Effect of Tacticity on London Dispersive Surface Energy, Polar Free Energy and Lewis Acid-Base Surface Energies of Poly Methyl Methacrylate by Inverse Gas Chromatography
Abstract
1. Introduction
2. Methods and Materials
- -
- Two kinds of probe molecules were used:
- -
- Non-polar molecules such as the n-alkanes () such as n-pentane (), n-hexane (), n-heptane (), n-octane (), and n-nonane ()
- -
- Polar molecules, divided into three groups:
- ➢
- Lewis’s acid solvents such as dichloromethane, chloroform, and carbon tetrachloride
- ➢
- Basic solvent such as ethyl acetate, diethyl ether, tetrahydrofuran (THF)
- ➢
- Amphoteric molecule such as toluene
3. Experimental Results
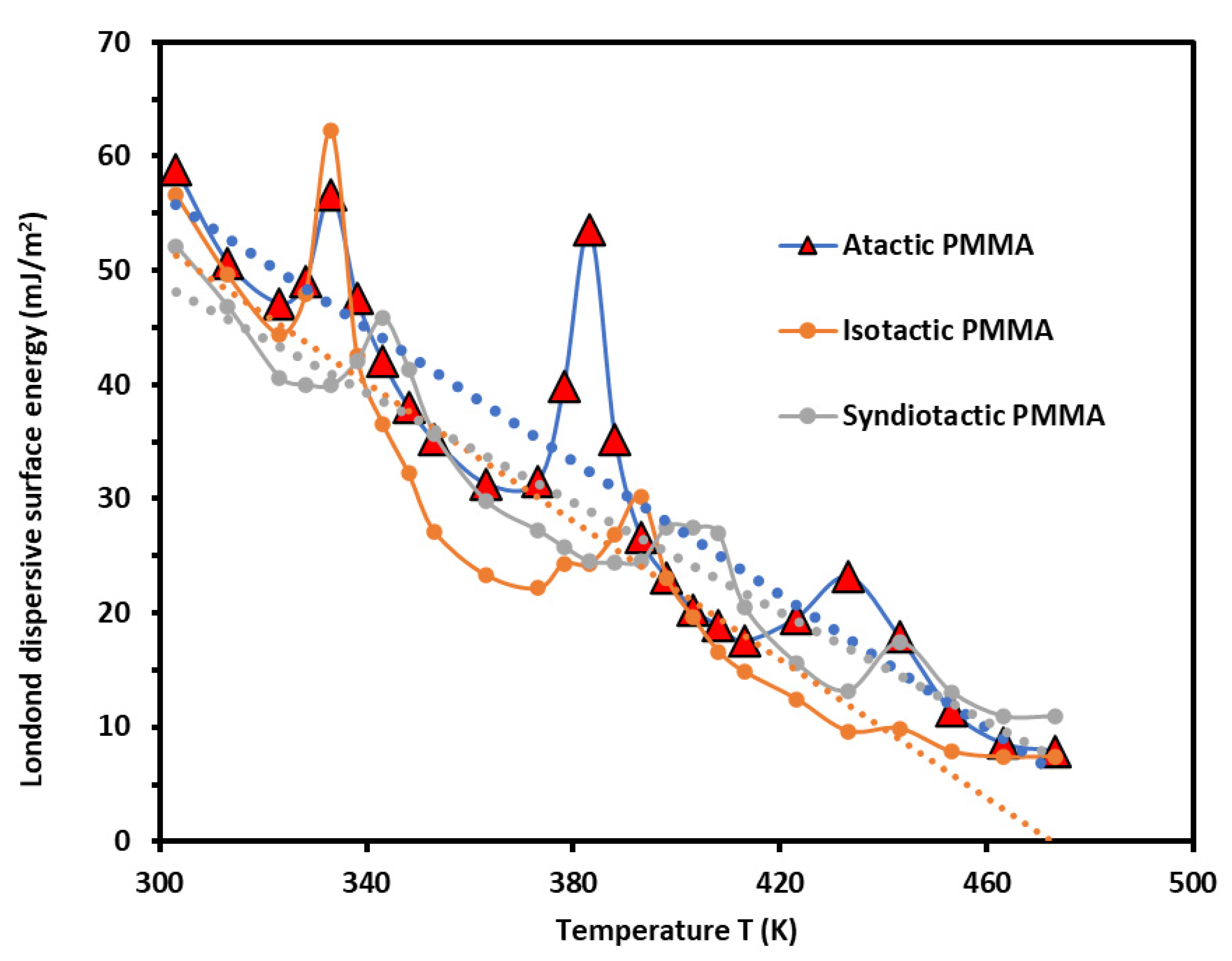
3.1. London Dispersive Component of Surface Energy of PMMA Polymers
- -
- The beta-relaxation ;
- -
- The glass transition ;
- -
- The liquid–liquid transitions .
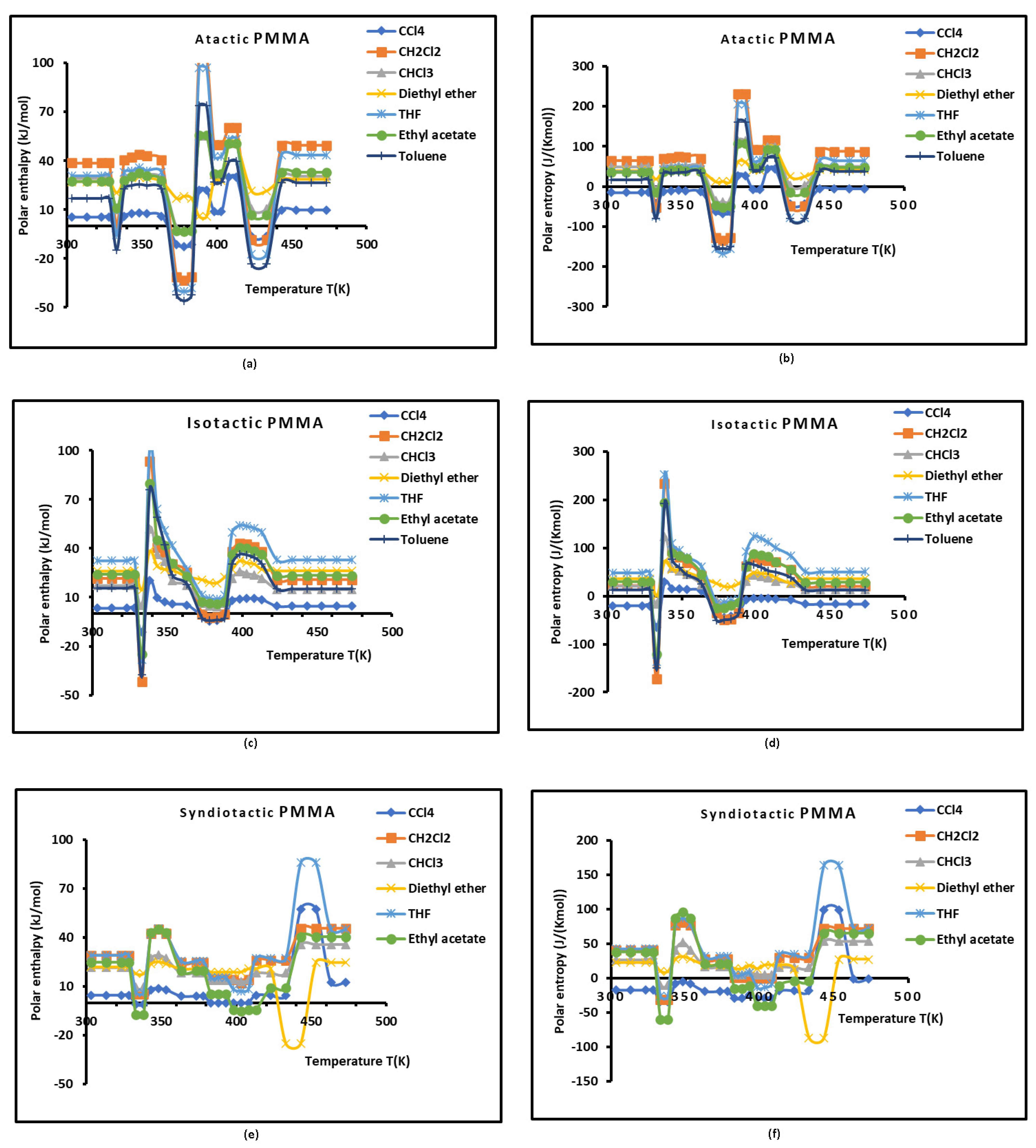
3.2. Polar Free Surface Energy of Atactic, Isotactic, and Syndiotactic PMMAs
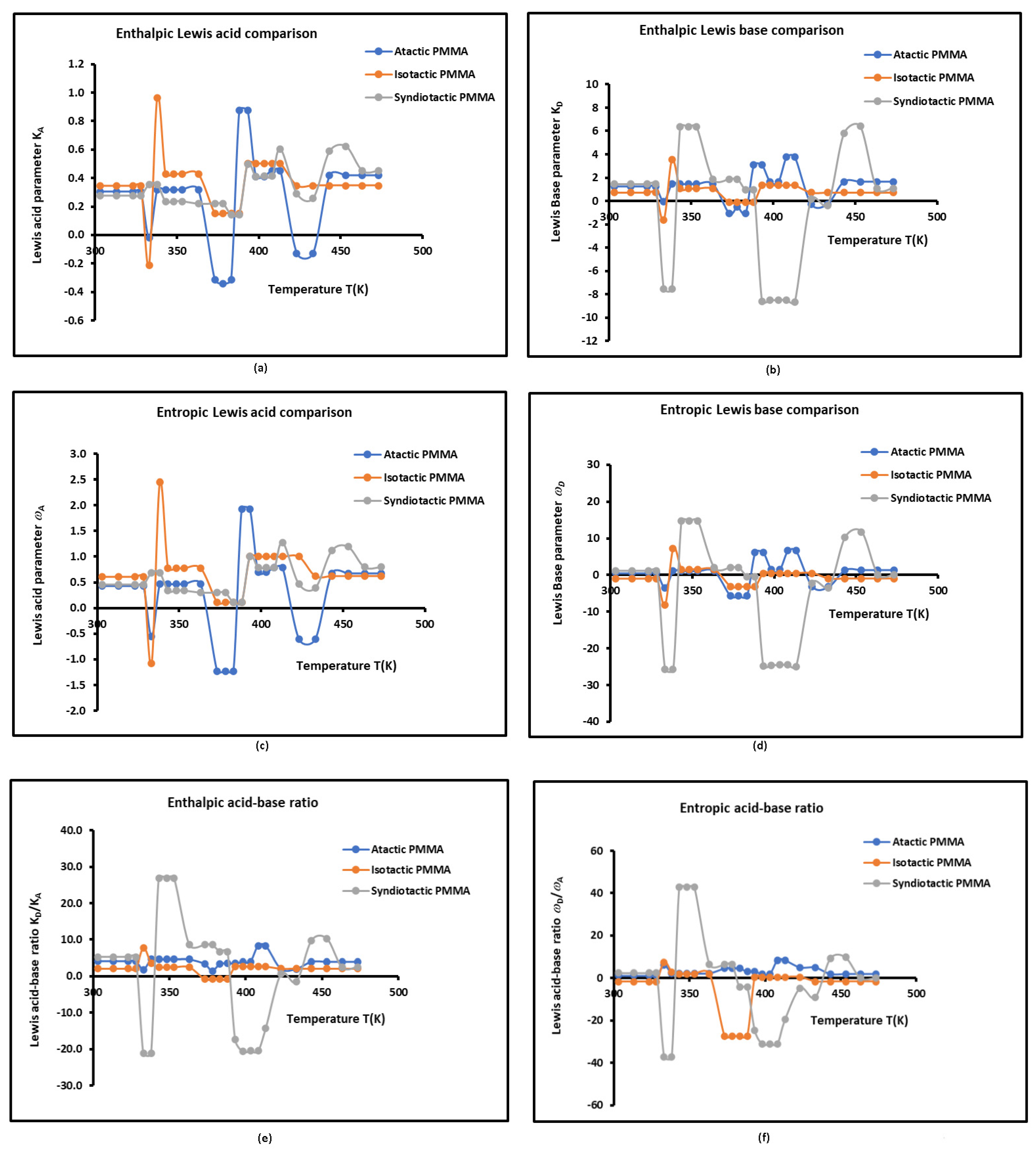
3.3. Lewis’s Acid-Base Properties
- -
- The three examined polymers are more basic than acidic in Lewis terms. The basicity of the tactic PMMA is about four times greater than its acidity outside the neighborhood of the transition temperatures of the polymer (Table S5 and Figure 4). Whereas, isotactic PMMA is about 2 to 2.5 times more basic than acid, while syndiotactic PMMA exhibits the highest basicity by showing a surface 5 to 8 times more basic than acid, of course outside the neighborhood of the transition temperatures of the polymer (Table S5 and Figure 4).
- -
- The Lewis acid parameter of atactic PMMA is comparable to that of isotactic PMMA with a slightly higher value in the isotactic PMMA but greater than that of syndiotactic PMMA. However, the basicity of the latter polymer is higher than that of atactic and isotactic PMMAs. In fact, the alternate acrylate groups present in syndiotactic PMMA principal chain confer the highest basicity of this polymer, whereas the methyl groups in isotactic and atactic PMMAs give the highest Lewis acidity.
- -
- The Lewis amphoteric character given by the values of and which are shown in Table S5 and Figure 4, led to the conclusion that the highest amphoteric surface is obtained with the syndiotactic PMMA followed by the atactic PMMA and isotactic PMMA, certainly due to the highest activity of the surface groups of syndiotactic PMMA.
- -
- Once again, the curves plotted in Figure 4 showed the three transition temperatures and sudden changes around each transition temperature with a drop in the values of the different acid-base parameters at these transition temperatures of the three polymers. The different parameters rapidly increase after reaching the different minima to be stabilized on positive parameter stages between two transition temperatures of PMMA.
- -
- The changes in the values of the different acid-base parameters in atactic PMMA are higher than those that happened in isotactic and syndiotactic PMMAs. This is due to the random distribution of the acrylate groups in the PMMA principal chain.
- -
- -
- Results proved an important effect of the tacticity polymer on the Lewis acid-base parameters and the polar surface interactions between the polymers and the organic solvents.
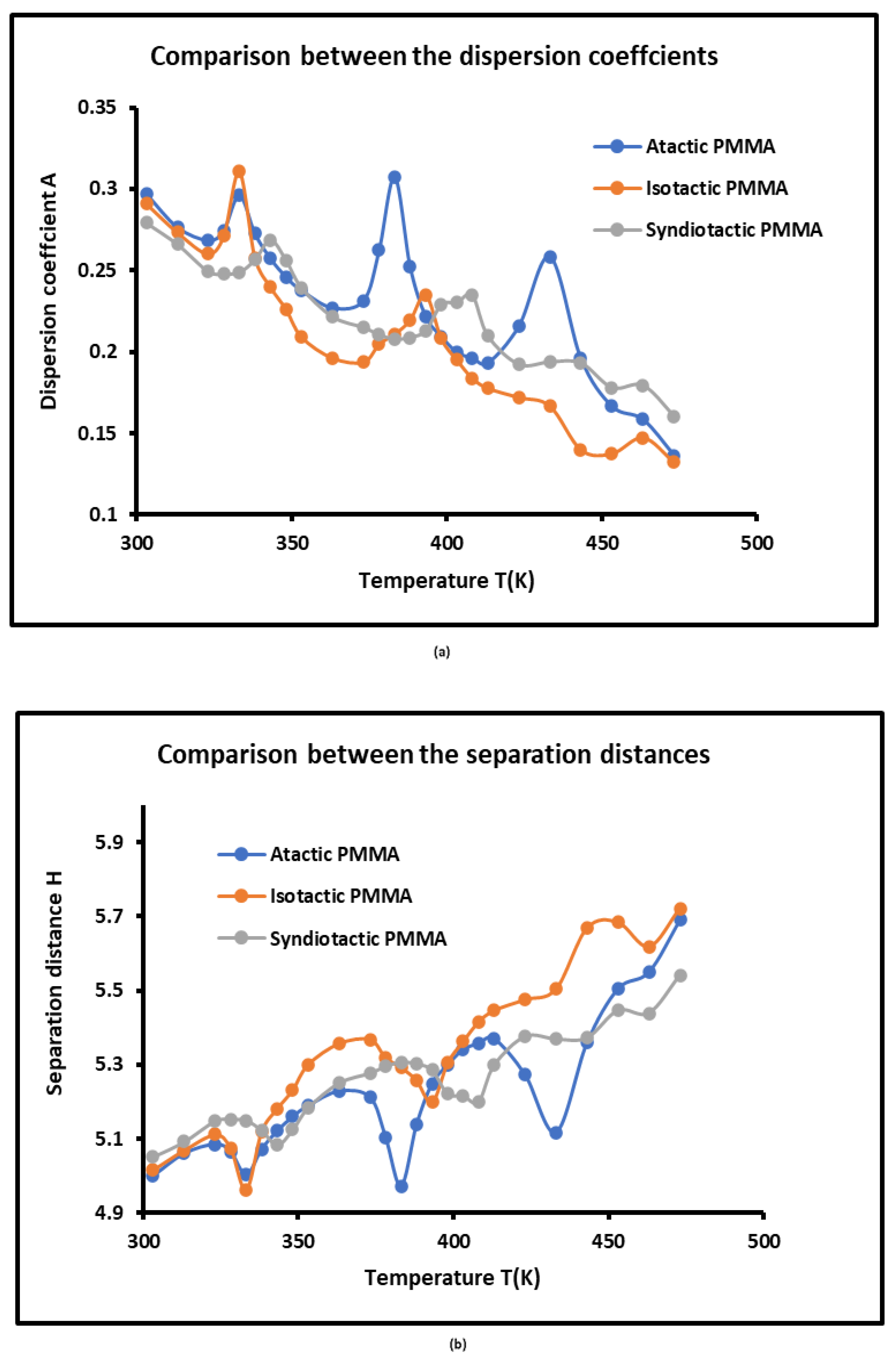
3.4. London Dispersive Free Energies of PMMAs and Dispersion Factor
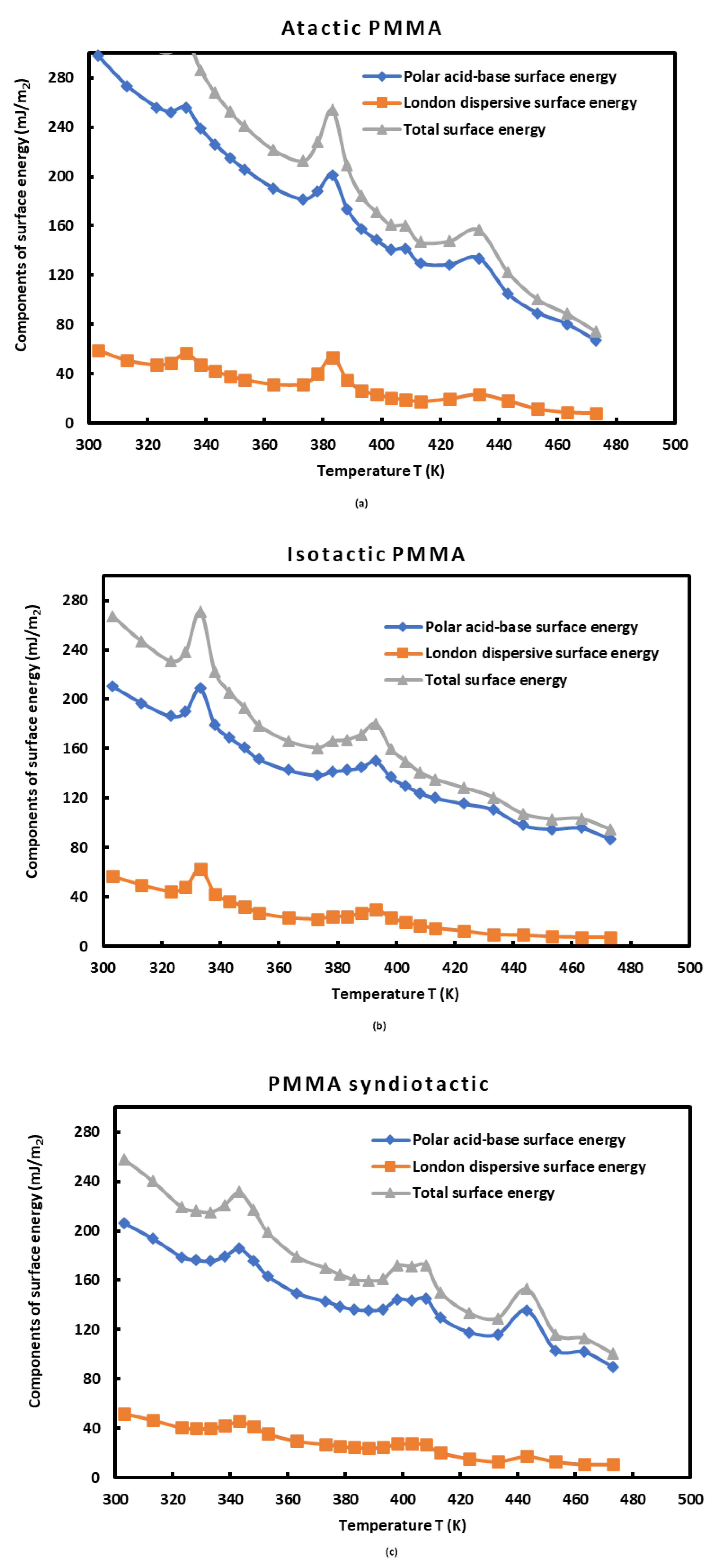
3.5. Lewis Acid-Base Surface Energies of PMMAs and Polar Component of the Surface Energy of Polar Molecules
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Miri, M.J.; Pritchard, B.P.; Cheng, H.N. A versatile approach for modeling and simulating the tacticity of polymers. J. Mol. Model. 2011, 17, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.C.; Prydderch, H.; Jimaja, S.; Bexis, P.; Becker, M.L.; Dove, A.P. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. [Google Scholar] [CrossRef]
- Tsuruta, T.; Kawakami, Y. 33-Anionic Ring-opening Polymerization: Stereospecificity for Epoxides, Episulfides and Lactones. Compr. Polym. Sci. Suppl. 1989, 3, 489–500. [Google Scholar] [CrossRef]
- Izzo, L.; Griffiths, P.C.; Nilmini, R.; King, S.M.; Wallom, K.L.; Ferguson, E.L. Ruth Duncan, Impact of polymer tacticity on the physico-chemical behaviour of polymers proposed as therapeutics. Int. J. Pharm. 2011, 408, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadi, K.; Westrik, J.B.H.M.; Vogiatzis, G.; van Breemen, L.; Anderson, P.; Hütter, M. Physical ageing of polystyrene: Does tacticity play a role? Macromolecules 2019, 52, 5948–5954. [Google Scholar] [CrossRef] [PubMed]
- Chat, K.; Tu, W.; Unni, A.B.; Adrjanowicz, K. Influence of Tacticity on the Glass-Transition Dynamics of Poly(methyl methacrylate) (PMMA) under Elevated Pressure and Geometrical Nanoconfinement. Macromolecules 2021, 54, 8526–8537. [Google Scholar] [CrossRef]
- Cheng, G.; Barriere, T. Effect of viscoplasticity on microfluidic cavity filling efficiency of a thermoplastic polymer in hot-embossing process. Int. J. Adv. Manuf. Technol. 2019, 103, 549–565. [Google Scholar] [CrossRef]
- Adrjanowicz, K.; Paluch, M. Discharge of the Nanopore Confinement Effect on the Glass Transition Dynamics via Viscous Flow. Phys. Rev. Lett. 2019, 122, 176101. [Google Scholar] [CrossRef] [PubMed]
- Chat, K.; Adrjanowicz, K. The Impact of the Molecular Weight on the Nonequilibrium Glass Transition Dynamics of Poly(Phenylmethyl Siloxane) in Cylindrical Nanopores. J. Phys. Chem. C 2020, 124, 22321–22330. [Google Scholar] [CrossRef]
- Floudas, G.; Paluch, M.; Grzybowski, A.; Ngai, K. Molecular Dynamics of Glass-Forming Systems; Advances in Dielectrics; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1. [Google Scholar]
- Karasz, F.E.; Bair, H.E.; O’Reilly, J.M. Thermal Properties of Atactic and Isotactic Polystyrene. J. Phys. Chem. A 1965, 69, 2657–2667. [Google Scholar] [CrossRef]
- Geng, K.; Tsui, O.K.C. Effects of Polymer Tacticity and Molecular Weight on the Glass Transition Temperature of Poly(Methyl Methacrylate) Films on Silica. Macromolecules 2016, 49, 2671–2678. [Google Scholar] [CrossRef]
- Ngai, K.L.; Paluch, M. Classification of Secondary Relaxation in Glass-Formers Based on Dynamic Properties. J. Chem. Phys. 2004, 120, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Cowie, J.M.G. Glass Transition Temperatures of Stereoblock, Isotactic and Atactic Polypropylenes of Various Chain Lengths. Eur. Polym. J. 1973, 9, 1041–1049. [Google Scholar] [CrossRef]
- Tretinnikov, O.N. Selective Accumulation of Functional Groups at the Film Surfaces of Stereoregular Poly(methyl methacrylate)s. Langmuir 1997, 13, 2988–2992. [Google Scholar] [CrossRef]
- Sharma, V.K.; Pethrick, R.A.; Affrossman, S. Poly(methyl methacrylate): Influence of tacticity on its use as an electron resist. Polymer 1982, 23, 1732–1736. [Google Scholar] [CrossRef]
- Jha, K.C.; Dhinojwala, A.; Tsige, M. Local Structure Contributions to Surface Tension of a Stereoregular Polymer. ACS Macro Lett. 2015, 4, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.C.; Zhu, H.; Dhinojwala, A.; Tsige, M. Molecular structure of poly(methyl methacrylate) surface II: Effect of stereoregularity examined through all-atom molecular dynamics. Langmuir ACS J. Surf. Colloids 2014, 30, 12775–12785. [Google Scholar] [CrossRef] [PubMed]
- Rungswang, W.; Jarumaneeroj, C.; Patthamasang, S.; Phiriyawirut, P.; Jirasukho, P.; Soontaranon, S.; Rugmai, S.; Hsiao, B.S. Influences of tacticity and molecular weight on crystallization kinetic and crystal morphology under isothermal crystallization: Evidence of tapering in lamellar width. Polymer 2019, 172, 41–51. [Google Scholar] [CrossRef]
- Kieber, R.J.; Silver, S.A.; Kennemur, J.G. Stereochemical effects on the mechanical and viscoelastic properties of renewable polyurethanes derived from isohexides and hydroxymethylfurfural. Polym. Chem. 2017, 8, 4822–4829. [Google Scholar] [CrossRef]
- Biswas, C.S.; Mitra, K.; Singh, S.; Ramesh, K.; Misra, N.; Maiti, B.; Panda, A.K.; Maiti, P.; Kamigaito, M.; Okamoto, Y.; et al. Study of the effect of isotacticity on some physical properties of poly(N-isopropylacrylamide). Colloid. Polym. Sci. 2015, 293, 1749–1757. [Google Scholar] [CrossRef]
- Hamieh, T.; Toufaily, J.; Mouneimné, A.B. Effect of the Tacticity of PMMA Adsorbed on Alumina and Silica on the Specific Entropy Change of Polymer by Inverse GC. Chromatographia 2011, 73, 99–107. [Google Scholar] [CrossRef]
- Hamieh, T.; Fadlallah, M.B.; Schultz, J. New approach to characterise physicochemical properties of solid substrates by inverse gas chromatography at infinite dilution: III. Determination of the acid–base properties of some solid substrates (polymers, oxides and carbon fibres): A new model. J. Chromatogr. A 2002, 969, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Okumura, Y.; Kitajima, H.; Seno, M.; Sato, T. Dual roles of alkyl alcohols as syndiotactic-specificity inducers and accelerators in the radical polymerization of N-isopropylacrylamide and some properties of syndiotactic poly(N-isopropylacrylamide). J. Polym. Sci. A Polym. Chem. 2006, 44, 4450–4460. [Google Scholar] [CrossRef]
- Hirano, T.; Miki, H.; Seno, M.; Sato, T. Effect of polymerization conditions on the syndiotactic-specificity in radical polymerization of N-isopropylacrylamide and fractionation of the obtained polymer according to the stereoregularity. Polymer 2005, 46, 5501–5505. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Panayiotou, C. Assessment of the thermodynamic properties of poly(2,2,2-trifluoroethyl methacrylate) by inverse gas chromatography. J. Chromatogr. A 2014, 1324, 207–214. [Google Scholar] [CrossRef]
- Saint-Flour, C.; Papirer, E.; Gas-solid chromatography. A method of measuring surface free energy characteristics of short carbon fibers. 1. Through adsorption isotherms. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 337–341. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Park, S.; Balard, H. Evaluation of specific interactions of solid surfaces by inverse gas chromatography. Chromatographia 1991, 31, 434–440. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A new topological index for molecular probes used in inverse gas chromatography for the surface nanorugosity evaluation, 2. Application for the Evaluation of the Solid Surface Specific Interaction Potential. J. Colloid. Interface Sci. 1997, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T. New Progress on London Dispersive Energy, Polar Surface Interactions, and Lewis’s Acid–Base Properties of Solid Surfaces. Molecules 2024, 29, 949. [Google Scholar] [CrossRef]
- Hamieh, T. Inverse Gas Chromatography to Characterize the Surface Properties of Solid Materials. Chem. Mater. 2024, 36, 2231–2244. [Google Scholar] [CrossRef]
- Hamieh, T. Study of the temperature effect on the surface area of model organic molecules, the dispersive surface energy and the surface properties of solids by inverse gas chromatography. J. Chromatogr. A 2020, 1627, 461372. [Google Scholar] [CrossRef]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33, 8–26. [Google Scholar] [CrossRef]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Plenum: New York, NY, USA, 1978. [Google Scholar]
- Riddle, F.L.; Fowkes, F.M. Spectral shifts in acid-base chemistry. Van der Waals contributions to acceptor numbers, Spectral shifts in acid-base chemistry. 1. van der Waals contributions to acceptor numbers. J. Am. Chem. Soc. 1990, 112, 3259–3264. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, Internet Version 2007, 87th ed.; Taylor and Francis: Boca Raton, FL, USA, 2007; Available online: http://www.hbcpnetbase.com (accessed on 1 November 2023).
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]




| Transition Temperature | |||
|---|---|---|---|
| Atactic PMMA | 60 °C | 110 °C | 160 °C |
| Isotactic PMMA | 60 °C | 120 °C | 170 °C |
| Syndiotactic PMMA | 70 °C | 130° C | 170 °C |
| Atactic PMMA | ||
|---|---|---|
| Equation of | R2 | Temperature interval |
| 0.935 | 303.15–333.15 K | |
| 0.9722 | 333.15–378.15 K | |
| 0.9477 | 378.15–423.15 K | |
| 0.9902 | 423.15–473.15 K | |
| Isotactic PMMA | ||
| Equation of | R2 | Temperature interval |
| 0.9398 | 303.15–333.15 K | |
| 0.9557 | 333.15–393.15 K | |
| 0.9925 | 393.15–443.15 K | |
| 0.9896 | 443.15–473.15 K | |
| Syndiotactic PMMA | ||
| Equation of | R2 | Temperature interval |
| 0.9485 | 303.15–343.15 K | |
| 0.9900 | 343.15–403.15 K | |
| 0.9569 | 403.15–443.15 K | |
| 1.000 | 443.15–473.15 K | |
| R2 | Temperature Interval | ||
|---|---|---|---|
| Atactic PMMA | 0.8368 | 303.15–473.15 K | |
| Isotactic PMMA | 0.8491 | 303.15–473.15 K | |
| Syndiotactic PMMA | 0.8368 | 303.15–473.15 K |
| Atactic PMMA | 144.37 | 494.1 | |
| Isotactic PMMA | 143.16 | 472.6 | |
| Syndiotactic PMMA | 120.8 | 503.8 |
| Atactic PMMA | Isotactic PMMA | Syndiotactic PMMA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T(K) | |||||||||
| 303.15 | 203.75 | 108.73 | 297.68 | 123.27 | 90.13 | 210.82 | 151.82 | 69.97 | 206.14 |
| 313.15 | 183.42 | 101.70 | 273.16 | 115.44 | 84.06 | 197.02 | 143.04 | 65.44 | 193.50 |
| 323.15 | 169.77 | 96.59 | 256.11 | 109.56 | 79.16 | 186.25 | 133.06 | 59.99 | 178.69 |
| 328.15 | 167.38 | 95.20 | 252.46 | 112.78 | 80.16 | 190.16 | 130.89 | 59.37 | 176.30 |
| 333.15 | 171.41 | 95.61 | 256.03 | 125.91 | 86.53 | 208.76 | 129.50 | 59.34 | 175.32 |
| 338.15 | 156.91 | 90.97 | 238.96 | 106.91 | 75.36 | 179.52 | 130.68 | 61.25 | 178.94 |
| 343.15 | 146.17 | 87.28 | 225.89 | 100.41 | 71.04 | 168.91 | 133.41 | 64.53 | 185.56 |
| 348.15 | 137.38 | 84.13 | 215.02 | 95.56 | 67.65 | 160.81 | 127.25 | 60.68 | 175.74 |
| 353.15 | 130.01 | 81.38 | 205.72 | 89.82 | 63.79 | 151.39 | 119.60 | 55.66 | 163.18 |
| 363.15 | 118.03 | 76.64 | 190.22 | 84.91 | 59.76 | 142.47 | 110.49 | 50.45 | 149.32 |
| 373.15 | 111.70 | 73.58 | 181.32 | 83.16 | 57.51 | 138.31 | 105.40 | 48.28 | 142.68 |
| 378.15 | 118.18 | 74.88 | 188.14 | 85.83 | 58.30 | 141.48 | 102.57 | 46.95 | 138.79 |
| 383.15 | 129.80 | 77.62 | 200.74 | 87.09 | 58.32 | 142.53 | 100.32 | 46.05 | 135.93 |
| 388.15 | 107.09 | 70.48 | 173.76 | 89.07 | 58.72 | 144.64 | 99.29 | 46.10 | 135.31 |
| 393.15 | 94.01 | 65.98 | 157.52 | 93.16 | 60.20 | 149.77 | 99.14 | 46.82 | 136.26 |
| 398.15 | 87.01 | 63.28 | 148.40 | 84.75 | 55.19 | 136.79 | 102.80 | 50.62 | 144.27 |
| 403.15 | 81.01 | 60.87 | 140.44 | 80.55 | 52.40 | 129.94 | 101.53 | 50.77 | 143.59 |
| 408.15 | 80.65 | 61.82 | 141.22 | 77.02 | 49.97 | 124.08 | 101.82 | 51.59 | 144.95 |
| 413.15 | 73.10 | 57.31 | 129.46 | 74.87 | 48.29 | 120.25 | 92.89 | 45.13 | 129.50 |
| 423.15 | 73.07 | 56.31 | 128.29 | 72.51 | 45.96 | 115.46 | 85.25 | 40.62 | 117.70 |
| 433.15 | 78.33 | 57.00 | 133.64 | 70.27 | 43.74 | 110.89 | 83.23 | 40.53 | 116.15 |
| 443.15 | 56.38 | 48.50 | 104.59 | 62.22 | 38.64 | 98.07 | 94.44 | 48.59 | 135.48 |
| 453.15 | 45.40 | 43.84 | 89.22 | 60.93 | 37.02 | 94.99 | 73.62 | 36.11 | 103.13 |
| 463.15 | 39.51 | 40.86 | 80.36 | 62.44 | 36.80 | 95.87 | 71.77 | 36.02 | 101.68 |
| 473.15 | 30.81 | 36.16 | 66.75 | 56.99 | 33.15 | 86.93 | 63.57 | 31.50 | 89.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamieh, T. Effect of Tacticity on London Dispersive Surface Energy, Polar Free Energy and Lewis Acid-Base Surface Energies of Poly Methyl Methacrylate by Inverse Gas Chromatography. Macromol 2024, 4, 356-375. https://doi.org/10.3390/macromol4020020
Hamieh T. Effect of Tacticity on London Dispersive Surface Energy, Polar Free Energy and Lewis Acid-Base Surface Energies of Poly Methyl Methacrylate by Inverse Gas Chromatography. Macromol. 2024; 4(2):356-375. https://doi.org/10.3390/macromol4020020
Chicago/Turabian StyleHamieh, Tayssir. 2024. "Effect of Tacticity on London Dispersive Surface Energy, Polar Free Energy and Lewis Acid-Base Surface Energies of Poly Methyl Methacrylate by Inverse Gas Chromatography" Macromol 4, no. 2: 356-375. https://doi.org/10.3390/macromol4020020
APA StyleHamieh, T. (2024). Effect of Tacticity on London Dispersive Surface Energy, Polar Free Energy and Lewis Acid-Base Surface Energies of Poly Methyl Methacrylate by Inverse Gas Chromatography. Macromol, 4(2), 356-375. https://doi.org/10.3390/macromol4020020







