Lignin as a Natural Antioxidant: Chemistry and Applications
Abstract
1. Introduction
2. Antioxidants
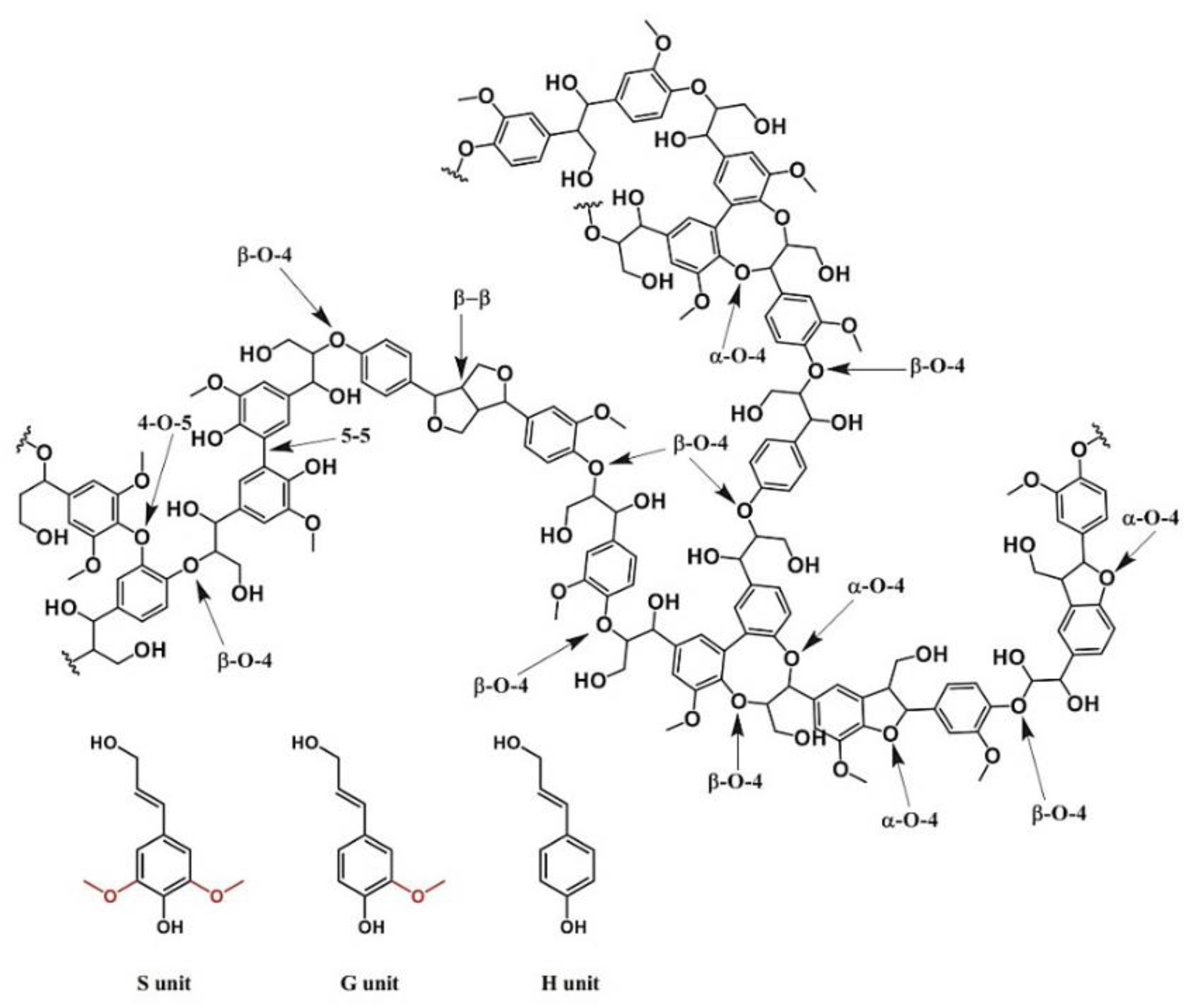
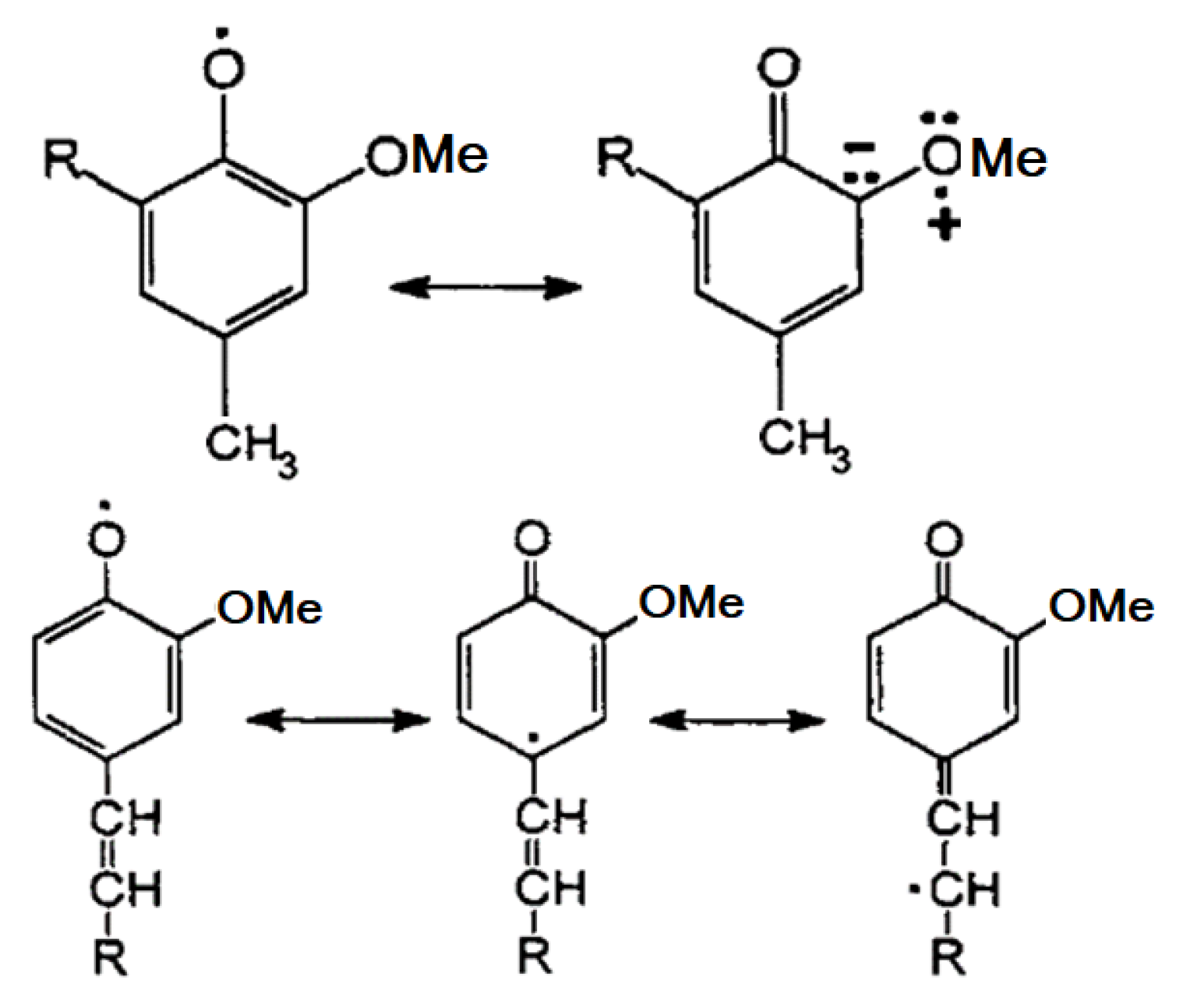
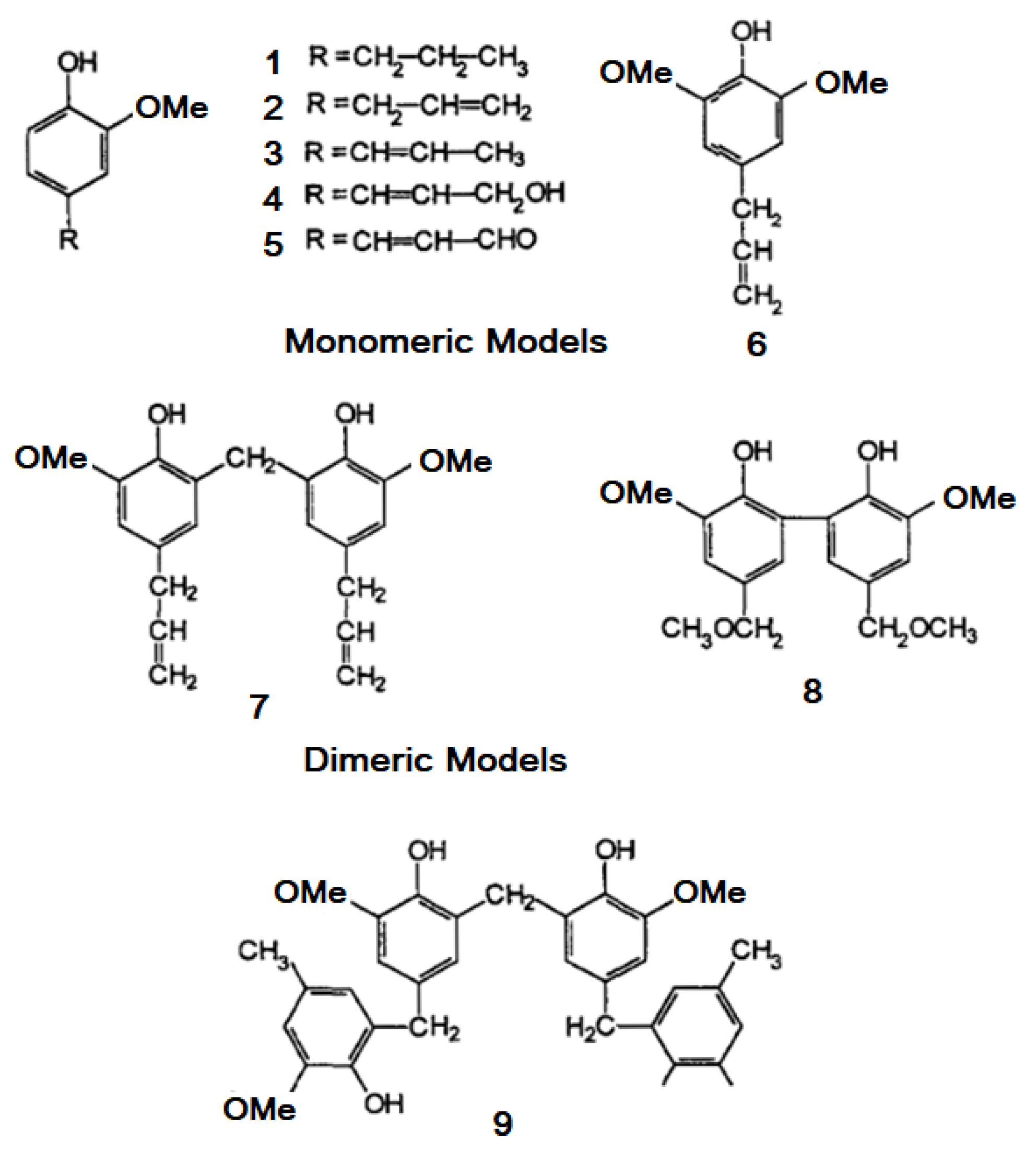
3. Chemistry of Lignin Antioxidant Properties
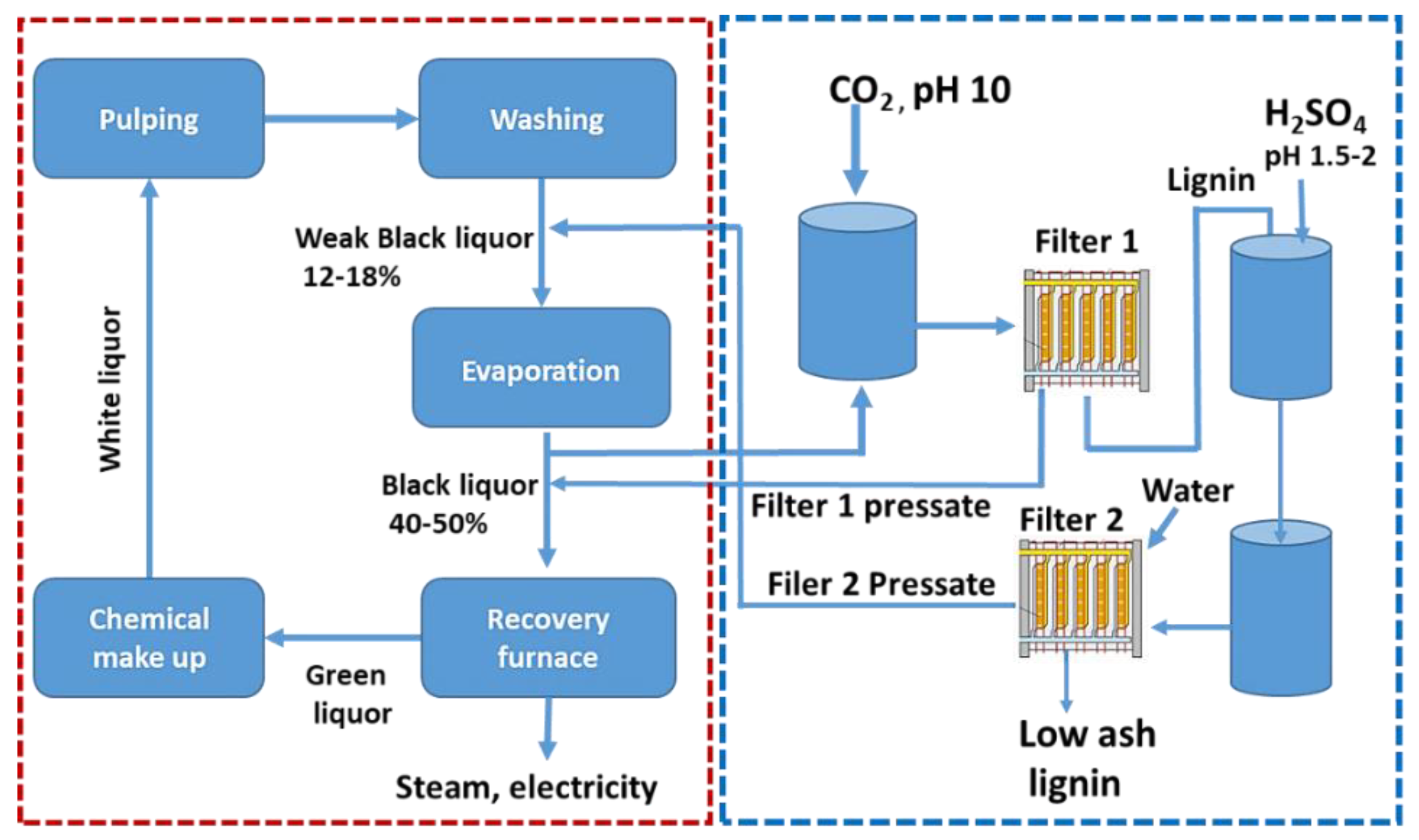
4. Antioxidant Activity of Technical Lignin
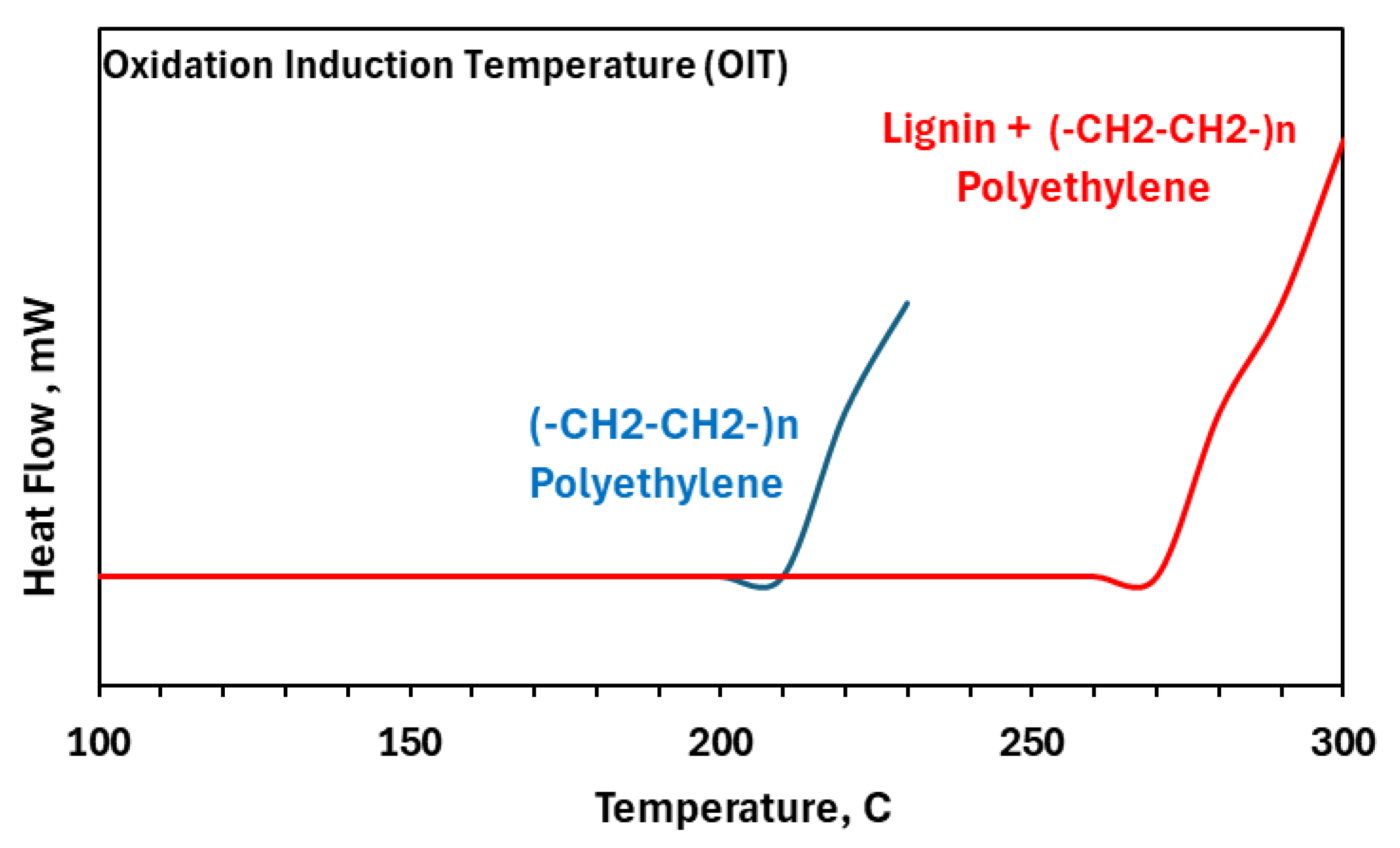
5. Lignin as an Antioxidant in the Lignin–Polymer Composite
6. Conclusions
Funding
Conflicts of Interest
References
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green. Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. A Comprehensive Approach for Quantitative Lignin Characterization by NMR Spectroscopy. J. Agric. Food Chem. 2004, 52, 1850–1860. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, G. Softwood kraft lignin: Raw material for the future. Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Dorrestijn, E.; Laarhoven, L.J.J.; Arends, I.W.C.E.; Mulder, P. Occurrence and reactivity of phenoxyl linkages in lignin and low rank coal. J. Anal. Appl. Pyrolysis 2000, 54, 153–192. [Google Scholar] [CrossRef]
- Crestini, C.; Melone, F.; Sette, M.; Saladino, R. Milled wood lignin: A linear oligomer. Biomacromolecules 2011, 12, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green. Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Kouisni, L.; Holt-Hindle, P.; Maki, K.; Paleologou, M. The LignoForce System: A new process for the production of high-quality lignin from black liquor. Pulp Pap. Canada 2012, 115, 18–22. [Google Scholar]
- Lake, M.A.; Blackburn, J.C. SLRPTM—An innovative Lignin-Recovery technology. Cellul. Chem. Technol. 2014, 48, 799–804. [Google Scholar]
- Gellerstedt, G.; Tomani, P.; Axegård, P.; Birgit, B. Integrated Forest Biorefineries—Challenges and Opportunities; Christopher, L., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 180–210. [Google Scholar]
- Kirkwood, T.B.L.; Austad, S.N. Why do we age? Nature 2000, 408, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Zhang, K.; Jiang, L.; Wong, H.Z.; Li, Z.; Zhang, Z.; Loh, X.J. Sustainable and Antioxidant Lignin-Polyester Copolymers and Nanofibers for Potential Healthcare Applications. ACS Sustain. Chem. Eng. 2017, 5, 6016–6025. [Google Scholar] [CrossRef]
- Arrigoni, O.; Mario, C.; Tullio, D. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta-Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- JMbah, C.; Orabueze, I.; HOkorie, N. Antioxidants Properties of Natural and Synthetic Chemical Compounds: Therapeutic Effects on Biological System. Acta Sci. Pharm. Sci. 2019, 3, 28–42. [Google Scholar]
- Liu, Z.Q. Chemical methods to evaluate antioxidant ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Shivakumar, A.; Yogendra Kumar, M.S. Critical Review on the Analytical Mechanistic Steps in the Evaluation of Antioxidant Activity. Crit. Rev. Anal. Chem. 2018, 48, 214–236. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Dizhbite, T.; Ponomarenko, J.; Andersone, A.; Dobele, G.; Lauberts, M.; Krasilnikova, J.; Mironova-Ulmane, N.; Telysheva, G. Role of paramagnetic polyconjugated clusters in lignin antioxidant activity (in vitro). IOP Conf. Ser. Mater. Sci. Eng. 2012, 38, 012033. [Google Scholar] [CrossRef]
- Barclay, L.; Xi, F.; Norris, J. Antioxidant properties of phenolic lignin model compounds. J. Wood Chem. Technol. 1997, 17, 73–90. [Google Scholar] [CrossRef]
- Qazi, S.S.; Li, D.; Briens, C.; Berruti, F.; Abou-Zaid, M.M. Antioxidant activity of the lignins derived from fluidized-bed fast pyrolysis. Molecules 2017, 22, 372. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Bikovens, O.; Telysheva, G. Characterization of softwood and hardwood lignoboost kraft lignins with emphasis on their antioxidant activity. BioResources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Lauberts, M.; Dizhbite, T.; Lauberte, L.; Jurkjane, V.; Telysheva, G. Antioxidant activity of various lignins and lignin-related phenylpropanoid units with high and low molecular weight. Holzforschung 2015, 69, 795–805. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Cromwell, G.R.; Hilborn, J.W. Photochemistry of a model lignin compound. Spin trapping of primary products and properties of an oligomer. Can. J. Chem. 1994, 72, 35–41. [Google Scholar] [CrossRef]
- Nsimba, R.Y.; West, N.; Boateng, A.A. Structure and Radical Scavenging Activity Relationships of Pyrolytic Lignins. J. Agric. Food Chem. 2012, 60, 12525–12530. [Google Scholar]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Cui, C.; Argyropoulos, D.S. Toward thermoplastic lignin polymers. Part 1. Selective masking of phenolic hydroxyl groups in kraft lignins via methylation and oxypropylation chemistries. Ind. Eng. Chem. Res. 2012, 51, 16713–16720. [Google Scholar] [CrossRef]
- Nenkova, S.; Radoykova, T.; Stanulov, K. Preparation and antioxidant properties of biomass low molecular phenolic compounds. J. Univ. Chem. Technol. Metall. 2011, 46, 109–120. [Google Scholar]
- Jiang, B.; Zhang, Y.; Zhao, H.; Guo, T.; Wu, W.; Jin, Y. Dataset on structure-antioxidant activity relationship of active oxygen catalytic lignin and lignin-carbohydrate complex. Data Br. 2019, 25, 104413. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Volperts, A.; Dobele, G.; Telysheva, G. Analytical pyrolysis—A tool for revealing of lignin structure-antioxidant activity relationship. J. Anal. Appl. Pyrolysis 2015, 113, 360–369. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Kocheva, L.S.; Borisenkov, M.F.; Karmanov, A.P.; Mishurov, V.P.; Spirikhin, L.V.; Monakov, Y.B. Structure and antioxidant characteristics of wheat and oat lignins. Russ. J. Appl. Chem. 2005, 78, 1343–1350. [Google Scholar] [CrossRef]
- Karmanov, A.P.; Borisenkov, M.F.; Kocheva, L.S. Chemical structure and antioxidant properties of lignins from conifer, broadleaf, and herbaceous plants. Chem. Nat. Compd. 2014, 50, 702–705. [Google Scholar] [CrossRef]
- Garcia, A.; Amendola, D.; Gonzalez, M.; Spigno, G.; Labidi, J. Lignin as a Natural Radical Scavenger. Study of the Antioxidant Capacity of Apple Tree Pruning Lignin Obtained by Different Methods. Chem. Eng. Trans. 2011, 24, 925–930. [Google Scholar]
- Kuzina, S.I.; Brezgunov, A.Y.; Dubinskii, A.A.; Mikhailov, A.I. Free radicals in the photolysis and radiolysis of polymers: IV. Radicals in γ- and UV-irradiated wood and lignin. High. Energy Chem. 2004, 38, 298–305. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Argyropoulos, D.S. Correlations of the antioxidant properties of softwood kraft lignin fractions with the thermal stability of its blends with polyethylene. ACS Sustain. Chem. Eng. 2015, 3, 349–356. [Google Scholar] [CrossRef]
- Thring, R.W.; Griffin, S.L. The heterogeneity of two Canadian kraft lignins. Can. J. Chem. 1995, 53, 629–634. [Google Scholar] [CrossRef]
- Jovanovic, S.; Steenken, S.; Simic, M.; Hara, Y. Antioxidant properties of flavonoids: Reduction potentials and electron transfer reactions of flavonoid radicals. In Flavonoids in Health and Disease; Rice-Evans, C.A., Packer, L., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 137–161. [Google Scholar]
- Nogueira, I.d.M.; Avelino, F.; de Oliveira, D.R.; Souza, N.F.; Rosa, M.F.; Mazzetto, S.E.; Lomonaco, D. Organic solvent fractionation of acetosolv palm oil lignin: The role of its structure on the antioxidant activity. Int. J. Biol. Macromol. 2019, 122, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, S.; Lapierre, C.; Monties, B. Utilization of Pine Kraft Lignin in Starch Composites: Impact of Structural Heterogeneity. J. Agric. Food Chem. 1998, 46, 2234–2240. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Ponomarenko, J.; Dizhbite, T.; Andersone, A.; Gosselink, R.J.; van der Putten, J.; Lauberts, M.; Telysheva, G. Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrolysis 2013, 103, 78–85. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K. Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid. Polym. Sci. 2015, 293, 2585–2592. [Google Scholar] [CrossRef]
- Hussin, M.H.; Rahim, A.A.; Mohamad Ibrahim, M.N.; Yemloul, M.; Perrin, D.; Brosse, N. Investigation on the structure and antioxidant properties of modified lignin obtained by different combinative processes of oil palm fronds (OPF) biomass. Ind. Crops Prod. 2014, 52, 544–551. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Sadeghifar, H.; Ragauskas, A.; Ragauskas, A.; Ragauskas, A.; Ragauskas, A. Perspective on Technical Lignin Fractionation. ACS Sustain. Chem. Eng. 2020, 8, 8086–8101. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, M.; Zu, Y.; Liu, W.; Yang, L.; Zhang, Y.; Zhao, X.; Zhang, X.; Zhang, X.; Li, W. Comparative antioxidant activity of nanoscale lignin prepared by a supercritical antisolvent (SAS) process with non-nanoscale lignin. Food Chem. 2012, 135, 63–67. [Google Scholar] [CrossRef]
- Trevisan, H.; Rezende, C.A. Pure, stable and highly antioxidant lignin nanoparticles from elephant grass. Ind. Crops Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green. Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro- To nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yuan, Q.; Cheng, G. Controlled Preparation of Corncob Lignin Nanoparticles and their Size-Dependent Antioxidant Properties: Toward High Value Utilization of Lignin. ACS Sustain. Chem. Eng. 2019, 7, 17166–17174. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Qin, L.; Ge, Y. Enhancing Antioxidant Performance of Lignin by Enzymatic Treatment with Laccase. ACS Sustain. Chem. Eng. 2018, 6, 2591–2595. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, G.; Chen, H.; Lang, Q. The effect of degradation of soda lignin using Pd/SO42−/ZrO2 as a catalyst: Improved reactivity and antioxidant activity. Polymers 2019, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Johnston, P.A.; Lee, J.H.; Smith, R.G.; Brown, R.C. Improving Lignin Homogeneity and Functionality via Ethanolysis for Production of Antioxidants. ACS Sustain. Chem. Eng. 2019, 7, 3520–3526. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, X.; Ma, G.; Qian, Y.; Qiu, X.; Ruan, T. Improving antioxidant activity of lignin by hydrogenolysis. Ind. Crops Prod. 2018, 125, 228–235. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Zhao, H.; Guo, T.; Wu, W.; Jin, Y. Structure-antioxidant activity relationship of active oxygen catalytic lignin and lignin-carbohydrate complex. Int. J. Biol. Macromol. 2019, 139, 21–29. [Google Scholar] [CrossRef]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L.; Grillo, G.; et al. Lignin e Derived antioxidants as value-added products obtained under cavitation treatments of the wheat straw processing for sugar production. J. Clean. Prod. 2021, 303, 126369. [Google Scholar] [CrossRef]
- Toriz, G.; Ramos, J.; Young, R.A. Lignin-polypropylene composites. II. Plasma modification of kraft lignin and particulate polypropylene. J. Appl. Polym. Sci. 2004, 91, 1920–1926. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Li, H.Q.; Zaitseva, A.; Pokki, J.-P.; Marina St, H.; Alopaeus, V.; Sixta, H. Effect of Softwood Kraft Lignin Fractionation on the Dispersion of Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2013, 52, 6311–6317. [Google Scholar]
- Sadeghifar, H.; Argyropoulos, D.S. Macroscopic Behavior of Kraft Lignin Fractions: Melt Stability Considerations for Lignin-Polyethylene Blends. ACS Sustain. Chem. Eng. 2016, 4, 5160–5166. [Google Scholar] [CrossRef]
- Guilhen, A.; Gadioli, R.; Fernandes, F.C.; Waldman, W.R.; Aurelio De Paoli, M. High-density green polyethylene biocomposite reinforced with cellulose fibers and using lignin as antioxidant. J. Appl. Polym. Sci. 2017, 134, 45219. [Google Scholar] [CrossRef]
- Chen, K.; Ye, D.; Gu, S.; Zhou, Y. Thermal-oxidative effect of Kraft lignin antioxidant in polypropylene: Uncovering the key factor using correlation analysis model. Int. J. Biol. Macromol. 2018, 107 Pt A, 478–485. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Alzagameem, A.; Rehahn, M.; Schulze, M. Antioxidant activity of unmodified kraft and organosolv lignins to be used as sustainable components for polyurethane coatings. J. Coat. Technol. Res. 2019, 16, 1543–1552. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Yan, Z.; Lu, T.; Liu, R.; Han, X.; Cai, C.; Zhao, S.; Wang, H. Preparation of lignin-based filling antioxidant and its application in styrene-butadiene rubber. J. Appl. Polym. Sci. 2021, 138, 51281. [Google Scholar] [CrossRef]
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering Poly(lactide)-Lignin Nanofibers with Antioxidant Activity for Biomedical Application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Jiang, S.; Kai, D.; Dou, Q.Q.; Loh, X.J. Multi-arm carriers composed of an antioxidant lignin core and poly(glycidyl methacrylate-co-poly(ethylene glycol)methacrylate) derivative arms for highly efficient gene delivery. J. Mater. Chem. B 2015, 3, 6897–6904. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant pla composites containing lignin for 3D printing applications: A potential material for healthcare applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Crouvisier-Urion, K.; Bodart, P.R.; Winckler, P.; Raya, J.; Gougeon, R.D.; Cayot, P.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Biobased Composite Films from Chitosan and Lignin: Antioxidant Activity Related to Structure and Moisture. ACS Sustain. Chem. Eng. 2016, 4, 6371–6381. [Google Scholar] [CrossRef]
- Tan, S.; Liu, D.; Qian, Y.; Wang, J.; Huang, J.; Yi, C.; Qiu, X.; Qin, Y. Towards better UV-blocking and antioxidant performance of varnish via additives based on lignin and its colloids. Holzforschung 2019, 73, 485–491. [Google Scholar] [CrossRef]
- Larson, R.A.; Sharma, B.K.; Marley, K.A.; Kunwar, B.; Murali, D.; Scott, J. Potential antioxidants for biodiesel from a softwood lignin pyrolyzate. Ind. Crops Prod. 2017, 109, 476–482. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Li, B.; Fan, J.; Chang, J. Effects of lignins on antioxidant biodiesel production in supercritical methanol. Energy Fuels 2011, 25, 2746–2748. [Google Scholar] [CrossRef]
- Li, Y.; Mlynar, J.; Sarkanan, S. The first 85% Kraft lignin-based thermoplastics. J. Polym. Sci. 1997, 35, 1899–1910. [Google Scholar] [CrossRef]
- de Oliveira, W.; Glasser, W.G. Multiphase materials with lignins. 11 starlike copolymers with caprolactone. Macromolecules 1994, 27, 5–11. [Google Scholar] [CrossRef]
- Cathala, B.; Saake, B.; Faix, O.; Monties, B. Association behaviour of lignins and lignin model compounds studied by multidetector size-exclusion chromatography. J. Chromatogr. A 2003, 1020, 229–239. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Gregorová, A.; Košíková, B.; Moravčík, R. Stabilization effect of lignin in natural rubber. Polym. Degrad. Stab. 2006, 91, 229–233. [Google Scholar] [CrossRef]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Zoia, L.; Orlandi, M.; Zanini, F.; Elegir, G. Structural characterization and antioxidant activity evaluation of lignins from rice husk. J. Agric. Food Chem. 2010, 58, 10049–10055. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ge, Y. Antioxidant activities of lignin extracted from sugarcane bagasse via different chemical procedures. Int. J. Biol. Macromol. 2012, 51, 1116–1120. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, W.; Yang, L.; Zu, Y.; Zu, B.; Zhu, M.; Zhang, Y.; Zhang, X.; Zhang, R.; Sun, Z.; et al. Investigation of the effects of different organosolv pulping methods on antioxidant capacity and extraction efficiency of lignin. Food Chem. 2012, 131, 313–317. [Google Scholar] [CrossRef]
- Ye, D.; Li, S.; Lu, X.; Zhang, X.; Rojas, O.J. Antioxidant and Thermal Stabilization of Polypropylene by Addition of Butylated Lignin at Low Loadings. ACS Sustain. Chem. Eng. 2016, 4, 5248–5257. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, antioxidant and swelling behaviour of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.; Bruni, G.; Kozanecki, M.; Kenny, J.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
| Categories | Properties | Delignification Process | |||||
|---|---|---|---|---|---|---|---|
| Induline Kraft | Soda p-1000 | Alcell | OS-W | OS-P | OS-S | ||
| Functional groups, mmol/1 g lignin, calculated by 31P NMR | Aliphatic-OH | 1.79 | 1.311.26 | 1.04 | 1.27 | 0.87 | 1.43 |
| 5-Substituted OH | 1.31 | 1.73 | 1.68 | 1.24 | 1.83 | 1.21 | |
| Guaiacyl OH | 1.3 | 0.73 | 0.58 | 0.92 | 0.58 | 1.44 | |
| P-Hydroxyphenyle OH | 0.16 | 0.4 | 0.11 | 0.38 | 0.18 | 0.08 | |
| Total Ph-OH | 1.77 | 2.86 | 3.3 | 2.54 | 2.59 | 2.73 | |
| COOH | 0.33 | 0.8 | 0.22 | 0.21 | 0.07 | 0.06 | |
| Molecular weight | Mw | 4290 | 3270 | 2580 | 1960 | 2180 | 2030 |
| Mn | 530 | 620 | 600 | 450 | 570 | 420 | |
| Polydispersity (PD) | 8.1 | 5.2 | 4.3 | 4.4 | 3.8 | 4.9 | |
| a: Precursors, S, G, H (Molar percentage (S + G + H = 100) b: Linkage (Number per 100 aromatic units (S + G) | B-O-4 a | 6.1 | 3.4 | 5.3 | 4.3 | 0.1 | 0.00 |
| B-5 a | 0.3 | 0.00 | 0.8 | 4.5 | 1.8 | 3.3 | |
| B-B a | 1.00 | 0.7 | 2.8 | 0.1 | 1.1 | 0.2 | |
| Sinapyl alcohol (S) % b | 0.00 | 50 | 63 | 39 | 53 | 0.00 | |
| Coniferyl Alcohol (H) % b | 97 | 39 | 37 | 58 | 47 | 100 | |
| P-Comaryl alcohol (P) % b | 3 | 11 | 0.00 | 3 | 0.00 | 0.00 | |
| S/G ratio | 0.00 | 1.3 | 1.7 | 0.7 | 1.2 | 0.00 | |
| H/G ratio | 0.00 | 0.3 | 0.00 | 0.1 | 0.00 | 0.00 | |
| Symbol | Name |
|---|---|
| RS● | Thiyl radical |
| O2● | Superoxide anion radical |
| OH● | Hydroxyl radical |
| RO● | Alkoxyl radical |
| ROO● | Peroxyl radical |
| P● | Hydrogen peroxide |
| LO● | Lipid hydroperoxide |
| LOO● | Lipid peroxyl radical |
| NO● | Nitric oxide radical |
| NO2 | Nitrogen dioxide radical |
| Lignin Model | Kinh a, M−1 s−1 × 10−4 | K, Relative | n |
|---|---|---|---|
| (1) R = -CH2-CH-CH3 | 3.07 | 1.69 | 1.68 |
| (2) R = -CH2-CH=CH2 | 3.33 | 1.83 | 1.62 |
| (3) R = -CH=CH-CH3 | 7.19 | 3.95 | 1.64 |
| (4) R = -CH=CHCH2OH | 7.74 | 4.25 | 1.69 |
| (5) R-CH=CH-CHO | 3.58 | 2 | 1.68 |
| (6) | 7.45 | 4.09 | 1.73 |
| (7) | 9.76 | 5.36 | 3.19 |
| (8) | 8.45 | 4.48 | 3.25 |
| (9) | 14.1 | 7.75 | 6.38 |
| BHT | 1.82 | 1 | 1.84 |
| Lignin Model | Antiradical Power, ARP a | Amount of Reduced b DPPH• | |
|---|---|---|---|
| 1. R1 = CH2-CH-CH3, R2 = H | 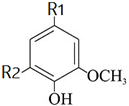 | 3.5 | 1.75 |
| 2. R1 = -CH2-CH=CH2, R2 = H | 4 | 2 | |
| 3. R1 = -CH=CH-CH3, R2 = H | 2.2 | 1 | |
| 4. R1 = -CH=CH-CH2OH, R2 = H | 4.2 | 2.1 | |
| 5. R1 = -CH=CH-CHO, R2 = H | 1.9 | 0.97 | |
| 6. R1, R2 = H | 2.6 | 1.3 | |
| 7. R1 = -CHO-CH2-CH3, R2 = H | 0.2 | 0.1 | |
| 8. R1 = -CO-CH2-CH3, R2 = -OCH3 | 0.5 | 0.2 | |
| 9. R1 = -CHOH-CH2-CH3, R2 = H | 3 | 1.5 | |
| Structural Parameter | Pearson’s Correlation Coefficient (n = 50, α = 0.05) | |
|---|---|---|
| DPPH• | ABTS•+ | |
| G + S phenol, % | 0.52 | −0.20 |
| CH2 in the α-position of the side chain, % | 0.53 | 0.33 |
| Oxygen containing group (α-C=O) in the side chains, % | −0.42 | −0.25 |
| Number of atoms in the π-conjugated system | −0.40 | −0.50 |
| Carbohydrates, % | −0.21 | 0.61 |
| OCH3 per C9 | 0.14 | 0.01 |
| Mw (g/mol) | 0.04 | −0.08 |
| Lignin | Wood | OH Phen. % | OH Aliph. % | OCH3, % | Mw | APR a | K2 b, M−1 s−1 |
|---|---|---|---|---|---|---|---|
| Acid-soluble | Aspen | 5.1 | 3.3 | 21.5 | 1980 | 1.5 | 8600 |
| Alkaline | Spruce | 5.0 | 3.5 | 15.1 | 2200 | 0.5 | 2000 |
| Birch | 4.0 | 6.9 | 17.4 | 2990 | 1.0 | 5600 | |
| Aspen | 3.6 | 5.2 | 18.1 | 3100 | 1.1 | 6100 | |
| Ethanol | Aspen | 2.0 | 4.5 | 25.0 | 1870 | 0.6 | 2600 |
| MWL | Spruce | 3.1 | 8.4 | 15.3 | 7500 | 0.2 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghifar, H.; Ragauskas, A.J. Lignin as a Natural Antioxidant: Chemistry and Applications. Macromol 2025, 5, 5. https://doi.org/10.3390/macromol5010005
Sadeghifar H, Ragauskas AJ. Lignin as a Natural Antioxidant: Chemistry and Applications. Macromol. 2025; 5(1):5. https://doi.org/10.3390/macromol5010005
Chicago/Turabian StyleSadeghifar, Hasan, and Arthur J. Ragauskas. 2025. "Lignin as a Natural Antioxidant: Chemistry and Applications" Macromol 5, no. 1: 5. https://doi.org/10.3390/macromol5010005
APA StyleSadeghifar, H., & Ragauskas, A. J. (2025). Lignin as a Natural Antioxidant: Chemistry and Applications. Macromol, 5(1), 5. https://doi.org/10.3390/macromol5010005








