Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products
Abstract
1. Introduction
2. Methodology
3. Non-Conventional Starch Sources
4. Starch Extraction from Non-Conventional Sources
5. Morphological and Chemical Properties
6. Nutritional and Functional Characteristics of Non-Conventional Starches
| Type | Characteristic | Description | Sources | Figure | References |
|---|---|---|---|---|---|
| RS I—Resistant Starch Type 1 | Physically inaccessible starch | Barrier effect of cell walls or protein isolation. | Coarsely ground or whole grains (Durum wheat), legumes, and seeds | 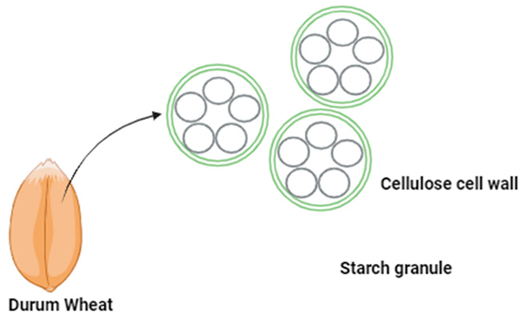 | [86,87,88] |
| RS II—Resistant Starch Type 2 | Granular starch with B or C polymorph | Naturally occurring resistant starch granules. | High-amylose corn starch, raw potato, green bananas, some legumes like brown lentils (Lens culinaris Medikus), high-amylose starches |  | [75,89] |
| RS III—Resistant Starch Type 3 | Retrograded starch | Resulting from crystallization formed during cooling and storage after starch granule gelatinization. | Cooked and cooled starch-rich foods |  | [73,89,90] |
| RS IV—Resistant Starch Type 4 | Chemically modified starches | Introduction of chemical functional groups to starch | Cross-linked starch and octenyl succinate starch, carboxymethylated starch |  | [40,86] |
| RS V—Resistant Starch Type 5 | Amylose-lipid complex | Resulting from the interaction between starch and lipids, where amylose and the long-branched chains of amylopectin form single-helix complexes with free fatty acids. | High-amylose starch complexed with stearic acid |  | [87,89,90] |
7. Thermal Properties of Non-Conventional Starches
8. Modification of Non-Conventional Starches
9. Recent Applications in Food and Non-Food Products
10. Final Remarks and Perspectives
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Golberg, A. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Shetty, P.R.; Batchu, U.R.; Buddana, S.K.; Rao, K.S.; Penna, S. A comprehensive review on α-D-Glucans: Structural and functional diversity, derivatization and bioapplications. Carbohydr. Res. 2021, 503, 108297. [Google Scholar] [CrossRef]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef] [PubMed]
- Makroo, H.A.; Naqash, S.; Saxena, J.; Sharma, S.; Majid, D.; Dar, B.N. Recovery and characteristics of starches from unconventional sources and their potential applications: A review. Appl. Food Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology, 3rd ed.; Academic Press: New York, NY, USA, 2009. [Google Scholar]
- Obadi, M.; Qi, Y.; Xu, B. High-amylose maize starch: Structure, properties, modifications and industrial applications. Carbohydr. Polym. 2023, 299, 120185. [Google Scholar] [CrossRef] [PubMed]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch modification for non-food, industrial applications: Market intelligence and critical review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef]
- Kishore, A.; Patil, R.J.; Singh, A.; Pati, K. Jicama (Pachyrhizus spp.) a nonconventional starch: A review on isolation, composition, structure, properties, modifications and its application. Int. J. Biol. Macromol. 2023, 258, 129095. [Google Scholar] [CrossRef]
- Henning, F.G.; Ito, V.C.; Demiate, I.M.; Lacerda, L.G. Non-conventional starches for biodegradable films: A review focussing on characterisation and recent applications in food packaging. Carbohydr. Polym. Technol. Appl. 2022, 4, 100157. [Google Scholar] [CrossRef]
- Santana, Á.L.; Meireles, M.A.A. New starches are the trend for industry applications: A review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- Salazar-Irrazabal, M.D.; Ramirez-Tixe, E.E.; Velasquez-Barreto, F.F.; Bello-Pérez, L.A. Avocado seed starch: Effect of the variety on molecular, physicochemical, and digestibility characteristics. Int. J. Biol. Macromol. 2023, 247, 125746. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-conventional starch sources. Curr. Opin. Food Sci. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Tosif, M.M.; Bains, A.; Sadh, P.K.; Sarangi, P.K.; Kaushik, R.; Burla, S.V.S.; Chawla, P.; Sridhar, K. Loquat seed starch—Emerging source of non-conventional starch: Structure, properties, and novel applications. Int. J. Biol. Macromol. 2023, 244, 125230. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Fakhouri, F.M.; De Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Chen, C.; Li, G.; Hemar, Y.; Corke, H.; Zhu, F. Physicochemical properties and molecular structure of lotus seed starch. Carbohydr. Polym. 2023, 305, 120515. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Bangar, S.P. Chapter 11—Quinoa starch: Extraction, physicochemical properties, functionality and potential applications. In Non-Conventional Starch Sources: Properties, Functionality, and Applications; Academic Press: Cambridge, MA, USA, 2023; pp. 3–344. [Google Scholar] [CrossRef]
- Miranda, B.M.; Almeida, V.O.; Terstegen, T.; Hundschell, C.; Flöter, E.; Silva, F.A.; Fernandes, K.F.; Wagemans, A.; Ulbrich, M. The microstructure of the starch from the underutilized seed of jaboticaba (Plinia cauliflora). Food Chem. 2023, 423, 136145. [Google Scholar] [CrossRef]
- Valencia, G.A.; Moraes, I.C.F.; Lourenco, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.D.A. Physicochemical, morphological, and functional properties of flour and starch from peach palm (Bactris gasipaes K.) fruit. Starch-Stärke 2014, 67, 163–173. [Google Scholar] [CrossRef]
- Páramo-Calderón, D.E.; Vázquez-León, L.A.; Palma-Rodríguez, H.M.; Utrilla-Coello, R.G.; Vargas-Torres, A.; Meza-Nieto, M.A.; Romero-Cortes, T.; Aparicio-Saguilán, A. Effect of high-energy mechanical milling on the physicochemical and rheological properties of chayotextle (Sechium edule Sw.) starch. Food Chem. 2023, 427, 136720. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch extraction potential from plantain peel wastes. J. Environ. Chem. Eng. 2017, 5, 4980–4985. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Beraldo, A.L.; Costa, M.S.; Boas, F.V.; Franco, C.M.L.; Clerici, M.T.P.S. Bambusa vulgaris starch: Characterization and technological properties. Food Res. Int. 2020, 132, 109102. [Google Scholar] [CrossRef]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Cereda, M.P.; Vilpoux, O.F. Chapter 2—Cultivation of arrowroot (Maranta arundinacea) in Brazil—Planting material from rooted stems, preserving rhizomes for starch production. In Varieties and Landraces: Cultural Practices and Traditional Uses; Cereda, M.P., Vilpoux, O.F., Eds.; Underground Starchy Crops of South American Origin; Academic Press: Cambridge, MA, USA, 2023; Volume 2, pp. 19–34. [Google Scholar] [CrossRef]
- Lovera, M.; Pérez, E.; Laurentin, A. Digestibility of starches isolated from stem and root tubers of arracacha, cassava, cush–cush yam, potato and taro. Carbohydr. Polym. 2017, 176, 50–55. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Cui, S.W.; Jin, Z.; Zhang, T. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res. Int. 2012, 49, 303–310. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Singh, S.; Saxena, D.C. Effect of heat-moisture and acid treatment on physicochemical, pasting, thermal and morphological properties of Horse Chestnut (Aesculus indica) starch. Food Hydrocoll. 2016, 57, 103–113. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Borges, L.A.; Ferreira, N.L.B.; Clerici, M.T.P.S. Seaweed as a Potential New Source for Starch, Produced in the Sea: A Short Review. Starch-Stärke 2022, 75, 2200130. [Google Scholar] [CrossRef]
- Da Silva, L.R.; De Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rices. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.P.; Carpiné, D.; Alves, F.E.S.B.; Barbi, R.C.T.; De Melo, A.M.; Ikeda, M.; Ribaniet, R.H. Thermal, structural, morphological and bioactive characterization of acid and neutral modified loquat (Eriobotrya japonica lindl.) seed starch and its by-products. J. Therm. Anal. Calorim. 2021, 147, 6721–6737. [Google Scholar] [CrossRef]
- Silveira, T.M.G.; Tapia-Blácido, D.R. Is isolating starch from the residue of annatto pigment extraction feasible? Food Hydrocoll. 2018, 77, 117–125. [Google Scholar] [CrossRef]
- De Castro, D.S.; Moreira, I.D.S.; Silva, L.M.D.M.; Lima, J.P.; Da Silva, W.P.; Gomes, J.P.; De Figueirêdo, R.M.F. Isolation and characterization of starch from pitomba endocarp. Food Res. Int. 2018, 124, 181–187. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; Teixeira, G.L.; Hornung, P.S.; Ávila, S.; Hoffmann-Ribani, R. Eriobotrya japonica seed as a new source of starch: Assessment of phenolic compounds, antioxidant activity, thermal, rheological and morphological properties. Food Hydrocoll. 2018, 77, 646–658. [Google Scholar] [CrossRef]
- Kringel, D.H.; El Halal, S.L.M.; Zavareze, E.D.R.; Dias, A.R.G. Methods for the Extraction of Roots, Tubers, Pulses, Pseudocereals, and other Unconventional Starches Sources: A Review. Starch-Stärke 2020, 72, 1900234. [Google Scholar] [CrossRef]
- Pires, M.B.; Amante, E.R.; De Oliveira Petkowicz, C.L.; Esmerino, E.A.; Da Cruz Rodrigues, A.M.; Da Silva, L.H.M.M.B. Impact of extraction methods and genotypes on the properties of starch from peach palm (Bactris gasipaes Kunth) fruits. LWT Food Sci. Technol. 2021, 150, 111983. [Google Scholar] [CrossRef]
- De Melo, A.M.; Barbi, R.C.T.; Costa, B.P.; Ikeda, M.; Alves, F.E.S.B.; Carpiné, D.; Ribani, R.H. Thermal, antioxidant, morphological and bioactive properties of starchy material extracted from the bacupari (Garcinia brasiliensis (Mart.)) seed using aqueous and alkaline maceration. J. Therm. Anal. Calorim. 2022, 147, 12313–12328. [Google Scholar] [CrossRef]
- Tulu, E.D.; Duraisamy, R.; Kebede, B.H.; Tura, A.M. Anchote (Coccinia abyssinica) starch extraction, characterization and bioethanol generation from its pulp/waste. Heliyon 2023, 9, e14320. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.H.F.; Pontes, K.V.; Fialho, R.L.; Fakhouri, F.M. Extraction and characterization of the starch present in the avocado seed (Persea americana Mill) for future applications. J. Agric. Food Res. 2022, 8, 100303. [Google Scholar] [CrossRef]
- Chi, C.; He, Y.; Xiao, X.; Chen, B.; Zhou, Y.; Tan, X.; Ji, Z.; Zhang, Y.; Liu, P. A novel very small granular starch from Chlorella sp. MBFJNU-17. Int. J. Biol. Macromol. 2023, 225, 557–564. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Beraldo, A.L.; Costa, M.S.; Boas, F.V.; Franco, C.M.L.; Clerici, M.T.P.S. Physicochemical and structural properties of starch from young bamboo culm of Bambusa tuldoides. Food Hydrocoll. 2019, 87, 101–107. [Google Scholar] [CrossRef]
- Wang, N.; Shi, N.; Fei, H.; Liu, Y.; Zhang, Y.; Li, Z.; Ruan, C.; Zhang, D. Physicochemical, structural, and digestive properties of pea starch obtained via ultrasonic-assisted alkali extraction. Ultrason. Sonochem. 2022, 89, 106136. [Google Scholar] [CrossRef] [PubMed]
- Felisberto, M.H.F.; Costa, M.S.; Boas, F.V.; Leivas, C.L.; Franco, C.M.L.; de Souza, S.M.; Clerici, M.T.P.S.; Cordeiro, L.M.C. Characterization and technological properties of peach palm (Bactris gasipaes var. gasipaes) fruit starch. Food Res. Int. 2020, 89, 109569. [Google Scholar] [CrossRef] [PubMed]
- Bento, J.A.C.; Ferreira, K.C.; De Oliveira, A.L.M.; Lião, L.M.; Caliari, M.; Júnior, M.S.S. Extraction, characterization and technological properties of white garland-lily starch. Int. J. Biol. Macromol. 2019, 135, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kong, L.; Du, B.; Xu, B. Morphological and physicochemical characterization of starches isolated from chestnuts cultivated in different regions of China. Int. J. Biol. Macromol. 2019, 130, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Mojel, R.; Li, G. Structure of black pepper (Piper nigrum) starch. Food Hydrocoll. 2017, 71, 102–107. [Google Scholar] [CrossRef]
- Li, X.; Xia, Y.; Gao, W.; Jiang, Q.; Guo, H.; Cao, J.; Huang, L.; Xiao, P. Evaluation of three traditional Chinese medicine (TCM) starches and potential application in health product industry. Food Hydrocoll. 2014, 40, 196–202. [Google Scholar] [CrossRef]
- Patino-Rodriguez, O.; Agama-Acevedo, E.; Ramos-Lopez, G.; Bello-Perez, L.A. Unripe mango kernel starch: Partial characterization. Food Hydrocoll. 2020, 101, 105512. [Google Scholar] [CrossRef]
- Ai, Y.; Gong, L.; Reed, M.; Huang, J.; Zhang, Y.; Jane, J.L. Characterization of starch from bamboo seeds. Starch-Stärke 2016, 68, 131–139. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Zeng, J.; Zang, J.; Liang, Q.; Tang, D.; Wang, Z.; Yin, Z. Digestive and Physicochemical Properties of Small Granular Starch from Euryale ferox Seeds Growing in Yugan of China. Food Biophys. 2022, 17, 126–135. [Google Scholar] [CrossRef]
- Barros, D.R.; Carvalho, A.P.M.G.; Da Silva, E.O.; Sampaio, U.M.; De Souza, S.M.; Sanches, E.A.; De Souza Sant’Ana, A.; Clerici, M.T.P.S.; Campelo, P.H. Ariá (Goeppertia allouia) Brazilian Amazon tuber as a non-conventional starch source for foods. Int. J. Biol. Macromol. 2020, 168, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Canto-Pinto, J.C.; Reyes-Pérez, E.; Pérez-Pacheco, E.; Ríos-Soberanis, C.R.; Chim-Chi, Y.A.; Lira-Maas, J.D.; Estrada-León, R.J.; Dzul-Cervantes, M.A.A.; Mina-Hernández, J.H. A Novel Starch from Talisia floresii Standl Seeds: Characterization of Its Physicochemical, Structural and Thermal Properties. Polymers 2023, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ye, F.; Chen, T.; Li, M.; Zhao, G. Structural characteristics and physicochemical properties of starches from winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch. ex Poir.). Food Hydrocoll. 2022, 122, 107115. [Google Scholar] [CrossRef]
- Farias, F.D.A.C.; De Souza Moretti, M.M.; Costa, M.S.; BordignonJunior, S.E.; Cavalcante, K.B.; Boscolo, M.; Gomes, E.; Franco, C.M.L.; da Silva, R. Structural and physicochemical characteristics of taioba starch in comparison with cassava starch and its potential for ethanol production. Ind. Crops Prod. 2020, 157, 112825. [Google Scholar] [CrossRef]
- Ghoshal, G.; Kaushal, K. Extraction, characterization, physicochemical and rheological properties of two different varieties of chickpea starch. Legume Sci. 2020, 2, e17. [Google Scholar] [CrossRef]
- Ferreira, S.; Araujo, T.; Souza, N.; Rodrigues, L.; Lisboa, H.M.; Pasquali, M.; Trindade, G.; Rocha, A.P. Physicochemical, morphological and antioxidant properties of spray-dried mango kernel starch. J. Agric. Food Res. 2019, 1, 100012. [Google Scholar] [CrossRef]
- Santana, Á.L.; Zabot, G.L.; Osorio-Tobón, J.F.; Johner, J.C.; Coelho, A.S.; Schmiele, M.; Steel, C.J.; Meireles, M.A.A. Starch recovery from turmeric wastes using supercritical technology. J. Food Eng. 2017, 214, 266–276. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Blazek, J.; Copeland, L. Pasting and swelling properties of wheat flour and starch in relation to amylose content. Carbohydr. Polym. 2008, 71, 380–387. [Google Scholar] [CrossRef]
- Vithu, P.; Dash, S.K.; Rayaguru, K.; Panda, M.K.; Nedunchezhiyan, M. Optimization of starch isolation process for sweet potato 804 and characterization of the prepared starch. J. Food Meas. Charact. 2020, 14, 1520–1532. [Google Scholar] [CrossRef]
- Yu, W.; Tan, X.; Zou, W.; Hu, Z.; Fox, G.P.; Gidley, M.J.; Gilbert, R.G. Relationships between protein content, starch molecular 807 structure and grain size in barley. Carbohydr. Polym. 2017, 155, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Clerici, M.T.P.S.; Sampaio, U.M.; Schmiele, M. Identification and Analysis of Starch. In Starches for Food Application; Clerici, M.T.P.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 23–69. [Google Scholar] [CrossRef]
- Dhull, S.B.; Chandak, A.; Chawla, P.; Goksen, G.; Rose, P.K.; Rani, J. Modifications of native lotus (Nelumbo nucifera G.) rhizome starch and its overall characterization: A review. Int. J. Biol. Macromol. 2023, 253, 127543. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.; El Halal, S.L.M.; Dias, A.R.G.; Da Rosa Zavareze, E. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef]
- Cai, J.; Cai, C.; Man, J.; Yang, Y.; Zhang, F.; Wei, C. Crystalline and structural properties of acid-modified lotus rhizome C-type starch. Carbohydr. Polym. 2014, 102, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Tovar, J. Update of the concept of type 5 resistant starch (RS5): Self-assembled starch V-type complexes. Trends Food Sci. Technol. 2021, 109, 711–724. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Hernández, H.A.R.; Gutiérrez, T.J.; Bello-Pérez, L.A. Can starch-polyphenol V-type complexes be considered as resistant starch? Food Hydrocoll. 2022, 124 Pt A, 107226. [Google Scholar] [CrossRef]
- Da Costa, F.J.O.G.; Leivas, C.L.; Waszczynskyj, N.; De Godoi, R.C.B.; Helm, C.V.; Colman, T.A.D.; Schnitzler, E. Characterisation of native starches of seeds of Araucaria angustifolia from four germplasm collections. Therm. Acta 2013, 565, 172–177. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Starch-Stärke 2012, 64, 382–391. [Google Scholar] [CrossRef]
- Dhull, S.B.; Chandak, A.; Collins, M.N.; Bangar, S.P.; Chawla, P.; Singh, A. Lotus seed starch: A novel functional ingredient with promising properties and applications in food—A review. Starch-Stärke 2022, 74, 2200064. [Google Scholar] [CrossRef]
- Cai, C.; Lin, L.; Man, J.; Zhao, L.; Wang, Z.; Wei, C. Different structural properties of high-amylose maize starch fractions varying in granule size. J. Agric. Food. Chem. 2014, 62, 11711–11721. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Okumus, B.N.; Tacer-Caba, Z.; Kahraman, K.; Nilufer-Erdil, D. Resistant starch type V formation in brown lentil (Lens culinaris Medikus) starch with different lipids/fatty acids. Food Chem. 2018, 240, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S.K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2019, 150, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.K.; Lucey, A.; Walter, J.; Zannini, E.; Arendt, E.K. Resistant starch-An accessible fiber ingredient acceptable to the Western palate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2930–2955. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Gomes, P.T.G.; Morais, L.A.; Rocha, T.S. Resistant Starch Production, Properties and Potential Application in Gluten-Free Products. In The Food Industry: Perceptions, Practices and Future Prospects, 1st ed.; Santos, D.T., Castillo-Torres, A., Giovani, B.M.C., Eds.; Nova Science Publisher: New York, NY, USA, 2021; Volume 1, pp. 83–118. [Google Scholar]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2018, 276, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hamaker, B.R. Banana starch structure and digestibility. Carbohydr. Polym. 2012, 87, 1552–1558. [Google Scholar] [CrossRef]
- Purwandari, F.A.; Westerbos, C.; Lee, K.; Fogliano, V.; Capuano, E. Proximate composition, microstructure, and protein and starch digestibility of seven collections of Jack bean (Canavalia ensiformis) with different optimal cooking times. Food Res. Int. 2023, 170, 112956. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhang, Y.; Jiang, H.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Molecular structure and digestibility of banana flour and starch. Food Hydrocoll. 2017, 72, 219–227. [Google Scholar] [CrossRef]
- Chavez-Salazar, A.; Alvarez-Barreto, C.I.; Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Castellanos-Galeano, F.J. Drying processes of OSA-modified plantain starch trigger changes in its functional properties and digestibility. LWT Food Sci. Technol. 2022, 154, 112846. [Google Scholar] [CrossRef]
- Gani, A.; Ashwar, B.A.; Akhter, G.; Gani, A.; Shah, A.; Masoodi, F.A.; Wani, I.A. Resistant starch from five Himalayan rice cultivars and Horse chestnut: Extraction method optimization and characterization. Sci. Rep. 2020, 10, 4097. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Kantamraju, P.; Jha, S.; Sundarrao, G.S.; Bhowmik, A.; Chakdar, H.; Mandal, S.; Sahana, N.; Roy, B.; Bhattacharya, P.M.; et al. Evaluation of indigenous aromatic rice cultivars from sub-Himalayan Terai region of India for nutritional attributes and blast resistance. Sci. Rep. 2021, 11, 4786. [Google Scholar] [CrossRef]
- Öztürk, S.; Mutlu, S. Physicochemical Properties, Modifications, and Applications of Resistant Starches. In Starches for Food Application; Clerici, M.T.P.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 297–332. [Google Scholar] [CrossRef]
- Thompson, M.S.; Yan, T.H.; Saari, N.; Sarbini, S.R.M.S. A review: Resistant starch, a promising prebiotic for obesity and weight management. Food Biosci. 2022, 50 Pt A, 101965. [Google Scholar] [CrossRef]
- Kraithong, S.; Wang, S.; Junejo, S.A.; Fu, X.; Theppawong, A.; Zhang, B.; Huang, Q. Type 1 resistant starch: Nutritional properties and industry applications. Food Hydrocoll. 2022, 125, 107369. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Resistant starches: A smart alternative for the development of functional bread and other starch-based foods. Food Hydrocoll. 2021, 121, 106949. [Google Scholar] [CrossRef]
- Yashini, M.; Khushbu, S.; Madhurima, N.; Sunil, C.K.; Mahendran, R.; Venkatachalapathy, N. Thermal properties of different types of starch: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 4373–4396. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Zhu, F.; Mojel, R.; Li, G. Physicochemical properties of black pepper (Piper nigrum) starch. Carbohydr. Polym. 2018, 181, 986–993. [Google Scholar] [CrossRef]
- Zia-ud-Din; Xiong, H.; Fei, P. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Magallanes-Cruz, P.A.; Duque-Buitrago, L.F.; Martínez-Ruiz, N.R. Native and modified starches from underutilized seeds: Characteristics, functional properties and potential applications. Food Res. Int. 2023, 169, 112875. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Underutilized and unconventional starches: Why should we care? Trends Food Sci. Technol. 2020, 100, 363–373. [Google Scholar] [CrossRef]
- Colussi, R.; Pinto, V.Z.; El Halal, S.L.M.; Biduski, B.; Prietto, L.; Castilhos, D.D.; Zavareze, E.R.; Dias, A.R.G. Acetylated rice starches films with different levels of amylose: Mechanical, water vapor barrier, thermal, and biodegradability properties. Food Chem. 2017, 221, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Sit, N. Comprehensive review on single and dual modification of starch: Methods, properties and applications. Int. J. Biol. Macromol. 2023, 253, 126952. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, C.; Li, Z.; Li, Z.; Gu, Z.; Ban, X.; Hong, Y.; Cheng, L.; Kong, H. Highly-branched modification of starch: An enzymatic approach to regulating its properties. Food Hydrocoll. 2024, 147 Pt B, 109433. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; BeMiller, J.N. Advances in conversion of natural biopolymers: A reactive extrusion (REX)–enzyme-combined strategy for starch/protein-based food processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Chen, Z.; Zhong, Q. Enzymatically modified starch with low digestibility produced from amylopectin by sequential amylosucrase and pullulanase treatments. Food Hydrocoll. 2019, 95, 195–202. [Google Scholar] [CrossRef]
- Hong, J.; An, D.; Liu, C.; Li, L.; Han, Z.; Guan, E.; Xu, B.; Zheng, X.; Bian, K. Rheological, textural, and digestible properties of fresh noodles: Influence of starch esterified by conventional and pulsed electric field-assisted dual technique with full range of amylose content. J. Food Process Preserv. 2020, 44, e14567. [Google Scholar] [CrossRef]
- Zhang, C.; Lim, S.T. Physical modification of various starches by partial gelatinization and freeze-thawing with xanthan gum. Food Hydrocoll. 2021, 111, 106210. [Google Scholar] [CrossRef]
- Van Hung, P.; Duyen, T.T.M.; Van Thanh, H.; Widiastuti, D.; An, N.T.H. Starch digestibility and quality of cookies made from acid and heat-moisture treated sweet potato starch and wheat flour composites. J. Food Meas. Charact. 2021, 15, 3045–3051. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Roldan-Cruz, C.A.; Chavez-Esquivel, G.; Vernon-Carter, E.J.; Bello-Perez, L.A.; Alvarez-Ramirez, J. In vitro digestibility of ultrasound-treated corn starch. Starch-Stärke 2017, 69, 1700040. [Google Scholar] [CrossRef]
- Wu, Z.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. Nonthermal physical modification of starch: An overview of recent research into structure and property alterations. Int. J. Biol. Macromol. 2022, 203, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, Q.Y.; Wu, M.; Jiang, W.; Qian, J.Y.; Rao, S.Q.; Zhang, L.; Li, Q.; Zhang, C. Effect of pulsed electric field on properties and multi-scale structure of japonica rice starch. LWT Food Sci. Technol. 2019, 116, 108515. [Google Scholar] [CrossRef]
- Tao, H.; Huang, J.S.; Xie, Q.T.; Zou, Y.M.; Wang, H.L.; Wu, X.Y.; Xu, X.M. Effect of multiple freezing-thawing cycles on structural and functional properties of starch granules isolated from soft and hard wheat. Food Chem. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.E.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A comprehensive review on starch: Structure, modification, and applications in slow/controlled-release fertilizers in agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Chakraborty, I.N.P.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An insight into the gelatinization properties influencing the modified starches used in food industry: A review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.C.; BeMiller, J.N. Location of sites of reaction within starch granules. Cereal Chem. 2001, 78, 173–180. [Google Scholar] [CrossRef]
- López, O.V.; Zaritzky, N.E.; García, M.A. Physicochemical characterization of chemically modified corn starches related to rheological behavior, retrogradation and film forming capacity. J. Food Eng. 2010, 100, 160–168. [Google Scholar] [CrossRef]
- Cornelia, M.; Christianti, A. Utilization of modified starch from avocado (Persea americana Mill.) seed in cream soup production. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012074. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Rios, N.S.; Dos Santos, E.S. Modification of red rice starch by a combination of hydrothermal pretreatments and α-amylase hydrolysis. Carbohydr. Polym. 2022, 296, 119963. [Google Scholar] [CrossRef]
- Souza, E.L.; Santos, L.F.P.; de Abreu Barreto, G.; Leal, I.L.; Oliveira, F.O.; dos Santos, L.M.C.; Ribeiro, C.D.F.; Rezende, C.S.M.; Machado, B.A.S. Development and characterization of panettones enriched with bioactive compound powder produced from Shiraz grape by-product (Vitis vinifera L.) and arrowroot starch (Maranta arundinaceae L.). Food Chem. Adv. 2023, 2, 100220. [Google Scholar] [CrossRef]
- Spada, J.C.; Noreña, C.P.Z.; Marczak, L.D.F.; Tessaro, I.C. Study on the stability of β-carotene microencapsulated with pinhão (Araucaria angustifolia seeds) starch. Carbohydr. Polym. 2012, 89, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, P.; Asadi, G.; Nafchi, A.M.; Ardebili, S.M.S. The Effects of Dually Modified (Hydrolyzed-Hydroxypropylated) Sago Starch on the Quality Characteristics of Frozen Dough and Bread. Starch-Stärke 2023, 75, 2200183. [Google Scholar] [CrossRef]
- Bajaj, R.; Singh, N.; Kaur, A. Properties of octenyl succinic anhydride (OSA) modified starches and their application in low fat mayonnaise. Int. J. Biol. Macromol. 2019, 131, 147–157. [Google Scholar] [CrossRef]
- Escobar, M.C.; Van Tassell, M.L.; Martínez-Bustos, F.; Singh, M.; Castaño-Tostado, E.; Amaya-Llano, S.L.; Miller, M.J. Characterization of a Panela cheese with added probiotics and fava bean starch. J. Dairy Sci. 2012, 95, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; She, Z.; Hou, D.; Wang, J.; Lan, T.; Lv, X.; Zhang, Y.; Sun, X.; Ma, T. Effect of partial substitution of wheat flour with kiwi starch on dough rheology, microstructure, the quality attributes and shelf life of Chinese steamed bread. Int. J. Biol. Macromol. 2024, 258, 128920. [Google Scholar] [CrossRef]
- Zarroug, Y.; Boulares, M.; Mejri, J.; Slimi, B.; Hamdaoui, G.; Djebi, S.; Saidi, F.; Nasri, H.; Sfayhi, D.T.; Kharrat, M. Extraction and Characterization of Tunisian Quercus ilex Starch and Its Effect on Fermented Dairy Product Quality. Int. J. Anal. Chem. 2020, 2020, 8868673. [Google Scholar] [CrossRef] [PubMed]
- Zarroug, Y.; Boulares, M.; Sfayhi, D.; Slimi, B.; Stiti, B.; Zaieni, K.; Nefissi, S.; Kharrat, M. Structural and Physicochemical Properties of Tunisian Quercus suber L. Starches for Custard Formulation: A Comparative Study. Polymers 2022, 14, 556. [Google Scholar] [CrossRef]
- Menon, R.; Padmaja, G.; Jyothi, A.N.; Asha, V.; Sajeev, M.S. Gluten-free starch noodles from sweet potato with reduced starch digestibility and enhanced protein content. J. Food Sci. Technol. 2016, 53, 3532–3542. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Puentes, A.; Rincón, S.; García-Gurrola, A.; Zepeda, A.; Calvo-López, A.D.; Martínez-Bustos, F. Development of a third-generation snack with type 4 resistant sorghum starch: Physicochemical and sensorial properties. Food Biosci. 2019, 32, 100474. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Y.; Lee, Y.E.J.; Chan, A.; Lee, P.R.; Yang, H. Effect of starch addition on the physicochemical properties, molecular interactions, structures, and in vitro digestibility of the plant-based egg analogues. Food Chem. 2023, 403, 134390. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Tan, C.P.; Li, C.; Fu, X.; Huang, Q. Octenylsuccinate quinoa starch granule-stabilized Pickering emulsion gels: Preparation, microstructure and gelling mechanism. Food Hydrocoll. 2019, 91, 40–47. [Google Scholar] [CrossRef]
- Yaowiwat, N.; Madmusa, N.; Yimsuwan, K. Potential of Thai aromatic fruit (Artocarpus species) seed as an alternative natural starch for compact powder. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124940. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaksmi, M.; Govindaraj, V.; Anisha, M.; Vigneshwari, N.; Gokul, M.; Nithila, E.E.; Bebin, M.; Prasath, T.A.; Chezhiyan, P. Synthesis and Characterization of Banana Peel Starch-based Bioplastic for Intravenous Tubes Preparation. Mater. Today Commun. 2022, 33, 104464. [Google Scholar] [CrossRef]
- Dularia, C.; Sinhmar, A.; Thory, R.; Pathera, A.K.; Nain, V. Development of starch nanoparticles based composite films from non-conventional source—Water chestnut (Trapa bispinosa). Int. J. Biol. Macromol. 2019, 136, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R.P. Citric acid esterified Glutinous Assam bora rice starch enhances disintegration and dissolution efficiency of model drug. Int. J. Biol. Macromol. 2023, 227, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.; Gibril, M.; Sithole, B.; Ramjugernath, D.; Chavan, R.; Chunilall, V.; Gounden, N. Valorisation of avocado seeds: Extraction and characterisation of starch for textile applications. Clean Technol. Environ. Policy 2018, 23, 581–595. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Romainor, A.N.; Pang, S.C.; Lee, B.K.; Hwang, S.S. pH-responsive starch-citrate nanoparticles for controlled release of paracetamol. Starch-Stärke 2019, 71, 1800336. [Google Scholar] [CrossRef]
- Yusoff, M.S.; Aziz, H.A.; Alazaiza, M.Y.; Rui, L.M. Potential use of oil palm trunk starch as coagulant and coagulant aid in semi-aerobic landfill leachate treatment. Water Qual. Res. J. 2019, 54, 203–219. [Google Scholar] [CrossRef]
- Awodi, P.S.; Ogbonna, J.C.; Nwagu, T.N. Bioconversion of mango (Mangifera indica) seed kernel starch into bioethanol using various fermentation techniques. Heliyon 2022, 8, e09707. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Jin, Y.; Liu, J.; Qin, X.; Liu, W.; Guo, L. Highly efficient fermentation of glycerol and 1, 3-propanediol using a novel starch as feedstock. Food Biosc. 2022, 46, 101521. [Google Scholar] [CrossRef]
- Baeza, P.; Pasten, B.; Concha, J.; Ojeda, J. Removal of thiophene and 4, 6-dimethyldibenzothiophene by adsorption on different kinds of starches. Water Air Soil Pollut. 2021, 232, 395. [Google Scholar] [CrossRef]


| Botanical Source | Extraction Method | Yield (%) | Morphology | Granule Size | Amylose Content (%) | Proximal Composition (%) | Crystallinity | References |
|---|---|---|---|---|---|---|---|---|
| Jabuticaba Seed (Plinia cauliflora) | H2O | 22.65 | Trimodal—smooth surface and irregular shape | 2–10 µm | 34.50 | M: 7.19, P: 1.19, L: 118, A: N. | Type C—35.4% Peaks at 2θ = 5.69°, 15.11°, 17.02°, and 23.19° | [17] |
| Pineapple Stems (Ananas comosus) | H2O (1:1) | 30 | Semi-angular with partially rounded segments | 3–14 μm | 35.15 | M: 10.64, P: 0.71, L: 0.55, A: 0.54 | Type A—Peaks at 2θ = 12.8°, 14.6°, 15.4°, and 19.8°. | [22] |
| Red Rice (Oryza sativa) | H2O (1:2) | 35.7–47.0 | Polyhedral with irregular shapes, acute angles, and edges | 15–30 µm | 25.75 | M: 6.89, P: 3.73, L: 0.51, A: 0.80 | Type A—25.50% Peaks at 2θ = 15.3°, 17.1°, 18.2°, and 23.5° | [29] |
| Black Rice (Oryza sativa) | M: 13.29, P: 2.60, L: 0.28, A: 0.68 | |||||||
| Annatto Seeds (Bixa orellana L.) | H2O | 19.0 | Oval, spherical, smooth surface, no aggregates | 5–20 µm | 27.8 | M: 12.5, P: 14.7, L: 3.4, A: 5.8 | Type C—H2O = 35.4%, NaOH = 41.6% Peaks at 2θ = 10°, 15°, 17°, 20°, 23°, and 26° | [30] |
| NaOH (0.25%) | 32.0 | 23.9 | M: 11.2, P: 17.4, L: 6.5, A: 3.8 | |||||
| Loquat Seed (Eriobotrya japonica) | H2O (1:15) | N | Oval and cylindrical | 29.05–43.66 μm | 10.53 | M: 7.36, P: 0.61, L: 0.80, A: 0.36 | Type C—24.30% Peaks at 2θ = 15°, 17°, 22°, and 23°. | [32] |
| Microalga Chlorella sp. MBFJNU-17 | Na2S2O5 (0.45%) | N | Unimodal—smooth surface with irregularities and ellipsoid shape | 400–1300 nm | 13.45 | M: N, P: 0.96, L: 0.23, A: 0.91 | Type A and V—25.93% Peaks at 2θ = 15°, 17°, 18°, 20°, and 23° | [38] |
| Bamboo Stem (Bambusa tuldoides) | Na2S2O5 (0.2%) | 2.51–3.55 | Polyhedral with rounded and spherical shapes | 5–12 µm | 19.26 to 33.35 | M: 7.25–7.89, P: 3.12–4.66, L: 0.23–0.61, A: 1.47–5.46 | Type A—22.07 to 26.42% Peaks at 2θ = 15°, 17°, 18°, 20°, and 23° | [39] |
| Pea (Pisum sativum L.) | NaOH (0.33%) | 53.20 | Rounded or elliptical with a smooth surface | 130–160 µm | 72.91 | M: N, P: 0.29, L: N, A: N | Type C—Peaks at 2θ = 15.35°, 17.38°, 18.28°, and 22.95° | [40] |
| Peach Palm (Bactris gasipaes var. gasipaes)—pulp | H2O (1:3) | N | Bimodal—irregular shapes (oval, conical, and spherical) | 5.2–12.5 µm | 18.92 | M: 10.76, P: 0.54, L: 2.69, A: 0.19 | Type C—23.56% Peaks at 2θ = 5.3°, 15.1°, 17.2°, 18.0°, 21.4°, and 23° | [41] |
| White Ginger Rhizome (Hedychium coronarium J. Koenig) | Na2S2O5 (0.02%) | 22.0 | Flat, thin, and smooth | E: 2–6 μm, C: 12–38 μm | 59.16 | M: 10.13, P: 0.97, L: 0.84, A: 0.28 | Type B—19.30% Peaks at 2θ = 16.9°, 21.9°, 23.8°, 14.6°, and 19.4° | [42] |
| Chestnut (Castanea mollissima Blume) | Na2SO3 (0.025 M) | N | Oval to spherical, elliptical, smooth surfaces, and edges | 1.2–517.2 μm | 34.17 | M: 13.5, P: 0.35, L: N, A: N | Type C—Peaks at 2θ = 5.6°, 15°, 17°, 22–24° | [43] |
| Black Pepper (Piper nigrum) | Na2SO3 (0.5%) | N | Polygonal and polyhedral with irregular shapes and smooth surface | Dm: 4.05 μm | 23.8 | M: N, P: N, L: N, A: N | Type A—21.50% Peaks at 2θ = 15°, 17°, and 23° | [44] |
| White Pepper (Piper nigrum) | Dm: 3.57 μm | 25.6 | M: N, P: N, L: N, A: N | Type A—21.18% Peaks at 2θ = 15°, 17°, and 23° | ||||
| Ginkgo Seeds (Ginkgo biloba L.) | H2O | N | Spherical and elliptical | 5–20 μm | 30.5 | M: 9.30, P: 0.44, L: 0.42, A: N | Type C—42.4% Peaks at 2θ = 5.6°, 15.2°, 17.1°, 22.1°, and 24.4° | [45] |
| Orchid Tuber (Bletilla striata (Thunb.) Reichb.) | Spherical with irregularities | 3–20 μm | 16.7 | M: 9.06, P: 0.61, L: 0.40, A: N | Type C—20.7% Peaks at 2θ = 5.6°, 15.2°, 17.1°, 22.1°, and 24.4° | |||
| Flower Tuber (Angelica dahurica (Fisch. ex Hoffm.) Benth.) | Irregular, spherical with smooth surfaces | 2–16 µm | 21.3 | M: 10.1, P: 0.46, L: 0.44, A: N | Type C—20.7% Peaks at 2θ = 5.6°, 15.2°, 17.1°, 22.1°, and 24.4° | |||
| Bacuri Seed (Garcinia brasiliensis (Mart.)) Green | NaOH (0.25%) | 40.56 | Oval with a smooth surface | 42.21–56.88 μm | 10.13 | M: 8.88, P: 3.15, L: 3.58, A: 0.46 | Type C—NaOH = 15.68%, H2O = 13.49 Peaks at 2θ = 15°, 17°, and 23° | [35] |
| H2O | 44.42 | 13.07 | M: 9.49, P: 4.28, L: 2.38, A: 0.15 | |||||
| Green Mang Seed (Mangifera indica L.) | Na2SO3 (1%) | N | Unimodal—oval and spherical | 50 μm | 23.00 | M: N, P: N, L: N, A: N | Type A—26.02% Peaks at 2θ = 15°, 17°, 18° and 23°. | [46] |
| Bamboo Seeds (Phyllostachys heterocycla var. Pubescens (Mazel) Ohwi) | H2O | N | Polyhedral with irregular shapes and edges | Dm: 5.0 µm | 24.1 | M: N, P: 18.6, L: 1.1, A: N | Type A—32.1% Peaks at 2θ = 15°, 17.1°, 17.5°, 18°, and 23°. | [47] |
| Lotus Seeds (Euryale ferox) | NaOH (0.17%) | N | Unimodal—polyhedral and irregular shape | 0.50–5.60 μm | 45.85 | M: 11.67, P: 0.09, L: 0.13, A: 0.08 | Type A—38.84% Peaks at 2θ = 15°, 17°, 18°, and 23°. | [48] |
| Ariá (Goeppertia allouia) | H2O | 11 | Unimodal—spherical with irregular sizes and smooth surface | 15–40 μm | 39 | M: 8.45, P: 2.04, L: 0.39, A: 0.15 | Type C—32% Peaks at 2θ = 5.8°, 10.4°, 18°, 18.5°, and 23°. | [49] |
| Gold Whisker Seed (Talisia floresii Standl) | NaHSO3 (0.1%) | N | Spherical, uniform, and fracture-free | 10–25 μm | 33.6 | M: 9.49, P: ND, L: 1.60, A: 1.17 | Type C—32% Peaks at 2θ = 15°, 17°, 18°, 23°, and 24°. | [50] |
| Pumpkin (Cucurbita maxima Duch.) | H2O | N | Spherical, polyhedral with irregular granules, and dome-shaped | 10–20 μm | 30.17 | M: 15.90, P: 0.16, L: 1.01, A: 0.19 | Type B—28.64% Peaks at 2θ = 5.6°, 15°, 17.2°, 19.8°, 22.3°, and 24.0°. | [51] |
| Pumpkin (Cucurbita moschata Duch. ex Poir.) | 15–30 μm | 21.35 | M: 14.69, P: 0.47, L: 1.38, A: 0.29 | Type B—31.31% Peaks at 2θ = 5.6°, 15°, 17.2°, 19.8°, 22.3°, and 24.0°. | ||||
| Cocoyam Root (Xanthosoma sagittifolium) | H2O | N | Polyhedral, oval, and irregular at the ends | 2–14.55 μm | 21.80 | M: 12.53, P: 0.17, L: 0.26, A: 0.55 | Type A—38.3% Peaks at 2θ = 17°, 18°, and 23°. | [52] |
| Chickpea (Cicer arietinum L.) | NaOH (0.05%) | 28.4 | Oval, small, and spherical with a smooth surface | 2–30 μm | 30.2 | M: 10.7, P: 0.75, L: N, A: 0.06 | Type C—ND Peaks at 2θ = 6.5°, 15°, 18°, and 23°. | [53] |
| Mango Seeds (Tommy Atkins) | NaHSO3 (0.5%) | N | Oval, disc-shaped, and spherical | 3.6–19.3 μm | 25.26 | N | Type A and V—28.3% Peaks at 2θ = 5.8°, 12.3°, 15.2°, 17.3°, 18.1°, 20.3°, and 23.2°. | [54] |
| Botanical Source | Starch | Quantity Applied | Applied Matrices | Technological Effect | References |
|---|---|---|---|---|---|
| Avocado Seed (Persea americana Mill.) | Native and Modified—Cross-linking (sodium tripolyphosphate at 6%) | 25 | Instant Soup |
| [115] |
| Arrowroot (Maranta arundinaceae L.) | Native | 10, 15, 20% | Panettone |
| [116] |
| Pine Nut Seeds (Araucaria angustifolia) | Native and Modified (Acid hydrolysis—HCl) | Native Starch, 6 Dextrose, and 12 Dextrose hydrolyzed starch | β-Carotene Preservation |
| [117] |
| Sago (Metroxylon sp.) | Native and Modified (Hydrolyzed-Hydroxypropylated) | 10% wheat flour substitution with native, hydrolyzed, hydroxypropylated, and doubly modified starch | Frozen Dough and Hamburger Buns |
| [118] |
| Sweet Potato (Ipomoea batatas) and Red Bean (Phaseolus vulgaris) | Chemically Modified (3%, octenyl succinic anhydride (OSA)) | Up to 75% oil substitution | Mayonnaise |
| [119] |
| Fava Bean (Vicia faba L.) | Native | 3% | Panela Cheese |
| [120] |
| Sweet Potato (Ipomoea batatas L.) | Modified: Chemically (citric acid 0.2 M) and Physically (moisture content of 30% at 110 °C for 8 h.) | 20% wheat flour substitution with physically and chemically modified starch | Biscuit |
| [104] |
| Kiwi (Actinidia deliciosa ‘Huayou’) | Native | 10–20% wheat flour substitution with native kiwi starch | Chinese Steamed Bread |
| [121] |
| Acorn (Quercus ilex) | Native | 0.5, 1, 2, 3% | Fermented Dairy Beverage |
| [122] |
| Acorn (Quercus Suber L.) | Native (extraction with H2O and NaOH 0.3%) | 100% commercial corn starch substitution | Cream |
| [123] |
| Sweet Potato (Sree Arun) | Native | 10, 20, 30% | Noodles |
| [124] |
| White Sorghum (Sorghum bicolor (L.) Moench) | Modified—Physically (extrusion) and chemically (phosphorylation with sodium trimetaphosphate and sodium tripolyphosphate) | 17% | Extruded Snacks |
| [125] |
| Glutinous Rice (Oryza sativa) | Native | 4, 6% | Plant-Based Egg Analog |
| [126] |
| Quinoa Seeds (Chenopodium quinoa) | Modified (Chemically—Octenyl Succinate at 1, 3, 5%) | - | Pickering Emulsion |
| [127] |
| Non-Food Matrices | |||||
| Champedak seeds (Artocarpus integer) and jackfruit seeds (Artocarpus heterophyllus L.) | Native | Partial substitution of 65.3% rice starch and 20% talc | Compact powder |
| [128] |
| Taro Root (Xanthosoma sagittifolium) | Native | 100% | Bioethanol |
| [52] |
| Banana Peel (Musa spp.) | Native | 1.5 to 5.7% | Intravenous Tubes |
| [129] |
| Water Chestnut (Trapa bispinosa) | Modified (Acid Hydrolysis—HCL 3.16 M) | 0.5, 1, 2, 5, and 10% | Composite films |
| [130] |
| Rice (Assam bora) | Native and Modified Chemically (citric acid 40%) | 100% | Model Medication (Paracetamol) |
| [131] |
| Avocado Seed (Persea americana Mill) | Native | 100% | Textile application in cotton threads |
| [132] |
| Green Bananas (Musa paradisiaca L.) | Native and Chemically Modified (Acetylation with 0.33% substitution) | 100% | Nanocarriers for curcumin for oral drug delivery |
| [133] |
| Sago | Native and Modified (Esterification—citric acid 1% (w/v)) | - | Nanocarrier for Paracetamol |
| [134] |
| Palm Trunk (Elaeis guineensis) | Native | 500 mg·L−1 | Coagulant |
| [135] |
| Mango (Mangifera indica) | Native | 7 g | Bioethanol |
| [136] |
| Duckweed (Landoltia punctata) | Native | 100 g | Glycerol Production |
| [137] |
| Green Banana (Musa paradisiaca L.) and Dessert Banana (Musa cavendishii) | Native | 0.1 g | Adsorbent |
| [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, H.J.M.; Barcia, M.T.; Schmiele, M. Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products. Macromol 2024, 4, 886-909. https://doi.org/10.3390/macromol4040052
Carvalho HJM, Barcia MT, Schmiele M. Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products. Macromol. 2024; 4(4):886-909. https://doi.org/10.3390/macromol4040052
Chicago/Turabian StyleCarvalho, Hugo José Martins, Milene Teixeira Barcia, and Marcio Schmiele. 2024. "Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products" Macromol 4, no. 4: 886-909. https://doi.org/10.3390/macromol4040052
APA StyleCarvalho, H. J. M., Barcia, M. T., & Schmiele, M. (2024). Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products. Macromol, 4(4), 886-909. https://doi.org/10.3390/macromol4040052







