Development of a Polyethylene Breathable Packaging Film with Modified Microcrystalline Cellulose for Fresh Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
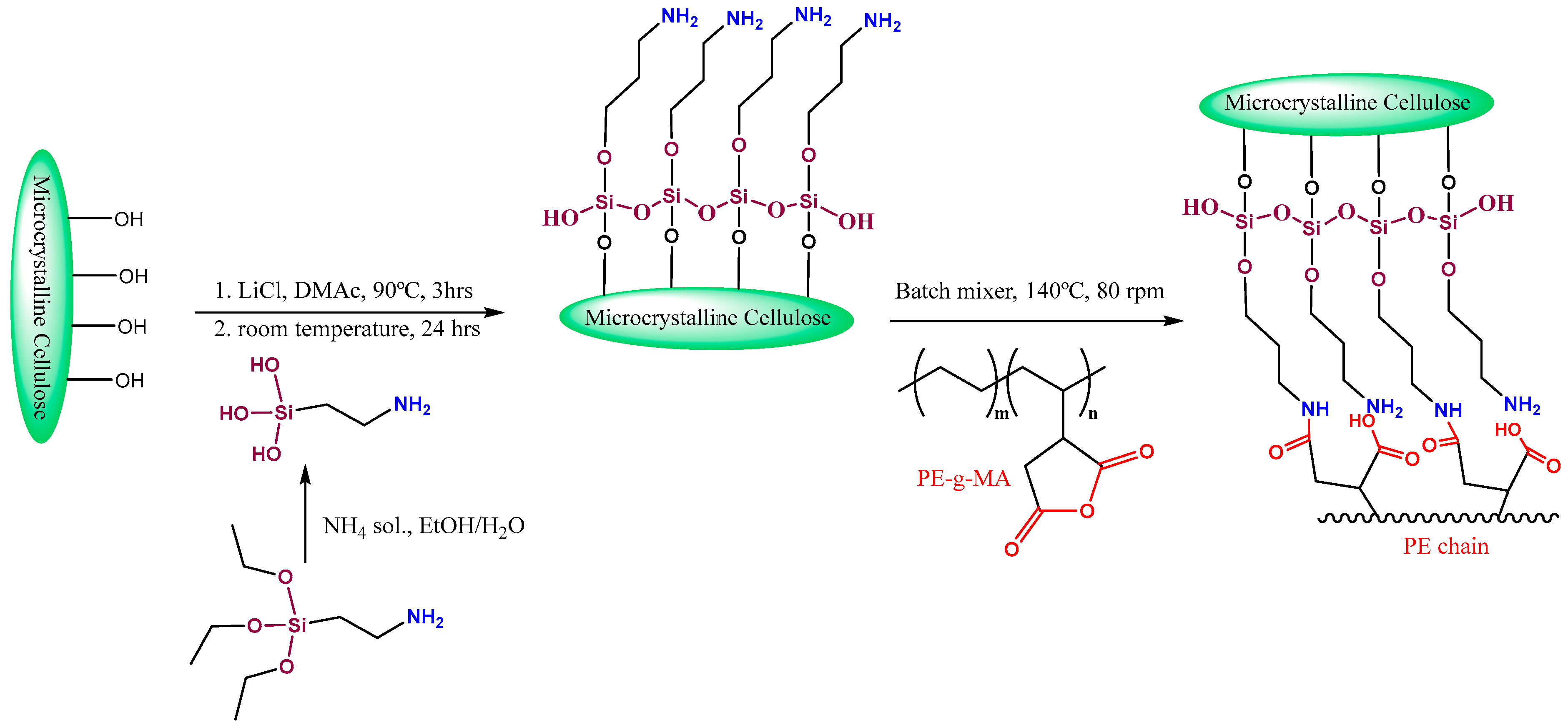
2.2.1. Microcrystalline Cellulose Modification with 3-Aminopropyltriethoxysilane
2.2.2. Microcomposite Preparation
2.3. Characterization
2.3.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.2. Thermal Analysis
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Energy Dispersive X-ray (EDX) Analysis
2.3.5. Dynamic Mechanical Analysis (DMA)
2.3.6. Contact Angle Measurements (CA)
2.3.7. Water Vapor Permeability (WVP)
2.3.8. Statistical Analysis
3. Results and Discussion
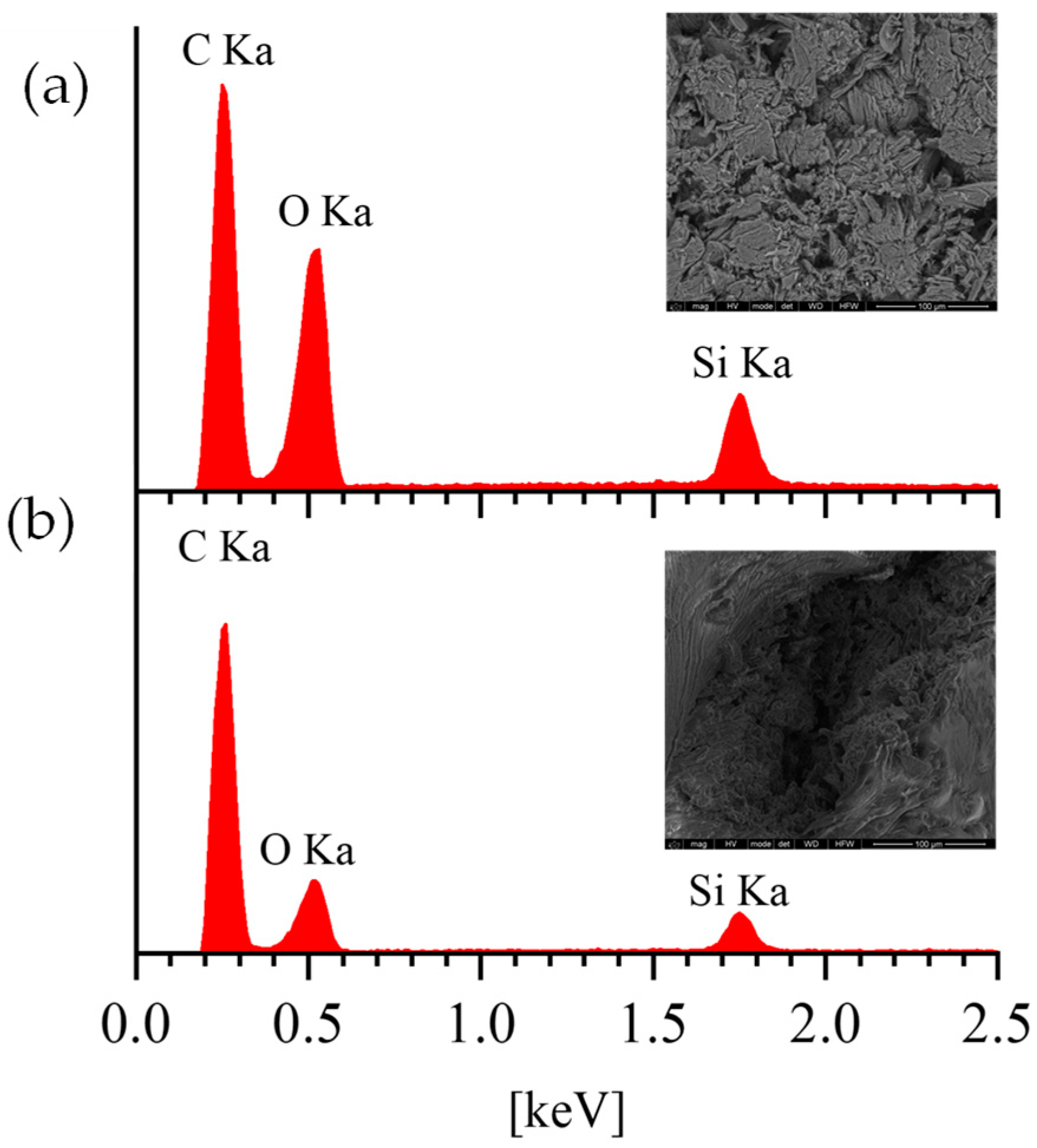
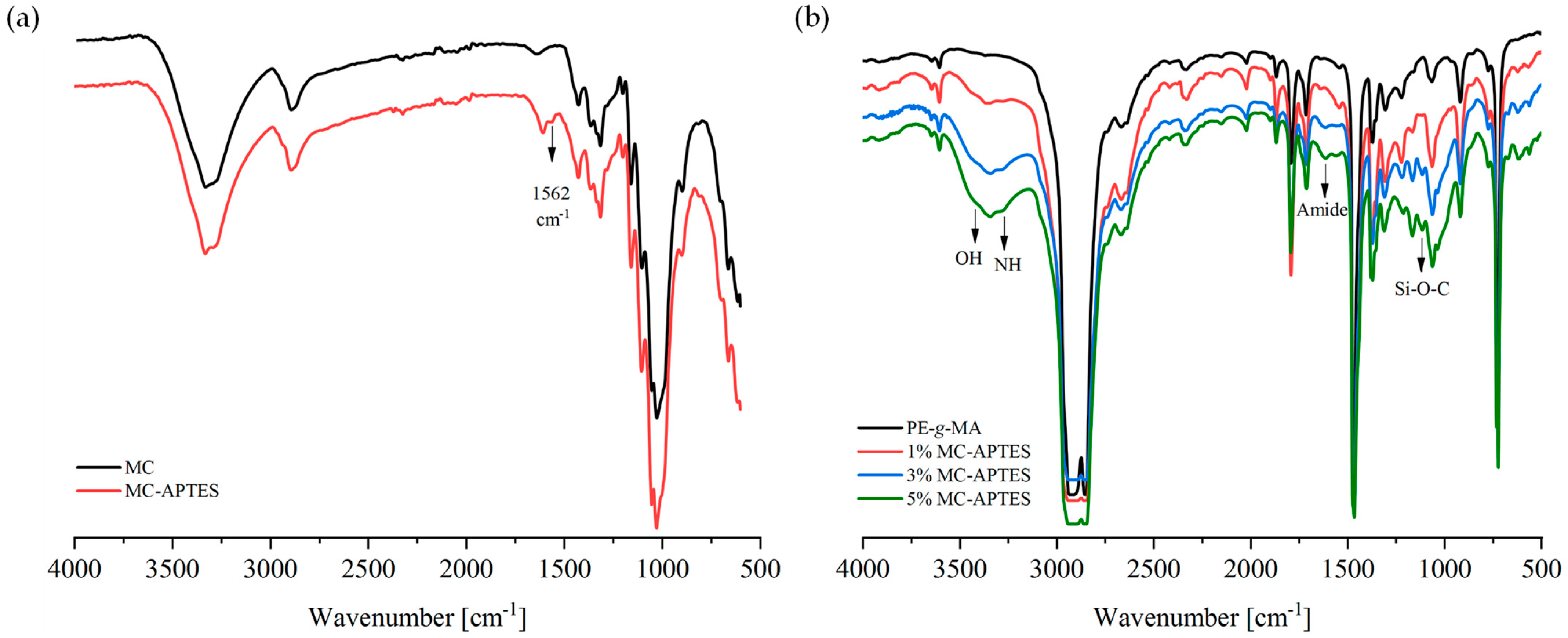
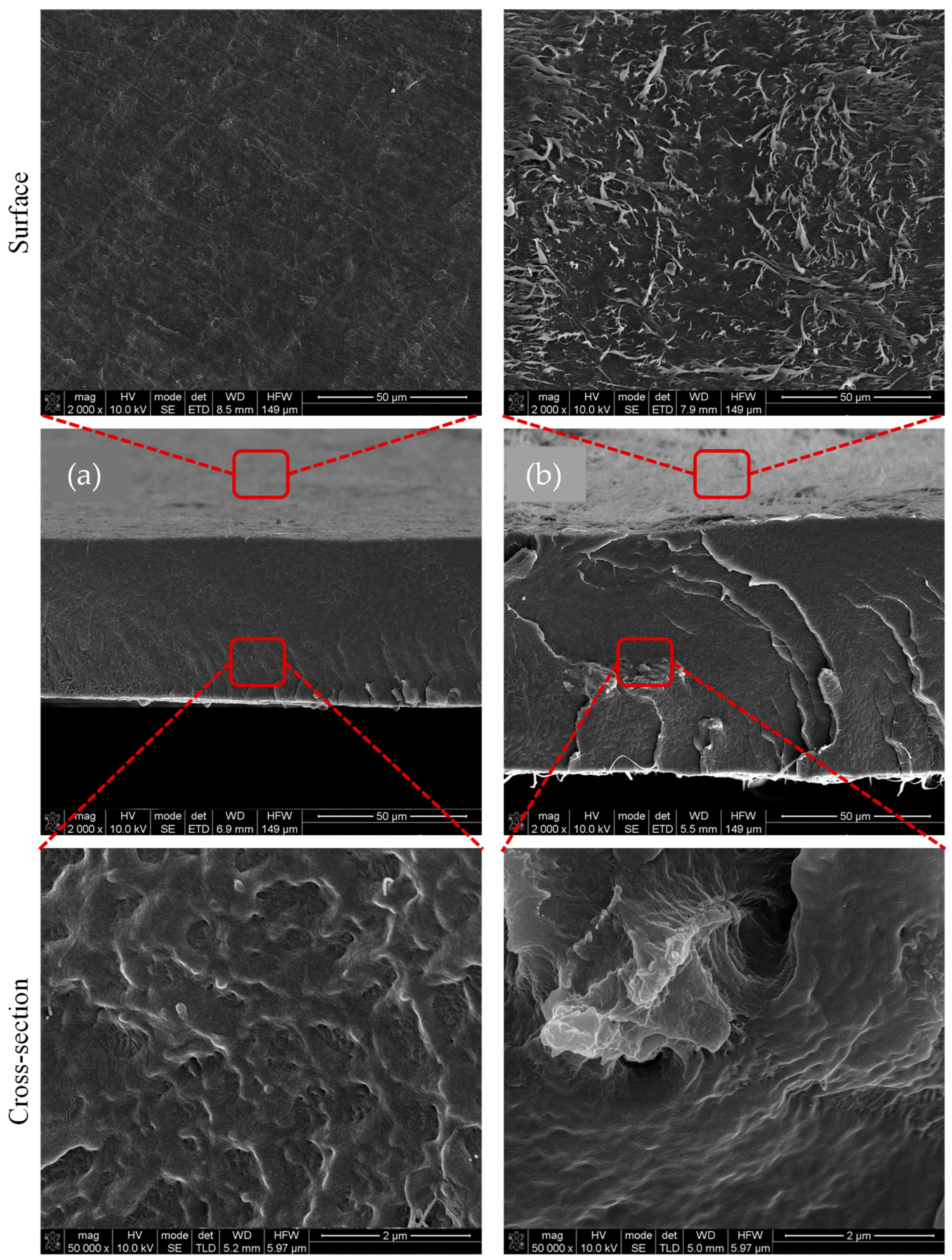
3.1. Structural and Morphological Analysis
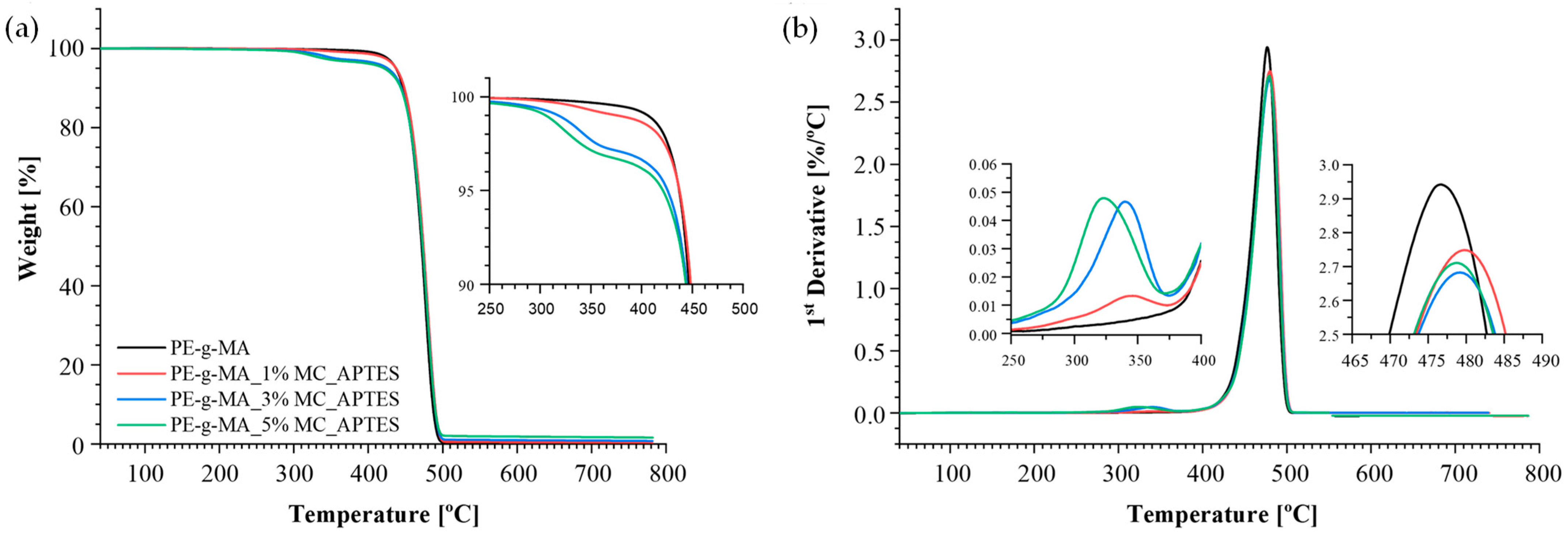
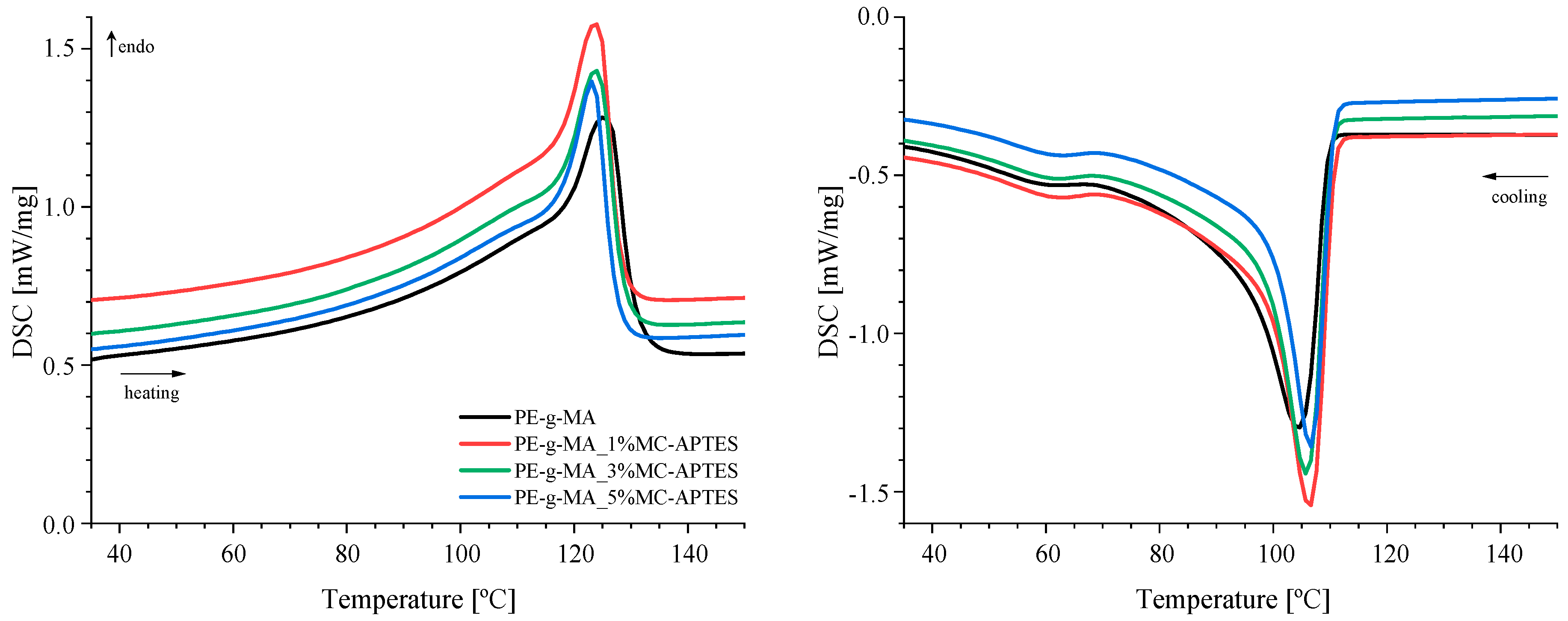
3.2. Thermal and Mechanical Analysis
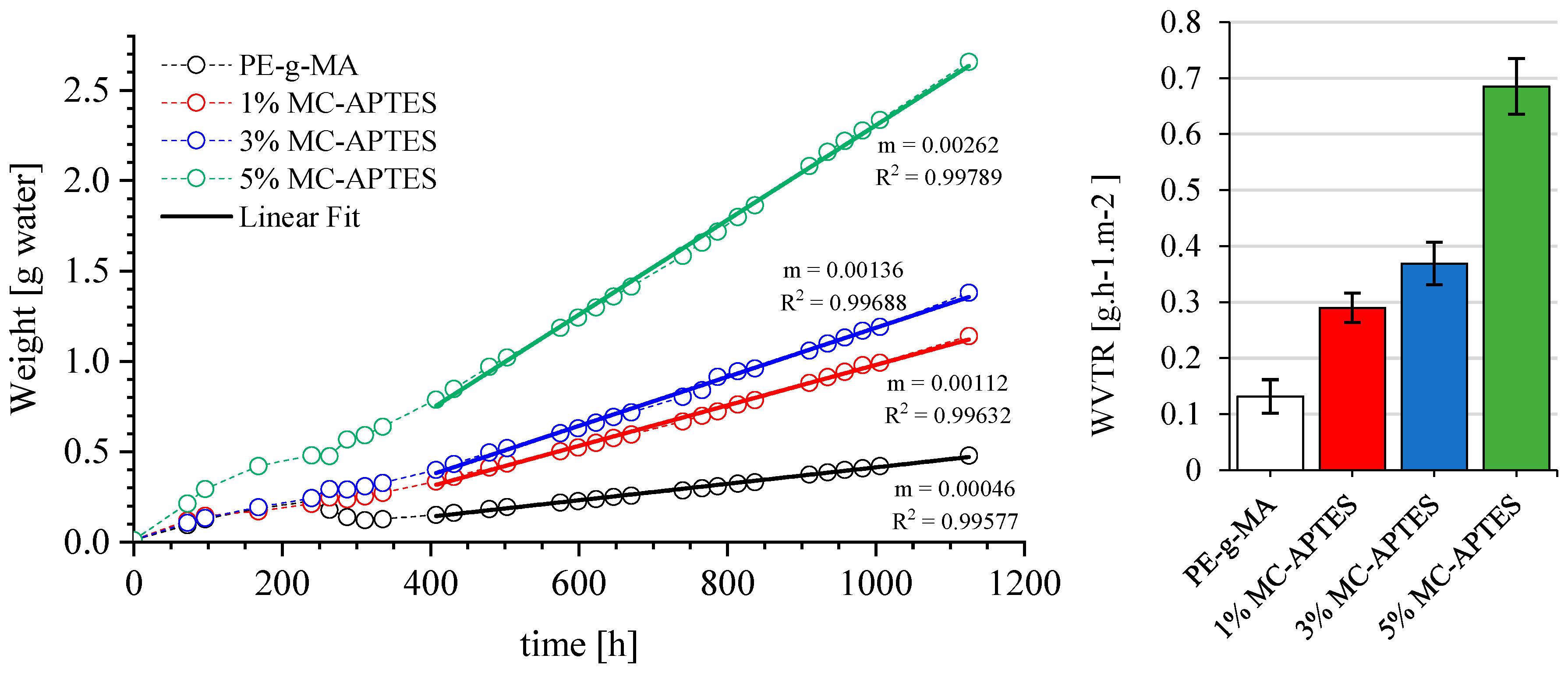
3.3. Water Affinity Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Czerwiński, K.; Rydzkowski, T.; Wróblewska-Krepsztul, J.; Thakur, V.K. Towards Impact of Modified Atmosphere Packaging (MAP) on Shelf-Life of Polymer-Film-Packed Food Products: Challenges and Sustainable Developments. Coatings 2021, 11, 1504. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.-J.; Opara, U.L. Modified Atmosphere Packaging Technology of Fresh and Fresh-cut Produce and the Microbial Consequences—A Review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hamad, W. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Yano, H.; Omura, H.; Honma, Y.; Okumura, H.; Sano, H.; Nakatsubo, F. Designing cellulose nanofiber surface for high density polyethylene reinforcement. Cellulose 2018, 25, 3351–3362. [Google Scholar] [CrossRef]
- Zimniewska, M.; Wladyka-Przybylak, M.; Mankowski, J. Cellulosic Bast Fibers, Their Structure and Properties Suitable for Composite Applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites—Green Chemistry and Technology, 1st ed.; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 97–119. [Google Scholar]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-reinforced polymer composites: Manufacturing, properties, and applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Mohit, H.; Arul Mozhi Selvan, V. A comprehensive review on surface modification, structure interface and bonding mechanism of plant cellulose fiber reinforced polymer based composites. Compos. Interfaces 2018, 25, 629–667. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, M.; Jafari, S.; van de Ven, T. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Jayasuriya, C.K. Interfacial Bonding in Polymer–Ceramic Nanocomposites. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, S.-M.; Ma, M.-G.; Zhu, J.; Sun, R.-C. Synthesis and characterization of cellulose-silica composite fiber in ethanol/water mixed solvents. Bioresources 2011, 6, 1186–1195. [Google Scholar] [CrossRef]
- Tarani, E.; Arvanitidis, I.; Christofilos, D.; Bikiaris, D.N.; Chrissafis, K.; Vourlias, G. Calculation of the degree of crystallinity of HDPE/GNPs nanocomposites by using various experimental techniques: A comparative study. J. Mater. Sci. 2023, 58, 1621–1639. [Google Scholar] [CrossRef]
- Mirabella, F.; Bafna, A. Determination of the crystallinity of polyethylene/-olefin copolymers by thermal analysis: Relationship of the heat of fusion of 100% polyethylene crystal and the density. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1637–1643. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2015.
- Kotov, N.; Raus, V.; Dybal, J. Intermolecular Interactions in N,N-Dimethylacetamide without and with LiCl Studied by Infrared Spectroscopy and Quantum Chemical Model Calculations. J. Phys. Chem. B 2018, 122, 8921–8930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, X.; Bao, J.; Xie, J.; Tang, X.; Che, J.; Ma, Y.; Tong, J. Characterization of Silane Treated and Untreated Natural Cellulosic Fibre from Corn Stalk Waste as Potential Reinforcement in Polymer Composites. Carbohydr. Polym. 2019, 218, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Oksman, K. The use of silane technology in crosslinking polyethylene/wood flour composites. Composites Part A Appl. Sci. Manuf. 2006, 37, 752–765. [Google Scholar] [CrossRef]
- Lomakin, S.M.; Rogovina, S.Z.; Grachev, A.V.; Prut, E.V.; Alexanyan, C.V. Thermal degradation of biodegradable blends of polyethylene with cellulose and ethylcellulose. Thermochim. Acta 2011, 521, 66–73. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. BioResources 2017, 12, 91. [Google Scholar] [CrossRef]
- Dang, X.; Cao, X.; Ke, L.; Ma, Y.; An, J.; Wang, F. Combination of cellulose nanofibers and chain-end-functionalized polyethylene and their applications in nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45387. [Google Scholar] [CrossRef]
- da S. Junior, O.G.; de Melo, R.P.; do B.C. Sales, R.; Ayres, E.; de O. Patricio, P.S. Processing and characterization of polyethylene/starch/curauá composites: Potential for application as thermal insulated coating. J. Build. Eng. 2017, 11, 178–186. [Google Scholar]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of Food Packaging Based on Nanocellulose: Current Advances and Challenges. Nanomaterials 2020, 10, 1726. [Google Scholar] [CrossRef] [PubMed]



| Composition Code | Weight (%) | Processing Conditions | |||
|---|---|---|---|---|---|
| PE-g-MA | MA-APTES | Tm (°C) | Rotors Speed (rpm) | tmixing (min) | |
| PE-g-MA_1% MC-APTES | 99 | 1 | 140 | 80 | 2.5 mixing + 7 reaction |
| PE-g-MA_3% MC-APTES | 97 | 3 | |||
| PE-g-MA_5% MC-APTES | 95 | 5 | |||
| Sample | ΔHm [J/g] | Χc [%] | Tm [°C] | Tc [°C] |
|---|---|---|---|---|
| PE-g-MA | 123.9 | 42.3 | 125 | 105 |
| 1% MC-APTES | 126.8 | 43.7 | 124 | 107 |
| 3% MC-APTES | 120.3 | 42.3 | 124 | 106 |
| 5% MC-APTES | 111.8 | 40.2 | 123 | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.V.; Castro, M.C.R.; Soares, A.M.S.; Melro, L.; Machado, A.V. Development of a Polyethylene Breathable Packaging Film with Modified Microcrystalline Cellulose for Fresh Products. Macromol 2024, 4, 269-281. https://doi.org/10.3390/macromol4020015
Rodrigues PV, Castro MCR, Soares AMS, Melro L, Machado AV. Development of a Polyethylene Breathable Packaging Film with Modified Microcrystalline Cellulose for Fresh Products. Macromol. 2024; 4(2):269-281. https://doi.org/10.3390/macromol4020015
Chicago/Turabian StyleRodrigues, Pedro V., M. Cidália R. Castro, Ana M. S. Soares, Liliana Melro, and Ana V. Machado. 2024. "Development of a Polyethylene Breathable Packaging Film with Modified Microcrystalline Cellulose for Fresh Products" Macromol 4, no. 2: 269-281. https://doi.org/10.3390/macromol4020015
APA StyleRodrigues, P. V., Castro, M. C. R., Soares, A. M. S., Melro, L., & Machado, A. V. (2024). Development of a Polyethylene Breathable Packaging Film with Modified Microcrystalline Cellulose for Fresh Products. Macromol, 4(2), 269-281. https://doi.org/10.3390/macromol4020015







