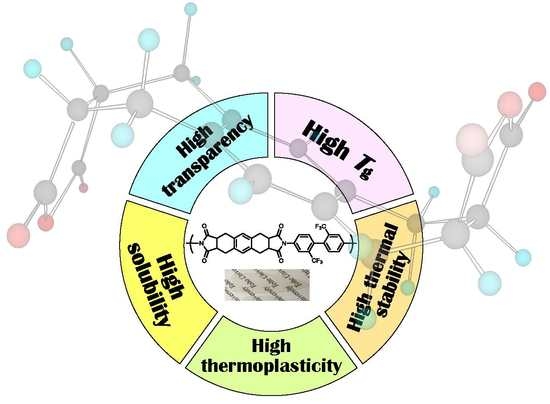
Colorless Polyimides Derived from Octahydro-2,3,6,7-anthracenetetracarboxylic Dianhydride
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
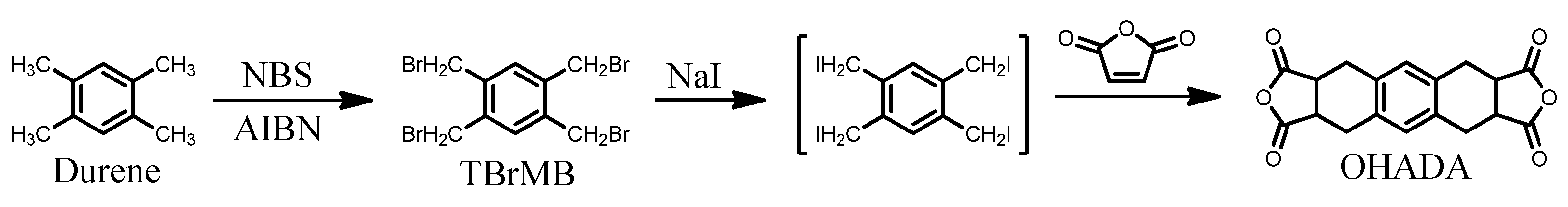
2.1.1. Synthesis of OHADA Prototype
2.1.2. Synthesis of Amide-Type Diamine
2.1.3. Common Monomers
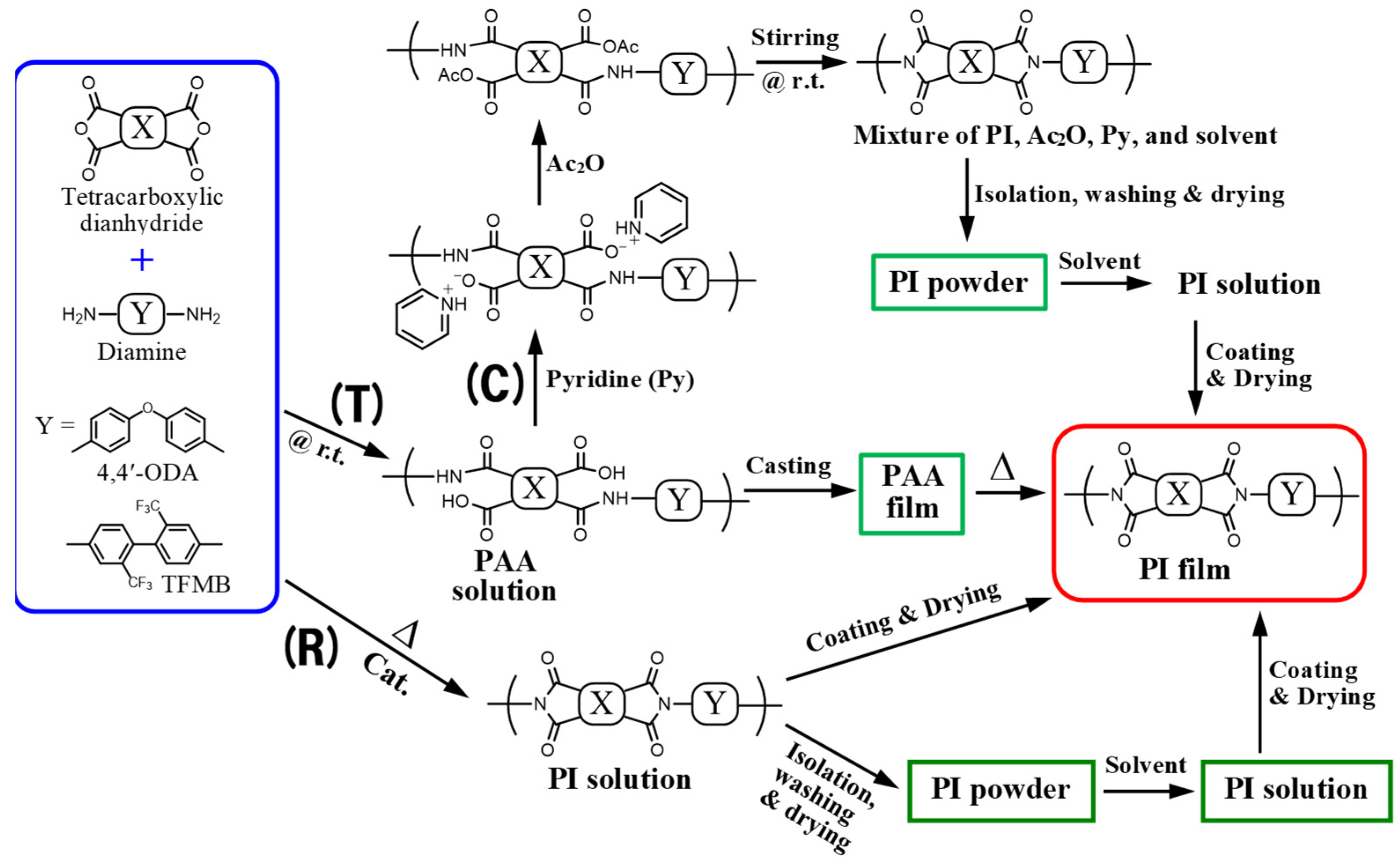
2.1.4. Polymerization, Imidization, and PI Film Preparation
2.2. Measurements
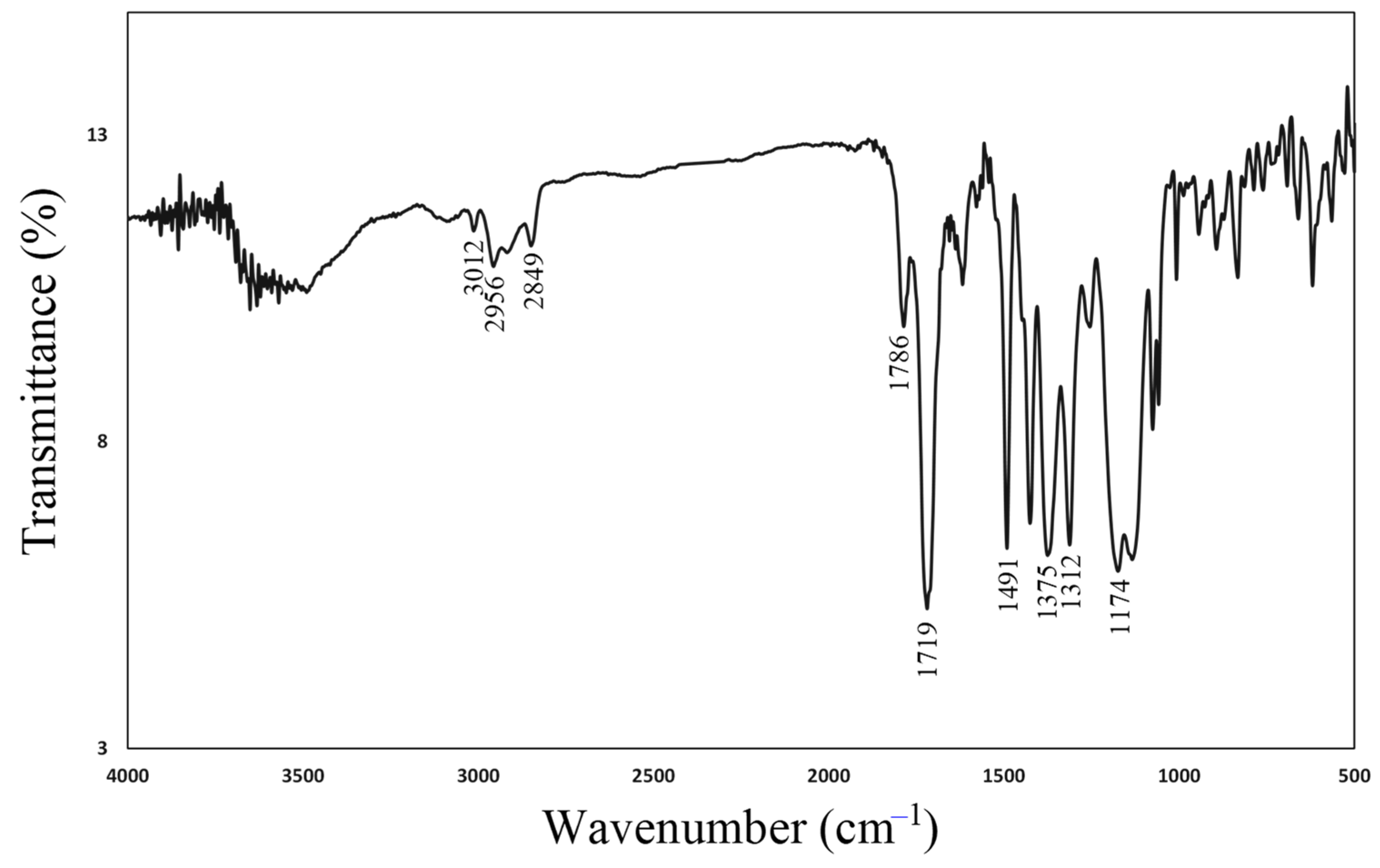
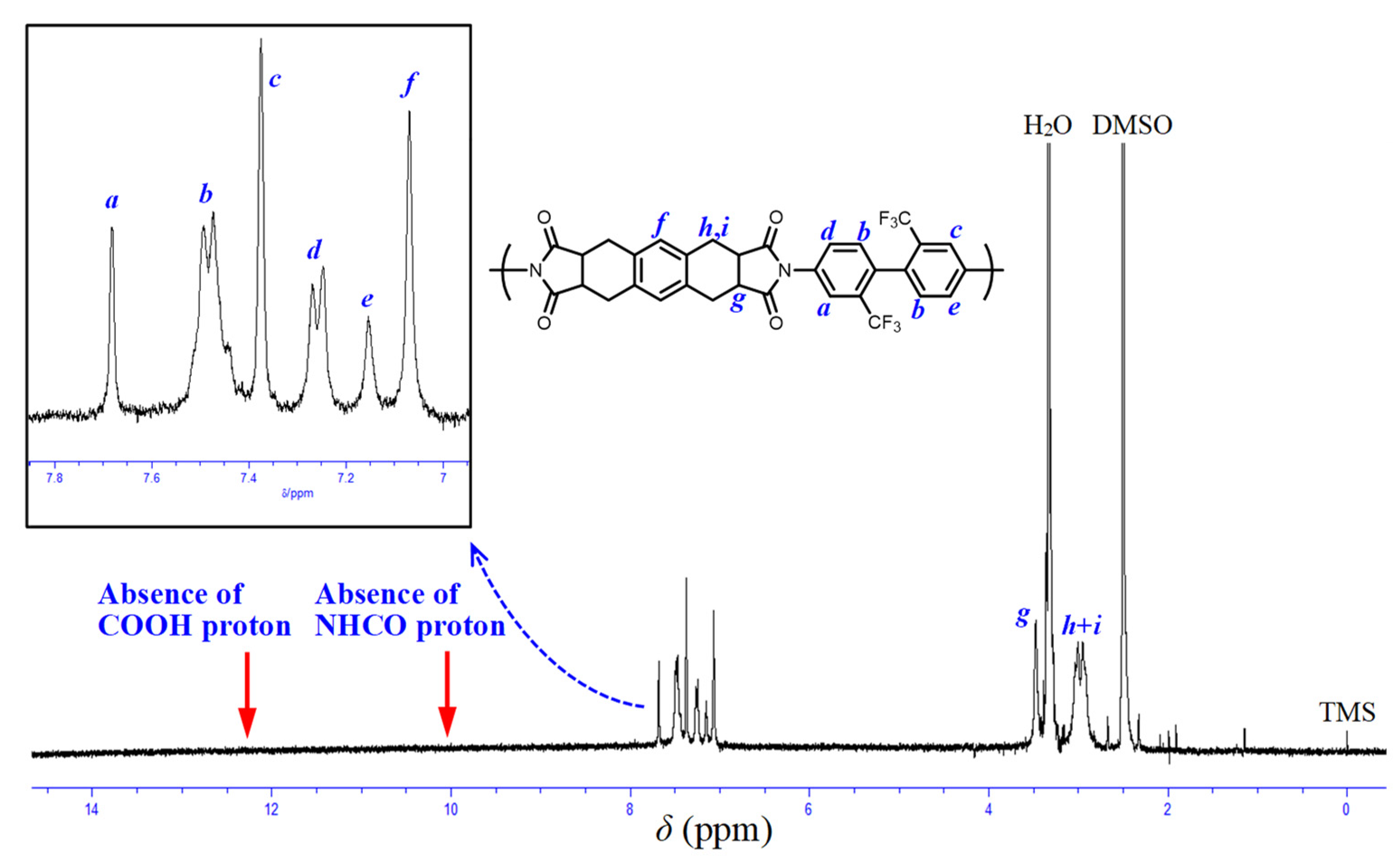
2.2.1. Structural Characterization of the Monomers and Polymers
2.2.2. Reduced Viscosities and Molecular Weights
2.2.3. Linear Coefficients of Thermal Expansion (CTEs)
2.2.4. Heat Resistance
2.2.5. Optical Transparency
2.2.6. Birefringence
2.2.7. Mechanical Properties
2.2.8. Solubility Test
3. Results and Discussion
3.1. Approaches for Obtaining Colorless OHADA
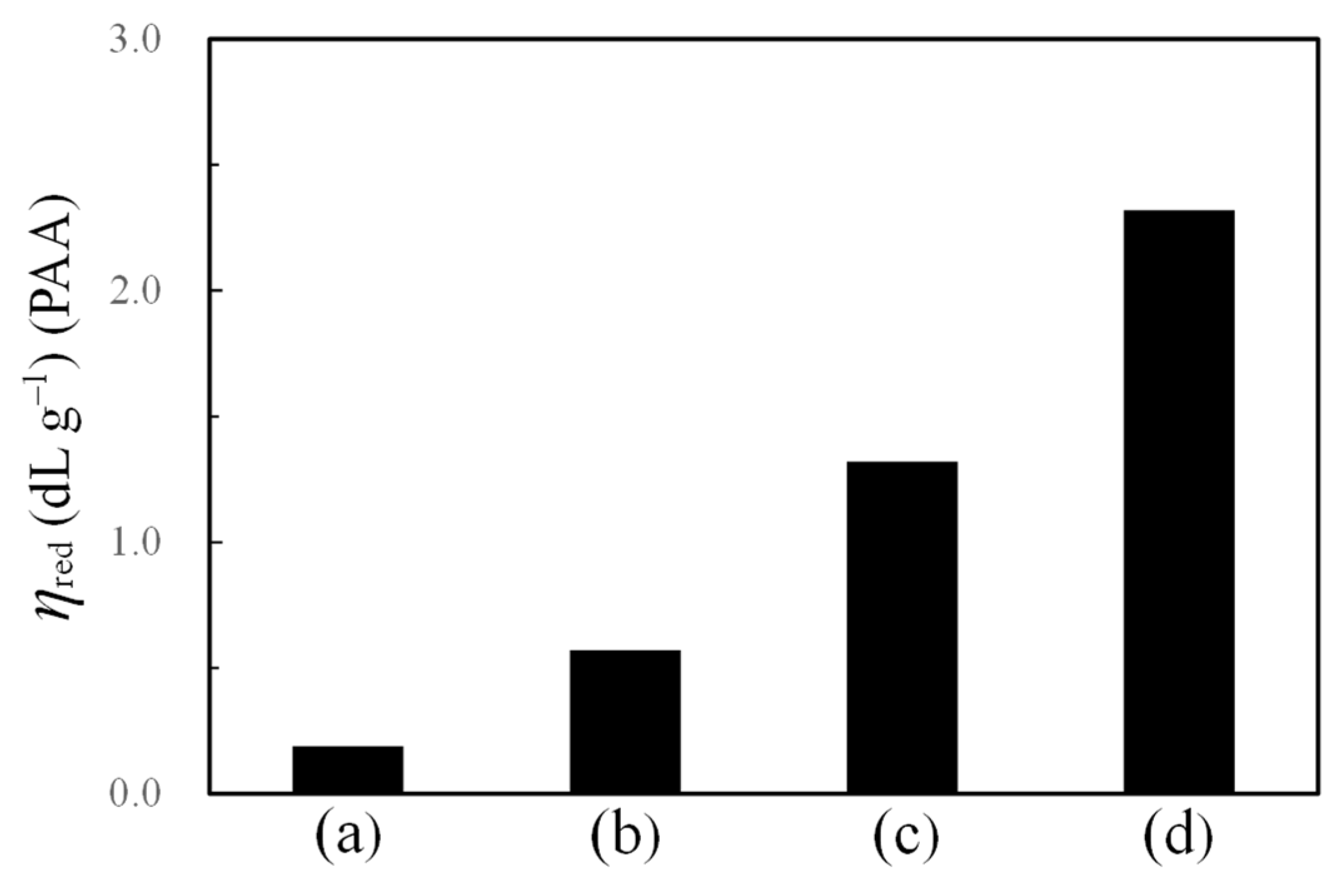
3.1.1. Decolorization Processes of the OHADA Prototype
3.1.2. Simplified Process for Obtaining Colorless OHADA (Method B)
3.2. Estimated Steric Structure of OHADA
3.3. Polyaddition Reactivity of OHADA with Aromatic Diamines
3.4. Polymerizability of OHADA with Diamines during the Modified One-Pot Process
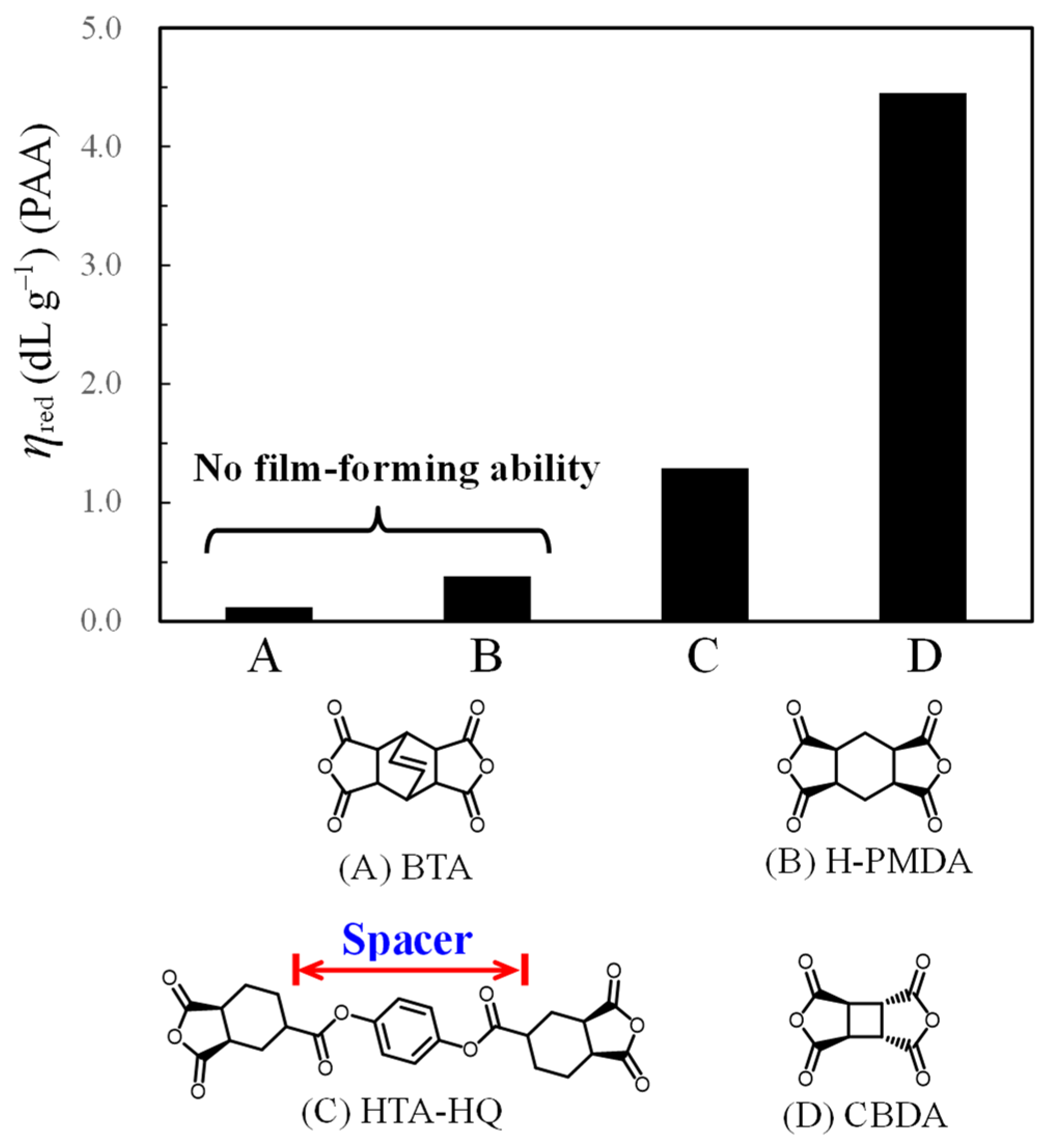
3.5. Solubility of OHADA-Based PIs
3.6. Properties of OHADA-Based PI Films
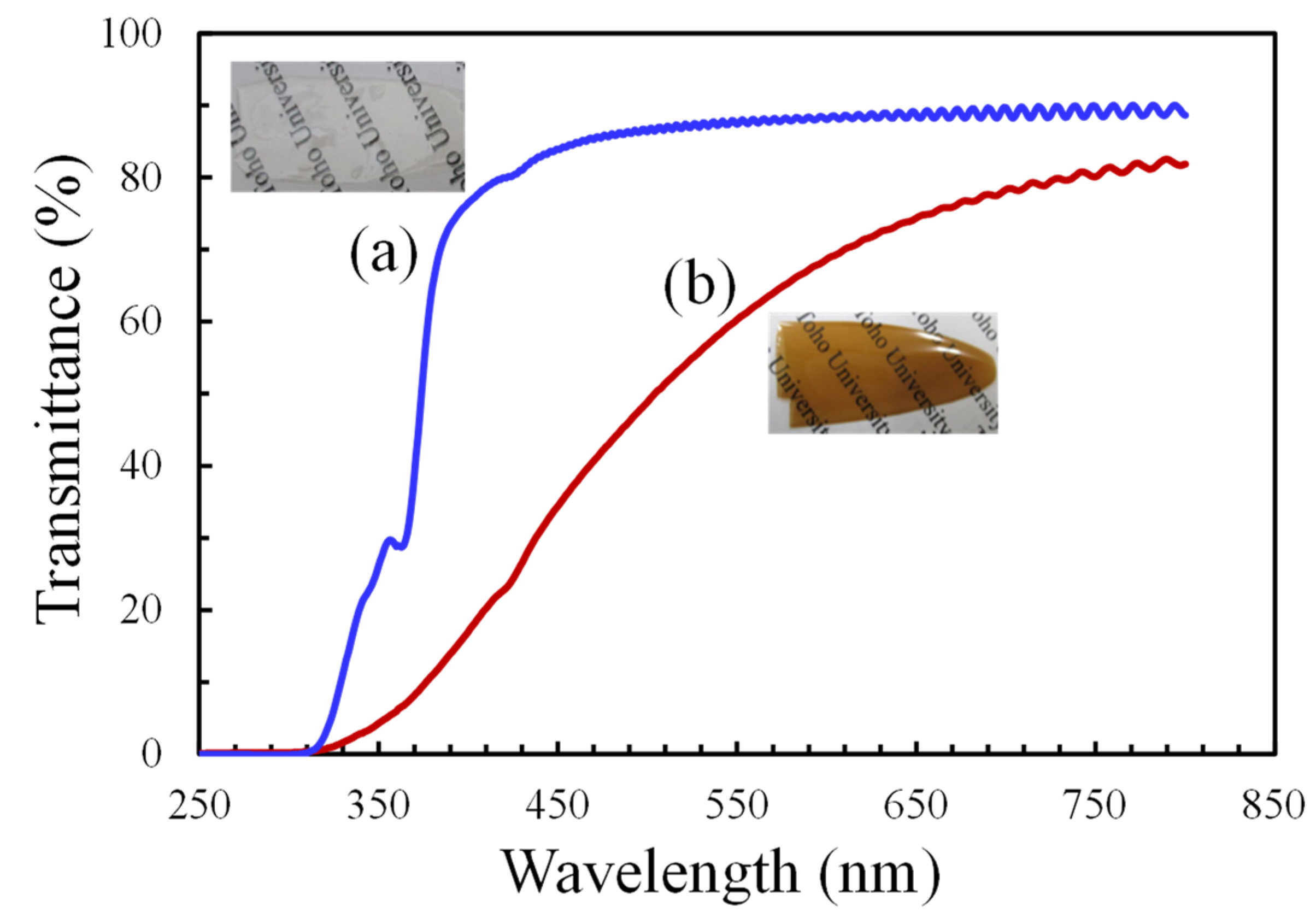
3.6.1. Effect of OHADA Decolorization
3.6.2. Features of the Properties of OHADA-Based PIs
3.6.3. Another Feature: Thermoplasticity
3.6.4. Modification of CBDA/TFMB Using OHADA as the Comonomer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumitomo Chemical Co., Ltd., Poly(ether sulfone). Available online: http://www.sumitomo-chem.co.jp/sep/english/products/pes/index.html (accessed on 20 March 2023).
- Cassidy, P.E. Thermally Stable Polymers: Syntheses and Properties; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Mittal, K.L. (Ed.) Polyimides: Synthesis, Characterization, and Applications; Plenum Press: New York, NY, USA, 1984; Volume 1–2. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. (Eds.) Polyimides: Thermally Stable Polymers; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Feger, C.; Khojasteh, M.M.; McGrath, J.E. (Eds.) Polyimides: Materials, Chemistry and Characterization; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Sroog, C.E. Polyimide. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Abadie, M.J.M.; Sillion, B. (Eds.) Polyimides and Other High-Temperature Polymers; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Bessonov, M.I.; Zubkov, V.A. (Eds.) Polyamic Acid and Polyimides: Synthesis, Transformation and Structure; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Feger, C.; Khojasteh, M.M.; Htoo, M.S. (Eds.) Advances in Polyimide Science and Technology; Technomic Publishing: Lancaster, PA, USA, 1993. [Google Scholar]
- Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Sachdev, H.S.; Khojasteh, M.M.; Feger, C. (Eds.) Advances in Polyimides and Low Dielectric Polymers; Society of Plastic Engineers: New York, NY, USA, 1997. [Google Scholar]
- Hergenrother, P.M. The Use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- Ando, S.; Ueda, M.; Kakimoto, M.; Kochi, M.; Takeichi, T.; Hasegawa, M.; Yokota, R. (Eds.) The Latest Polyimides: Fundamentals and Applications, 2nd ed.; NTS: Tokyo, Japan, 2010. [Google Scholar]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Yang, S.Y. (Ed.) Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Chemical Industry Press: Shanghai, China; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Diaham, S. (Ed.) Polyimide for Electronic and Electrical Engineering Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficient of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Volksen, W.; Cha, H.J.; Sanchez, M.I.; Yoon, D.Y. Polyimides derived from nonaromatic monomers: Synthesis, characterization and potential applications. React. Funct. Polym. 1996, 30, 61–69. [Google Scholar] [CrossRef]
- Seino, H.; Sasaki, T.; Mochizuki, A.; Ueda, M. Synthesis of fully aliphatic polyimides. High Perform. Polym. 1999, 11, 255–262. [Google Scholar] [CrossRef]
- Mathews, A.S.; Kim, I.; Ha, C.S. Synthesis, characterization, and properties of fully aliphatic polyimides and their derivatives for microelectronics and optoelectronics applications. Macromol. Res. 2007, 15, 114–128. [Google Scholar] [CrossRef]
- Tsuda, Y.; Etou, K.; Hiyoshi, N.; Nishikawa, M.; Matsuki, Y.; Bessho, N. Soluble copolyimides based on 2,3,5-tricarboxycyclopentyl acetic dianhydride and conventional aromatic tetracarboxylic dianhydrides. Polym. J. 1998, 30, 222–228. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Suzuki, H.; Abe, T.; Takaishi, K.; Narita, M.; Hamada, F. The synthesis and X-ray structure of 1,2,3,4-cyclobutane tetracarboxylic dianhydride and the preparation of a new type of polyimide showing excellent transparency and heat resistance. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 108–116. [Google Scholar] [CrossRef]
- Matsumoto, T. Nonaromatic polyimides derived from cycloaliphatic monomers. Macromolecules 1999, 32, 4933–4939. [Google Scholar] [CrossRef]
- Li, J.; Kato, J.; Kudo, K.; Shiraishi, S. Synthesis and properties of novel soluble polyimides having an unsymmetric spiro tricyclic dianhydride unit. Macromol. Chem. Phys. 2000, 201, 2289–2297. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Ishii, J.; Yamaguchi, S.; Takezawa, E.; Kagayama, T.; Ishikawa, A. Colorless polyimides derived from 1S,2S,4R,5R-cyclohexanetetracarboxylic dianhydride, self-orientation behavior during solution casting, and their optoelectronic applications. Polymer 2014, 55, 4693–4708. [Google Scholar] [CrossRef]
- Shiotani, A.; Shimazaki, H.; Matsuo, M. Preparation of transparent polyimides derived from cis- and trans-dicyclohexyl-3,3′,4,4′--tetracarboxylic dianhydrides. Macromol. Mater. Eng. 2001, 286, 434–441. [Google Scholar] [CrossRef]
- Guo, Y.; Song, H.; Zhai, L.; Liu, J.; Yang, S. Synthesis and characterization of novel semi-alicyclic polyimides from methyl-substituted tetralin dianhydride and aromatic diamines. Polym. J. 2012, 44, 718–723. [Google Scholar] [CrossRef]
- Fang, X.; Yang, Z.; Zhang, S.; Gao, L.; Ding, M. Synthesis and properties of polyimides derived from cis- and trans-1,2,3,4-cyclohexanetetracarboxylic dianhydrides. Polymer 2004, 45, 2539–2549. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, D.; Du, H.; Wu, X.; Zhang, Y.; Tan, Y.; Wu, L.; Liu, J.; Zhang, X. Reduced coefficients of linear thermal expansion of colorless and transparent semi-alicyclic polyimide films via incorporation of rigid-rod amide moiety. Preparation and properties. Polymers 2020, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Wang, Y.; Mu, H.; Wang, Z.; Cheng, H.; Zhao, F.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless polyimides derived from an alicyclic tetracarboxylic dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, L.; Bai, L.; He, M.; Wang, C.; Mo, S.; Fan, L. Synthesis and characterization of optically transparent semi-aromatic polyimide films with low fluorine content. Polymer 2019, 163, 106–114. [Google Scholar] [CrossRef]
- Hu, X.; Mu, H.; Wang, Y.; Wang, Z.; Yan, J. Colorless polyimides derived from isomeric dicyclohexyl-tetracarboxylic dianhydrides for optoelectronic applications. Polymer 2018, 134, 8–19. [Google Scholar] [CrossRef]
- Hasegawa, M.; Watanabe, Y.; Tsukuda, S.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ichikawa, K.; Takahashi, S.; Ishii, J. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride: Strategies to reduce the coefficients of thermal expansion by maximizing the spontaneous chain orientation behavior during solution casting. Polymers 2022, 14, 1131. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Kumar, S.V.; Lee, J.H.; Oh, S.Y.; Chung, C.M. Preparation of robust, flexible, transparent films from partially aliphatic copolyimide. Macromol. Res. 2015, 23, 566–573. [Google Scholar] [CrossRef]
- Tang, Y.; Li, L.; Ma, K.; Chen, G.; Wang, W.; Fang, X. Transparent and organosoluble cardo polyimides with different trans/cis ratios of 1,4-diaminocyclohexane via aromatic nucleophilic substitution polymerization. Polym. Int. 2018, 67, 598–605. [Google Scholar] [CrossRef]
- Miao, J.; Hu, X.; Wang, X.; Meng, X.; Wang, Z.; Yan, J. Colorless polyimides derived from adamantane-containing diamine. Polym. Chem. 2021, 11, 6009–6016. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Ma, X.; Ghanem, B.S.; Pinnau, I. Synthesis and characterization of organo-soluble polyimides derived from alicyclic dianhydrides and a dihydroxyl-functionalized spirobisindane diamine. ACS Appl. Polym. Mat. 2019, 1, 63–69. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Hasegawa, M.; Koyanaka, M. Polyimides containing trans-1,4-cyclohexane unit. Polymerizability of their precursors and low-CTE and low-K and High-Tg properties. High Perform. Polym. 2003, 15, 47–64. [Google Scholar] [CrossRef]
- Hoshino, K.; Sato, H.; Ishii, J.; Hasegawa, M. Solution-processable colorless polyimides derived from novel cycloaliphatic tetracarboxylic dianhydride (3). Polym. Prepr. Jpn. 2019, 68, 1Pf090. [Google Scholar]
- Kim, Y.S.; Jung, J.C. Synthesis and characterization of polyimides from 9,10-diphenyl-1,2,3,4,5,6,7,8-octahydro-2,3,6,7-anthracenetetracarboxylic 2,3:6,7-dianhydride. Polym. Bull. 2002, 48, 327–335. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, J.C. Synthesis and properties of polyimides derived from 9,10-dialkyloxy-1,2,3,4,5,6,7,8-octahydro-2,3,6,7-anthracenetetracarboxylic 2,3:6,7-dianhydrides. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1764–1774. [Google Scholar] [CrossRef]
- Chi, J.H.; Shin, G.J.; Kim, Y.S.; Jung, J.C. Synthesis of new alicyclic polyimides by Diels-Alder polymerization. J. Appl. Polym. Sci. 2007, 106, 3823–3832. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and properties of novel asymmetric biphenyl type polyimides. homo- and copolymers and blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Hasegawa, M.; Matano, T.; Shindo, Y.; Sugimura, T. Spontaneous molecular orientation of polyimides induced by thermal imidization (2). In-plane orientation. Macromolecules 1996, 29, 7897–7909. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Wada, Y. Approaches to improve the film ductility of colorless cycloaliphatic polyimides. Polym. Adv. Technol. 2018, 29, 921–933. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hoshino, Y.; Katsura, N.; Ishii, J. Superheat resistant polymers with low coefficients of thermal expansion. Polymer 2017, 111, 91–102. [Google Scholar] [CrossRef]
- Baklagina, Y.G.; Milevskaya, I.S.; Efanova, N.V.; Sidorovich, A.V.; Zubkov, V.A. Structure of rigid-chain polyimides from pyromellitic dianhydride. Vysokomol. Soedin. 1976, A18, 1235–1242. [Google Scholar]
- Obata, Y.; Okuyama, K.; Kurihara, S.; Kitano, Y.; Jinda, T. X-ray Structure Analysis of an Aromatic Polyimide. Macromolecules 1995, 28, 1547–1551. [Google Scholar] [CrossRef]
- Hasegawa, M.; Koseki, K. Poly(ester imide)s possessing low CTE and low water absorption. High Perform. Polym. 2006, 18, 697–717. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horii, S. Low-CTE polyimides derived from 2,3,6,7-naphthalenetetracarboxylic dianhydride. Polym. J. 2007, 39, 610–621. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takahashi, S.; Tsukuda, S.; Hirai, T.; Ishii, J.; Yamashina, Y.; Kawamura, Y. Symmetric and asymmetric spiro-type colorless poly(ester imide)s with low coefficients of thermal expansion, high glass transition temperatures, and excellent solution-processability. Polymer 2019, 169, 167–184. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kaneki, T.; Tsukui, M.; Okubo, N.; Ishii, J. High-temperature polymers overcoming the trade-off between excellent thermoplasticity and low thermal expansion properties. Polymer 2016, 99, 292–306. [Google Scholar] [CrossRef]
- Takekoshi, T.; Wirth, J.G.; Heath, D.R.; Kochanowski, J.E.; Manello, J.S.; Webber, M.J. Polymer syntheses via aromatic nitro displacement reaction. J. Polym. Sci. Part A Polym. Chem. 1980, 18, 3069–3080. [Google Scholar] [CrossRef]

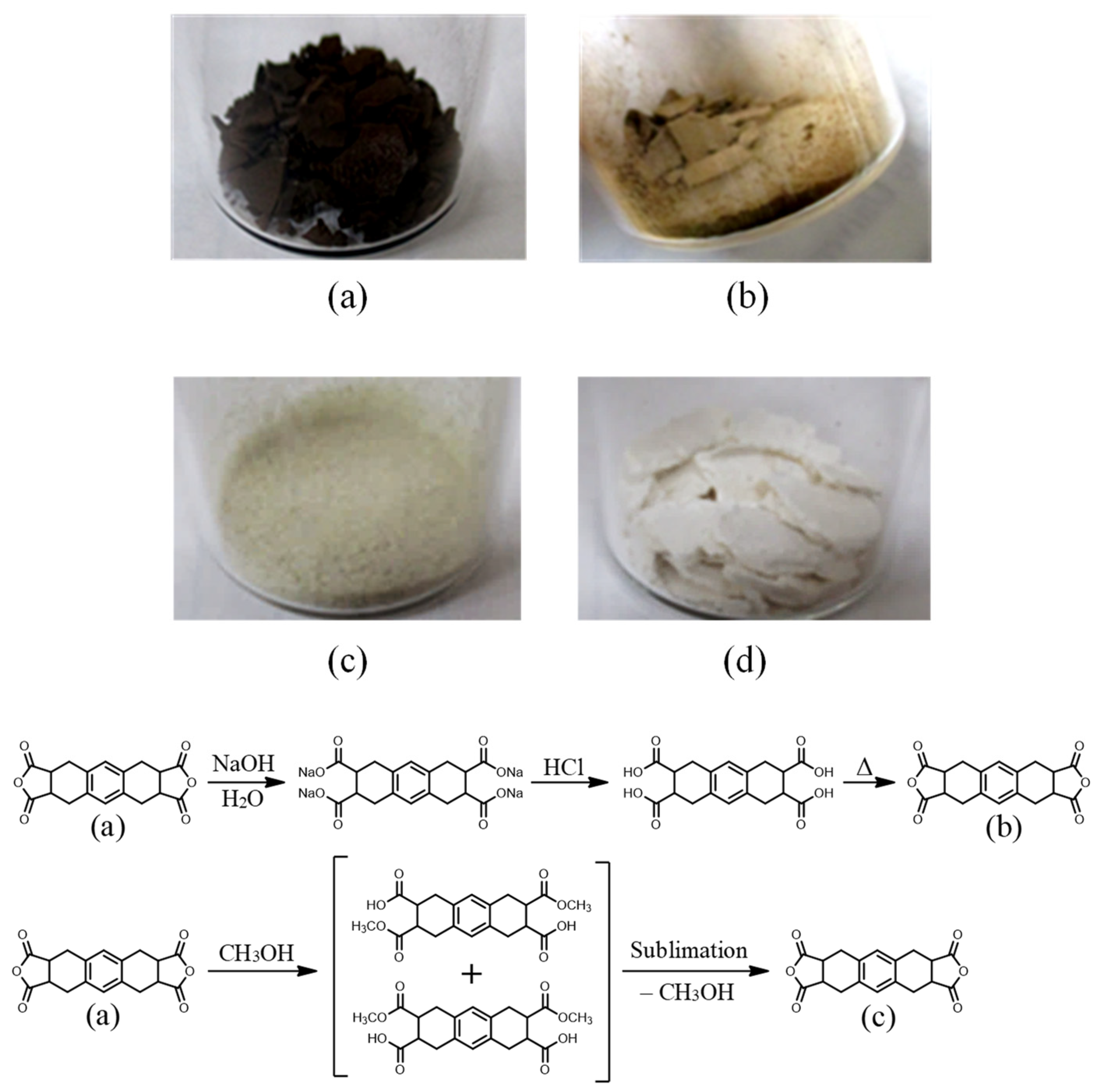
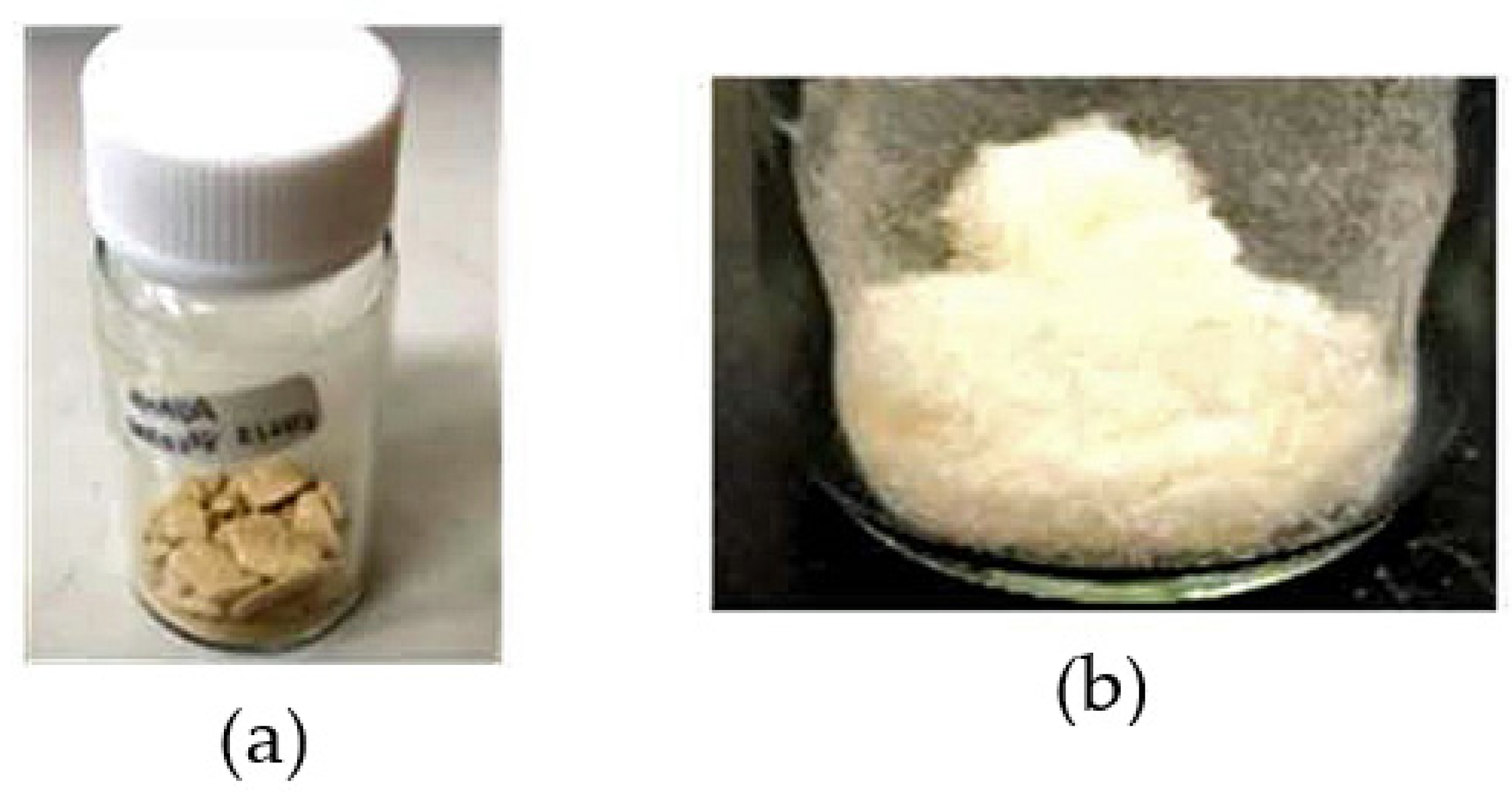


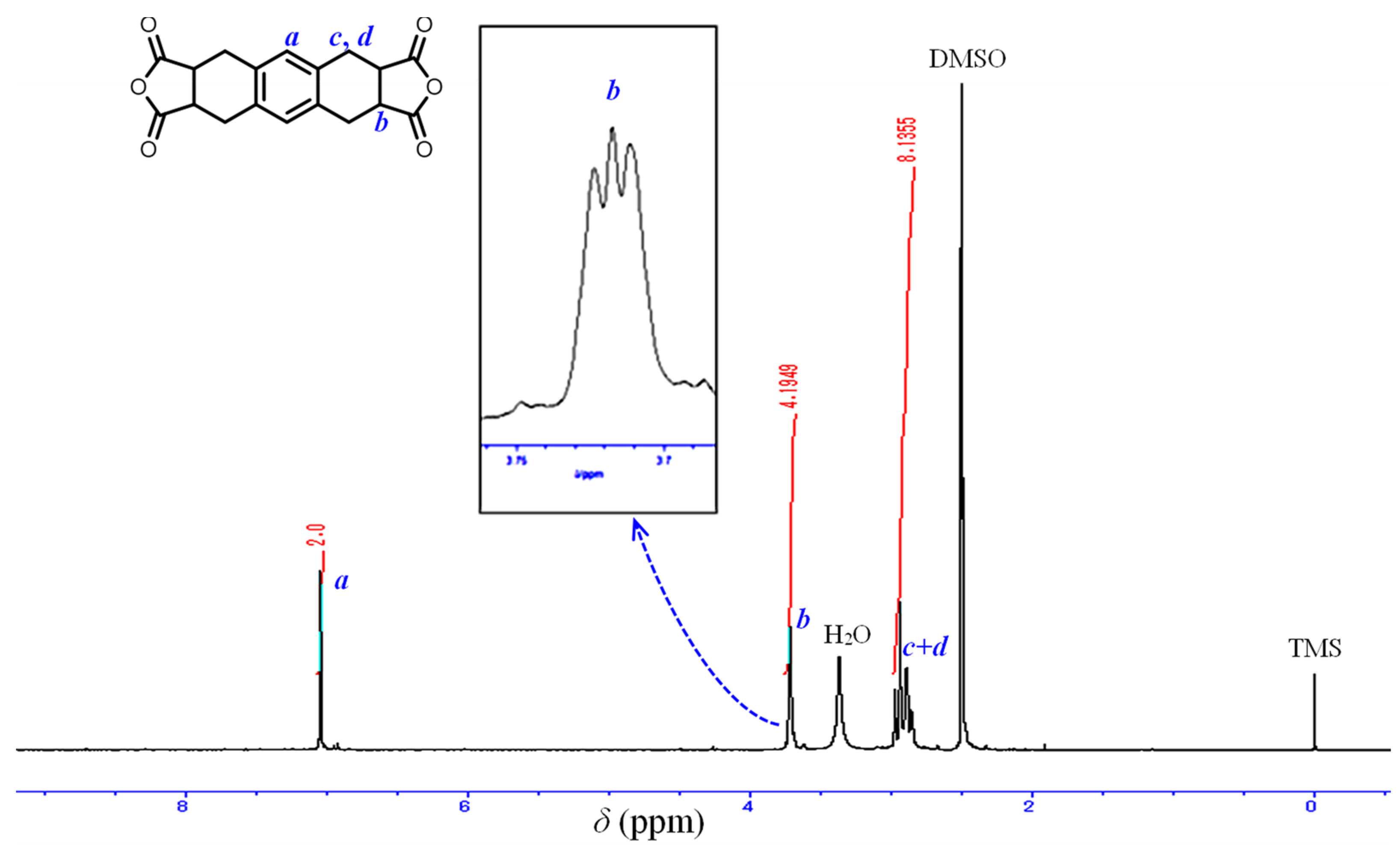





| System No. | Grade of OHADA | Diamine | Solvent | Solid Content (Initial → Final) (wt%) | Appearance of PAA Solution | ηinh (PAA) (dL g−1) | PI Film Formability |
|---|---|---|---|---|---|---|---|
| 1p | Colored (Prototype) | 4,4′-ODA | NMP | 50 → 12.5 | Homogeneous and black | 0.88 | Sufficient |
| 2p | Colored (Prototype) | TFMB | DMAc | 50 → 11.5 | Inhomogeneous and black | 0.24 | None (cracked) |
| 1 | Decolorized (Method A) | 4,4′-ODA | NMP | 30 → 17 | Homogeneous and pale brown | 1.32 | Sufficient |
| 3 | Decolorized (Method A) | DABA | NMP | 30 → 20 | Homogeneous and dark brown | 0.27 | None (cracked) |
| 4 | Decolorized (Method A) | AB-TFMB | NMP | 30 | Inhomogeneous | ---- | ---- |
| No. | Tetracarboxylic Dianhydride | Diamine | NMP | DMAc | DMF | DMSO | GBL | CF | ACT | TriGL |
|---|---|---|---|---|---|---|---|---|---|---|
| Heating Temperature (°C) at the 2nd Step | ||||||||||
| 150 | 150 | 140 | 150 | 150 | 50 | 50 | 150 | |||
| 2 | OHADA | TFMB | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 4 | OHADA | AB-TFMB | ++ | ++ | ++ | ++ | ++ | − | − | ++ |
| 7 | CBDA (50) OHADA (50) | TFMB | ++ | ++ | ++ | ++ | ++ | − | + | ++ |
| 8 | CBDA (25) OHADA (75) | TFMB | ++ | ++ | ++ | ++ | ++ | − | ++ | ++ |
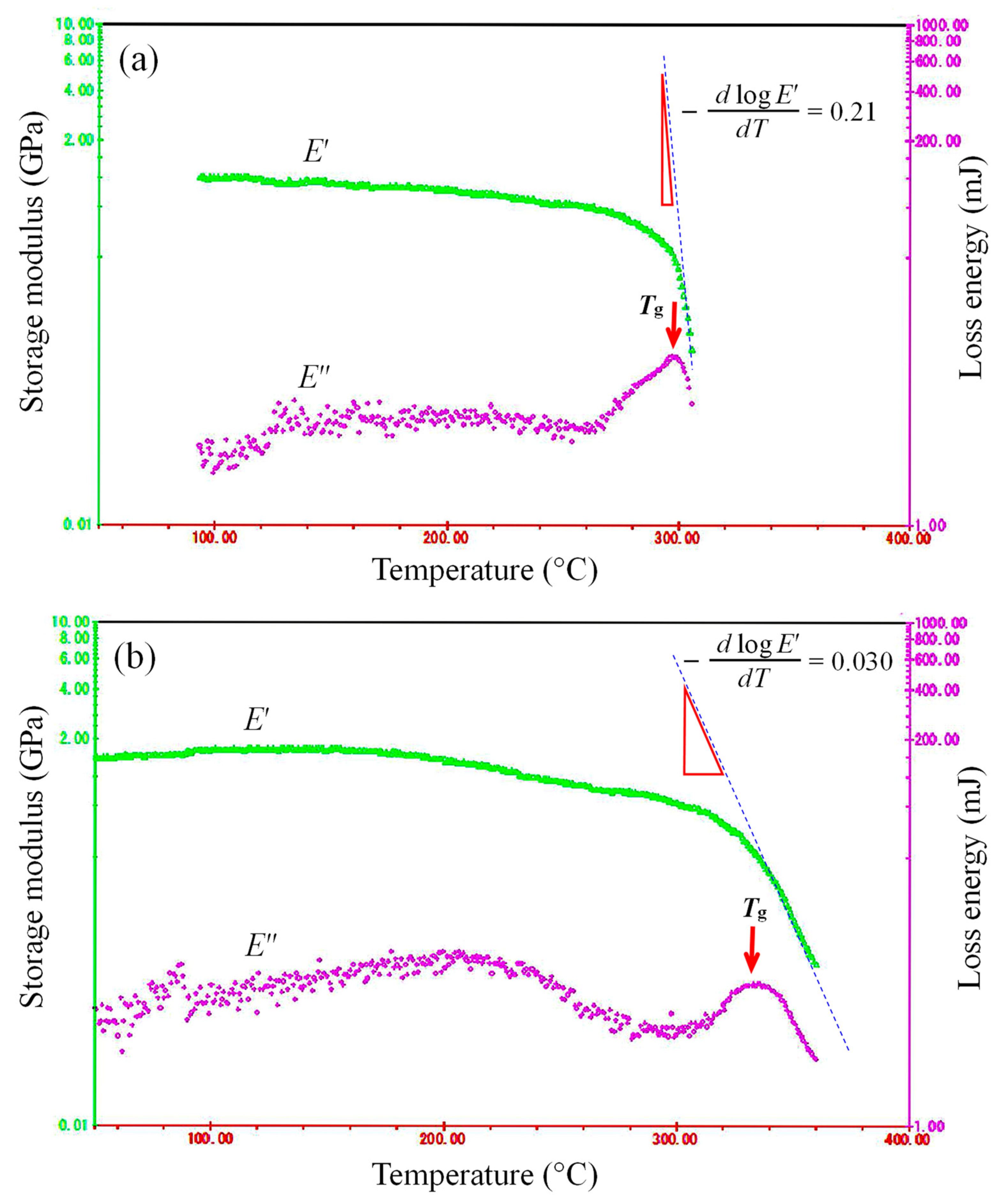
| No. | Diamine | ηred (dL g−1) | Route | T400 (%) | YI | λcut (nm) | Ttot (%) | Haze (%) | Δnth | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb ave/max (%) | σb (GPa) | Td5 (N2) (°C) | Td5 (air) (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1p | 4,4′-ODA | 0.88 a (NMP) | T | 6.1 | 51.4 | 392 | 74.9 | 5.64 | 0.004 | 310 c 304 d | 53.5 | 495 | 459 | |||
| 1 | 1.32 a (NMP) | T | 76.4 | 4.8 | 314 | 88.3 | 1.02 | 0.005 | 316 c 306 d | 52.5 | 2.58 | 4.0/5.5 | 0.060 | 478 | 446 | |
| 1 | 0.77 b (GBL) | C | 74.7 | 2.4 | 316 | 88.8 | 0.86 | 0.006 | 290 c 290 d | 53.8 | ---- | ---- | ||||
| 2 | TFMB | 0.51 b (GBL) | R | 82.4 | 2.7 | 293 | 89.5 | 2.77 | 0.013 | 304 c 298 d | 59.9 | 3.31 | 3.3/4.7 | 0.084 | 512 | 433 |
| 4 | AB-TFMB | 0.59 b (GBL) | R | 76.3 | 4.6 | 337 | 88.5 | 3.1 | 0.028 | 355 c | 48.7 41.5 e | 3.09 | 6.1/9.4 | 0.078 | 463 | 422 |
| 9 f | TFMB | 1.24 b (GBL) | R | 86.9 | 1.9 | 299 | 90.7 | 0.51 | 0.016 | 340 d | 57.1 | 3.13 | 7.4/8.9 | 0.13 | 468 | 433 |
| No. | CBDA (mol%) | OHADA (mol%) | ηred (PI) (dL g−1) | T400 (%) | YI | λcut (nm) | Ttot (%) | Haze (%) | Δnth | Tg a (°C) | CTE (ppm K−1) | E (GPa) | εb ave/max (%) | σb (GPa) | Td5 (N2) (°C) | Td5 (air) (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 100 | 0 | Gelation during the modified one-pot process in GBL | |||||||||||||
| 6 | 75 | 25 | Gelation during the modified one-pot process in GBL | |||||||||||||
| 7 | 50 | 50 | 0.98 (GBL) | 76.5 | 7.1 | 271 | 82.2 | 3.21 | 0.015 | 333 | 56.9 | 3.02 | 3.4/4.9 | 0.080 | 448 | 436 |
| 8 | 25 | 75 | 0.89 (GBL) | 69.3 | 10.5 | 261 | 78.4 | 4.43 | 0.014 | 323 | 58.8 | 2.94 | 3.6/4.8 | 0.086 | 461 | 409 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M.; Sato, H.; Hoshino, K.; Arao, Y.; Ishii, J. Colorless Polyimides Derived from Octahydro-2,3,6,7-anthracenetetracarboxylic Dianhydride. Macromol 2023, 3, 175-199. https://doi.org/10.3390/macromol3020011
Hasegawa M, Sato H, Hoshino K, Arao Y, Ishii J. Colorless Polyimides Derived from Octahydro-2,3,6,7-anthracenetetracarboxylic Dianhydride. Macromol. 2023; 3(2):175-199. https://doi.org/10.3390/macromol3020011
Chicago/Turabian StyleHasegawa, Masatoshi, Hiroki Sato, Katsuhisa Hoshino, Yasuhisa Arao, and Junichi Ishii. 2023. "Colorless Polyimides Derived from Octahydro-2,3,6,7-anthracenetetracarboxylic Dianhydride" Macromol 3, no. 2: 175-199. https://doi.org/10.3390/macromol3020011
APA StyleHasegawa, M., Sato, H., Hoshino, K., Arao, Y., & Ishii, J. (2023). Colorless Polyimides Derived from Octahydro-2,3,6,7-anthracenetetracarboxylic Dianhydride. Macromol, 3(2), 175-199. https://doi.org/10.3390/macromol3020011