roberts, G. (Eds.) A brief survey of the early contribution of European scientists to chitin knowledge. In Advances in Chitin Sciences; Jacques André Publ.: Lyon, France, 1996; pp. 1–9. [Google Scholar]
- Crini, G. Historical Landmarks in the Discovery of Chitin. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–47. ISBN 978-3-030-16538-3. [Google Scholar]
- Berezina, N. Production and Application of Chitin. Phys. Sci. Rev. 2016, 1, 20160048. [Google Scholar] [CrossRef]
- Arnold, N.D.; Brück, W.M.; Garbe, D.; Brück, T.B. Enzymatic Modification of Native Chitin and Conversion to Specialty Chemical Products. Mar. Drugs 2020, 18, 93. [Google Scholar] [CrossRef]
- Khor, H.; Wan, A.C.A. Chitin: Fulfilling a Biomaterials Promise, 2nd ed.; Elsevier Insights: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Jollès, P.; Muzzarelli, R.A.A. Chitin and Chitinases; Birkhäuser Verlag: Basel, Switzerland, 1999. [Google Scholar]
- Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173. [Google Scholar] [CrossRef]
- Karwatkar, P.A.; Kulkarni, S.J.; Goswami, A.K. Bionanomaterials in Food Systems: Sources, Synthesis, Properties and Opportunities. BioNanoScience 2025, 15, 5. [Google Scholar] [CrossRef]
- Li, C.; Shang, W.; Huang, Y.; Ge, J.; Ye, J.; Qu, X.; Guo, Q.; Wang, C.; Hu, P.; Liu, Y. Sodium Alginate/Chitosan Composite Scaffold Reinforced with Biodegradable Polyesters/Gelatin Nanofibers for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2025, 285, 138054. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Falgayrac, A.; Pellerin, V.; Terrol, C.; Fernandes, S.C. Turning Black Soldier Fly Rearing By-Products into Valuable Materials: Valorisation through Cchitin and Chitin Nanocrystals Production. Carbohydr. Polym. 2024, 344, 122545. [Google Scholar] [CrossRef]
- Olza, S.; Salaberria, A.M.; Alonso-Varona, A.; Samanta, A.; Fernandes, S.C.M. The Role of Nanochitin in Biologically-Active Matrices for Tissue Engineering-Where Do We Stand? J. Mat. Chem. B 2023, 11, 5630–5649. [Google Scholar] [CrossRef]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing Sustainable Agriculture Systems in Medicinal and Aromatic Plant Production by Using Chitosan and Chitin-Based Biostimulants. Plants 2023, 12, 2469. [Google Scholar] [CrossRef]
- Lindner, S.; Bonin, M.; Hellmann, M.J.; Moerschbacher, B.M. Three Intertwining Effects Guide the Mode of Action of Chitin Deacetylase De-and N-Acetylation Reactions. Carbohydr. Polym. 2025, 347, 122725. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.E.; Hekmat, O.; Schuttelkopf, A.W.; Shrestha, B.; Tokuyasu, K.; Withers, S.G.; van Aalten, D.M. Structure and Mechanism of Chitin Deacetylase from the Fungal Pathogen Colletotrichum lindemuthianum. Biochemistry 2006, 45, 9416–9426. [Google Scholar] [CrossRef]
- Harmsen, R.A.G.; Tuveng, T.R.; Antonsen, S.G.; Eijsink, V.G.H.; Sørlie, M. Can We Make Chitosan by Enzymatic Deacetylation of Chitin? Molecules 2019, 24, 3862. [Google Scholar] [CrossRef]
- Liang, B.C.; Song, W.; Xing, R.G.; Liu, S.; Yu, H.H.; Li, P.C. The Source, Activity Influencing Factors and Biological Activities for Future Development of Chitin Deacetylase. Carbohydr. Polym. 2023, 321, 121335. [Google Scholar] [CrossRef] [PubMed]
- Grifoll-Romero, L.; Pascual, S.; Aragunde, H.; Biarnés, X.; Planas, A. Chitin Deacetylases: Structures, Specificities, and Biotech Applications. Polymers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Dixit, A.; Gaware, S.; Agarwal, B. Chitosan as Excellent Bio-macromolecule with Myriad of Anti-activities in Biomedical Applications—A Review. Int. J. Biol. Macromol. 2023, 257, 128697. [Google Scholar] [CrossRef]
- Edo, G.I.; Yousif, E.; Al-Mashhadani, M.H. Chitosan: An Overview of Biological Activities, Derivatives, Properties, and Current Advancements in Biomedical Applications. Carbohydr. Res. 2024, 542, 109199. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, Chitosan, and Nanochitin: Extraction, Synthesis, and Applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef]
- Pandey, R.; Mathur, G. Current Trends in Chitosan Functionalization Methods and Their Applications. Starch-Stärke 2025, 77, 2300248. [Google Scholar] [CrossRef]
- Elnaggar, E.M.; Abusaif, M.S.; Abdel-Baky, Y.M.; Ragab, A.; Omer, A.M.; Ibrahim, I.; Ammar, Y.A. Insight Into Divergent Chemical Modifications of Chitosan Biopolymer: Review. Int. J. Biol. Macromol. 2024, 277, 134347. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Giuzio, F.; Saturnino, C.; Longo, P.; Sinicropi, M.S. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics 2023, 11, 320. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Sinani, G.; Sessevmez, M.; Şenel, S. Applications of Chitosan in Prevention and Treatment Strategies of Infectious Diseases. Pharmaceutics 2024, 16, 1201. [Google Scholar] [CrossRef]
- Mao, S.; Zeng, Y.; Ren, Y.; Ye, X.; Tian, J. Modification of Physicochemical, Antioxidant, and Antibacterial Properties of Chitosan Film with Curcumin-Loaded TA/Fe Nanoparticles. Food Hydrocoll. 2025, 160, 110722. [Google Scholar] [CrossRef]
- Shi, S.; Shi, W.; Zhou, B.; Qiu, S. Research and Application of Chitosan Nanoparticles in Orthopedic Infections. Int. J. Nanomed. 2024, 19, 6589–6602. [Google Scholar] [CrossRef]
- Conde, A.; Borges, S.; Baptista-Silva, S.; Veloso, T.; Pereira, J.L.; Ventura, S.P.M.; Pintado, M.M. A Crayfish Chitosan-based Bioactive Film to Treat Vaginal Infections: A Sustainable Approach. Int. J. Biol. Macromol. 2024, 277, 134460. [Google Scholar] [CrossRef]
- Azhar, F.; Naureen, H.; Shahnaz, G.; Hamdani, S.D.A.; Shareef, U.; Khattak, S.; Babar, M.M.; Rahdar, A.; Fathi-karkan, S.; Pandey, S. Chitosan Functionalized Skin-Adhesive Formulation for Ergotamine Delivery in Melanoma Treatment. BioNanoScience 2025, 15, 29. [Google Scholar] [CrossRef]
- Sharaf, M.; Liu, C.G. Chitin and Chitosan Applications in Medication Delivery Systems. In Chitin and Chitosan; Jenny Stanford Publishing: Singapore, 2025; pp. 283–319. ISBN 9781003589778. [Google Scholar]
- Tang, W.; Wang, J.; Hou, H.; Li, Y.; Wang, J.; Fu, J.; Lu, L.; Gao, D.; Liu, Z.; Zhao, F.; et al. Review: Application of Chitosan and Its Derivatives in Medical Materials. Int. J. Biol. Macromol. 2023, 240, 124398. [Google Scholar] [CrossRef]
- Di Cintio, F.; Argenziano, M.; Scomparin, A.; Capolla, S.; Busato, D.; Steffè, A.; Mangogna, A.; Sblattero, D.; Cavlli, R.; Macor, P.; et al. The Anti-Glypican 1 AT101 Antibody as Targeting Agent to Effectively Deliver Chitosan Nanobubbles to Glioblastoma Cells. Nanomedicine 2025, 20, 23–36. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Chevrier, A.; Horn-Bourque, D.; Alameh, M.G.; Lavertu, M. Chitosan Immunomodulation: Insights into Mechanisms of Action on Immune Cells and Signaling Pathways. RSC Adv. 2025, 15, 896–909. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, S.; Singh, S.; Wani, M.Y. Overcoming Resistance: Chitosan-modified Liposomes as Targeted Drug Carriers in the Fight against Multidrug Resistant Bacteria-A Review. Int. J. Biol. Macromol. 2024, 278, 135022. [Google Scholar] [CrossRef]
- Nguyen, M.T.P.; Escribà-Gelonch, M.; Hessel, V.; Coad, B.R. A Review of the Current and Future Prospects for Producing Bioplastic Films Made from Starch and Chitosan. ACS Sustain. Chem. Eng. 2024, 12, 1750–1768. [Google Scholar] [CrossRef]
- Paul, M.; Das Pramanik, S.; Sahoo, R.N.; Dey, Y.N.; Nayak, A.K. Dental Delivery Systems of Antimicrobial Drugs using Chitosan, Alginate, Dextran, Cellulose and Other Polysaccharides: A Review. Int. J. Biol. Macromol. 2023, 247, 125808. [Google Scholar] [CrossRef]
- Sarfraz, M.H.; Hayat, S.; Siddique, M.H.; Aslam, B.; Ashraf, A.; Saqalein, M.; Khurshid, M.; Sarfraz, M.F.; Afzal, M.; Muzammil, S. Chitosan Based Coatings and Films: A Perspective on Antimicrobial, Antioxidant, and Intelligent Food Packaging. Prog. Org. Coat. 2024, 188, 108235. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Q.; Liu, Y.; Jiang, M. Effects of Chitosan Guanidine on Blood Glucose Regulation and Gut Microbiota in T2DM. Int. J. Biol. Macromol. 2024, 279, 135422. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Mariconda, A.; D’Amato, A.; Checconi, P.; Aquaro, S.; Longo, P.; Sinicropi, M.S. Chitosan-Based Schiff Bases (CSBs) and Their Metal Complexes: Promising Antimicrobial Agents. Molecules 2025, 30, 207. [Google Scholar] [CrossRef]
- Shrestha, R.; Thenissery, A.; Khupse, R.; Rajashekara, G. Strategies for the Preparation of Chitosan Derivatives for Antimicrobial, Drug Delivery, and Agricultural Applications: A Review. Molecules 2023, 28, 7659. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Khubiev, O.M.; Egorov, A.R.; Kirichuk, A.A.; Khrustalev, V.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Antibacterial Films for Biomedical and Food Applications. Int. J. Mol. Sci. 2023, 24, 10738. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved Hydrophobicity, Antibacterial and Mechanical Properties of Polyvinyl Alcohol/Quaternary Chitosan Composite Films for Antibacterial Packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef] [PubMed]
- Narciso, F.; Cardoso, S.; Monge, N.; Lourenço, M.; Martin, V.; Duarte, N.; Santos, C.; Gomes, P.; Bettencourt, A.; Ribeiro, I.A. 3D-Printed Biosurfactant-Chitosan Antibacterial Coating for the Prevention of Silicone-Based Associated Infections. Colloids Surf. B Biointerfaces 2023, 230, 113486. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, X.; Cheng, H.; Yang, D.; Xu, W.; Hu, Y.; Song, Y.; Zeng, G. Type I Photodynamic Antimicrobial Therapy: Principles, Progress, and Future Perspectives. Acta Biomater. 2024, 177, 1–19. [Google Scholar] [CrossRef]
- Tang, W.; Ye, L.; Han, T.; He, J.; Liu, J. Effect of Chitosan with Different Molecular Weights on the Freeze-Thaw Stability of Gluten Protein: Protein Structures, Functional Characteristics, and Cryo-Protective Mechanism. Food Hydrocoll. 2025, 160, 110763. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of The Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic Chitosan Derivatives as Potential Antifungals: A Review of Structural Optimization and Applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Petroudi, S.H.H.; Ojagh, S.M.; Romanazzi, G. Toward Understanding the Antibacterial Mechanism of Chitosan: Experimental Approach and In Silico Analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Kumar, M.; Ahmad, N.; Ge, Y.; Mahmood, S. Recent Applications of Chitosan and Its Derivatives in Antibacterial, Anticancer, Wound Healing, and Tissue Engineering Fields. Polymers 2024, 16, 1351. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Akdaşçi, E.; Duman, H.; Eker, F.; Bechelany, M.; Karav, S. Chitosan and Its Nanoparticles: A Multifaceted Approach to Antibacterial Applications. Nanomaterials 2025, 15, 126. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Liang, H.; Meng, N.; Zhou, N. Fabrication of Antimicrobial Chitosan/ZnO Nanoparticles/Lecithin-Montmorillonite Films for Food Packaging Application. Food Hydrocoll. 2025, 159, 110686. [Google Scholar] [CrossRef]
- Villani, S.; De Matteis, V.; Calcagnile, M.; Cascione, M.; Pellegrino, P.; Vincenti, L.; Demitri, C.; Alifano, P.; Rinaldi, R. Tuning Antibacterial Efficacy against Pseudomonas aeruginosa by Using Green AgNPs in Chitosan Thin Films as a Plastic Alternative. Int. J. Biol. Macromol. 2025, 285, 138277. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Venkatesan, R.; Dhilipkumar, T.; Mayakrishnan, V.; Raorane, C.J.; Sana, S.S.; Ansari, M.A.; Kim, S.C. Enhancing Cherry Tomato Packaging: Evaluation of Physicochemical, Mechanical, and Antibacterial Properties in Tannic Acid and Gallic Acid Crosslinked Cellulose/Chitosan Blend Films. Int. J. Biol. Macromol. 2025, 285, 138276. [Google Scholar] [CrossRef]
- Liang, F.; Li, X.; Li, X.; Liu, X.; Pan, R.; Su, W.; Li, C.; Liu, K. Preparation and Characterization of a Chitosan-Gentamicin Derivative and Its Effect on Tropical Fruit Preservation. Food Hydrocoll. 2025, 158, 110485. [Google Scholar] [CrossRef]
- Ma, J.; Huang, X.; Jin, L.; Xu, Q. Effect of Dialdehyde Nanocellulose-Tannin Fillers on Antioxidant, Antibacterial, Mechanical and Barrier Properties of Chitosan Films for Cherry Tomato Preservation. Food Chem. 2025, 463, 141274. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, D.; Xu, L.; Sun, J.; Zhong, X.; Xie, X.; Yu, M.; Shi, L.; Liu, Y. Comprehensive Study on Malus ‘Donald Wyman’ Crabapple Extract: Its Characterization, Impact on Chitosan Film Properties, and Preservation Efficacy in Grapes. LWT 2025, 215, 117163. [Google Scholar] [CrossRef]
- Tabrizi, L.; Hughes, D.F.; Pryce, M.T. Covalent Organic Frameworks: Advancing Antimicrobial Photodynamic Therapy for Next-Generation Treatments. Coord. Chem. Rev. 2025, 528, 216424. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, J.; Yuan, X.; Cheng, X. Chitosan-BODIPY Fluorescent Composite Materials for Photodynamical Antibacterial and Therapy. Int. J. Biol. Macromol. 2025, 286, 1382. [Google Scholar] [CrossRef]
- Luo, H.; Xu, H.; Zhang, H.; Li, X.; Wu, Q.; Gao, T. Photodynamic Therapy Combined with Quaternized Chitosan Antibacterial Strategy for Instant and Prolonged Bacterial Infection Treatment. Carbohydr. Polym. 2025, 352, 123147. [Google Scholar] [CrossRef]
- Soleimani, F.; Karimi, A.R.; Hadizadeh, M. Multifunctional Riboflavin/Chitosan-Based Dianhydride Crosslinked Hydrogels: Photodynamic Therapy, Antioxidant, and Antibacterial Properties. Next Res. 2025, 2, 100139. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhao, W.; Zhu, L.; Hong, L.; Cui, K.; Yu, N.; Chen, Z.; Wen, M. Chitosan-Based Hydrogel Incorporated with Polydopamine and Protoporphyrin for Photothermal-Oxidation Sterilization of Bacteria-Infected Wound Therapy. J. Colloid Interf. Sci. 2025, 678, 89–100. [Google Scholar] [CrossRef]
- Liu, T.; Lei, H.; Qu, L.; Zhu, C.; Ma, X.; Fan, D. Algae-Inspired Chitosan-Pullulan-Based Multifunctional Hydrogel for Enhanced Wound Healing. Carbohydr. Polym. 2025, 347, 122751. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Ji, P.; Hao, L.; Sun, C.; Mao, H.; Gu, Z. Self-assembling chitosan based injectable and expandable sponge with antimicrobial property for hemostasis and wound healing. Carbohydr. Polym. 2025, 347, 122699. [Google Scholar] [CrossRef]
- Krajišnik, D.; Uskoković-Marković, S.; Daković, A. Chitosan–Clay Mineral Nanocomposites with Antibacterial Activity for Biomedical Application: Advantages and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 10377. [Google Scholar] [CrossRef]
- Goel, S.; Bano, Y. Chitosan-based nanofibrous membranes for antibacterial filter applications. In Antimicrobial Materials and Coatings; Woodhead Publishing: Cambridge, UK, 2025; pp. 425–447. [Google Scholar] [CrossRef]
- Cicciu, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; D’Amato, A.; Lauria, G.; Saturnino, C.; Andreu, I.; Longo, P.; Sinicropi, M.S. Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries. Antibiotics 2023, 12, 112. [Google Scholar] [CrossRef]
- Qu, S.; Ma, X.; Yu, S.; Wang, R. Chitosan as a Biomaterial for the Prevention and Treatment of Dental Caries: Antibacterial Effect, Biomimetic Mineralization, and Drug Delivery. Front. Bioeng. Biotechnol. 2023, 11, 1234758. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, J.; Paczkowska-Walendowska, M.; Rył, A.; Karpiński, T.M.; Miklaszewski, A.; Swora-Cwynar, E.; Leśna, M.; Cielecka-Piontek, J. Azithromycin-Loaded Nanoparticles Incorporated in Chitosan-Based Soft Hydrogels: A Novel Approach for Dental Drug Delivery. Pharmaceutics 2025, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Ehsanfar, N.; Qomi, M.; Youseftabar-Miri, L.; Divsar, F. Adsorption and Controlled Release of Isotretinoin and Adapalene Retinoid Drugs from Water Using Chitosan-Modified Manganese Ferrite Nanoparticles. Int. J. Environ. Anal. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Alakayleh, Z. From Inactive Biomass in Removing Amoxicillin to New Active Chitosan-Biomass Composite Adsorbents. Results Eng. 2025, 25, 103709. [Google Scholar] [CrossRef]
- Alyasi, H.; Wahib, S.; Tong, Y.; Gomez, T.; Mahmoud, K.A. Magnetic MXene Chitosan-Lignosulfonate Composite (Fe3O4@MCLS) for the Reductive Removal of Cr(VI) and Other Heavy Metals from Water. J. Hazard. Mat. Adv. 2025, 17, 100536. [Google Scholar] [CrossRef]
- Yu, C.; Nghiem, L.D.; Zou, L. Catalytic chitosan/MXene/GO Nanocomposite Membrane for Removing Dye and Heavy Metals. Desalination 2025, 594, 118313. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Mei, A.; Peng, L.; Sun, J. Novel Chitosan/Lignin Hydrogel Prepared by the Mannich Reaction for Pb(II) and Cu(II) Removal from Aqueous Solution. Int. J. Biol. Macromol. 2025, 285, 138177. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, X.; Zhou, Y.; Ma, S.; Zhao, C.; Liu, S. Effective Removal of Copper(II) Ion from Polluted Water Using Ferric Oxide-Chitosan Composite: Kinetic, Equilibrium and Adsorption Mechanism Studies. Water Air Soil Pollut. 2025, 236, 27. [Google Scholar] [CrossRef]
- Ibrahim, A.; Eldemerdash, U.N.; Elkady, M. Breakthrough Continuous Adsorption of Cationic Heavy Metal and Anionic Dye Using Economical Polyurethane Waste-Based Bio-Filters. J. Water Process Eng. 2025, 70, 106982. [Google Scholar] [CrossRef]
- Allahkarami, E.; Maleki, S.; Azadmehr, A.; Aghayan, S.; Allahkarami, E. Fabricating Nepheline Syenite-Chitosan Composite for Heavy Metals Uptake: Mechanism Insight via Statistical Physics Modeling. Sep. Purif. Technol. 2025, 354, 129152. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, Y.; Feng, Z.; Ren, Q.; Wang, Y.; Chen, X.; Li, Y.; Wang, Y. Immobilization of Novel Bifunctional Polymer with Amide and Amidoxime Groups in Biochar-Chitosan Composite Gels for Enhancing Uranium(VI) Uptake. Sep. Purif. Technol. 2025, 354, 128891. [Google Scholar] [CrossRef]
- Zhong, J.; Cao, Y.; Zhu, J.; Wang, Y.; Yu, B.; Li, J.; Huang, J. Facile Construction of Phenolic Hydroxyl Anchored Covalent Organic Frameworks/Chitosan Composite Aerogels for Efficient Adsorption of Pb(II) from Water. Sep. Purif. Technol. 2025, 354, 129087. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Batool, F.; Mohyuddin, A.; Goh, H.H.; Othman, M.H.D.; Aziz, F.; Anouzla, A.; Al-Hazmi, H.E.; Chew, K.W. Chitosan-Coated Coconut Shell Composite: A Solution for Treatment of Cr(III)-Contaminated Tannery Wastewater. J. Taiwan Inst. Chem. Eng. 2024, 166, 105478. [Google Scholar] [CrossRef]
- Boughanmi, R.; Oelmann, M.; Steinbach, C.; Schwarz, S. Sustainable Polyelectrolyte Complexes of Pectin and Chitosan as Adsorbents for Heavy Metal Ions from Surface Water. J. Polym. Sci. 2024, 63, 133–145. [Google Scholar] [CrossRef]
- Tri, L.M.; Thi Mai Huong, P.; Thi Huong, N. Magnetic Chitosan Nanocomposites Derived from Industrial Solid Waste: A Promising Approach for Arsenic(III) Remediation. ACS Omega 2025, in press. [Google Scholar] [CrossRef]
- A Worldwide Market with a Strong Demand. Available online: http://sflyproteins.com/a-worldwide-market-with-a-strong-demand/ (accessed on 23 March 2025).
- Chitosan Market—By Source (Shrimps, Prawns, Crabs, Lobsters, Fungi), By End-User (Water Treatment, Cosmetics & Toiletries, Food & Beverage, Healthcare/Medical, Agrochemical, Biotechnology) & Forecast, 2021–2027. Available online: https://www.gminsights.com/industry-analysis/chitosan-market (accessed on 23 March 2025).
- Oyatogun, G.M.; Esan, T.A.; Akpan, E.I.; Adeosun, S.O.; Popoola, A.P.I.; Imasogie, B.I.; Soboyejo, W.O.; Afonja, A.A.; Ibitoye, S.A.; Abere, V.D.; et al. Chitin, chitosan, marine to market. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–376. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Sustainable Production of Chitosan. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Studies in Systems, Decision and Control; Królczyk, G., Wzorek, M., Król, A., Kochan, O., Su, J., Kacprzyk, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 198. [Google Scholar] [CrossRef]


| Contaminant | Ref. | |
|---|---|---|
| Pharmaceuticals | Retinoids (isotretinoin, adapalene) | [129] |
| Amoxicillin | [130] | |
| Dyes | Methylene blue | [132] |
| Congo red | [135] | |
| Metals | Cu(II) | [132,133,134,140] |
| Co(II) | [132] | |
| Mn(II) | [135] | |
| Pb(II) | [133,138] | |
| Ni(II) | [136] | |
| Cd(II) | [136] | |
| U(VI) | [137] | |
| Cr(VI) | [131] | |
| Cr(III) | [139] | |
| As(III) | [141] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarcelli, E.; Catalano, A.; Iacopetta, D.; Ceramella, J.; Sinicropi, M.S.; Aiello, F. Chitin, Chitosan and Its Derivatives: Antimicrobials and/or Mitigators of Water. Macromol 2025, 5, 15. https://doi.org/10.3390/macromol5020015
Scarcelli E, Catalano A, Iacopetta D, Ceramella J, Sinicropi MS, Aiello F. Chitin, Chitosan and Its Derivatives: Antimicrobials and/or Mitigators of Water. Macromol. 2025; 5(2):15. https://doi.org/10.3390/macromol5020015
Chicago/Turabian StyleScarcelli, Eva, Alessia Catalano, Domenico Iacopetta, Jessica Ceramella, Maria Stefania Sinicropi, and Francesca Aiello. 2025. "Chitin, Chitosan and Its Derivatives: Antimicrobials and/or Mitigators of Water" Macromol 5, no. 2: 15. https://doi.org/10.3390/macromol5020015
APA StyleScarcelli, E., Catalano, A., Iacopetta, D., Ceramella, J., Sinicropi, M. S., & Aiello, F. (2025). Chitin, Chitosan and Its Derivatives: Antimicrobials and/or Mitigators of Water. Macromol, 5(2), 15. https://doi.org/10.3390/macromol5020015










