The Influence of the Molecular Weight of Poly(Ethylene Oxide) on the Hydrolytic Degradation and Physical Properties of Polycaprolactone Binary Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hot Melt Extrusion Conditions
2.3. Attenuated Total Reflectance Fourier-transform Infrared Spectroscopy
2.4. Differential Scanning Calorimetry
2.5. Melt Flow Index
2.6. Macrostructure Observation
2.7. Scanning Electron Microscopy (SEM)
2.8. Dynamic Mechanical Analysis
2.9. Rheological Analysis
2.10. Accelerated Degradation Studies
2.11. Contact Angle
3. Results and Discussion
3.1. Filament Manufacturing and Melt Flow
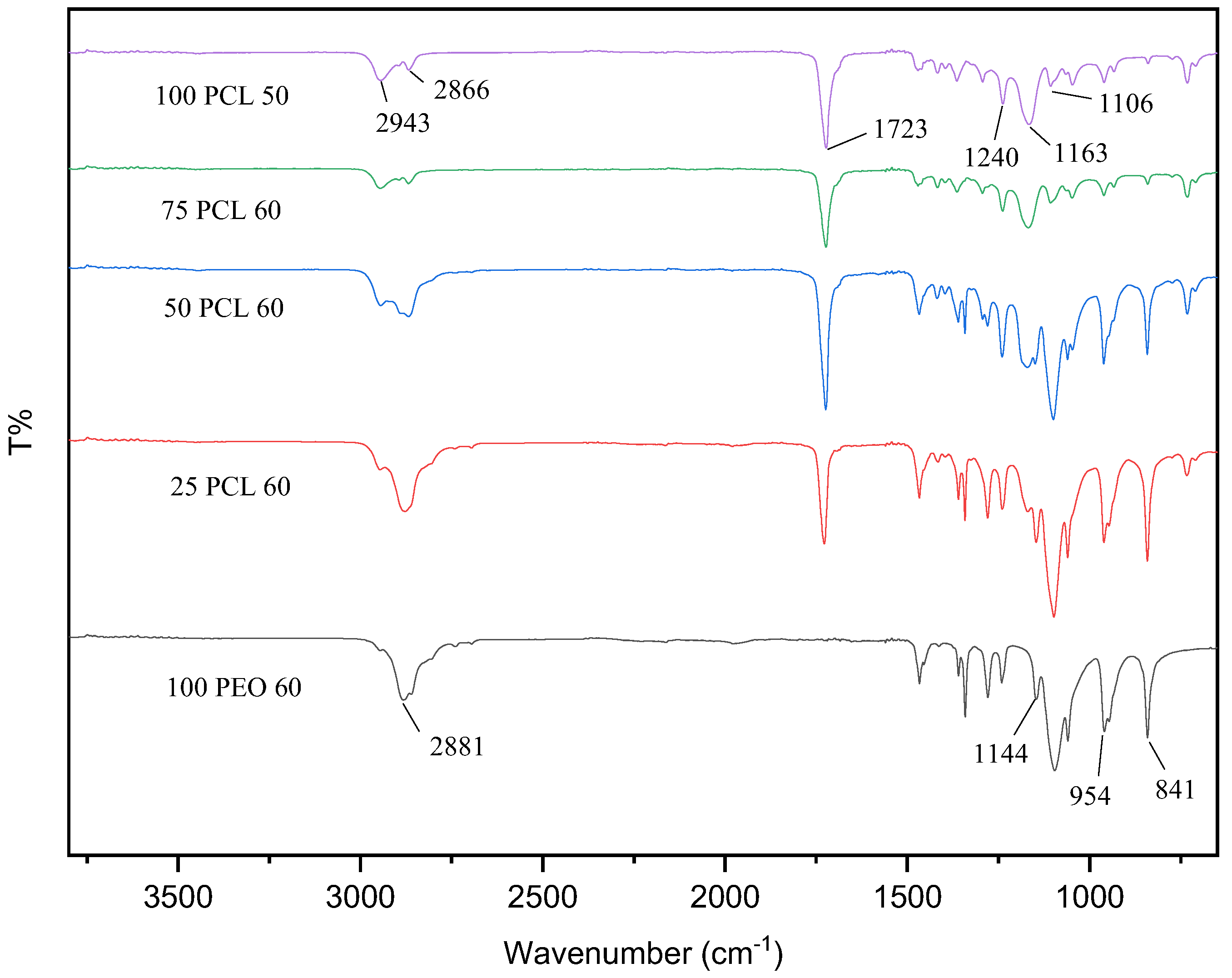
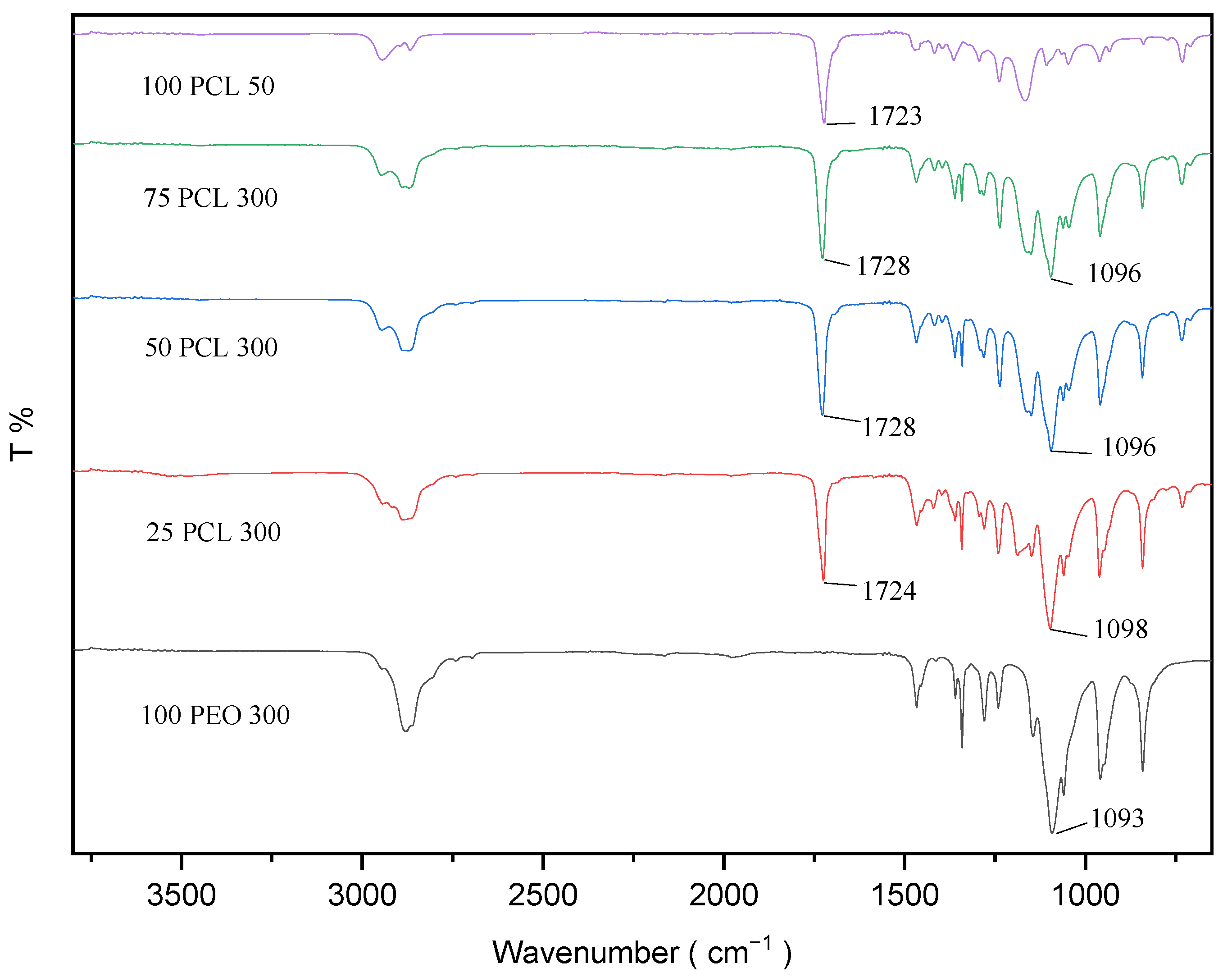
3.2. Fourier-transform Infrared (FTIR) Spectroscopy
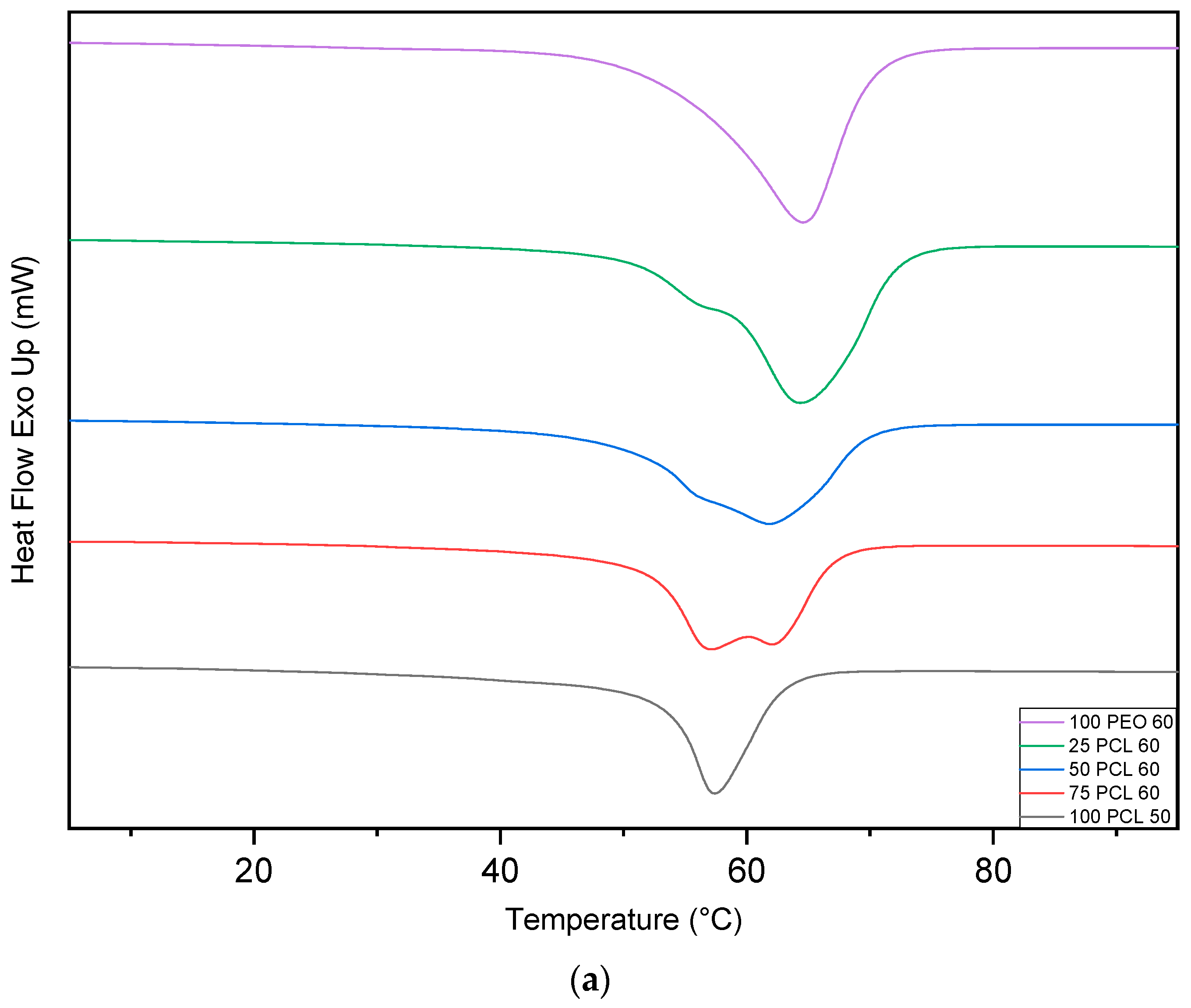
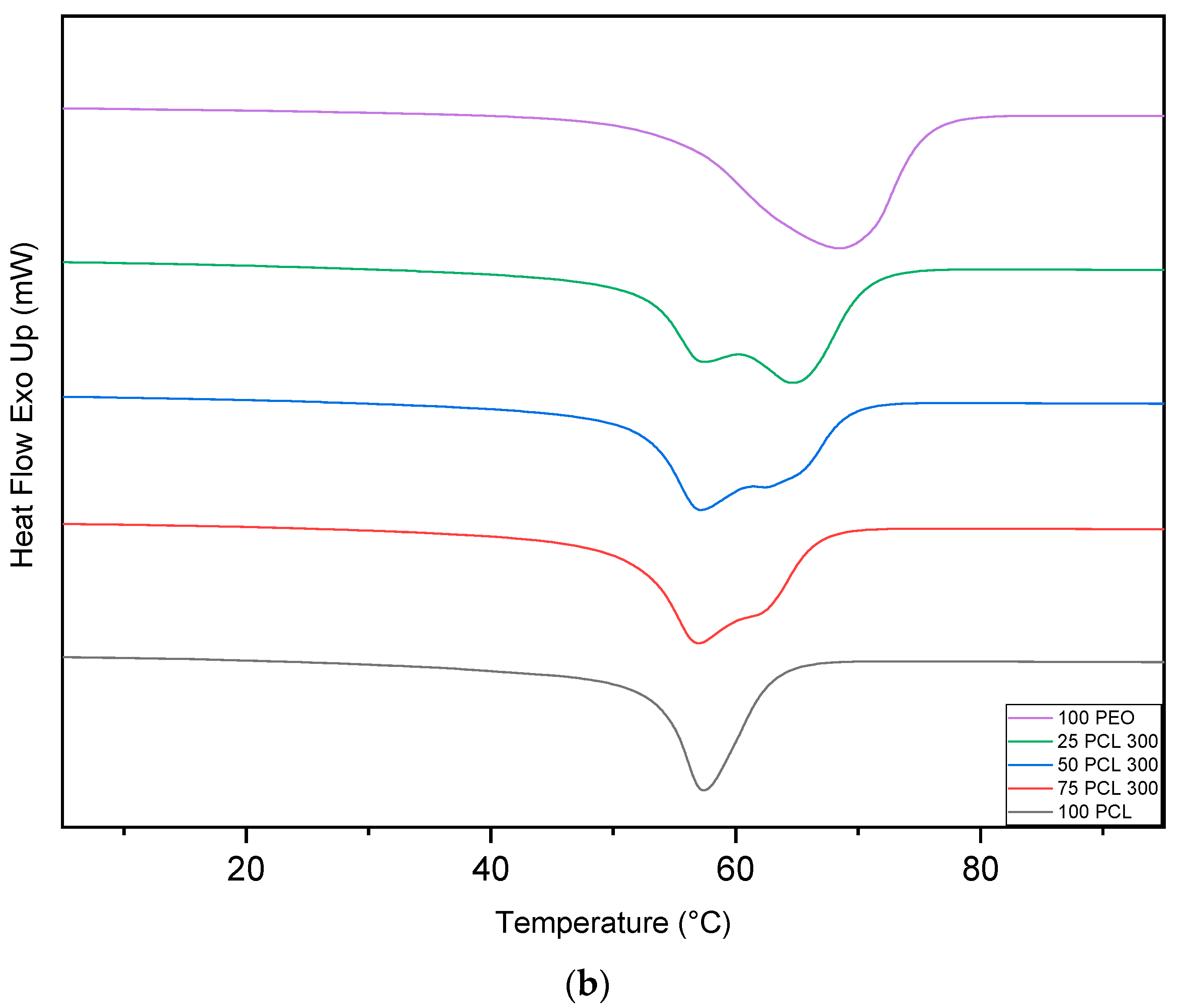
3.3. Differential Scanning Calorimetry (DSC)
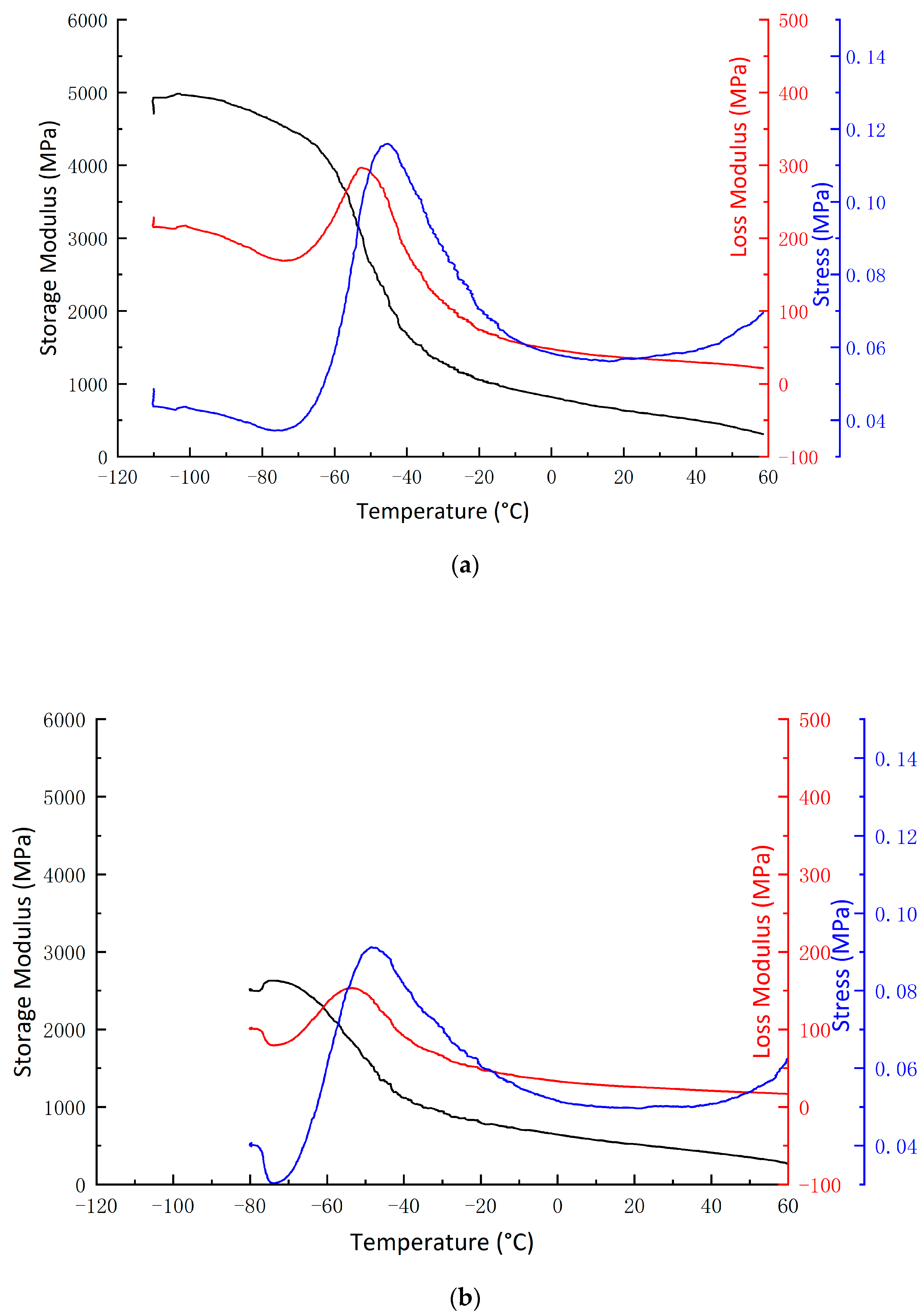
3.4. Dynamic Mechanical Analysis (DMA)
3.5. Rheometry
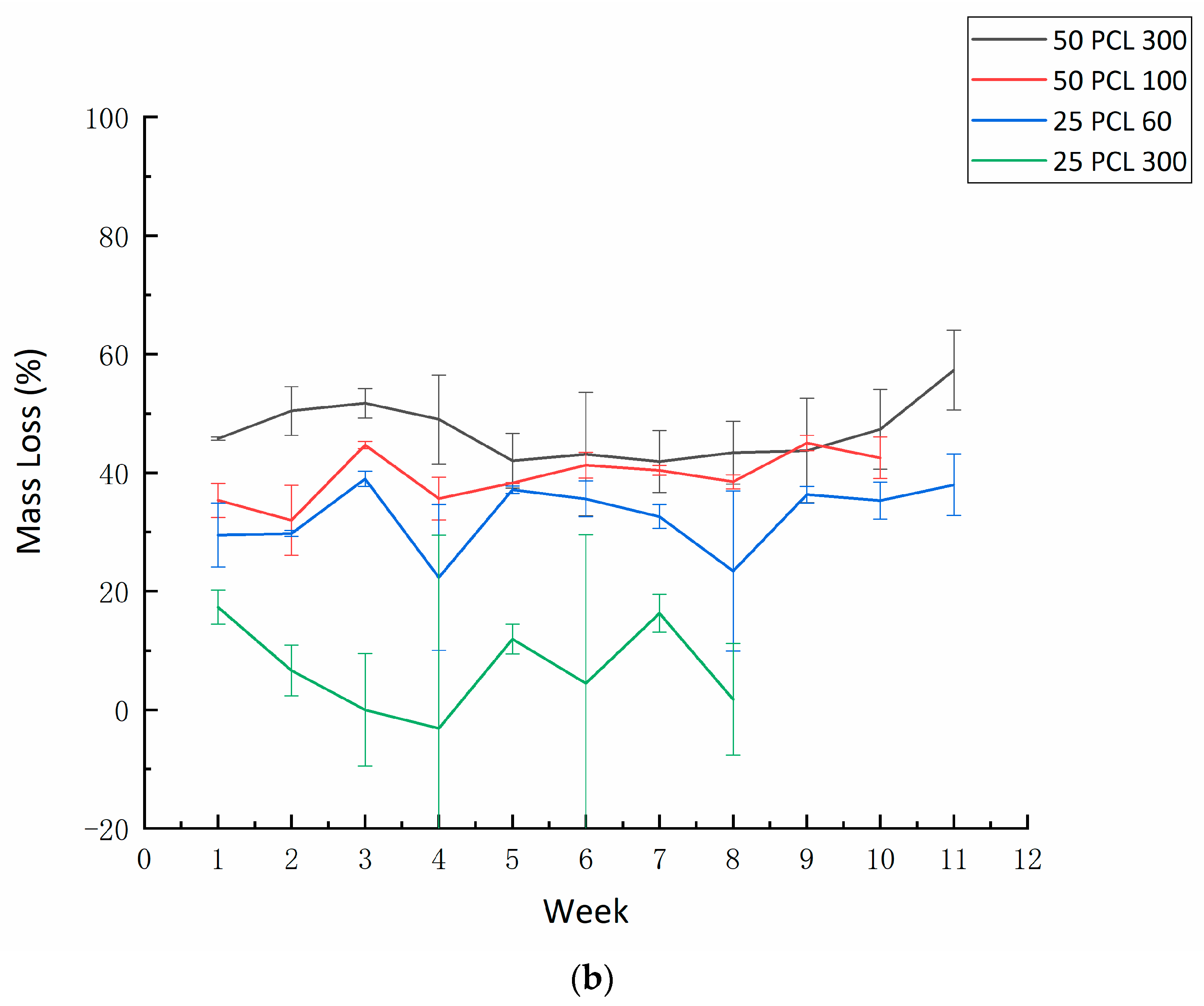
3.6. Degradation
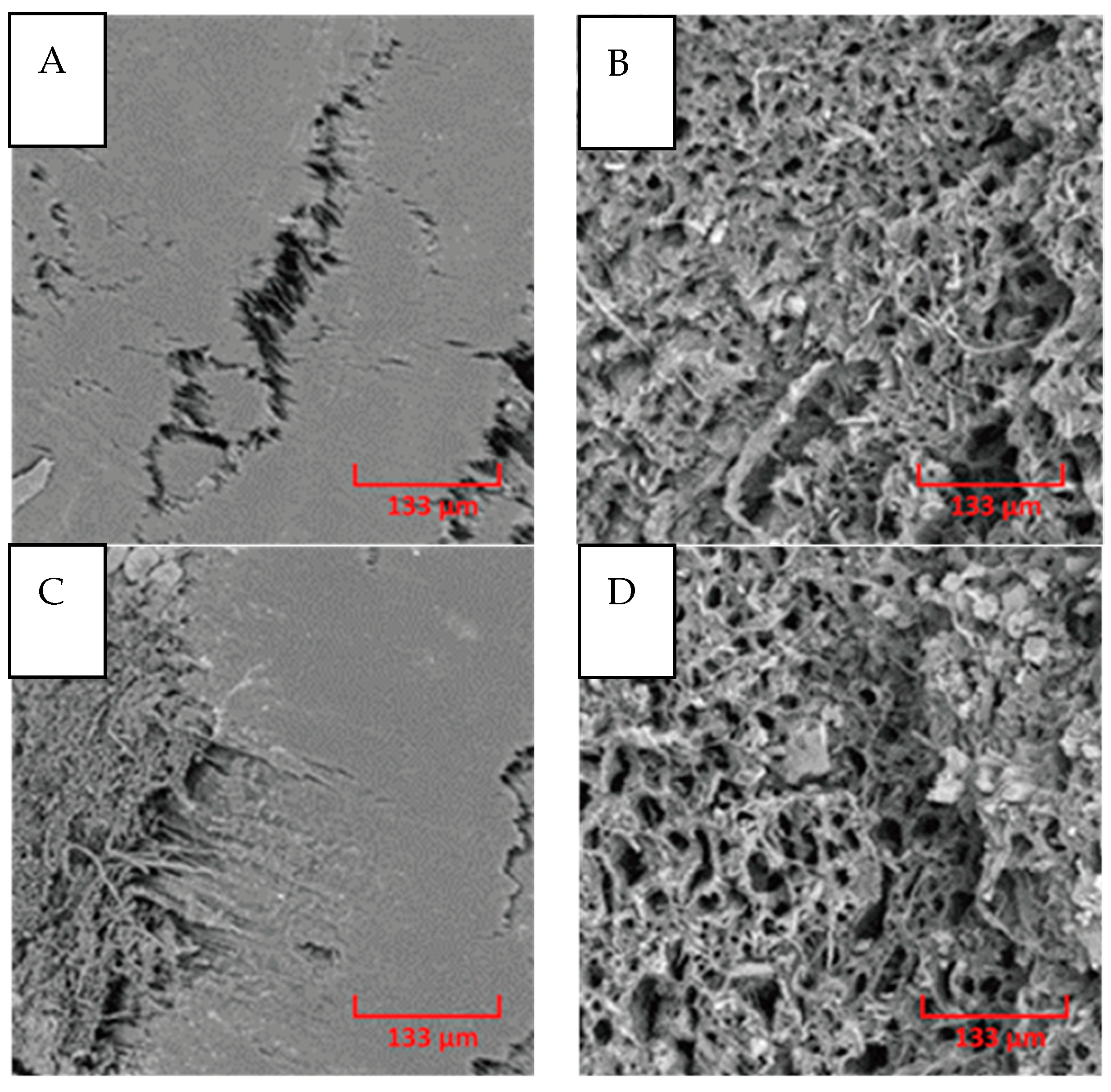
3.7. Filament Morphology
3.8. Contact Angle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Capêto, A.P.; Azevedo-Silva, J.; Sousa, S.; Pintado, M.; Guimarães, A.S.; Oliveira, A.L.S. Synthesis of Bio-Based Polyester from Microbial Lipidic Residue Intended for Biomedical Application. Int. J. Mol. Sci. 2023, 24, 4419. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Dana, H.R. Poly lactic acid (PLA) polymers: From properties to biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1117–1130. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Golbaten-Mofrad, H.; Najmoddin, N.; Goodarzi, V.; Shavandi, A.; Chen, W.-H. Reinforced conductive polyester based on itaconic acids, glycerol and polypyrrole with potential for electroconductive tissue restoration. Synth. Met. 2023, 293, 117238. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, K.; Huang, C.; Hu, S.; Xu, H.; Fuenmayor, E.; Cao, Z.; Major, l. Parameter optimization for PETG/ABS bilayer tensile specimens in material extrusion 3D printing through orthogonal method. Int. J. Adv. Manuf. Technol. 2023, 127, 447–458. [Google Scholar] [CrossRef]
- Gong, K.; Liu, H.; Huang, C.; Jiang, Q.; Xu, H.; Cao, Z.; Fuenmayor, E.; Major, l. Mass Customization of Polylactic Acid (PLA) Parts via a Hybrid Manufacturing Process. Polymers 2022, 14, 5413. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Dana, H.R.; Ebrahimi, F. Synthesis, properties, and applications of polylactic ACID-BASED polymers. Polym. Eng. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste—Its applications and degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Assad, H.; Assad, A.; Kumar, A. Recent Developments in 3D Bio-Printing and Its Biomedical Applications. Pharmaceutics 2023, 15, 255. [Google Scholar] [CrossRef] [PubMed]
- Baei, P.; Daemi, H.; Aramesh, F.; Baharvand, H.; Eslaminejad, M.B. Advances in mechanically robust and biomimetic polysaccharide-based constructs for cartilage tissue engineering. Carbohydr. Polym. 2023, 308, 120650. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, J.; Khaliq, N.U.; Meng, F.; Oucherif, K.A.; Xue, J.; Horava, S.D.; Cox, A.L.; Richard, C.A.; Swinney, M.R.; et al. Development of poly(lactide-co-glycolide) microparticles for sustained delivery of meloxicam. J. Control. Release 2023, 353, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt electrowriting of PLA, PCL, and composite PLA/PCL scaffolds for tissue engineering application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Comparison of fused-filament fabrication to direct compression and injection molding in the manufacture of oral tablets. Int. J. Pharm. 2019, 558, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, E.; O’Donnell, C.; Gately, N.; Doran, P.; Devine, D.; Lyons, J.G.; McConville, C.; Major, I. Mass-customization of oral tablets via the combination of 3D printing and injection molding. Int. J. Pharm. 2019, 569, 118611. [Google Scholar] [CrossRef]
- Xu, H.; Ebrahimi, F.; Gong, K.; Cao, Z.; Fuenmayor, E.; Major, I. Hybrid Manufacturing of Oral Solid Dosage Forms via Overprinting of Injection-Molded Tablet Substrates. Pharmaceutics 2023, 15, 507. [Google Scholar] [CrossRef]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef]
- Adarkwa, E.; Roy, A.; Ohodnicki, J.; Lee, B.; Kumta, P.N.; Desai, S. 3D printing of drug-eluting bioactive multifunctional coatings for orthopedic applications. Int. J. Bioprinting 2023, 9, 661. [Google Scholar] [CrossRef]
- Früh, A.; Rolauffs, B.; Seidenstuecker, M. Parametric Numerical Modeling and Fabrication of PCL Scaffolds for Bone Tissue Engineering Applications. Appl. Sci. 2022, 12, 12280. [Google Scholar] [CrossRef]
- Shi, W.; Shen, Z.; Bian, L.; Gao, Y.; Wu, Y.; Zhao, F.; Tang, H.; Lu, X. A mussel-bioinspired coating strategy to licence PCL osteoinductive activity for repairing bone defects. J. Mater. Res. Technol. 2022, 18, 2025–2036. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Y.; Qian, Y.; Chen, X.; Ouyang, Y.; Yuan, W.-E. 3D structured self-powered PVDF/PCL scaffolds for peripheral nerve regeneration. Nano Energy 2020, 69, 104411. [Google Scholar] [CrossRef]
- Yao, X.; Zhan, L.; Yan, Z.; Li, J.; Kong, L.; Wang, X.; Xiao, H.; Jiang, H.; Huang, C.; Ouyang, Y.; et al. Non-electric bioelectrical analog strategy by a biophysical-driven nano-micro spatial anisotropic scaffold for regulating stem cell niche and tissue regeneration in a neuronal therapy. Bioact. Mater. 2023, 20, 319–338. [Google Scholar] [CrossRef]
- Ayran, M.; Dirican, A.Y.; Saatcioglu, E.; Ulag, S.; Sahin, A.; Aksu, B.; Croitoru, A.-M.; Ficai, D.; Gunduz, O.; Ficai, A. 3D-Printed PCL Scaffolds Combined with Juglone for Skin Tissue Engineering. Bioengineering 2022, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef]
- Asghari, F.; Faradonbeh, D.R.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, E.; Sherafat, Z.; Zebarjad, S.M.; Khodaei, A.; Yavari, S.A. Stimuli-responsive piezoelectricity in electrospun polycaprolactone (PCL)/Polyvinylidene fluoride (PVDF) fibrous scaffolds for bone regeneration. J. Mater. Res. Technol. 2023, 23, 379–390. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Derayat, P.; Pourmanouchehri, Z.; Kahrarian, M.; Samimi, Z.; Hajialyani, M.; Bahrami, G.; Hosseinzadeh, L.; Rashidi, K.; Tajehmiri, A.; et al. Characterization and evaluation of antibacterial and wound healing activity of naringenin-loaded polyethylene glycol/polycaprolactone electrospun nanofibers. J. Drug Deliv. Sci. Technol. 2023, 81, 104182. [Google Scholar] [CrossRef]
- Khunová, V.; Kováčová, M.; Olejniková, P.; Ondreáš, F.; Špitalský, Z.; Ghosal, K.; Berkeš, D. Antibacterial Electrospun Polycaprolactone Nanofibers Reinforced by Halloysite Nanotubes for Tissue Engineering. Polymers 2022, 14, 746. [Google Scholar] [CrossRef]
- Adamu, M.; Sumaila, M.; Dauda, M.; Ause, T. Production and optimization of novel rice husk ash reinforced polycaprolactone/hydroxyapatite composite for bone regeneration using grey relational analysis. Sci. Afr. 2023, 19, e01563. [Google Scholar] [CrossRef]
- Youssef, S.H.; Kim, S.; Khetan, R.; Afinjuomo, F.; Song, Y.; Garg, S. The development of 5-fluorouracil biodegradable implants: A comparative study of PCL/PLGA blends. J. Drug Deliv. Sci. Technol. 2023, 81, 104300. [Google Scholar] [CrossRef]
- Goranov, V.; Shelyakova, T.; De Santis, R.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, G.-I.; Bonatti, A.F.; De Maria, C.; Naseem, R.; Melo, P.; Coelho, C.; Vozzi, G.; Dalgarno, K.; Quadros, P.; Vitale-Brovarone, C.; et al. Promotion of In Vitro Osteogenic Activity by Melt Extrusion-Based PLLA/PCL/PHBV Scaffolds Enriched with Nano-Hydroxyapatite and Strontium Substituted Nano-Hydroxyapatite. Polymers 2023, 15, 1052. [Google Scholar] [CrossRef] [PubMed]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based on PLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar] [CrossRef]
- Kennedy, S.W.; Choudhury, N.R.; Parthasarathy, R. 3D printing soft tissue scaffolds using Poly(caprolactone). Bioprinting 2023, 30, e00259. [Google Scholar] [CrossRef]
- Cetiner, B.; Dundar, G.S.; Yusufoglu, Y.; Okan, B.S. Sustainable Engineered Design and Scalable Manufacturing of Upcycled Graphene Reinforced Polylactic Acid/Polyurethane Blend Composites Having Shape Memory Behavior. Polymers 2023, 15, 1085. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.-H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 34108. [Google Scholar] [CrossRef]
- Anilkumar, K.; Jinisha, B.; Manoj, M.; Jayalekshmi, S. Poly(ethylene oxide) (PEO)—Poly(vinyl pyrrolidone) (PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur. Polym. J. 2017, 89, 249–262. [Google Scholar] [CrossRef]
- Qiu, Z.; Fujinami, S.; Komura, M.; Nakajima, K.; Ikehara, T.; Nishi, T. Miscibility and crystallization of poly(ethylene succinate)/poly(vinyl phenol) blends. Polymer 2004, 45, 4515–4521. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Khodaverdi, E.; Oroojalian, F.; Rahmanian-Devin, P.; Hashemi, S.H.M.; Omidkhah, N.; Asare-Addo, K.; Nokhodchi, A.; Kamali, H. Preparation of porous PCL-PEG-PCL scaffolds using supercritical carbon dioxide. Int. J. Pharm. 2023, 631, 122507. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Youssef, A.A.A.; Senapati, S.; Tripathi, S.; Bandari, S.; Majumdar, S.; Repka, M.A. Formulation development and in Vitro–Ex vivo characterization of hot-melt extruded ciprofloxacin hydrochloride inserts for ocular applications: Part I. Int. J. Pharm. 2023, 630, 122423. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Minghetti, P.; Casiraghi, A.; Montanari, L. Characterization of nifedipine solid dispersions. Int. J. Pharm. 2002, 242, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.-M.; Huang, Y.-F.; Ren, Y.; Wang, P.; Zhao, B.; Li, J.-H.; Xu, J.-Z.; Li, Z.-M. Toward biomimetic porous poly(ε-caprolactone) scaffolds: Structural evolution and morphological control during solid phase extrusion. Compos. Sci. Technol. 2018, 156, 192–202. [Google Scholar] [CrossRef]
- Jakić, M. Miscibility of Poly(Vinyl Chloride) with Poly(Ethylene Oxide) of Different Molecular Weights. Chem. Biochem. Eng. Q. 2016, 30, 61–71. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Lin, C.-L.; Chang, F.-C. Phase Behavior and Hydrogen Bonding in Ternary Polymer Blends of Phenolic Resin/Poly(ethylene oxide)/Poly(ε-caprolactone). Macromolecules 2002, 35, 278–285. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Joshi, M.K.; Lee, J.; Maharjan, B.; Ko, S.W.; Park, C.H.; Kim, C.S. Heterogeneous electrospun polycaprolactone/polyethylene glycol membranes with improved wettability, biocompatibility, and mineralization. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 105–113. [Google Scholar] [CrossRef]
- Aho, J.; Boetker, J.P.; Baldursdottir, S.; Rantanen, J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int. J. Pharm. 2015, 494, 623–642. [Google Scholar] [CrossRef]
- Valdés, A.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Jiménez, A.; Garrigós, M.C. Effect of Almond Skin Waste and Glycidyl Methacrylate on Mechanical and Color Properties of Poly(ε-caprolactone)/Poly(lactic acid) Blends. Polymers 2023, 15, 104. [Google Scholar] [CrossRef]
- Hameed, M.M.A.; Khan, S.A.P.M.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun nanofibers for drug delivery applications: Methods and mechanism. Polym. Adv. Technol. 2023, 34, 6–23. [Google Scholar] [CrossRef]
- van Kampen, E.E.M.; Ayyoubi, S.; Willemsteijn, L.; van Bommel, K.J.C.; Ruijgrok, E.J. The Quest for Child-Friendly Carrier Materials Used in the 3D Semi-Solid Extrusion Printing of Medicines. Pharmaceutics 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Abu-Serie, M.M.; El-Gendi, H.; El-Fakharany, E.M. Fabrication, characterization and in vitro evaluation of disulfiram-loaded cellulose acetate/poly(ethylene oxide) nanofiber scaffold for breast and colon cancer cell lines treatment. Int. J. Biol. Macromol. 2022, 204, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Mawuntu, V.J.; Yusuf, Y. Porous structure engineering of bioceramic hydroxyapatite-based scaffolds using PVA, PVP, and PEO as polymeric porogens. J. Asian Ceram. Soc. 2019, 7, 161–169. [Google Scholar] [CrossRef]
- Pooshidani, Y.; Zoghi, N.; Rajabi, M.; Nazarpak, M.H.; Hassannejad, Z. Fabrication and evaluation of porous and conductive nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2021, 32, 46. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Heseltine, P.L.; Bayram, C.; Gultekinoglu, M.; Homer-Vanniasinkam, S.; Ulubayram, K.; Edirisinghe, M. Facile One-Pot Method for All Aqueous Green Formation of Biocompatible Silk Fibroin-Poly(Ethylene Oxide) Fibers for Use in Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Mfoafo, K.; Kwon, Y.; Omidi, Y.; Omidian, H. Contemporary applications of thermogelling PEO-PPO-PEO triblock copolymers. J. Drug Deliv. Sci. Technol. 2022, 70, 103182. [Google Scholar] [CrossRef]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical processing of PCL and PCL-PEG blends to produce improved PCL-based porous scaffolds. J. Supercrit. Fluids 2022, 186, 105611. [Google Scholar] [CrossRef]
- Sailema-Palate, G.P.; Vidaurre, A.; Campillo-Fernández, A.; Castilla-Cortázar, I. A comparative study on Poly(ε-caprolactone) film degradation at extreme pH values. Polym. Degrad. Stab. 2016, 130, 118–125. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, J.; Guo, H.; Guo, X.; Wang, X.; Shen, Y.; Li, Q. Fabrication of fibrillated and interconnected porous poly(ε-caprolactone) vascular tissue engineering scaffolds by microcellular foaming and polymer leaching. RSC Adv. 2020, 10, 10055–10066. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Garcia, R.; Schindler, C.; Dean, D.; Pogwizd, S.M.; Singh, R.; Vohra, Y.K.; Thomas, V. Fibro-porous poliglecaprone/polycaprolactone conduits: Synergistic effect of composition and in vitro degradation on mechanical properties. Polym. Int. 2015, 64, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.; Albadarin, A.B.; Sajjia, M.; Mangwandi, C.; Kuhs, M.; Collins, M.N.; Walker, G.M. Effect of poly ethylene glycol on the mechanical and thermal properties of bioactive poly(ε-caprolactone) melt extrudates for pharmaceutical applications. Int. J. Pharm. 2016, 500, 179–186. [Google Scholar] [CrossRef] [PubMed]







| Name | PCL 50,000 (wt %) | PEO 60,000 (wt %) | PEO 300,000 (wt %) |
|---|---|---|---|
| 100 PCL 50 | 100 | - | - |
| 75 PCL 60 | 75 | 25 | - |
| 50 PCL 60 | 50 | 50 | - |
| 25 PCL 60 | 25 | 75 | - |
| 100 PEO 60 | 0 | 100 | - |
| 75 PCL 300 | 75 | - | 25 |
| 50 PCL 300 | 50 | - | 50 |
| 25 PCL 300 | 25 | - | 75 |
| 100 PEO 300 | 0 | - | 100 |
| Blend | MFI (g/10 min) | Torque N/m |
|---|---|---|
| 100 PCL | 16.00 ± 1 | 8 |
| 75 PCL 60 | 15.00 ± 1 | 8 |
| 50 PCL 60 | 9.00 ± 1 | 8 |
| 25 PCL 60 | 4.5 ± 1 | 9 |
| 100 PEO 60 | 1.500 ± 1 | 11 |
| 75 PCL 300 | 3.5 ± 1 | 13 |
| 50 PCL 300 | 1.2 ± 1 | 14 |
| 25 PCL 300 | 0.8 ± 1 | 11 |
| 100 PEO 300 | 0 | 16 |
| Batch | Contact Angulation (°) |
|---|---|
| 100 PCL 50 | 74.1 ± 0.9 |
| 75 PCL 60 | 54.1 ± 3.5 |
| 75 PCL 300 | 69.4 ± 1.2 |
| 50 PCL 60 | 44.9 ± 1.1 |
| 50 PCL 300 | 66.9 ± 1.5 |
| 25 PCL 60 | 49.0 ± 4.6 |
| 25 PCL 300 | 50.6 ± 1.0 |
| 100 PEO 60 | 51.9 ± 2.4 |
| 100 PEO 300 | 37.4 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalton, M.; Ebrahimi, F.; Xu, H.; Gong, K.; Fehrenbach, G.; Fuenmayor, E.; Murphy, E.J.; Major, I. The Influence of the Molecular Weight of Poly(Ethylene Oxide) on the Hydrolytic Degradation and Physical Properties of Polycaprolactone Binary Blends. Macromol 2023, 3, 431-450. https://doi.org/10.3390/macromol3030026
Dalton M, Ebrahimi F, Xu H, Gong K, Fehrenbach G, Fuenmayor E, Murphy EJ, Major I. The Influence of the Molecular Weight of Poly(Ethylene Oxide) on the Hydrolytic Degradation and Physical Properties of Polycaprolactone Binary Blends. Macromol. 2023; 3(3):431-450. https://doi.org/10.3390/macromol3030026
Chicago/Turabian StyleDalton, Maurice, Farnoosh Ebrahimi, Han Xu, Ke Gong, Gustavo Fehrenbach, Evert Fuenmayor, Emma J. Murphy, and Ian Major. 2023. "The Influence of the Molecular Weight of Poly(Ethylene Oxide) on the Hydrolytic Degradation and Physical Properties of Polycaprolactone Binary Blends" Macromol 3, no. 3: 431-450. https://doi.org/10.3390/macromol3030026
APA StyleDalton, M., Ebrahimi, F., Xu, H., Gong, K., Fehrenbach, G., Fuenmayor, E., Murphy, E. J., & Major, I. (2023). The Influence of the Molecular Weight of Poly(Ethylene Oxide) on the Hydrolytic Degradation and Physical Properties of Polycaprolactone Binary Blends. Macromol, 3(3), 431-450. https://doi.org/10.3390/macromol3030026







