The Use of Common Bean and Mesquite Pods Flours as Partial Substitute of Semolina, Impact of Their Proteins and Polysaccharides in the Physical, Chemical, and Microstructural Characteristics of Spaghetti Pasta
Abstract
1. Introduction
2. Materials and Methods
3. Results
- Protein digestibility
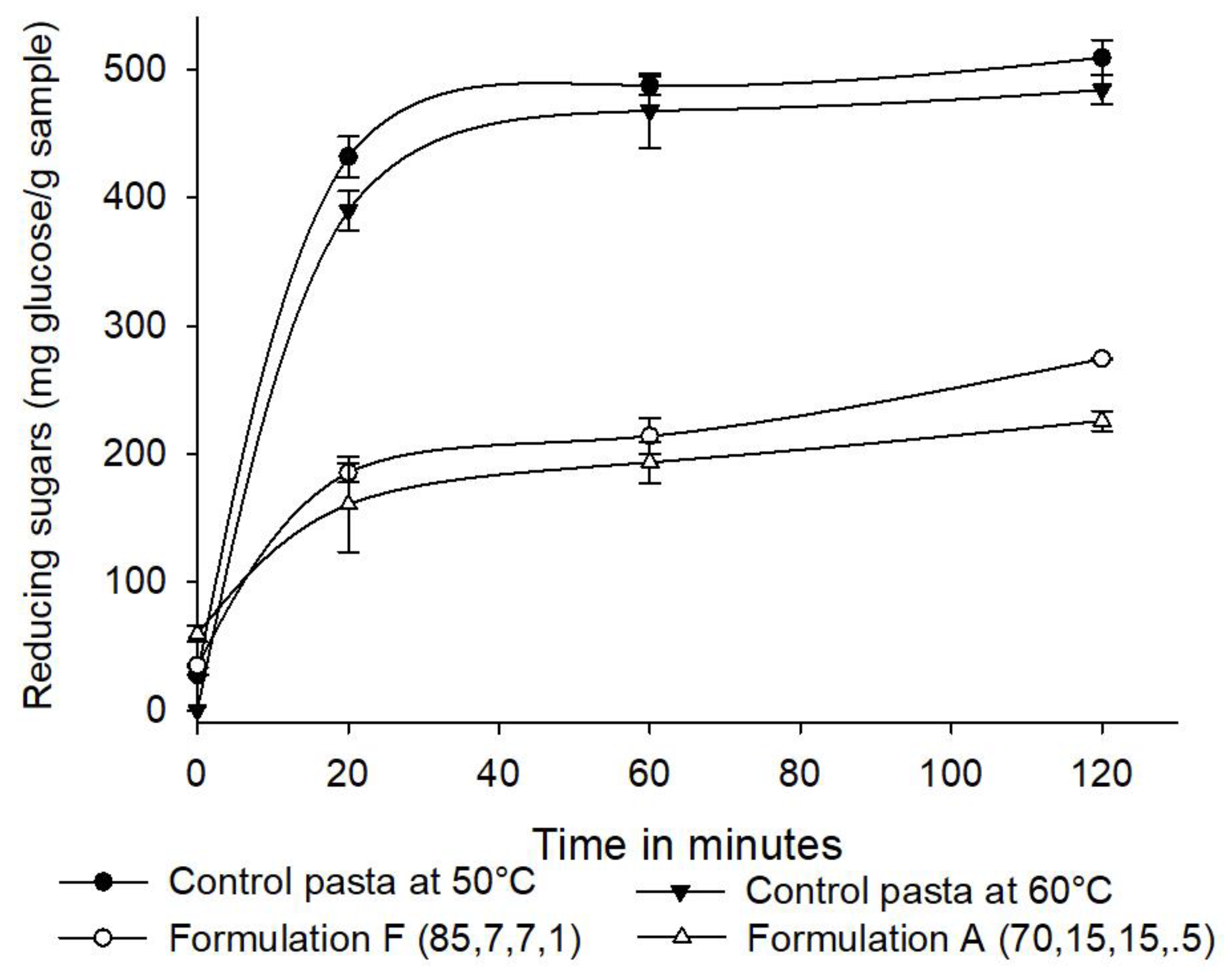
- Starch digestibility
4. Discussion
- Proximate analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickie, J. Delizia!: The Epic History of the Italians and Their Food; Sceptre: Hachette, UK, 2007. [Google Scholar]
- NOM F-23-S-Pasta de Harina de Trigo y/o Semolina para sopa y sus Variedades. 1980. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4857471&fecha=24/07/1980#gsc.tab=0 (accessed on 5 February 2025).
- Henciya, S.; Seturaman, P.; James, A.R.; Tsai, Y.-H.; Nikam, R.; Wu, Y.-C.; Dahms, H.-U.; Chang, F.R. Biopharmaceutical potentials of Prosopis spp. (Mimosaceae, Leguminosa). J. Food Drug Anal. 2016, 25, 187–196. [Google Scholar] [CrossRef]
- Burkart, A.E. A Monograph of the Genus prosopis (Leguminosae Subfam. Mimosoideae). J. Arnold Arbor. 1976, 57, 450–525. [Google Scholar] [CrossRef]
- Comisión Nacional de Zonas Áridas. (n.d.). CONAZA. Retrieved 18 November 2022. Available online: https://www.gob.mx/conaza (accessed on 22 October 2024).
- Igwe, C.U.; Ojiako, O.A.; Anugweje, K.C.; Nwaogu, L.A.; Ujowundu, C.O. Amino acid profile of raw and locally processed seeds of Prosopis africana and Ricinus communis: Potential antidotes to protein malnutrition. Funct. Foods Health Dis. 2012, 2, 107–114. [Google Scholar] [CrossRef]
- Sciammaro, L.; Ferrero, C.; Puppo, M. Gluten-free baked muffins developed with Prosopis alba flour. LWT 2018, 98, 568–576. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Korus, A.; Juszczak, L. Mesquite (Prosopis L.) as a functional ingredient in gluten-free dough and bread. LWT 2022, 168, 113957. [Google Scholar] [CrossRef]
- López-Franco, Y.L.; Goycoolea, F.M.; Valdez, M.A.; Calderón De La Barca, A.M. Goma de Mezquite: Alternativa de uso industrial. Interciencia 2006, 31, 183–189. [Google Scholar]
- Cuevas-Bernardino, J.C.; Leyva-Gutierrez, F.M.; Vernon-Carter, E.J.; Lobato-Calleros, C.; Román-Guerrero, A.; Davidov-Pardo, G. Formation of biopolymer complexes composed of pea protein and mesquite gum—Impact of quercetin addition on their physical and chemical stability. Food Hydrocoll. 2017, 77, 736–745. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2019, 60, 2593–2605. [Google Scholar] [CrossRef]
- Leterme, P.; Monmart, T.; Baudart, E. Amino acid composition of pea (Pisum sativum) proteins and protein profile of pea flour. J. Sci. Food Agric. 1990, 53, 107–110. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Varela, A.; Domínguez-Timón, F.; Tovar, C.A.; Moreno, H.M.; Borderías, A.J.; Díaz, M.T. Comparison of Bioactive Compounds Content and Techno-Functional Properties of Pea and Bean Flours and their Protein Isolates. Plant Foods Hum. Nutr. 2020, 75, 642–650. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pagliaro, A.; Del Nobile, M.A.; Conte, A.; Melilli, M.G. Lentil Fortified Spaghetti: Technological Properties and Nutritional Characterization. Foods 2020, 10, 4. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Goñi, I.; Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Towards an updated methodology for measurement of dietary fiber, including associated polyphenols, in food and beverages. Food Res. Int. 2009, 42, 840–846. [Google Scholar] [CrossRef]
- Anton, A.A.; Fulcher, R.G.; Arntfield, S.D. Physical and nutritional impact of fortification of corn starch-based extruded snacks with common bean (Phaseolus vulgaris L.) flour: Effects of bean addition and extrusion cooking. Food Chem. 2009, 113, 989–996. [Google Scholar] [CrossRef]
- Gasparre, N.; Betoret, E.; Rosell, C.M. Quality Indicators and Heat Damage of Dried and Cooked Gluten Free Spaghetti. Plant Foods Hum. Nutr. 2019, 74, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Tudoricǎ, C.M.; Kuri, V.; Brennan, C.S. Nutritional and Physicochemical Characteristics of Dietary Fiber Enriched Pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Padalino, L.; Mastromatteo, M.; Lecce, L.; Cozzolino, F.; Del Nobile, M. Manufacture and characterization of gluten-free spaghetti enriched with vegetable flour. J. Cereal Sci. 2013, 57, 333–342. [Google Scholar] [CrossRef]
- Sozer, N.; Dalgıç, A.C.; Kaya, A. Thermal, textural and cooking properties of spaghetti enriched with resistant starch. J. Food Eng. 2007, 81, 476–484. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, H.F.; Peplinski, A.J. Gelatinization of Corn Grits by Roll Cooking, Extrusion Cooking and Steaming. Starch-Starke 1969, 22, 130–135. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC–Orbitrap-MS approach. J. Sci. Food Agric. 2018, 99, 1038–1045. [Google Scholar] [CrossRef]
- Garcia-Valle, D.E.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Structural characteristics and in vitro starch digestibility of pasta made with durum wheat semolina and chickpea flour. LWT 2021, 145, 111347. [Google Scholar] [CrossRef]
- Manno, D.; Filippo, E.; Serra, A.; Negro, C.; De Bellis, L.; Miceli, A. The influence of inulin addition on the morphological and structural properties of durum wheat pasta. Int. J. Food Sci. Technol. 2009, 44, 2218–2224. [Google Scholar] [CrossRef]
- Sissons, M.; Aravind, N.; Fellows, C.M. Quality of Fiber-Enriched Spaghetti Containing Microbial Transglutaminase. Cereal Chem. 2010, 87, 57–64. [Google Scholar] [CrossRef]
- Rachman, A.; Brennan, M.A.; Morton, J.; Torrico, D.; Brennan, C.S. In-vitro digestibility, protein digestibility corrected amino acid, and sensory properties of banana-cassava gluten-free pasta with soy protein isolate and egg white protein addition. Food Sci. Hum. Wellness 2023, 12, 520–527. [Google Scholar] [CrossRef]
- Manai–Djebali, H.; Oueslati, I.; Nouairi, I.; Taamalli, A.; Nait-Mohamed, S.; Mliki, A.; Ghorbel, A. Chemical composition of durum wheat kernels: Impact of the growing location. Euro-Mediterranean J. Environ. Integr. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Campos, C.C.Z.; García-Martínez, J.E.; Chavira, J.S.; Valdés, J.A.A.; Morales, M.A.M.; Mellado, M. Chemical composition and nutritional value of leaves and pods of Leucaena leucocephala, Prosopis laevigata and Acacia farnesiana in a xerophilous shrubland. Emir. J. Food Agric. 2020, 32, 723. [Google Scholar] [CrossRef]
- Teterycz, D.; Sobota, A.; Zarzycki, P.; Latoch, A. Legume flour as a natural colouring component in pasta production. J. Food Sci. Technol. 2020, 57, 301–309. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Szymańska-Chargot, M.; Krawęcka, A.; Sobota, A.; Miś, A. Effect of moisturizing pre-treatment of dietary fibre preparations on formation of gluten network during model dough mixing—A study with application of FT-IR and FT-Raman spectroscopy. LWT 2020, 121, 108959. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. FTIR studies of gluten matrix dehydration after fibre polysaccharide addition. Food Chem. 2018, 252, 198–206. [Google Scholar] [CrossRef]
- Azpeitia, L.G.; Labrada-Delgado, G.J.; Montalvo-González, E.; Loza-Cornejo, S. Caracteres morfométricos y anatómicos de frutos y semillas de una población de Prosopis laevigata (Fabaceae) en Lagos de Moreno, Jalisco, México. Acta Bot. Mex. 2022, 129–135, 2057. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Brennan, M.; Darwish, A.M.G.; Brennan, C. Physicochemical, texture and sensorial evaluation of pasta enriched with chickpea flour and protein isolate. Ann. Agric. Sci. 2020, 65, 28–34. [Google Scholar] [CrossRef]
- Gallegos-Infante, J.; Rocha-Guzman, N.; Gonzalez-Laredo, R.; Ochoa-Martínez, L.; Corzo, N.; Bello-Perez, L.; Medina-Torres, L.; Peralta-Alvarez, L. Quality of spaghetti pasta containing Mexican common bean flour (Phaseolus vulgaris L.). Food Chem. 2010, 119, 1544–1549. [Google Scholar] [CrossRef]
- Laleg, K.; Barron, C.; Cordelle, S.; Schlich, P.; Walrand, S.; Micard, V. How the structure, nutritional and sensory attributes of pasta made from legume flour is affected by the proportion of legume protein. LWT Food Sci. Technol. 2016, 79, 471–478. [Google Scholar] [CrossRef]
- Turco, I.; Bacchetti, T.; Bender, C.; Zimmermann, B.; Oboh, G.; Ferretti, G. Polyphenol content and glycemic load of pasta enriched with Faba bean flour. Funct. Foods Health Dis. 2016, 6, 291. [Google Scholar] [CrossRef]
- Li, G.L.; Zhu, F. Functional Properties of Starch. In Starch and Starchy Food Products, 1st ed.; Taylor & Francis Group: London, UK, 2023. [Google Scholar] [CrossRef]
- Peressini, D.; Cavarape, A.; Brennan, M.A.; Gao, J.; Brennan, C.S. Viscoelastic properties of durum wheat doughs enriched with soluble dietary fibres in relation to pasta-making performance and glycaemic response of spaghetti. Food Hydrocoll. 2019, 102, 105613. [Google Scholar] [CrossRef]
- Ronda, F.; Pérez-Quirce, S.; Villanueva, M. Rheological Properties of Gluten-Free Bread Doughs: Relationship with Bread Quality; Elsevier eBooks: Amsterdam, The Netherlands, 2017; pp. 297–334. [Google Scholar] [CrossRef]
- Biernacka, B.; Dziki, D.; Różyło, R.; Wójcik, M.; Miś, A.; Romankiewicz, D.; Krzysiak, Z. Relationship between the properties of raw and cooked spaghetti—New indices for pasta quality evaluation. Int. Agrophys. 2018, 32, 217–223. [Google Scholar] [CrossRef]
- Feige, M.; Braakman, I.; Hendershot, L.M. Disulfide Bonds in Protein Folding and Stability. In Oxidative Folding of Proteins: Basic Principles, Cellular Regulation and Engineerin, 9th ed.; The Royal Society of Chemistry: London, UK, 2018; pp. 3–23. [Google Scholar]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Assaf, A.; Thouand, G.; Le-Bail, A. Study of structural changes of gluten proteins during bread dough mixing by Raman spectroscopy. Food Chem. 2021, 358, 129916. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Wang, X.; Ren, T.; Ma, Z.; Li, X.; Xu, B.; Hu, X. Interpreting the correlation between repeated sheeting process and wheat noodle qualities: From water molecules movement perspective. LWT 2021, 151, 112219. [Google Scholar] [CrossRef]
- Kim, E.H.-J.; Petrie, J.R.; Motoi, L.; Morgenstern, M.P.; Sutton, K.H.; Mishra, S.; Simmons, L.D. Effect of Structural and Physicochemical Characteristics of the Protein Matrix in Pasta on In Vitro Starch Digestibility. Food Biophys. 2008, 3, 229–234. [Google Scholar] [CrossRef]
- Desai, A.S.; Brennan, M.A.; Brennan, C.S. Effect of Fortification with Fish (Pseudophycis bachus) Powder on Nutritional Quality of Durum Wheat Pasta. Foods 2018, 7, 62. [Google Scholar] [CrossRef]
- Osipova, G.; Koryachkina, S.; Koryachkin, V.; Seregina, T.; Zhugina, A. Effects of protein-containing additives on pasta quality and biological value. Food Raw Mater. 2019, 7, 60–66. [Google Scholar] [CrossRef]
- Pérez-Beltrán, Y.E.; Tovar, J.; Sáyago-Ayerdi, S.G. Influence of the Food Matrix on the Digestibility and Metabolic Responses to Starchy Foods; CRC Press eBooks: Boca Raton, FL, USA, 2022; pp. 291–322. [Google Scholar] [CrossRef]
- Jang, H.L.; Bae, I.Y.; Lee, H.G. In vitro starch digestibility of noodles with various cereal flours and hydrocolloids. LWT 2015, 63, 122–128. [Google Scholar] [CrossRef]
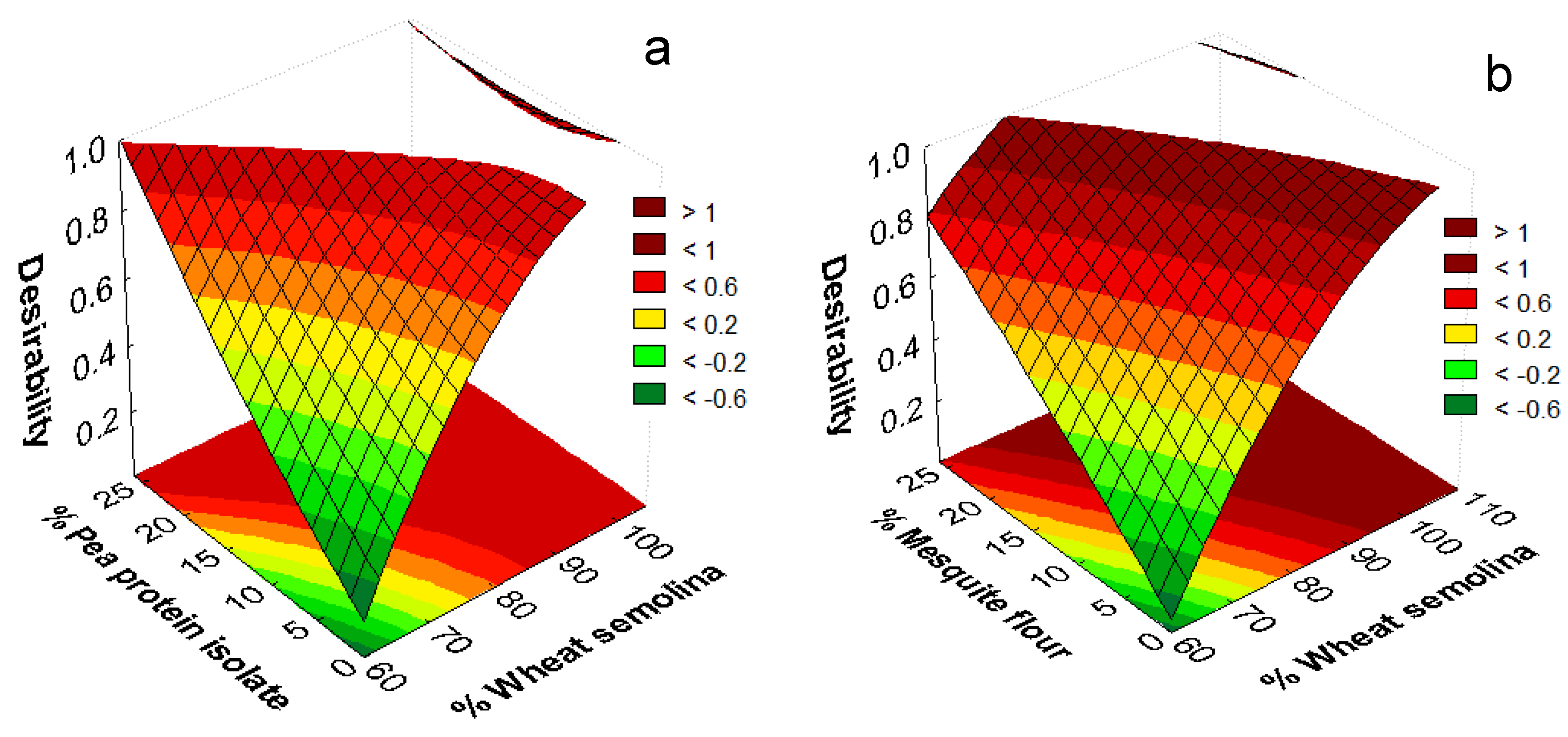




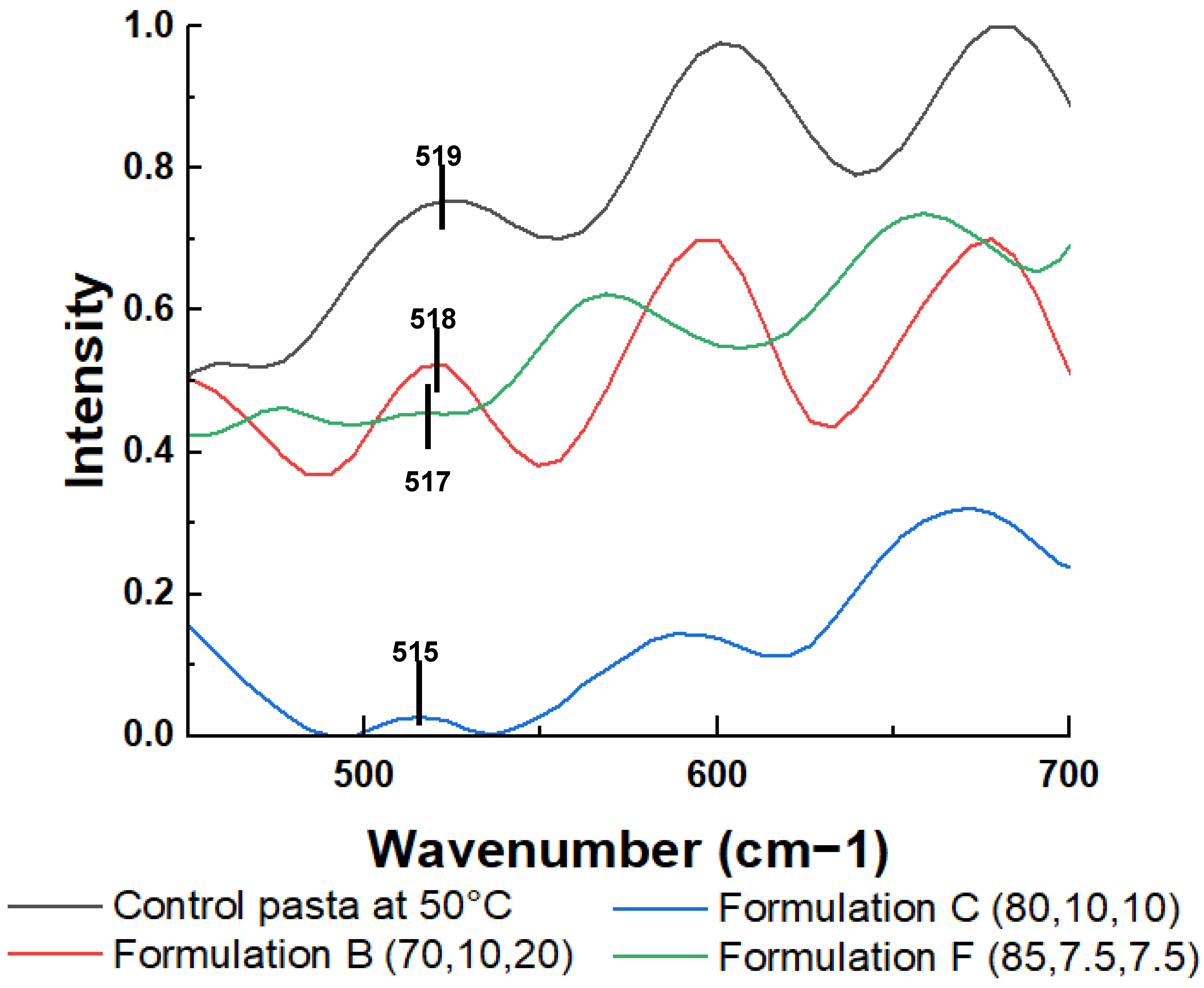
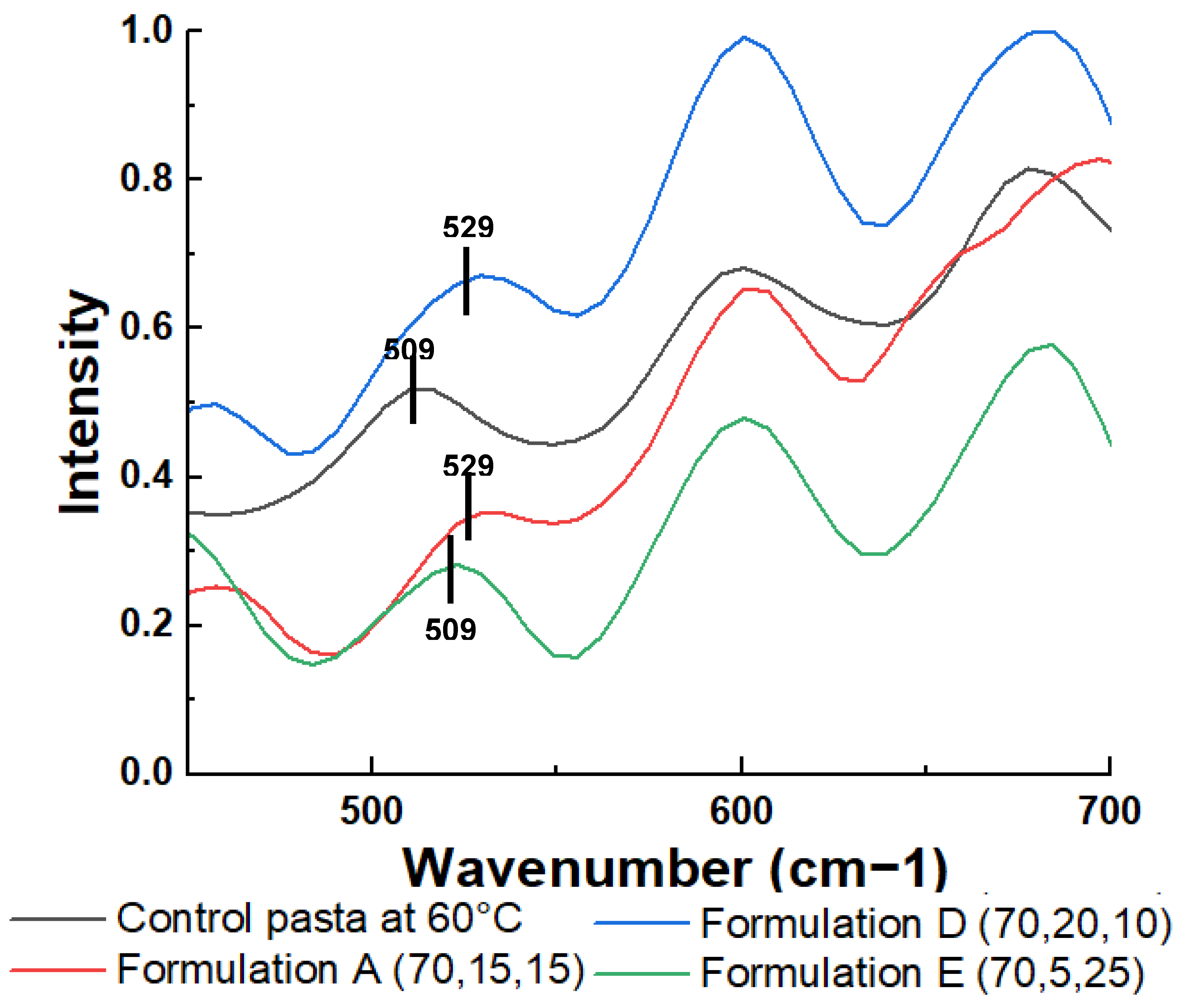





| Sample Name | Semolina (% w/w) | Pea Protein Isolate (% w/w) | Mesquite Flour (% w/w) | Gum Rate * | Drying Temperature (°C) |
|---|---|---|---|---|---|
| Control50 | 100 | 0 | 0 | 0 | 50 |
| Control60 | 100 | 0 | 0 | 0 | 60 |
| A | 70 | 15 | 15 | 0.5 | 60 |
| B | 70 | 10 | 20 | 0.5 | 50 |
| C | 80 | 10 | 10 | 0.5 | 50 |
| D | 70 | 20 | 10 | 1 | 60 |
| E | 70 | 5 | 25 | 1 | 60 |
| F | 85 | 7.5 | 7.5 | 1 | 50 |
| Main Ingredients | Drying Temp (°C) | Protein (% w/w) | Lipids (% w/w) | Carbohydrates (% w/w) | Ash (% w/w) | Humidity (% w/w) |
|---|---|---|---|---|---|---|
| Semolina | NA | 9.18 ± 0.50 | 1.34 ± 0.43 | 77.69 ± 2.23 | 0.73 ± 0.05 | 11.06 ± 0.60 |
| Mesquite flour | NA | 11.91 ± 0.00 * | 2.55 ± 0.06 | 74.37 ± 0.89 | 4.24 ± 0.00 * | 6.93 ± 0.57 * |
| Pea protein isolate (PPI) | NA | 73.59 ± 2.32 * | 0.55 ± 0.18 | 14.91 ± 4.25 * | 3.72 ± 0.05 * | 7.23 ± 0.46 * |
| Spaghetti samples | ||||||
| Control50 | 50 | 9.30 ± 0.75 | 0.27 ± 0.05 | 81.77 ± 1.63 | 0.61 ± 0.18 | 8.16 ± 0.32 |
| Control60 | 60 | 8.64 ± 0.37 | 0.45 ± 0.07 | 82.15 ± 0.39 | 0.78 ± 0.02 | 7.95 ± 0.03 |
| A | 60 | 17.25 ± 0.76 * | 0.42 ± 0.12 | 74.74 ± 0.60 * | 1.72 ± 0.00 * | 7.57 ± 0.04 * |
| B | 50 | 13.25 ± 0.37 * | 0.55 ± 0.03 * | 77.04 ± 0.90 * | 1.85 ± 0.10 * | 6.98 ± 0.11 * |
| C | 50 | 14.86 ± 1.10 * | 0.76 ± 0.03 * | 74.82 ± 1.90 * | 1.39 ± 0.01 * | 7.31 ± 0.19 |
| D | 60 | 20.97 ± 0.01 * | 0.66 ± 0.07 | 69.62 ± 0.28 * | 1.75 ± 0.02 * | 7.26 ± 0.15 * |
| E | 60 | 10.88 ± 0.75 | 0.57 ± 0.10 | 79.39 ± 1.45 | 1.84 ± 0.02 * | 8.15 ± 0.21 |
| F | 50 | 12.78 ± 0.41 * | 0.27 ± 0.08 | 78.43 ± 0.38 | 1.31 ± 0.01 * | 7.20 ± 0.04 |
| Spaghetti Samples | Drying Temperature (°C) | Phosphate Buffer + NaCl Solubility (%) | Phosphate Buffer + Urea Solubility (%) | Phosphate Buffer + Urea + DTT Solubility (%) |
|---|---|---|---|---|
| Control50 | 50 | 4.91 ± 0.18 | 9.26 ± 0.03 | 11.54 ± 0.53 |
| Control60 | 60 | 3.71 ± 0.10 | 5.71 ± 0.21 | 9.17 ± 0.01 |
| A | 60 | 7.93 ± 0.12 * | 5.94 ± 0.4 * | 7.38 ± 0.52 * |
| B | 50 | 8.66 ± 0.10 * | 8.46 ± 0.7 * | 9.30 ± 0.09 |
| C | 50 | 6.32 ± 0.20 * | 6.15 ± 0.47 | 6.90 ± 0.32 * |
| D | 60 | 4.48 ± 0.13 | 4.76 ± 0.1 * | 8.21 ± 0.04 * |
| E | 60 | 12.98 ± 0.1 * | 11.4 ± 0.5 * | 14.28 ± 0.27 * |
| F | 50 | 6.41 ± 0.06 * | 7.73 ± 0.0 * | 11.91 ± 0.04 * |
| Formulation | % Protein Cooked Pasta | Protein Digestibility | Protein Availability |
|---|---|---|---|
| Control pasta at 50 °C | 11.20 ± 0.65 | 80.32 ± 0.25 | 8.99 ± 0.02 |
| Formulation F (85, 7.5, 7.5, 1) 1 | 15.74 ± 0.40 * | 80.77 ± 0.12 | 12.71 ± 0.02 * |
| Control pasta at 60 °C | 11.04 ± 0.38 | 79.33 ± 0.12 | 8.75 ± 0.01 |
| Formulation A (70, 15, 15, 0.5) 1 | 17.76 ±0.79 * | 80.5 ± 0.00 * | 14.29 ± 0.00 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Lozano, A.; Gallegos-Infante, J.-A.; Chaírez-Ramírez, M.H.; Rocha-Guzmán, N.-E.; Moreno-Jiménez, M.R.; Ochoa-Martínez, L.-A.; Fierro, I.V.; Castañeda, V.L.; Medina-Torres, L. The Use of Common Bean and Mesquite Pods Flours as Partial Substitute of Semolina, Impact of Their Proteins and Polysaccharides in the Physical, Chemical, and Microstructural Characteristics of Spaghetti Pasta. Macromol 2025, 5, 8. https://doi.org/10.3390/macromol5010008
Pérez-Lozano A, Gallegos-Infante J-A, Chaírez-Ramírez MH, Rocha-Guzmán N-E, Moreno-Jiménez MR, Ochoa-Martínez L-A, Fierro IV, Castañeda VL, Medina-Torres L. The Use of Common Bean and Mesquite Pods Flours as Partial Substitute of Semolina, Impact of Their Proteins and Polysaccharides in the Physical, Chemical, and Microstructural Characteristics of Spaghetti Pasta. Macromol. 2025; 5(1):8. https://doi.org/10.3390/macromol5010008
Chicago/Turabian StylePérez-Lozano, Alejandro, José-Alberto Gallegos-Infante, Manuel Humberto Chaírez-Ramírez, Nuria-Elizabeth Rocha-Guzmán, Martha Rocío Moreno-Jiménez, Luz-Araceli Ochoa-Martínez, Ignacio Villanueva Fierro, Verónica Loera Castañeda, and Luis Medina-Torres. 2025. "The Use of Common Bean and Mesquite Pods Flours as Partial Substitute of Semolina, Impact of Their Proteins and Polysaccharides in the Physical, Chemical, and Microstructural Characteristics of Spaghetti Pasta" Macromol 5, no. 1: 8. https://doi.org/10.3390/macromol5010008
APA StylePérez-Lozano, A., Gallegos-Infante, J.-A., Chaírez-Ramírez, M. H., Rocha-Guzmán, N.-E., Moreno-Jiménez, M. R., Ochoa-Martínez, L.-A., Fierro, I. V., Castañeda, V. L., & Medina-Torres, L. (2025). The Use of Common Bean and Mesquite Pods Flours as Partial Substitute of Semolina, Impact of Their Proteins and Polysaccharides in the Physical, Chemical, and Microstructural Characteristics of Spaghetti Pasta. Macromol, 5(1), 8. https://doi.org/10.3390/macromol5010008






