1. Introduction
Cyanoacrylate adhesives are particularly attractive in recent years because of their quick polymerization at room temperature, absence of hardener constituent, manual easy and safe application in a variety of substrates, or their availability in a range of viscosities. Colorless and transparent in nature, they fill some of the conditions that restorative adhesives for cultural heritage preservation and artifacts’ conservation require [
1,
2]. Polycyanocacrylates are known as “instant glues”, especially for glass and ceramic substrates, with poly(ethyl 2-cyanoacrylate) (PECA)—a macromolecule of the corresponding ester CH
2=C(CN)COOC
2H
5— the most popular among them.
Cyanoacrylate esters polymerize directly by certain mechanisms; anionic polymerization is the most common and in correspondence with their most widespread use as adhesion materials. As a rule, when they are used as glues, the process is started by nucleophile substances that already exist on the surfaces to be welded. For this reason, the hardening of the cyanoacrylate esters seems to take place spontaneously (self-polymerization at ambient conditions) when spread as thin film between surfaces [
3]. The most important feature in a cyanoacrylate molecule is the lack of electron density in the double bond between
α- and
β-carbon due to the influence of the electronegative groups —CN and —COOR. This means an excess of positive charge δ
+ locally to
β-carbon, which becomes susceptible to nucleophiles. Other factors through which the cyanoacrylate molecule becomes very active are the absence of substitutes in
β-carbon (no stereochemical inhibition) and the stabilization of the resulting negative charge on
α-carbon (delocalization). According to the above, the anionic mechanism of polymerization can be triggered even with very weak nucleophiles such as moisture water [
4]. The polymerization reaction is not affected by other molecular phenomena generally, but mainly by the fact that the negative charge of the developing anion is 97% located on the last monomer molecule of the chain [
5].
Thus, cyanoacrylate monomers carry out rapid polymerization with the simultaneous presence of suitable anionic initiators in an aprotic environment. Especially as welding materials, it should be noted that the role of the solvent that “frames” the hardening is covered by the monomers themselves in the existing liquid phase [
6]. What mostly happens when applying the cyanoacrylate glue is an attack of the monomer by OH
− [
7] of the pre-existing moisture and the formation of the carbanion, to which there is a cation nearby, probably H
+ (initiation). Free ions maintain and stabilize the charge of the carbanion which attacks as a nucleophile to the next monomer (propagation). The step of chain transfer at the anionic polymerization of ethyl 2–cyanoacrylate is less clear, but it is speculated that a water molecule can react with a “living” anionic chain, resulting in the production of an inert polymer chain and a hydroxyl ion (termination) [
8,
9]. If there are no impurities in the package or in the application surface, the hardening will end when all monomers are consumed; otherwise, strong acids are needed to achieve termination of the polymerization [
7].
In general, cyanoacrylate glues have utilitarian advantages, but lack in terms of stability and durability. However, since the behavior of the adhesion bond against the corrosive forces of the environment is the most important property of any glue [
10], they are worth being examined by experts. The polymer produced by ethyl 2-cyanoacrylate is a thermoplastic material, so it softens and flows at temperatures higher than its glass transition temperature T
g, which is in the range of 140–150 °C [
10], depending on the extent of polymerization, as in any polymer. For more industrial applications, polycyanoacrylates require more functional polymers than alkyl cyanoacrylates to create cross-links or mix of monomers for copolymerization; those two are the basic methods for provoking changes in the properties of the final material. It is known that polycyanoacrylate materials are susceptible to hydrolysis as a result of moisture effect, particularly when pH > 7 [
10]. Water molecules that pre-exist in the package (or are created by condensation in other cases) diffuse in the adhesive along with those adsorbed from the environment. Adhesives tend to accumulate moisture in this way in the welding line [
11]. Thus, polymers of high MW show remarkable sensitivity to the degradation of the macrochains in the presence of water but also due to the presence of bases such as ammonia, sodium hydroxide and some amines [
12].
Conservation of a cultural artifact is a work of prevention and protection carried out mainly on its material to avoid the restoration operation, if possible, which is a traumatic event. It aims to restore it to its original state, healing the damage it has suffered over time [
13]. A fundamental principle of conservation is that the process of restoration should stop when the speculation begins [
14]. Basic principles of cultural conservation are the minimum aesthetic intervention, the use of appropriate materials and methods, the complete documentation of all the work undertaken and the study to avoid or reduce future problems (according to Venice Charter 1964) [
14]. For an adhesive to be ideal for use in glass conservation, it must exhibit any of the following:
pe. hardening even at room temperature, reversibility, no reaction with common solvents and no affection by moisture, invisible intervention, no yellowness over time, no cracking during ageing and similar refractive index as that of the glass [
15].
Cyanoacrylate adhesives are used in glass conservation as “first aid” adhesives but have limited permanent application because they yellow easily under the influence of light and temperature [
16], dissolve in the alkaline conditions prevailing on most glass substrates, are unstable under heat and do not stand mechanical stresses. The heterogeneous nature of the surface from which curing starts leads to connections whose performance and mechanical properties are highly dependent on the physicochemical state and morphology of the bonded surfaces and the distance between them [
17]. In the bonding of cultural heritage objects, we aim for zero gap between the surfaces, yet gaps are practically about 10 μm. In irregular surface topographies, the range of gaps can vary from 50 to 500 μm. The retention of mechanical properties and the speed of curing of cyanoacrylate adhesives have proven to be insufficient for their effective application in such ranges [
17]. Any object that comes in contact with cyanoacrylate glue should be washed with weak acid solution, rinsed with acetone, and dried in the oven before application to avoid impurities that will disable the ions. There are few ageing studies of polycyanoacrylates to determine how stable they are in a museum environment [
18,
19]. In order to decide whether a commercial product is suitable for cultural glass conservations, a detailed characterization is preferred towards the aspects which would affect its performance.
An aspect that is of great interest to art conservators is the range of solvents applicable to clean artwork and also to remove adhesives. The most widely used chart in the selection of suitable solvents is the Teas solubility triangle. Presented by J.P. Teas in 1960, this equilateral triangle facilitates the conservator in selecting the solvent or preparing the solvent mixture for neighboring areas. Each side of the triangle represents the forces below by raising the polarity from right to left. The organic solvents, as well as water, are placed based on their three fractional solubility parameters, namely (a)dispersion forces (
Fd), (b) dry polarity forces (
Fp), and (c) hydrogen bonding forces (
Fh) [
20]. In any case, in situ solubility testing, performed by experienced conservators, is recommended in order to obtain the final decisions for removal solutions [
21].
It is indicated that glass has a coefficient of linear thermal expansion of 4.8·10
−6 grad
−1 and acrylic polymers of 70–80·10
−6 grad
−1. The difference between the coefficients is significant and that difference will result in a failed restoration upon heat applied. In order to reduce the value of the polymers’ coefficient, inert fillers are often added into adhesives to give relative compatibility between the polymers and the substrates of the artifacts. Polycyanoacrylate is a thermoplastic material, so it softens and flows at temperatures higher than the glass transition temperature T
g which is in the range of 140–150 °C [
3,
10]. Welding near such temperatures is forbidden, while in many cases the strength of the joint bond decreases at temperatures of 60–80 °C [
22] due to the remains of unreacted monomers. On the other hand, when time passes by, the most common cause of ageing is increases in temperature because this accelerates chemical reactions of the polymer with oxygen from the atmosphere. Increasing the temperature can have different effects on various polymers depending on the properties and the composition of the polymer. Properties that change mainly with thermal ageing are the color and reversibility of the polymers or their elasticity [
23].
The response of a polymeric restoration towards the conditions of the environment also delineates the temperature and humidity ranges in which it can be found. Under normal conditions, the glue demonstrates very good adhesion and tensile strength, resulting in a very strong bond of the welded surfaces. However, exposure to intense conditions leads to degradation of welding and ultimately to failure. It is well-known that epoxy resins present the highest endurance to most corrosive environments, retarding even yellowness [
24]. The relatively short duration of resistance of polycyanoacrylate glues is usually due to higher temperatures, moisture presence, solvents or aqueous basic solutions [
25]. Its restricted performance is attributed to the degradation of the polymer when exposed to the above. Furthermore, the polycyanoacrylates’ degradation may yield substances that will harm the artifact surface they come in touch with. The corrosive substances for objects of cultural interest, which may come from the glue, can be classified into two categories: (a) substances such as the monomer itself, formaldehyde, ethyl cyanoacetate and (b) substances such as traces of acid stabilizers from the production process and small amounts of carboxylic acids formed by the hydrolysis of monomers. Most of the above compounds belong to the so-called volatile organic compounds (VOCs), which are compounds with a relatively high vapor pressure at room temperature and are emitted in their gaseous state by many materials—among them, adhesives [
26]—and need to be controlled. Polymer tests in extreme conditions serve the investigation of their normal use in prolonged times. Accelerated artificial ageing experiments have been performed for at least the last 80 years in order to detect, over a short period of time, the changes caused in materials due to natural ageing. The real environment is quite complex, yet the devices used for these experiments may simulate solar radiation, humidity or temperature alone [
16].
Py–GC/MS is a method of analytical chemistry often applied in the field of polymers, where the sample is thermally degraded in the absence of oxygen into smaller and volatile fragments called pyrolysates. They are indicative because the degradation is instantaneous, and fragments rarely coalesce or undergo side reactions [
27]. The chemical affinity of pyrolysis with the stationary phase also determines the retention time, a parameter useful for identification through mass spectrometry data [
28]. From the pyrograms and chromatograms, conclusions are drawn regarding the composition and chemical structures of the polymeric sample as well as the degradation mechanisms and pyrolytic reactions [
29]. They are the “fingerprint” of the material because the fragmentation pattern in the pyrolysis chamber is reproducible if the method is repeated under identical conditions [
30].
The aim of this study is the evaluation of the thermal endurance of a commercial polycyanoacrylate adhesive capable of instant joining of glass surfaces, as a restoration means for glassy or ceramic artifacts of cultural heritage. The aged adhesives were studied in terms of solubility convenience and degradation tolerance, while their degradation products were evaluated as well.
2. Materials and Methods
2.1. Adhesive Polymerization and Samples Preparation
Loctite
® Super Attak Glass (3 g, Henkel, Henkel Hellas SA, Athens, Greece) is the commercial cyanoacrylate adhesive studied, with ethyl 2-cyanoacrylate (CAS# 7085-85-0) as the main monomer. It contains traces of triethyl
o-acetylcitrate (C
14H
22O
8, 20–40%) as a plasticizer, 2,2′-methylenebis(6-
tert-butyl-
p-cresol) (C
23H
32O
2, 0.1–1%) as a UV-stabilizer, and hydroquinone (C
6H
6O
2, 0.01–0.1%) as an inhibitor to suppress free-radical polymerization, as listed on the escorting sheet. It is a colorless liquid with low viscosity and a slight odor in pure form [
31]. Loctite
® Super Attak Glass adhesive was supplied in several packages (IDH 2051823), used as received, and stored in a cool, dry and shady place at ambient conditions [
32]. With proper sealing of a used package, it is possible to maintain the good condition of the product, both fluid and colorless, which is confirmed experimentally. The small packages help to be consumed in a short period of time.
After several trials with laboratory equipment in order to produce usable polycyanoacrylate films, with certain thickness, surface uniformity and easy detachment, the following procedure was set: A glue quantity was transferred in a square polyester mold of 3 × 5 × 0.7 cm size placed between two glass plates (5 mm thick) covered with PP sheets and gathered tightly with clamps, placed in a horizontal position in a cool and shady place (25 ± 1 °C, 50–60 RH%) for 4 days to polymerize thoroughly [
16]. Special care was taken to avoid air bubbles (the low viscosity of the glue facilitates the air entrapment). After the careful detachment, a colorless, transparent adhesive film was produced, the procedure was repeated as many times as needed to produce the films for the ageing ovens, whereas films were kept in a lightless environment (black sample bags), preventing any early ageing [
32]. The time periods required for cyanoacrylates to becomefully polymerized or samples to becomeaged under controlled conditions were determined by our previous FT-IR studies on the adhesive [
16,
32].
2.2. Samples Weathering
Two processes of accelerated ageing were performed on the polymerized adhesive samples by exposing them to intense conditions in terms of temperature and light. Thermal ageing was performed at 25 ± 0.1, 50 ± 0.1 and 75 ± 1 °C ovens (Memmert 200 D06058, Termaks B8000, Heraus ovens correspondingly, Germany) for 12, 7 and 5 weeks in total correspondingly, i.e., until the samples were severely influenced in terms of their flexural behavior when removed. In fact, the stay at 50 and 75 °C may be called thermal ageing of the glue, since the stay at 25 °C took place as a reference experiment to compare and no changes were noted for the 12 weeks (or 3 months) this investigation lasted. Two pieces of cured adhesive were placed in horizontal position onto microscopy glass slides for each ageing temperature and a part of them was removed to be tested when necessary [
32].
The UV-ageing process was performed in a closed Gallenkap oven (Cambridge, UK, OVR-400-010 G). In particular, the radiation emission of the apparatus is in the UV
C region (
λ = 254 nm) in room conditions (25 ± 1 °C and 50–55% RH). The oven provides 6 sockets (Panalight
®, ref. # TL 2001/KPP 2001) for Hg-lamps (OSRAM
®, Puritec HNS 8W, G5, G8T5/OF, RG3 HG, Italy) placed at a 10 cm distance from the shelf with the samples, irradiated for 48 h [
16].
2.3. Characterization of Weathered Samples
Regarding the solubility test, approximately 20 mg of the polymerized films were transferred into closed test tubes. Then, 1 mL of solvent was added at first (i.e., 2% w/v), observing the effect on the polymer after shaking. When the solvent did not influence immediately, the mixture was left for up to 1 h at room temperature. Then, up to 4 mL of solvent was added and in case of difficulty the ultrasound bath (Grant XUBA3) was used in combination with heating (60 °C) for 0.5 h. The solvents tested were EtOH (absolute, Scharlau, Spain Batch N° 12920902, μ = 1.69 D), acetone (pro analysi, Merck, Germany Batch N° 016Κ13993614, μ = 2.91 D), ACN (99.98%, Scharlau, Spain Batch N° 42783, μ = 3.84 D), CH2Cl2, (Sigma Aldrich, Louis, MO, USA, Batch N° 22.1330810.400, μ = 0.0), Hexane (>99%, Chem-Lab, Belgium Batch N° 20.4172911.800, μ = 0.0), ethyl acetate (Fluka puriss, Switzerland, LOT 41830, μ = 1.78 D), petroleum ether (mix of C5H12 and C6H14, Merck, Germany, LOT 90110, μ = 0.0 D), THF (Riedel-de-Häen, Holland, LOT 00040, μ = 1.63 D), toluene (p.a., Merck, Germany, Batch N° K36619525, μ = 0.36 D), and HCHO (37%, Scharlau, Spain Batch N° 63405, μ = 2.33 D). The solubility tests were repeated for the thermally aged and the UV-aged polycyanoacrylate samples by applying only the solvents that dissolved the neat samples immediately.
In a Diamond DSC calorimeter from Perkin Elmer (Waltham, MA, USA) with an Intercooler II (Perkin Elmer) cooling machine, N2 > 99.9% as purge gas (50 mL/min) and sample gas (20 mL/min), the thermal properties of the neat and weathered samples were studied. The instrument was calibrated with In and Zn standards and the results were processed with Pyris Software, ver. 8.0.0.0172 (2006 PerkinElmer, Inc.). A small amount of samples (3–5 mg) was accurately weighted on a Mettler Toledo A250 (±0.0001 g) analytical balance and transferred into tightly sealed Al pans of 50 µL volume (Part N°. B016-9321). An appropriate Perkin Elmer hammer was used to hermetically seal the sample pans. The sample and empty reference pans were transferred to the DSC oven under an inert atmosphere and the following heating program was applied: 1 min holding at 0 °C and heating from 0 to 280 °C with heating rate of 10 °C/min.
Pyrolysis experiments were carried out at a QP2010 Ultra GC/MS (Shimadzu, Japan) equipped with a Multi-Shot Pyrolyzer EGA/PY-3030D (Frontier, Japan) under inert conditions of flowing He. First, the EGA program (Ultra-Alloy tube, 2.5 m × 0.15 mm) was executed in order to investigate the thermal profile of the polycyanoacrylate adhesive. The method development included variations in the heating rate, the initial temperature, and the column flow until a well-distributed program was resulted. Eventually, heating from 50 to 500 °C with 50 °C/min rate was applied, all interfaces kept at 150 °C, split ratio at 100, linear velocity at 13.8 cm/s, inlet pressure at 98.5 kPa, total flow at 104 mL/min, column flow at 1.5 mL/min and purge flow at 3 mL/min. The MS settings were chosen at 200 °C for the ion source, m/z at 14–500 amu and 10,000 the scan rate with satisfactory vacuum in the detector. From these EGA profiles, the pyrolysis temperatures were chosen to be 190, 250, 300 and 420 °C.
Similarly, the SS pyrolyses were executed after some trials and tests occurred for the method development, on a Mega 5HT capillary column (Italy) of 30 m × 0.2 5mm × 0.25 μm geometry, non-polar in nature, covered with 95% dimethylsiloxane. At each selected temperature, pyrolysis occurred for 0.5 min, while interfaces’ temperatures were at 200 °C. The GC column oven remained at 50 °C for 1 min, heated until 300 °C at 10 °C/min, and then held for 4 min. The column flow at 0.7 mL/min, the purge flow of He at 3 mL/min, and the total flow at 38.8 mL/min resulted in a pressure of 28.4 kPa, while linear velocity yield of 30.4 cm/s. Several trials regarding the split and the column flow led to ratio 50, because the initial saturation was intense. The MS parameters involved the ion source stable at 200 °C, the detector voltage at 1.2 kV, the m/z range at 14–500, and the scan speed at 10,000. A tiny quantity of each specimen was required, transferred in cups (stainless steel, coated with molten SiO2). The evaluation of the results was carried out through GC/MS Lab Solutions, v.2.71 (Shimandzu, Japan 2011) and “Search analysis program and Data” (NIST11 ver.1.00, Shimandzu, 2011, Japan).
3. Results
The accelerated weathering of an adhesive material points to the prediction of its state in long future, so to the foreseeing of its tolerance and failures [
16]. This study involves the evaluation of aged samples of the polycyanoacrylate commercial adhesive Loctite
® Super Attak Glass in terms of its dissolution ability and degradation behavior. The polymerization kinetics and photooxidation kinetics of the very same adhesive have been studied in our previous works, whose results and conclusions have also been taken into consideration herein [
16,
32]. To begin with the macroscopical examination, the authors will try to provide the observations made so the readers follow the changes.
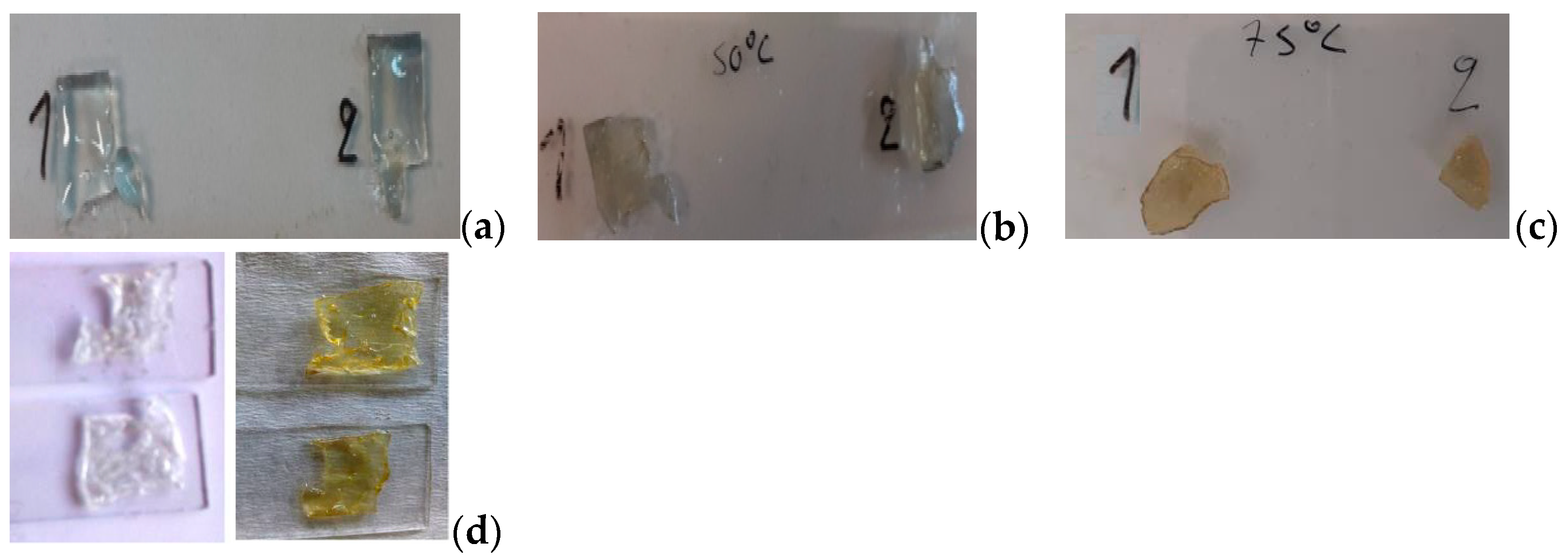
Figure 1a gives the image of the specimens right after polymerization where the adhesive produced is colorless, transparent, with no flaws into its mass (even at 0.7 cm thick, m ≈ 2 g), which verifies the good function of that adhesive for glass restoration, where those characteristics are essential. Next,
Figure 1b illustrates the changes when specimens were aged at 50 °C for 7 weeks in row, where the yellowish shade was evident (appeared during the last week) along with the dullness that occurred. The softening of the specimens was evident too, so they were let to cool naturally before use. The reason why the experiment ended at 7-weeks duration is because the specimens began to alter their shape when transferred.
Regarding the adhesive films under 75 °C thermal ageing,
Figure 1c illustrates the results after a 5-weeks-time heat. The samples turned yellow after 10 days of heat exposure, became soft, then hard after some days and finally brittle materials. The chromophore groups are formed when a part of it breaks under the influence of light, oxygen or moisture, resulting in yellowing of the adhesive and growth of cross-links which transform a soluble part of the polymer into insoluble. Chain cleavage reactions reduce the chains length of the polymer as well as its molecular weight [
8]. The experiment ended when cracking was enough and every movement cost with mass detachment and loss. It must be noted that the samples kept at 25 °C did not present any changes compared to the initial neat polymers, used as reference samples at every case.
As far as the UV-aged specimens are concerned, the detrimental effect of the irradiation is apparent with extensive yellowing (begun 3 h after exposure) and material softening. Their yellowing is due to absorption of blue or ultraviolet light by cyano groups resulting in the formation of double bonds between carbons or carbonyl groups (>C=O) along the polymer chains converting them into chromophores [
24]. The specimens did not lose their elasticity and remained easy to handle when they had been in the UVc chamber for 48 h in total. It is worth noting that even with the color change of the resin, the UV-aged films remained clear and transparent, as seen in
Figure 1d. After 6 h of exposure, the films became more elastic and stickier (after being kept in the environment for a few minutes it turned more rigid) [
16].
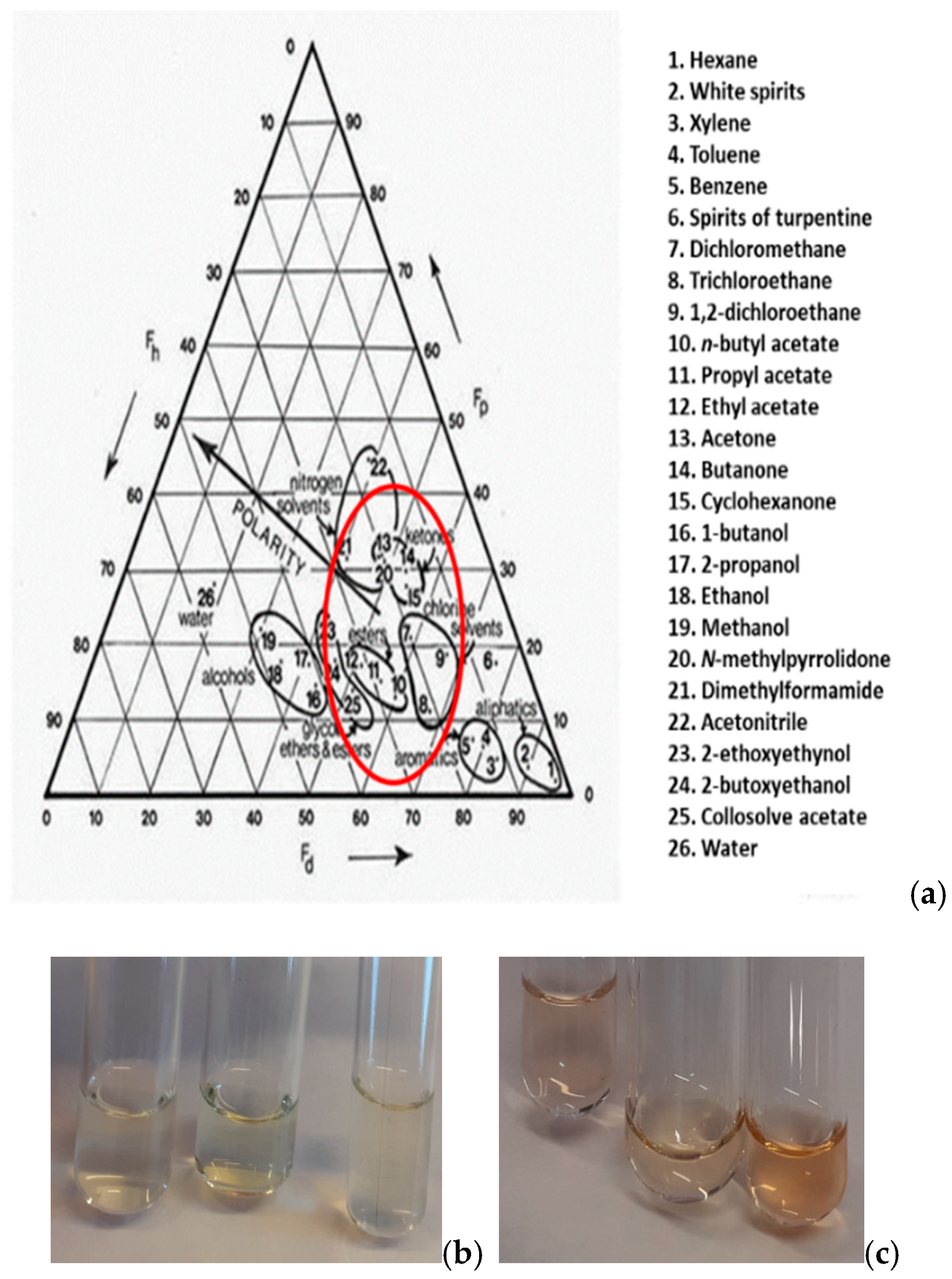
Solubility tests are essential for the conservators’ guidance regarding the solvent choices for removing old adhesives or temporary restorative materials.
Table 1 lists the observations of the authors when experimented with common solvents used in restoration of cultural heritage artifacts. To be noted, the substrate tolerance is of no concern in our case, since glass is durable to all organic solvents. Moreover, solvents from all categories were tested. In the case of HCHO, it was noticed that the polymer probably reacted with the solvent since the solution became dull. It was concluded that the polar solvents like acetone, ACN, ethyl acetate, and THF acted at once to dissolve the polycyanoacrylate samples, while the non-polar hexane, petroleum ether and toluene needed extra effort to affect the macromolecules. Thus, if we would like to draw an area of preferable solvents on the Teas Chart for this particular adhesive, we would give the red line seen in
Figure 2a. Analogously to the neat adhesive, solubility tests were repeated for the thermally aged and UV-aged samples. It was found that the aged polymer shows lower resistance to polar solvents. The difference noted for rigid, aged specimens is that swelling precedes the dissolution and that a yellow hue is apparent, as expected (
Figure 2b,c).
Next, the DSC proved to be a demanding technique to study the polymerized adhesive at first, since the thermograms of the neat materials showed only the melting of the polymer at 234 °C with a wide endotherm peak and Tg transition was hard to be detected. On the contrary, the aged adhesives corresponded to heat flow and showed both Tg and Tm temperatures. For the 12-weeks-kept reference sample at 25 °C, it was found that Tg = 116 °C, for the 7-weeks-aged sample at 50 °C, it was found that Tg = 87 °C, and for the 5-weeks-aged sample at 75 °C, it was found that Tg = 69 °C, while for all cases Tm remained at 229–234 °C (ΔHm < 0.1 J/g). Similarly, after 48 h of UV-ageing, the adhesive gave Tg = 80 °C and Tm at 234 °C as well. Beyond melting, at higher temperatures, the decomposition of the ester occurs.
The last and greater part of the results of this investigation regards the pyrolysis of the Loctite
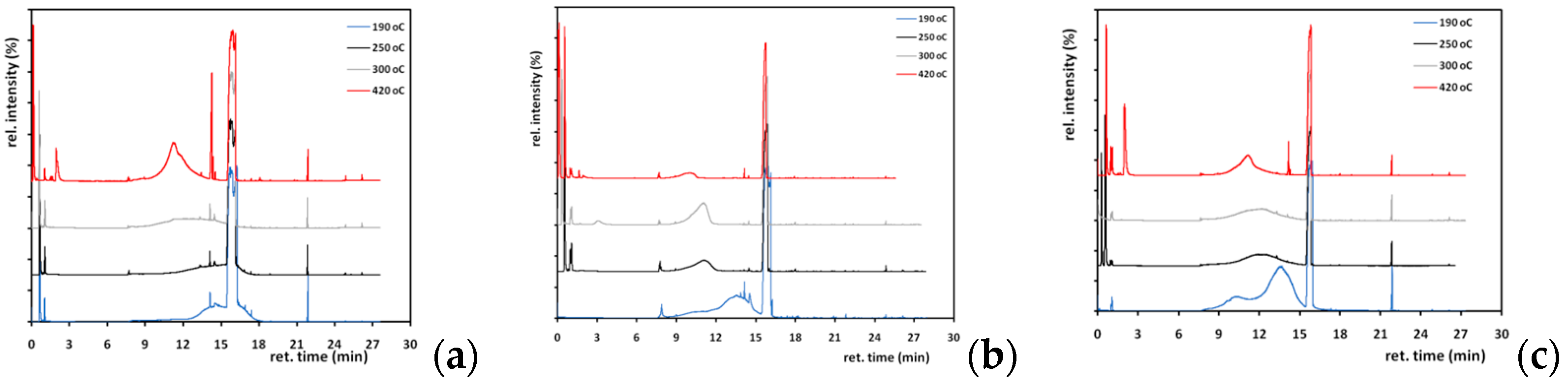
® Super Attak Glass polymerized adhesive along with its aged samples. The reason for those analyses is the identification of the decomposition products of the adhesive (since it was found that it is not tolerant of heat or photooxidation) and whether those products are harmful to the substrates applied or the staff handling them, in longtime terms. The EGA results are shown in
Figure 3 where the intensity of gas degradation products is charted vs. the temperature of the pyrolysis. The neat adhesive starts its decomposition at 190 °C, reaches a maximum at 250 °C, and lowers the emission at 300 °C, while a smaller emission occurs at 420 °C. The aged sample, as expected, presents a weaker profile with decomposition initiation at 110 °C, peaks at 230 and 245 °C, and conclusion of the great elution at 380 °C, whereas a second emission occurs at 420 °C as well (
Table 2). All aged samples behave similarly, so there is no point for separate reference. As seen, aged adhesive breaks down the macrochain bonds easier and the release of various components begins at lower temperatures. It is possible that photooxidation sensitizes the polymer, so the fission of the chain happens mainly due to quaternary carbon atoms [
34]. In addition, another qualitative difference is shown in the first 1–2 min of the program where the primary neat glue releases more volatile products, probably because the treated one has already lost them during ageing time.
Following the EGA recordings, the main SS analysis took place at four pyrolysis temperatures set by the aforementioned results, where the elution profile alters at 190, 250, 300 and 420 °C.
Figure 4 demonstrates the effect of pyrolysis temperature on the elution of fragments on both neat, 75 °C thermally aged and UV-aged adhesive. Fewer peaks are presented in the case of the UV-aged and thermally aged chromatograms, indicating fewer decomposition products compared to the fragments of neat adhesive. Plus, it is shown that certain peaks are repeated in all four chromatograms of each case, and thus a characteristic profile is shaped.
The main compounds detected and identified are presented in
Table 3 for the untreated neat material when pyrolyzed at 250 °C, in
Table 4 for the UV-treated adhesive when pyrolyzed at 300 °C, and in
Table 5 for the thermally treated specimen at 75 °C. A small variety of fragments were detected and identified, with
m/
z 28–318. The similarity ratio rises above 77% for all cases working on the NIST 2011 Library.
Regarding neat adhesive the main pyrolyzates in all pyrolysis temperatures included 2-methyl-1-heptene, 2-methyl-1-octene, 3,3-diethyl-cyclobutancarbonitrile, 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraen-1-ol, 4-methyl-1-hexene, 2-hexenal, 2-butenal and 3-methyl-pentane in a range of 14 to 25 min of elution. Butyl cyanoacetate was detected at 7.7 min while the aldehydes were detected only at pyrolysis of 420 °C.
Regarding UV-aged adhesive, the main pyrolyzates detected in all pyrolysis temperatures were butyl cyanoacetate, 2-methyl-1-octene, 2-methyl-1-hexene, n-hexadecenoic acid, butyl isocyanotoacetate, ethanedioic acid, 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraen-1-ol and squalene; at 420 °C of pyrolysis, the additional fragments 3,3-diethyl-cyclobutancarbonitrile, ethanedial, hydroxy acetonitrile and 1-butanol were detected.
Regarding thermally treated adhesive, the pyrolyzates hydrazine, nitrogen, acetonitrile hydroxyl, ethanedial, acetic acid, 1-butanol, butyl formate, butyl cyanoacetate, 2-hexenal, 2-butenal, 2-methyl-1-octene, 2-methyl-1-hexene, 3,3-diethyl -cyclobutancarbonitrile and supraene were detected as well.
The results presented in
Table 3,
Table 4 and
Table 5 represent the fragments produced by the degradation of the polymeric structure of the material. The authors give separately, in
Table 6, the molecules eluted and detected that correspond to the additives existing in many commercial products, like Loctite
® Super Attak Glass [
31], and are eluted unchanged in gaseous form. Among them, plasticizers, thickener, and stabilizer are detected, all assumed to exist in analogous adhesives.
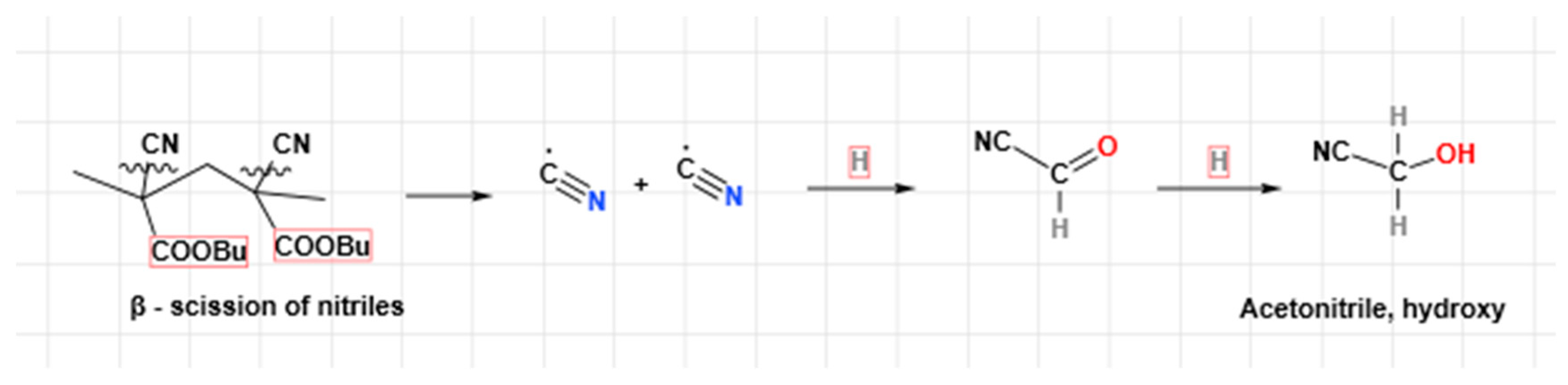
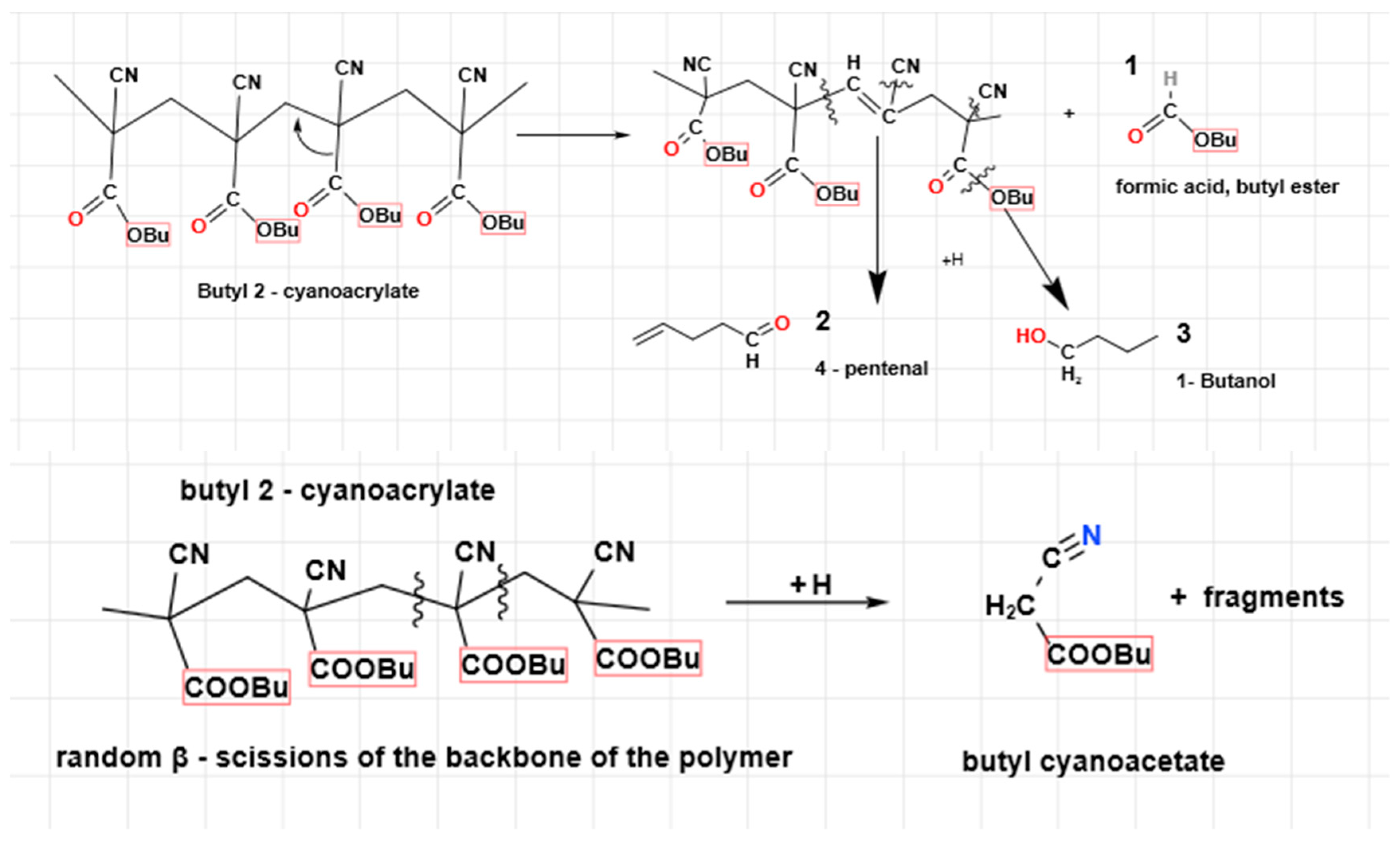
Finally, the authors have sketched some main decomposition reactions that could describe the degradation of the polymeric structure to smaller molecules that have been detected in GC/MS fragments. Let us take the poly(butyl 2-cyanoacrylate), PBCA, as the polymeric macromolecule for degradation. The unzipping occurs through H
+ abstraction, while the -COOBu and –CN groups are the first ones to leave the polymeric skeleton (
Figure 5). Theanalogous fragmentation for PECA is assumed, though its products are of lighter MW and smaller molecules are easier to be released. The butyl 2-cyanoacrylate is another ester often found with ethyl 2-cyanoacrylate in packaging, as a softener of the final polymer.
4. Discussion
Polycyanoacrylates are a type of polymer usually constituting adhesives that are commercialized under the label of “instant glues” and, due to the chemistry of the macromolecules, are simple to apply at ambient conditions, without the need of special equipment or an experienced operator. They only need clean surfaces and moisture to initiate the curing of the material. The polymer produced after little time is thermoplastic, not cross-linked, with transparency and good appearance. This is why conservators of cultural heritage working on glass or ceramic (mineral) artifacts apply polycyanoacrylate adhesives for rapid and safe temporary restorations [
1], even though cross-linked epoxy resins are the strongest restoration adhesives available. The usual practice involves the preceding of the instant glue for rapid solidification, and when the conservator is certain for the intervention, epoxy adhesive is the final choice over the polycyanoacrylate one [
18,
19]. This is why the reversibility rule is essential for the cultural heritage conservators.
As the case for all esters, cyanoacrylates also react towards water and moisture. The excessive exposure to moisture may results in hydrolytic degradation of the polymer and into detachment from the glassy surfaces (which also accumulate H
2O molecules). Cyanoacrylate glue is sensitive to alkaline hydrolysis which degrades the molecular weight of polymeric chains. In addition, various degradation products on the glue-substrate interface may provoke the decomposition reactions [
7]. Resistance to the corrosive effect of water or moisture is limited when the surfaces welded are both rigid, while welding is significantly improved if at least one of them is elastic [
35].
The results of the solubility tests demonstrate some polar solvents as the optimum way to remove the Loctite
® adhesive by dissolution, such as acetone, THF, ACN or CH
2Cl
2. Among them, the authors would propose the use of acetone, since it responded well not only in the case of the neat polymer but also for the UV-treated and thermally treated adhesive. Acetone is the most accessible and safe choice for the conservators and the substrates. Baric et al. removed a polycyanoacrylate restoration by applying acetone too, even though ageing may make polycyanoacrylates less dissoluble (in DMF for example) [
36]. As for HCHO, it led to the dullness of the solution when Loctite
® was added (
Table 1); it should be mentioned that HCHO traces exist already in the production of ethyl 2-cyanoacrylate in the reaction stages [
25,
37].
When heated, PECA depolymerizes producing gases such as formaldehyde and oxides of both nitrogen and carbon, which may be strong irritants to the lungs and eyes. The most abundant gas though is N
2, which is totally harmless and inert (form the condensation of –CN groups). This is why the degradation of polycyanoacrylate adhesives it worth studying in detail by applying pyrolysis. The thermal degradation and photooxidation may simulate the long-term exposure of the commercial adhesives to mild conditions of storage and exhibition. PECA may be applied in modified form containing some unsaturated monomers too, since the chemical composition of the polymer has an important influence upon its adhesion performance [
38]. PBCA, often also found in PECA adhesives, presents lower T
g = 130 °C for example. A key disadvantage of the polymerization of alkyl cyanoacrylates is their limitation to monofunctional monomers which do not cross-link in the stage of condensation. From the polymerization of 2-cyanoacrylate esters, linearly high molecular weight homopolymers are created which are not cross-linked. However, if unsaturated structures are added, extra heat after anionic polymerization will induce free radical polymerization between the unsaturated groups and cross-links will be formed [
3]. Estan-Cerezo et al. presented DSC theromograms with post-polymerized adhesives which showed higher endothermic peaks, meaning the initial curing was incomplete even at 90 °C [
23].
Application of polycyanoacrylates is favored at ambient conditions and their best performance is achieved near such temperatures. Otherwise, higher surrounding temperatures lead to failure, while in some cases the T
g decreases even at temperatures of 60–80 °C [
22], i.e., much lower than the theoretical T
g that applies to the pure polymer. Due to the onset of polymerization from the few water molecules found on surfaces, an amount of non-polymerized monomer remains, which laminates the layer of glue and reduces the glass transition temperature [
39]. This is why extra time is proposed for the reactions to proceed, without rising the curing temperature, as is the case for other restorative adhesives where higher temperature favors the degree of curing [
15]. In addition, the common poly(ethyl 2-cyanoacrylate) adhesive shows a decrease in adhesion capability at an exposure between 80–100 °C. This is because of the changes caused by the polymerization, with the help of heat towards the monomers remaining from the previous stage [
10]. This detailed hardening of monomers reduces resistance to shear and tensile stresses [
39]. The extremely fast curing rate of the glue is responsible for the high stresses generated at the point/surface of welding which make the polymer susceptible to physical or chemical degradation. For this reason, suitable plasticizers have been added in some adhesives, such as alkyl esters. In that way, they increase heat resistance of the studied polymers, which seem to connect with a cross-linked formation in the adhesive layer. The decrease in endurance also accelerates when the adhesive is stored at high temperatures [
25]. We have observed physical changes for thermally treated samples at 50 °C and even more at 75 °C, whereas the observations for the photooxidized glue are analogous (
Figure 1). The comparisons are made to the samples kept at ambient conditions for 3months, without noticing any visual alterations.
Whatever the corrosive cause, the depolymerization begins with the deprotonation of the end-monomer by quantities of nucleophiles that are located on the surface of the solid polymer and substrate [
10]. This proton is sufficiently acidic due to proximity to the strongly electronegative groups of –CN and –COO– and is easily detached from bases [
7]. Cut-away monomers leave behind a shorter chain and with subsequent “repolymerization” create smaller polymeric chains of smaller molecular weight [
40]. This process eventually leads to a dramatic reduction in the molecular weight of the initial polymer and therefore degradation. The linear structure of the poly(ethyl 2–cyanoacrylate) undoubtedly facilitates the degradation of its “backbone” through “unzipping” [
39]. During this mechanism of degradation, polycyanoacrylate material releases large numbers of monomers rather than fragments of oligomers due to the tertiary carbons that form the skeleton of its chains [
41,
42]. Polymers containing H in the
α-position do not break down in their monomers to a large extent, while materials like polycyanoacrylate and polymethacrylate esters are thermally degraded mainly in their monomers [
43]. This indicates that the degradation is a homogeneous unzipping process through polymer chains and that, moreover, the chain unzipping process is slow relative to chain-end activation [
4].
The museum environment is known to be stable and ideal for the objects exposed, with humidity, temperature, luminance, and even air consistency. After interacting with substances of substrates or air, light, or moisture, adhesives gradually cause alterations in surfaces and degradation of materials, processes that are largely determined by the temperature and relative humidity of the atmosphere [
37]. The production of HCHO for example is undesirable in addition to being toxic and harmful to health, and for that reason needs to be removed from the atmosphere. In addition, it has the ability to oxidize in the air and produce the corrosive HCOOH [
26]. Especially in the museum environment, HCOOH is one of the most corrosive substances, along with CH
3COOH, and can affect most materials such as metals, marbles and paper. Although the emissions of CH
3COOH are up to ten times larger in quantity, especially from wood, HCOOH infestation is carried out to a greater extent due to the strong stresses caused by its salts. The reduction in its concentrations is achieved by controlling the oxidizing agents of the atmosphere that will prevent an oxidative reaction towards HCHO [
37]. In the case of cyanoacrylate glue in particular, HCOOH is produced in two ways: (a) indirectly by the oxidation of formic acid, i.e., as a secondary product of its degradation, and (b) directly because of hydrolysis within its mass. The collections of cultural heritage objects act both as a source of emission and as a repository of carboxylic acids where they can cause irreversible heterogeneous reactions on their surfaces [
44] and synergize in erosion processes [
37]. The contribution of monomer to the production of undesired active substances occurs with its slow hydrolysis/oxidation rate, where the corresponding cyanoacrylate acid is formed [
10]. The production of cyanoacrylate esters also involves the HCHO. In the production process of monomers of cyanoacrylate glue and at the stage of condensation, piperidine is mainly used as a catalyst, thus it is possible that there are also traces of the substances with piperidine [
45].
In a cyanoacrylate glue, the cyanoacrylate monomers—80–99.9% wt, the suitable inhibitor for anionic polymerization—0.002–0.01% wt, an inhibitor for free radical polymerization—0.1–0.3% wt, a viscosity-increasing agent—0–20% wt, and a suitable stabilizer for avoiding ageing—0.01–0.5% wt [
46]. The additives are in small quantities as known, yet GC/MS performed in that study was capable of detecting them (
Table 6).
The degradation reactions in pyrolyzer yield most of the substances mentioned previously, either as decomposition molecules form polymeric fragmentation or by releasing the components already found in the commercial product. As seen in EGA of
Figure 3, the aged sample does not yield gases of low MW in the initiation of the pyrolysis (50–70 °C) as the freshly polymerized glue does. This indicates that those volatile products exist in the mass of the freshly-cured adhesive and are slowly released in the environment. The precaution towards VOCs should be considered, although, practically, tiny quantities of glues and adhesives are used in restorations surfaces and thus found exposed on jointing surfaces and lines. The volumes of volatiles are low, since as the small content of the commercial packages (only 3 g) declare, the quantities needed for every restoration are small, shaping just adhesion films between covering surfaces. The mass of the samples studied here and the extreme treating conditions applied mean to drawthe succeeding degradation profile of the adhesive.
Thus, the advantages presented by polycyanoacrylate adhesive Loctite® Super Attak Glass prevail undoubtedly when a rapid, easy, and short-term restoration is the case. The adhesive may be an auxiliary tool for the rapid bonding of glass/ceramic surfaces in mild conditions where it retains its good behavior (ambient conditions, internal room space).