Thermal and Mechanical Properties of Guaiacol–Fatty Acid–Sulfur Composites
Abstract
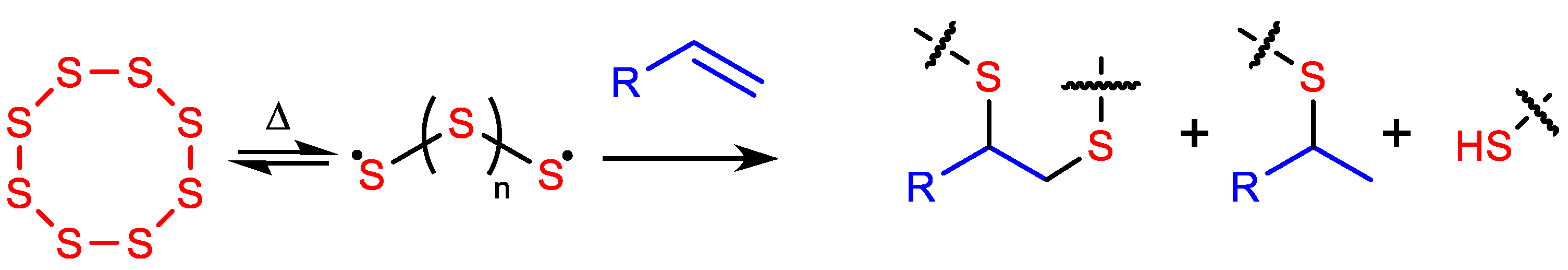
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials and Precautions
2.3. Synthesis
2.3.1. GO1S70
2.3.2. GO5S70
2.3.3. GO10S80
2.3.4. GL1S70
2.3.5. GL5S70
2.3.6. GL10S80
3. Results and Discussion
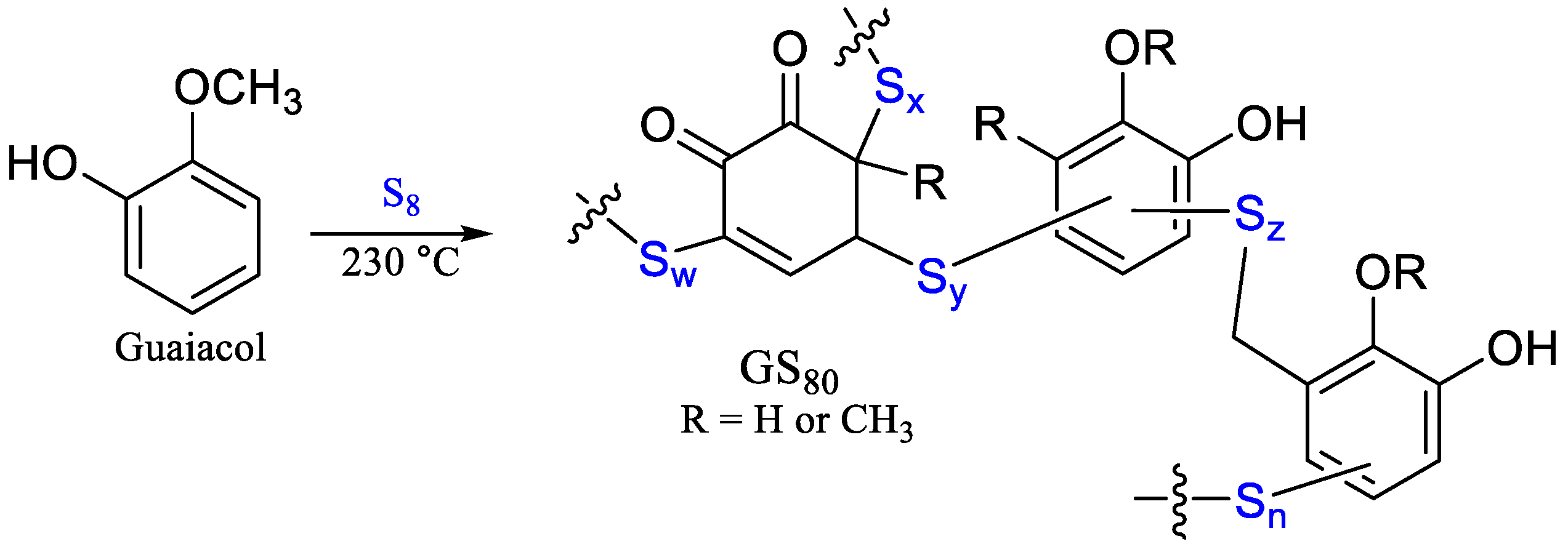
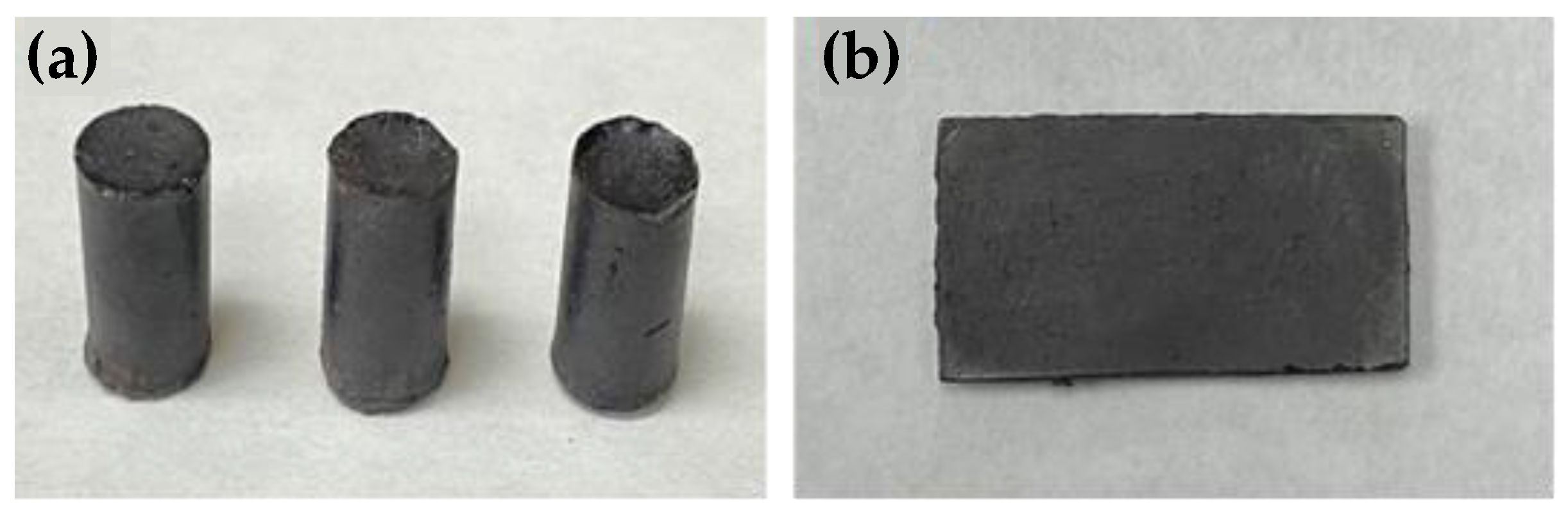
3.1. Synthesis and Surface Properties
3.2. Thermal Properties
3.3. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Merino-Saum, A.; Clement, J.; Wyss, R.; Baldi, M.G. Unpacking the Green Economy concept: A quantitative analysis of 140 definitions. J. Clean. Prod. 2020, 242, 118339. [Google Scholar] [CrossRef]
- Loiseau, E.; Saikku, L.; Antikainen, R.; Droste, N.; Hansjürgens, B.; Pitkänen, K.; Leskinen, P.; Kuikman, P.; Thomsen, M. Green economy and related concepts: An overview. J. Clean. Prod. 2016, 139, 361–371. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Maladeniya, C.P.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Durable Composites by Vulcanization of Oleyl-Esterified Lignin. RSC Adv. 2023, 13, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur-lignin copolymers. J. Mater. Chem. A 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of Lignin as a Sustainable Component of Structural Materials and Composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Thi, H.D.; Van Aelst, K.; Van den Bosch, S.; Katahira, R.; Beckham, G.T.; Sels, B.F.; Van Geem, K.M. Identification and quantification of lignin monomers and oligomers from reductive catalytic fractionation of pine wood with GC × GC–FID/MS. Green. Chem. 2022, 24, 191–206. [Google Scholar]
- Van Aelst, K.; Van Sinay, E.; Vangeel, T.; Cooreman, E.; Van den Bossche, G.; Renders, T.; Van Aelst, J.; Van den Bosch, S.; Sels, B. Reductive catalytic fractionation of pine wood: Elucidating and quantifying the molecular structures in the lignin oil. Chem. Sci. 2020, 11, 11498–11508. [Google Scholar] [CrossRef]
- Ebikade, E.O.; Samulewicz, N.; Xuan, S.; Sheehan, J.D.; Wu, C.; Vlachos, D.G. Reductive catalytic fractionation of agricultural residue and energy crop lignin and application of lignin oil in antimicrobials. Green. Chem. 2020, 22, 7435–7447. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, A.T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of corn stover lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Glass, R.S.; Char, K.; Pyun, J. Recent advances in the polymerization of elemental sulphur, inverse vulcanization and methods to obtain functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). Polym. Chem. 2019, 10, 4078–4105. [Google Scholar] [CrossRef]
- Kleine, T.S.; Glass, R.S.; Lichtenberger, D.L.; MacKay, M.E.; Char, K.; Norwood, R.A.; Pyun, J. 100th Anniversary of Macromolecular Science Viewpoint: High Refractive Index Polymers from Elemental Sulfur for Infrared Thermal Imaging and Optics. ACS Macro Lett. 2020, 9, 245–259. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Zhang, B.; Jiang, L.; Petcher, S.; Smith, J.A.; Parker, D.J.; Cooper, A.I.; Lei, J.; Hasell, T. Inverse vulcanized polymers with shape memory, enhanced mechanical properties, and vitrimer behavior. Angew. Chem. Int. Ed. 2020, 59, 13371–13378. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green. Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and Applications of Polymers Made by Inverse Vulcanization. Top. Curr. Chem. 2019, 377, 16. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Carbon-Negative Polymer Cements by Copolymerization of Waste Sulfur, Oleic Acid, and Pozzolan Cements. Sust. Chem. Pharm. 2020, 16, 100249. [Google Scholar] [CrossRef]
- Gutarowska, B.; Piotrowska, M.; Kozirog, A.; Berlowska, J.; Dziugan, P.; Kotynia, R.; Bielinski, D.; Anyszka, R.; Wreczycki, J. New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion. Materials 2019, 12, 2602. [Google Scholar] [CrossRef]
- Mohammed, S.; Poornima, V. Strength and durability study of sulphur concrete with replaced fine aggregate. Mater. Today Proc. 2018, 5, 23888–23897. [Google Scholar] [CrossRef]
- Mohamed, A.-M.O.; Gamal, M.E. Sulfur Concrete for the Construction Industry; Ross, J., Ed.; J. Ross Publishing: Fort Lauderdale, FL, USA, 2010; p. 424. [Google Scholar]
- Okumura, H.A. Early sulfur concrete installations. Concr. Int. 1998, 20, 72–75. [Google Scholar]
- Weber, H.H.; McBee, W.C.; Krabbe, E.A. Sulfur concrete composite materials for construction and maintenance. Mater. Perform. 1990, 29, 73–77. [Google Scholar]
- Pickard, S.S. Sulfur concrete for acid resistance. Chem. Eng. 1985, 92, 77–78+80. [Google Scholar]
- Smith, A.D.; Thiounn, T.; Lyles, E.W.; Kibler, E.K.; Smith, R.C.; Tennyson, A.G. Combining agriculture and energy industry waste products to yield recyclable, thermally healable copolymers of elemental sulfur and oleic acid. J. Poly. Sci. A 2019, 57, 1704–1710. [Google Scholar] [CrossRef]
- Michal, B.T.; Jaye, C.A.; Spencer, E.J.; Rowan, S.J. Inherently Photohealable and Thermal Shape-Memory Polydisulfide Networks. ACS Macro Lett. 2013, 2, 694–699. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; MacKay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Stimuli responsive self-healing polymers: Gels, elastomers and membranes. Polym. Chem. 2017, 8, 6464–6484. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Recycling and Self-Healing of Polybenzoxazines with Dynamic Sulfide Linkages. Sci. Rep. 2017, 7, 5207. [Google Scholar] [CrossRef]
- Takahashi, A.; Goseki, R.; Ito, K.; Otsuka, H. Thermally Healable and Reprocessable Bis(hindered amino)disulfide-Cross-Linked Polymethacrylate Networks. ACS Macro Lett. 2017, 6, 1280–1284. [Google Scholar] [CrossRef]
- Smith, A.D.; McMillin, C.D.; Smith, R.C.; Tennyson, A.G. Copolymers by Inverse Vulcanization of Sulfur with Pure or Technical Grade Unsaturated Fatty Acids. J. Poly. Sci. 2020, 58, 438–445. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sus. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 10035–10044. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef]
- Zhang, B.; Petcher, S.; Hasell, T. A ternary system for delayed curing inverse vulcanisation. Chem. Commun. 2019, 55, 10681–10684. [Google Scholar] [CrossRef]
- Westerman Clayton, R.; Walker Princess, M.; Jenkins Courtney, L. Synthesis of Terpolymers at Mild Temperatures Using Dynamic Sulfur Bonds in Poly(S-Divinylbenzene). J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Sahu, S.; Lochab, B. Facile Strategy for Room Temperature Knitting of Sulfur in Polybenzoxazine: A New Class of Solution Processable Copolymers. ACS Sustain. Chem. Eng. 2022, 10, 12355–12364. [Google Scholar] [CrossRef]
- Mann, M.; Pauling, P.J.; Tonkin, S.J.; Campbell, J.A.; Chalker, J.M. Chemically Activated S-S Metathesis for Adhesive-Free Bonding of Polysulfide Surfaces. Macromol. Chem. Phys. 2022, 223, 2100333. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem. A Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef]
- Jia, J.; Liu, J.; Wang, Z.-Q.; Liu, T.; Yan, P.; Gong, X.-Q.; Zhao, C.; Chen, L.; Miao, C.; Zhao, W.; et al. Photoinduced inverse vulcanization. Nat. Chem. 2022, 14, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.-J.; van der Laan, J.; Nguyen, N.A.; et al. New Infrared Transmitting Material via Inverse Vulcanization of Elemental Sulfur to Prepare High Refractive Index Polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li-S Batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Dirlam, P.T.; Simmonds, A.G.; Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Klever, A.O.; Florian, A.; Costanzo, P.J.; Theato, P.; Mackay, M.E.; et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li-S batteries. RSC Adv. 2015, 5, 24718–24722. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Poly. Sci. A 2015, 53, 173–177. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Combining Elemental Sulfur with Polybenzoxazines via Inverse Vulcanization. Macromolecules 2016, 49, 767–773. [Google Scholar] [CrossRef]
- Ng, P.C.; Hendry-Hofer, T.B.; Witeof, A.E.; Brenner, M.; Mahon, S.B.; Boss, G.R.; Haouzi, P.; Bebarta, V.S. Hydrogen sulfide toxicity: Mechanism of action, clinical presentation, and countermeasure development. J. Med. Toxicol. 2019, 15, 287–294. [Google Scholar] [CrossRef]
- Beauchamp, R.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Truong, D.H.; Eghbal, M.A.; Hindmarsh, W.; Roth, S.H.; O’Brien, P.J. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab. Rev. 2006, 38, 733–744. [Google Scholar] [CrossRef]
- Yoon, K.-B.; Ryu, H.M.; Lee, G.H.; Gopalan, A.I.; Sai-anand, G.; Lee, D.-E. Enhanced compressive strength of rammed earth walls stabilized with eco-friendly multi-functional polymeric system. Renew. Sustain. Energy Rev. 2021, 152, 111681. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green. Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Meyer, B. Solid allotropes of sulfur. Chem. Rev. 1964, 64, 429–451. [Google Scholar] [CrossRef]
- Meyer, B.; Oommen, T.V.; Jensen, D. Color of liquid sulfur. J. Phys. Chem. 1971, 75, 912–917. [Google Scholar] [CrossRef]
- Meyer, C.B.; Stroyer-Hansen, T.; Jensen, D.; Oommen, T.V. Color of liquid sulfur. J. Amer. Chem. Soc. 1971, 93, 1034–1035. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable Polysulfides for Oil Spill Remediation: Repurposing Industrial Waste for Environmental Benefit. Adv. Sust. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying Waste to Mercury: Inexpensive Sorbents Made from Sulfur and Recycled Cooking Oils. Chem. A Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem. A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef]
- Hanna, V.; Yan, P.; Petcher, S.; Hasell, T. Incorporation of fillers to modify the mechanical performance of inverse vulcanised polymers. Polym. Chem. 2022, 13, 3930–3937. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Dodd, L.J.; Hasell, T. Processable crosslinked terpolymers made from elemental sulfur with wide range of thermal and mechanical properties. ChemRxiv 2023, 1–20. [Google Scholar] [CrossRef]
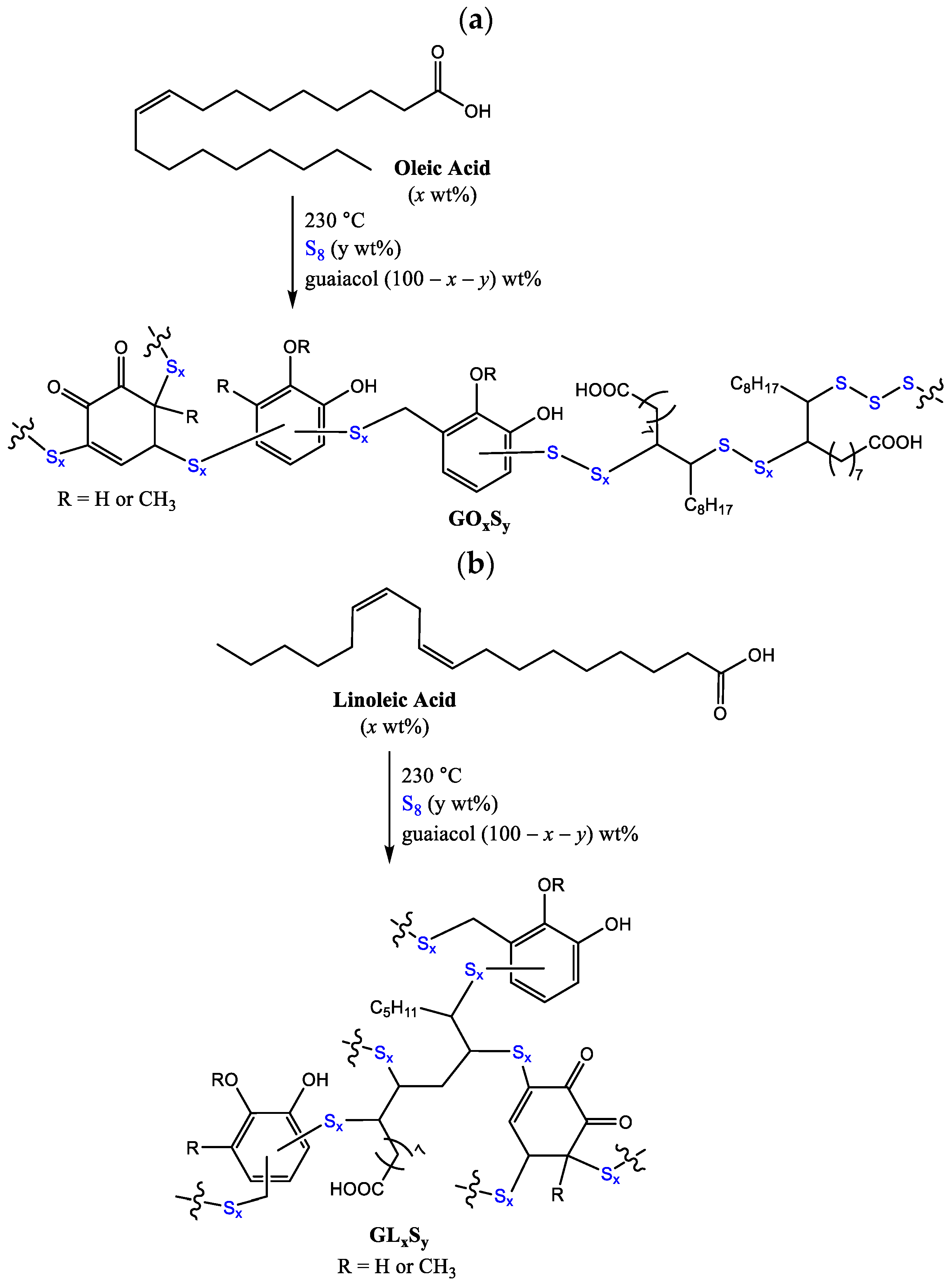


| Materials [a] | Guaiacol (wt%) | Oleic Acid (wt%) | Linoleic Acid (wt%) | Sulfur (wt%) | Reference |
|---|---|---|---|---|---|
| GO1S70 | 29 | 1 | 0 | 70 | This work |
| GO5S70 | 25 | 5 | 0 | 70 | This work |
| GO10S80 | 10 | 10 | 0 | 80 | This work |
| GL1S70 | 29 | 0 | 1 | 70 | This work |
| GL5S70 | 25 | 0 | 5 | 70 | This work |
| GL10S80 | 10 | 0 | 10 | 80 | This work |
| GS80 | 20 | 0 | 0 | 80 | [42] |
| ZOS90 [b] | 0 | 10 | 0 | 90 | [24,30] |
| ZPLS90 [b],[c] | 0 | 0 | 10 | 90 | [30] |
| ZPLS95 [b],[c] | 0 | 0 | 5 | 95 | [30] |
| ZPLS99 [b],[c] | 0 | 0 | 1 | 99 | [30] |
| Materials | Td/°C | Tg/°C | Tm/°C | Tcc/°C | Reference |
|---|---|---|---|---|---|
| S8 | 229 | NA | 119 | NA [a] | [2] |
| ZOS90 | 220 | –39 | 109 | NA [a] | [24,30] |
| ZPLS90 | 231 | –39 | 107 | 87 | [30] |
| ZPLS95 | 231 | –39 | 117 | 34 | [30] |
| ZPLS99 | 252 | –39 | 118 | NA [a] | [30] |
| GS80 | 264 | –30 | 107 | 57 | [42] |
| GO1S70 | 159 | –23 | 107 | 44 | This work |
| GO5S70 | 184 | –13 | 114 | NA [a] | This work |
| GO10S80 | 201 | –33 | 99 | NA [a] | This work |
| GL1S70 | 209 | –31 | 112 | 29 | This work |
| GL5S70 | 204 | –35 | 115 | 21 | This work |
| GL10S80 | 217 | –37 | 112 | NA [a] | This work |
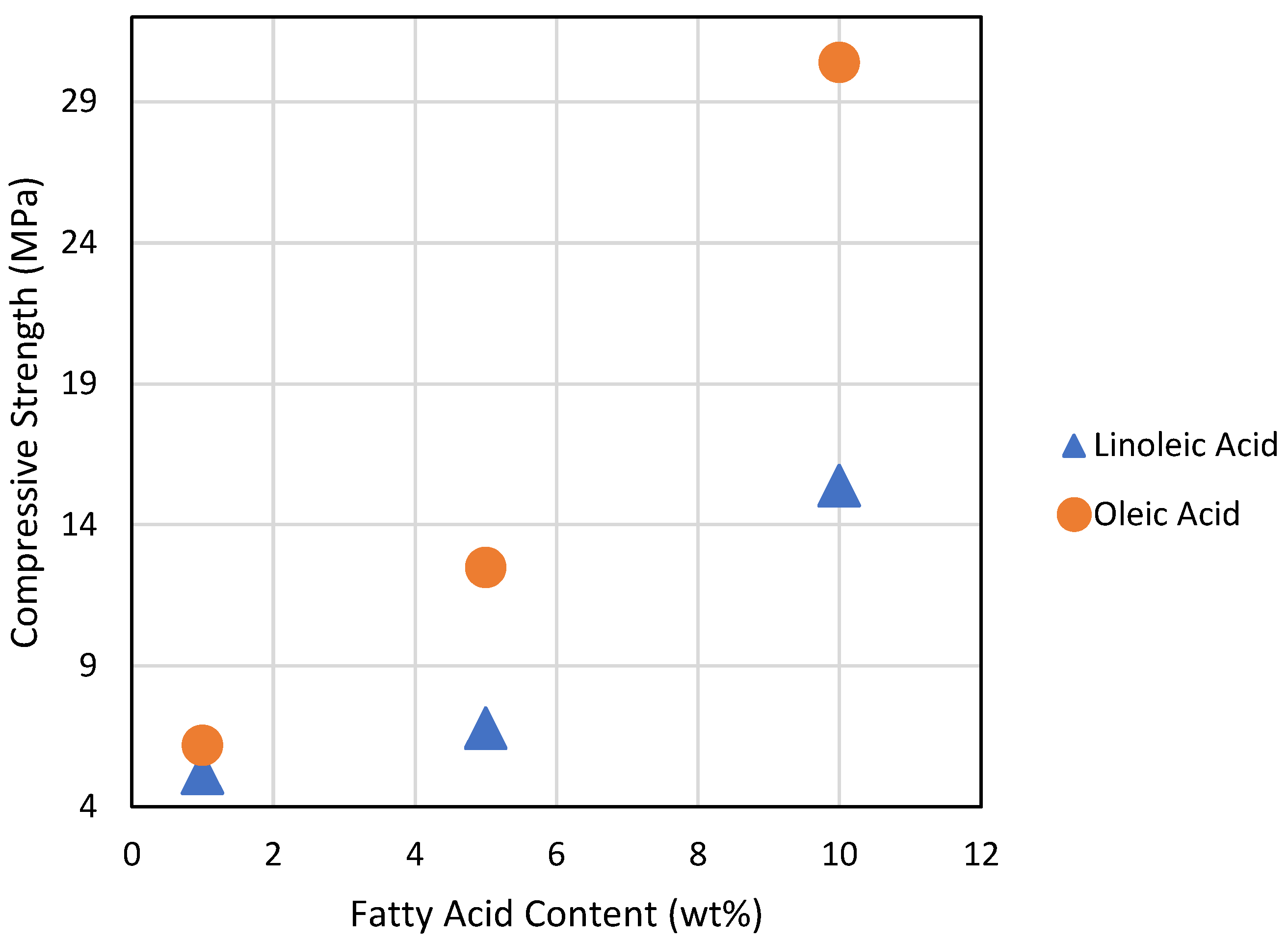
| Materials | Compressive Strength (MPa) | Flexural Strength (MPa) | Reference |
|---|---|---|---|
| S8 | ND [a] | <0.3 | [2] |
| GL10S80 | 15.4 ± 0.2 | 0.3 | This work |
| GO10S80 | 30.4 ± 0.5 | 0.3 | This work |
| GL5S70 | 6.8 ± 0.1 | 2.3 | This work |
| GO5S70 | 12.5 ± 0.2 | 1.9 | This work |
| GL1S70 | 5.2 ± 0.2 | 2.4 | This work |
| GO1S70 | 6.2 ± 0.1 | 1.9 | This work |
| GS80 | 3.2 ± 0.2 | ND | [42] |
| ZOS90 | 19.4 ± 1.8 | <0.3 | [24,30] |
| ZPLS90 | ND [a] | 2.0 | [30] |
| ZPLS95 | ND [a] | 1.6 | [30] |
| ZPLS99 | ND [a] | 1.3 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maladeniya, C.P.; Kapuge Dona, N.L.; Smith, A.D.; Smith, R.C. Thermal and Mechanical Properties of Guaiacol–Fatty Acid–Sulfur Composites. Macromol 2023, 3, 681-692. https://doi.org/10.3390/macromol3040038
Maladeniya CP, Kapuge Dona NL, Smith AD, Smith RC. Thermal and Mechanical Properties of Guaiacol–Fatty Acid–Sulfur Composites. Macromol. 2023; 3(4):681-692. https://doi.org/10.3390/macromol3040038
Chicago/Turabian StyleMaladeniya, Charini P., Nawoda L. Kapuge Dona, Ashlyn D. Smith, and Rhett C. Smith. 2023. "Thermal and Mechanical Properties of Guaiacol–Fatty Acid–Sulfur Composites" Macromol 3, no. 4: 681-692. https://doi.org/10.3390/macromol3040038
APA StyleMaladeniya, C. P., Kapuge Dona, N. L., Smith, A. D., & Smith, R. C. (2023). Thermal and Mechanical Properties of Guaiacol–Fatty Acid–Sulfur Composites. Macromol, 3(4), 681-692. https://doi.org/10.3390/macromol3040038








