A Review of Xyloglucan: Self-Aggregation, Hydrogel Formation, Mucoadhesion and Uses in Medical Devices
Abstract
1. Introduction
1.1. Basic Features of Hydrogels
1.2. Basic Features of XG
2. Colloidal Aspects of XG
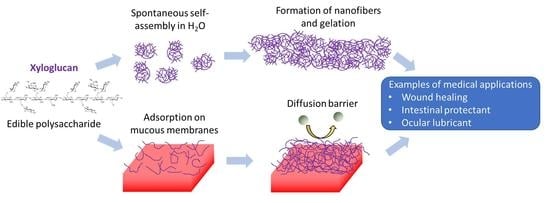
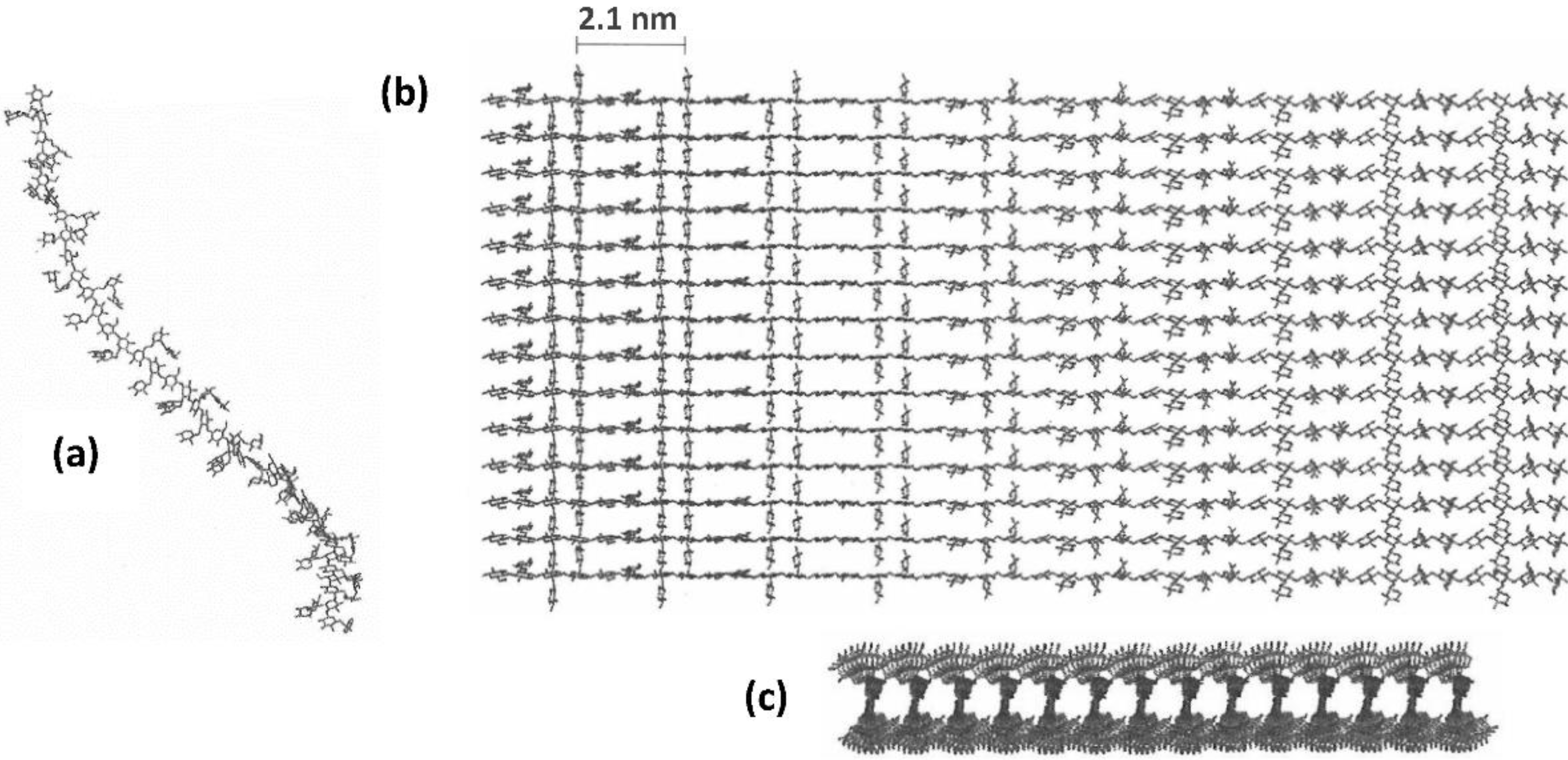
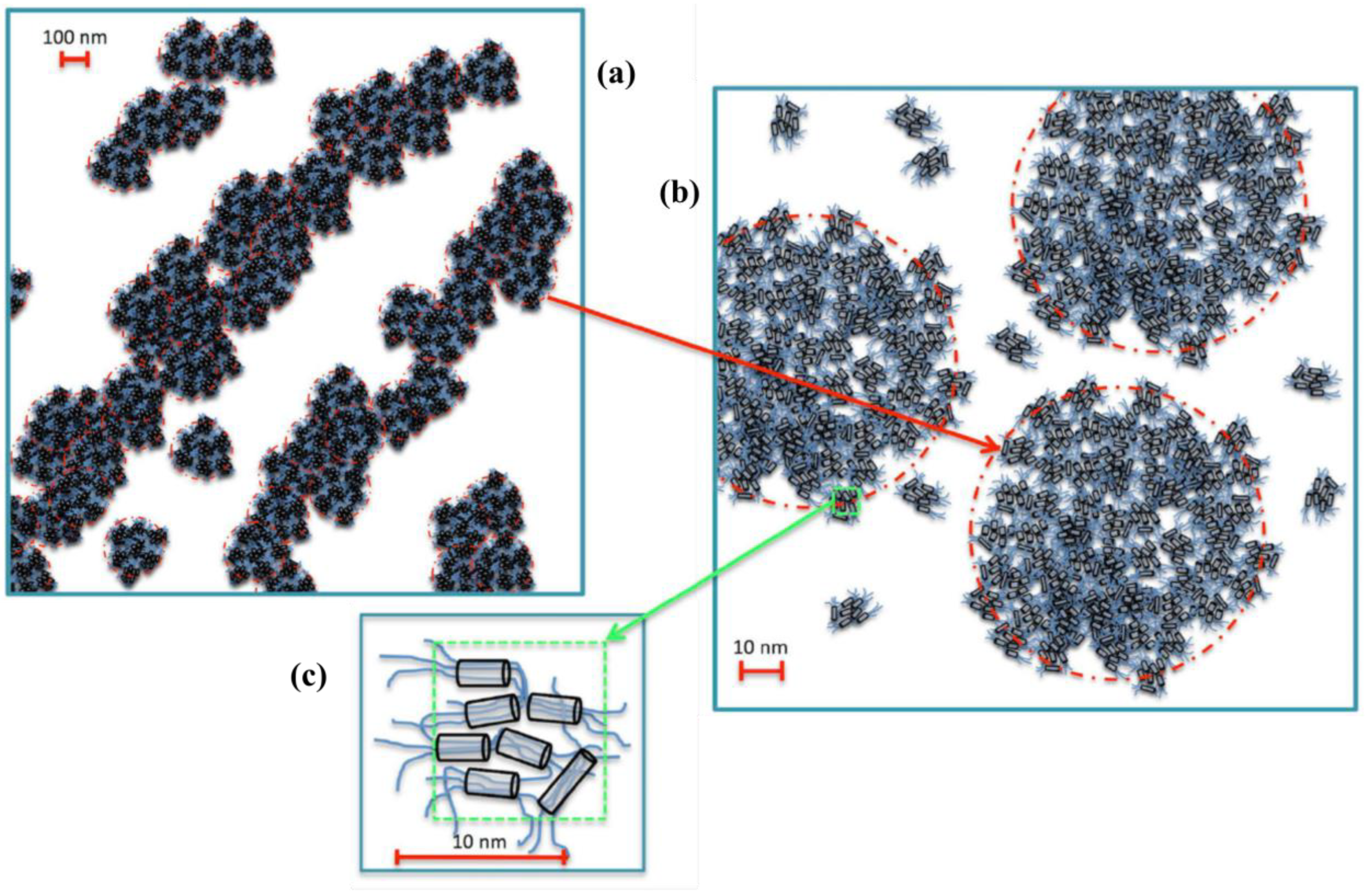
2.1. Self-Aggregation
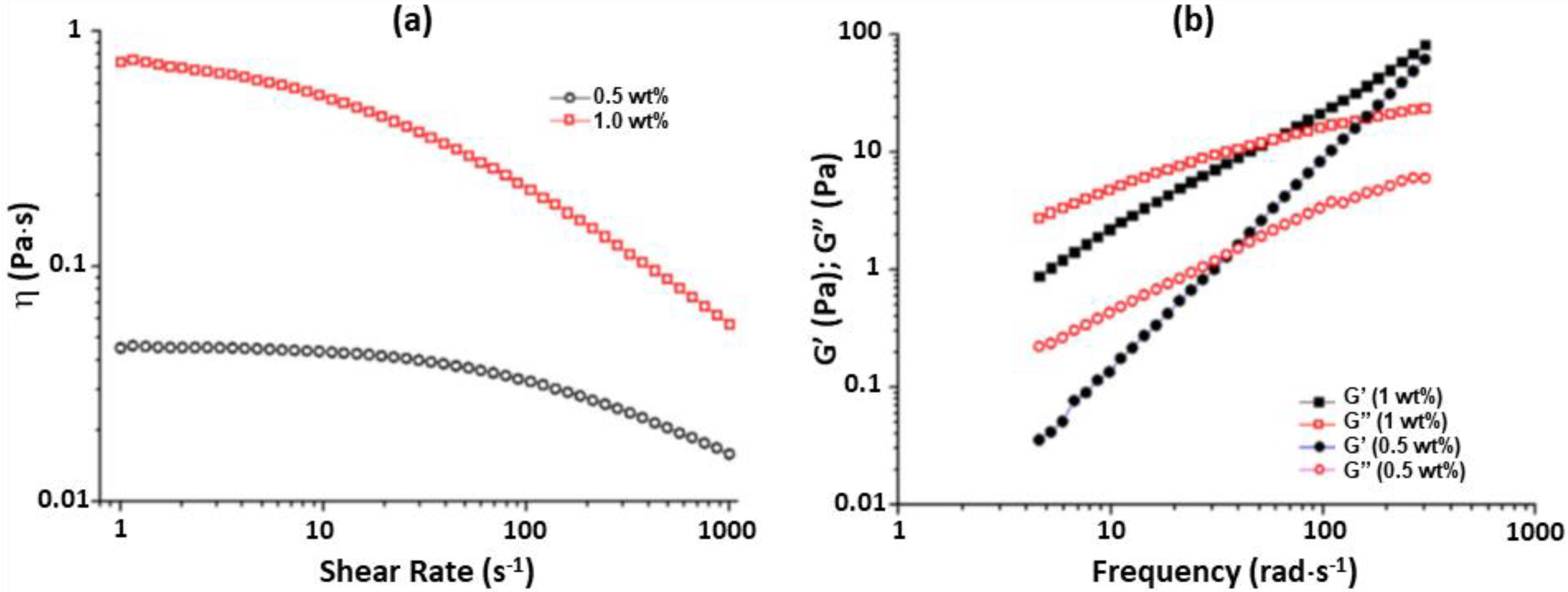
2.2. Xyloglucan Hydrogels
2.3. XG Films
2.4. Mucoadhesivity of XG
3. Potential Medical Applications of XG
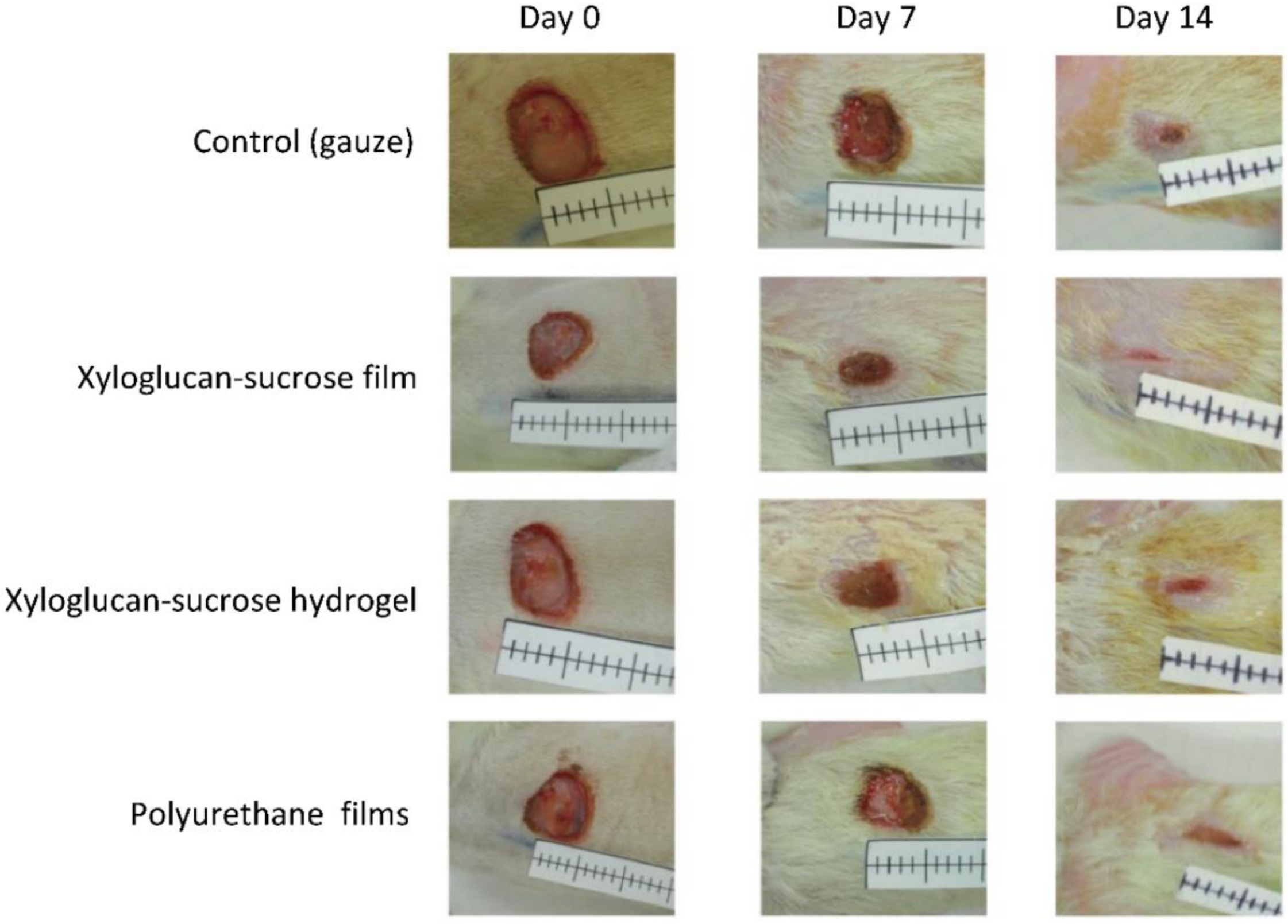
3.1. Material for Wound Dressings
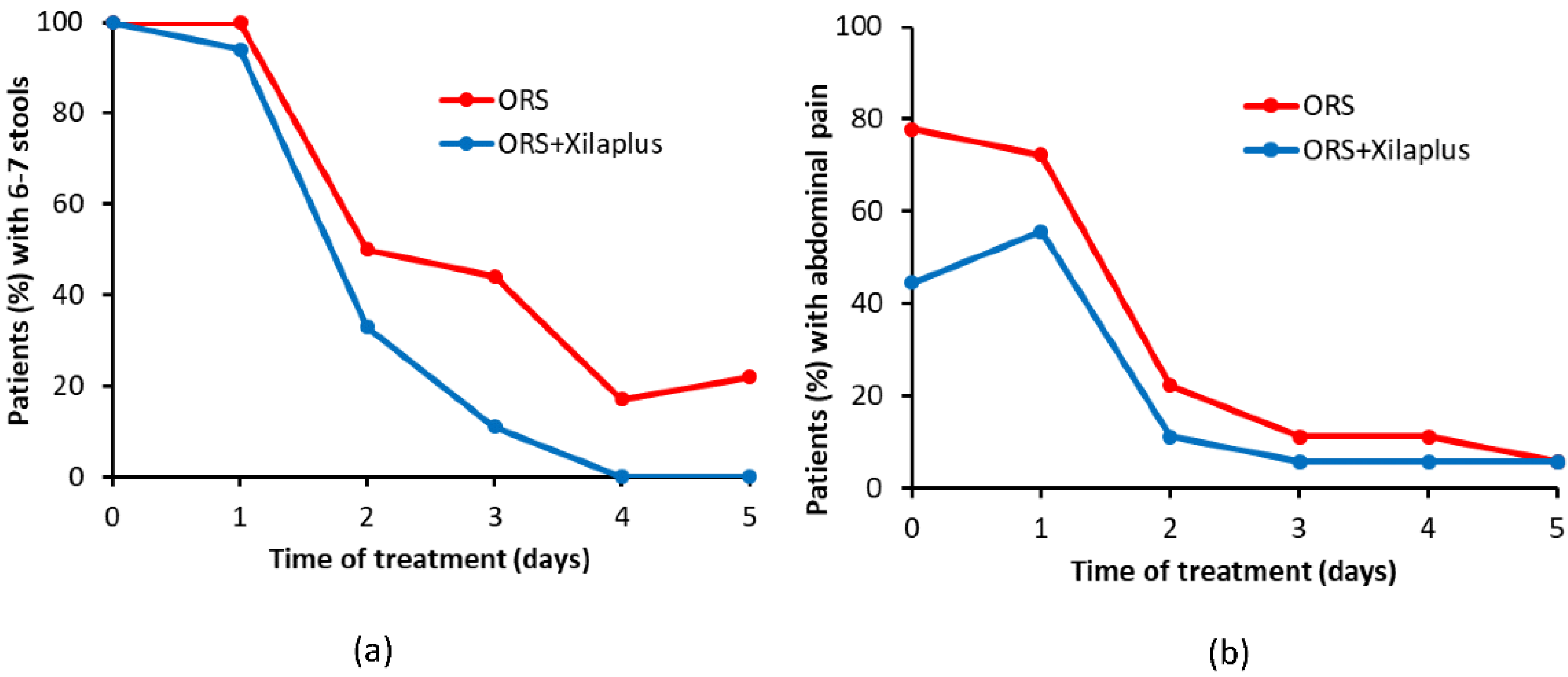
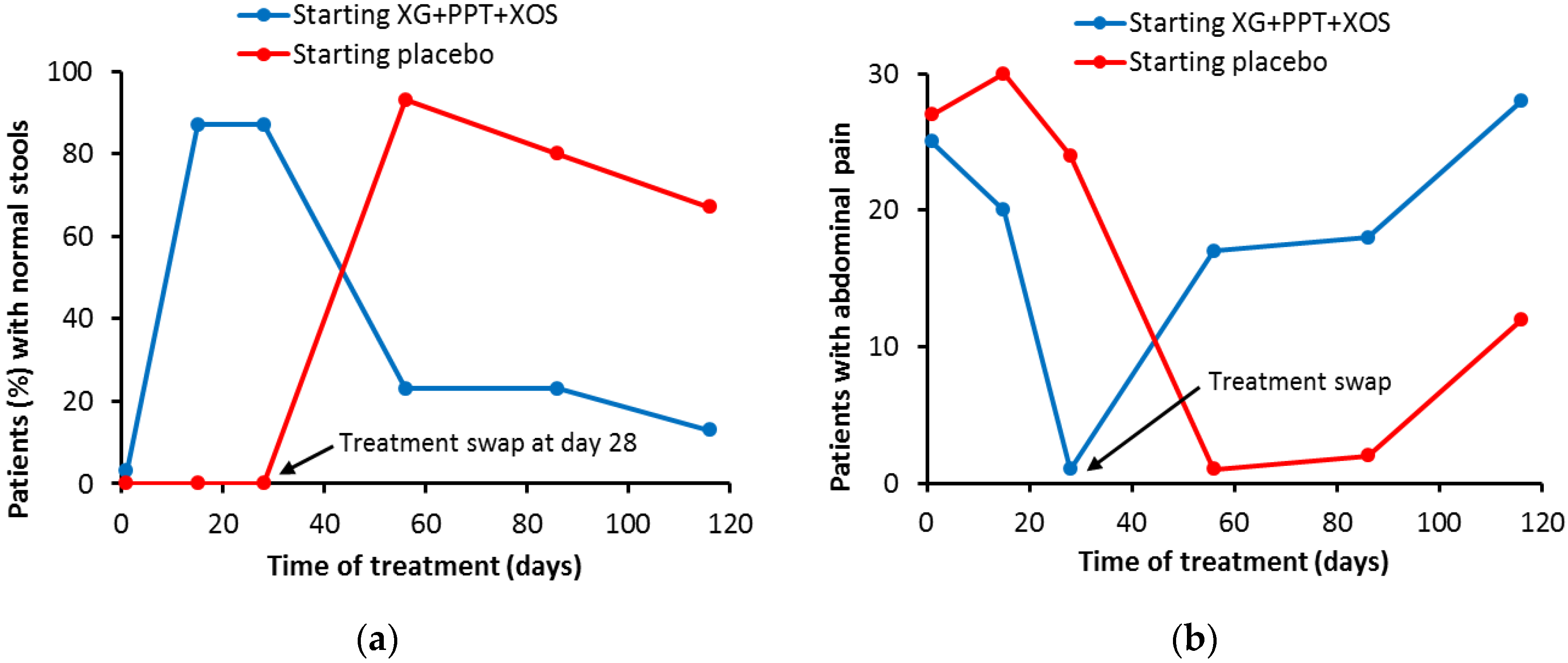
3.2. Mucosal Protectant
3.3. Ocular Lubricant
4. Concluding Remarks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
In Memoriam
References
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2021, 278, 118952. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterization and Applications. In Progress in Molecular and Environmental Bioengineering, from Analysis to Modelling and Technology Applications; Carpi, A., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Miras, J.; Liu, C.; Blomberg, E.; Thormann, E.; Vílchez, S.; Esquena, J. pH-Responsive Chitosan Nanofilms Crosslinked with Genipin. Colloids Surf. A 2021, 616, 126229. [Google Scholar] [CrossRef]
- Vílchez, S.; Samitier, V.; Porras, M.; Esquena, J.; Erra, P. Chitosan Hydrogels Covalently Crosslinked with a Natural Reagent. Tenside Surfactants Deterg. 2009, 46, 13–17. [Google Scholar] [CrossRef]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2020, 58, 2453–2466. [Google Scholar] [CrossRef]
- Filho, G.P.C.; Lima, M.E.G.B.; Rocha, H.A.D.O.; Moreira, S.M.G. Role of sulfated polysaccharides from seaweeds in bone regeneration: A systematic review. Carbohydr. Polym. 2022, 284, 119204. [Google Scholar] [CrossRef] [PubMed]
- Bashier, A.; Sharma, P.K.; Warsi, M.H. Extraction and characterization of xyloglucan (tamarind seed polysaccharide) as pharmaceutical excipient. Pharmacogn. J. 2016, 20, 17–19. [Google Scholar]
- Babu, P.R.; Mathai, M.T.; Julius, A.; Madhu, V. Dual property of tamarind seed polysaccharide aid wound healing. Int. J. Adv. Sci. An Technol. 2019, 28, 1130–1141. [Google Scholar]
- Dutta, P.; Giri, S.; Giri, T.K. Xyloglucan as green renewable biopolymer used in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2020, 160, 55–68. [Google Scholar] [CrossRef]
- Fry, S.C. The Structure and Functions of Xyloglucan. J. Exp. Bot. 1989, 40, 1–11. [Google Scholar] [CrossRef]
- Nishinari, K.; Takemasa, M.; Yamatoya, K.; Shirakawa, M. Xyloglucan. In Handbook of Hydrocolloids; Williams, P.A., Phillips, G.O., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Amsterdam, The Netherlands, 2009; pp. 535–566. [Google Scholar]
- Kochumalayil, J.; Sehaqui, H.; Zhou, Q.; Berglund, L.A. Tamarind seed xyloglucan—A thermostable high-performance biopolymer from non-food feedstock. J. Mater. Chem. 2010, 20, 4321–4327. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Joshi, A.A.; Patil, C.L.; Amale, P.D.; Patel, H.M.; Surana, S.J.; Belgamwar, V.S.; Chaudhari, K.S.; Pardeshi, C.V. Xyloglucan: A functional biomacromolecule for drug delivery applications. Int. J. Biol. Macromol. 2017, 104, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Teleman, A. Hemicelluloses and pectins. In Wood Chemistry and Wood Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; De Gruyter: Berlin, Germany, 2009; pp. 101–120. [Google Scholar]
- Shirakawa, M.; Yamatoya, K.; Nishinari, K. Tailoring of xyloglucan properties using an enzyme. Food Hydrocoll. 1998, 12, 25–28. [Google Scholar] [CrossRef]
- Gidley, M.J.; Lillford, P.J.; Rowlands, D.W.; Lang, P.; Dentini, M.; Crescenzi, V.; Edwards, M.; Fanutti, C.; Reid, J.G. Structure and solution properties of tamarind-seed polysaccharide. Carbohydr. Res. 1991, 214, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Arruda, I.R.; Albuquerque, P.B.; Santos, G.R.; Silva, A.G.; Mourão, P.A.; Correia, M.T.; Vicente, A.A.; Carneiro-Da-Cunha, M.G. Structure and rheological properties of a xyloglucan extracted from Hymenaea courbaril var. courbaril seeds. Int. J. Biol. Macromol. 2015, 73, 31–38. [Google Scholar] [CrossRef]
- Ieiri, D.; Kawamura, T.; Mimura, M.; Urakawa, H.; Yuguchi, Y.; Kajiwara, K. Solution Structure for Xyloglucans Extracted from Various Seeds. Sen’i Gakkaishi 2003, 59, 93–98. [Google Scholar] [CrossRef][Green Version]
- Singh, M.; Pahal, V.; Ahuja, D. Isolation and characterization of microfibrillated cellulose and nanofibrillated cellulose with “biomechanical hotspots”. Carbohydr. Polym. 2020, 234, 115827. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Immerzeel, P.; Hayashi, T. Xyloglucan: The Molecular Muscle of Trees. Ann. Bot. 2008, 102, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Ropitaux, M.; Bernard, S.; Follet-Gueye, M.L.; Vicré, M.; Boulogne, I.; Driouich, A. Xyloglucan and cellulose form molecular cross-bridges connecting root border cells in pea (Pisum sativum). Plant Physiol. Biochem. 2019, 139, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Krishna, S. Tamarind seed polysaccharide. Curr. Sci. 1947, 16, 256. [Google Scholar] [PubMed]
- Tauzin, A.S.; Kwiatkowski, K.J.; Orlovsky, N.I.; Smith, C.J.; Creagh, A.L.; Haynes, C.A.; Wawrzak, Z.; Brumer, H.; Koropatkin, N.M. Molecular Dissection of Xyloglucan Recognition in a Prominent Human Gut Symbiont. mBio 2016, 7, e02134-15. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; Nascimento, G.E.D.; Zhang, X.; Iacomini, M.; Cordeiro, L.M.; Hamaker, B.R. Soluble xyloglucan generates bigger bacterial community shifts than pectic polymers during in vitro fecal fermentation. Carbohydr. Polym. 2018, 206, 389–395. [Google Scholar] [CrossRef]
- Baker, J.; Duarte, M.; Holanda, D.; Kim, S. Friend or Foe? Impacts of Dietary Xylans, Xylooligosaccharides, and Xylanases on Intestinal Health and Growth Performance of Monogastric Animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef]
- Yamatoya, K.; Shirakawa, M.; Kuwano, K.; Suzuki, J.; Mitamura, T. Effects of hydrolyzed xyloglucan on lipid metabolism in rats. Food Hydrocoll. 1996, 10, 369–372. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, X.; Li, S.; Qin, W.; Huang, Z.; Luo, Y.; Li, H.; Wu, D.; Zhang, Q.; Zhao, Y.; et al. Possible beneficial effects of xyloglucan from its degradation by gut microbiota. Trends Food Sci. Technol. 2020, 97, 65–75. [Google Scholar] [CrossRef]
- Dilbaghi, N.; Ahuja, M.; Bernela, M.; Kumar, S.; Bhardwaj, P.; Kaur, H. Synthesis and characterization of novel amphiphilic tamarind seed xyloglucan-octenyl succinic anhydride conjugate. J. Polym. Res. 2020, 27, 236. [Google Scholar] [CrossRef]
- Lang, P.; Kajiwara, K. Investigations of the architecture of tamarind seed polysaccharide in aqueous solution by different scattering techniques. J. Biomater. Sci. Polym. Ed. 1993, 4, 517–518. [Google Scholar] [CrossRef]
- Lang, P.; Kajiwara, K.; Burchard, W. Investigations on the solution architecture of carboxylated tamarind seed polysaccharide by static and dynamic light scattering. Macromolecules 1993, 26, 3992–3998. [Google Scholar] [CrossRef]
- Urakawa, H.; Mimura, M.; Kajiwara, K. Diversity and Versatility of Plant Seed Xyloglucan. Trends Glycosci. Glycotechnol. 2002, 14, 355–376. [Google Scholar] [CrossRef][Green Version]
- Yamanaka, S.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K.; Shirakawa, M.; Yamatoya, K. Gelation of Enzymatically Degraded Xyloglucan Extracted from Tamarind Seed. Sen’i Gakkaishi 1999, 55, 528–532. [Google Scholar] [CrossRef]
- Dispenza, C.; Todaro, S.; Sabatino, M.A.; Martino, D.C.; Martorana, V.; Biagio, P.L.S.; Maffei, P.; Bulone, D. Multi-scale structural analysis of xyloglucan colloidal dispersions and hydro-alcoholic gels. Cellulose 2020, 27, 3025–3035. [Google Scholar] [CrossRef]
- Kozioł, A.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Evaluation of Structure and Assembly of Xyloglucan from Tamarind Seed (Tamarindus indica L.) with Atomic Force Microscopy. Food Biophys. 2015, 10, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Bishnoi, R.S.; Kumar, M.; Fenin, V.; Jain, C.P. Applications of Tamarind seeds Polysaccharide-based copolymers in Controlled Drug. Asian J. Pharm. Pharmacol. 2018, 4, 23–30. [Google Scholar] [CrossRef]
- Lang, P.; Masci, G.; Dentini, M.; Crescenzi, V.; Cooke, D.; Gidley, M.; Fanutti, C.; Reid, J. Tamarind seed polysaccharide: Preparation, characterisation and solution properties of carboxylated, sulphated and alkylaminated derivatives. Carbohydr. Polym. 1992, 17, 185–198. [Google Scholar] [CrossRef]
- Parikka, K.; Leppänen, A.-S.; Xu, C.; Pitkänen, L.; Eronen, P.; Österberg, M.; Brumer, H.; Willför, S.; Tenkanen, M. Functional and Anionic Cellulose-Interacting Polymers by Selective Chemo-Enzymatic Carboxylation of Galactose-Containing Polysaccharides. Biomacromolecules 2012, 13, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Mali, K.; Dhawale, S.C.; Dias, R.J. Extraction, Characterization and Functionalization of Tamarind Gum. Res. J. Pharm. Technol. 2019, 12, 1745. [Google Scholar] [CrossRef]
- Kaur, G.; Mahajan, M.; Bassi, P. Derivatized Polysaccharides: Preparation, Characterization, and Application as Bioadhesive Polymer for Drug Delivery. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 475–481. [Google Scholar] [CrossRef]
- Ajovalasit, A.; Caccami, M.C.; Amendola, S.; Sabatino, M.A.; Alotta, G.; Zingales, M.; Giacomazza, D.; Occhiuzzi, C.; Marrocco, G.; Dispenza, C. Development and characterization of xyloglucan-poly(vinyl alcohol) hydrogel membrane for Wireless Smart wound dressings. Eur. Polym. J. 2018, 106, 214–222. [Google Scholar] [CrossRef]
- Nandi, G.; Changder, A.; Ghosh, L.K. Graft-copolymer of polyacrylamide-tamarind seed gum: Synthesis, characterization and evaluation of flocculating potential in peroral paracetamol suspension. Carbohydr. Polym. 2019, 215, 213–225. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. I. Gelation. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. II. Trifunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3091–3096. [Google Scholar] [CrossRef]
- Stockmayer, W.H. Theory of Molecular Size Distribution and Gel Formation in Branched Polymers II. General Cross Linking. J. Chem. Phys. 1944, 12, 125–131. [Google Scholar] [CrossRef]
- Yamatoya, K.; Yamazaki, K.; Shirakawa, M.; Baba, O. Effects of xyloglucan with the partial removal of galactose on plasma lipid concentration. J. Funct. Foods 2011, 3, 275–279. [Google Scholar] [CrossRef]
- Nisbet, D.; Crompton, K.; Hamilton, S.; Shirakawa, S.; Prankerd, R.; Finkelstein, D.; Horne, M.; Forsythe, J. Morphology and gelation of thermosensitive xyloglucan hydrogels. Biophys. Chem. 2006, 121, 14–20. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.; Richard, C.; Bessodes, M.; Scherman, D.; Narita, T.; Ducouret, G.; Merten, O.W. Study on the sol-gel transition of xyloglucan hydrogels. Carbohydr. Polym. 2010, 80, 555–562. [Google Scholar] [CrossRef]
- Moneo-Sánchez, M.; Alonso-Chico, A.; Knox, J.P.; Dopico, B.; Labrador, E.; Martín, I. β-(1,4)-Galactan remodelling in Arabidopsis cell walls affects the xyloglucan structure during elongation. Planta 2018, 249, 351–362. [Google Scholar] [CrossRef]
- Nitta, Y.; Kim, B.S.; Nishinari, K.; Shirakawa, M.; Yamatoya, K.; Oomoto, T.; Asai, I. Synergistic gel formation of xyloglucan/gellan mixtures as studied by rheology, DSC, and circular dichroism. Biomacromolecules 2003, 4, 1654–1660. [Google Scholar] [CrossRef]
- Yamanaka, S.; Yuguchi, Y.; Urakawa, H.; Kajiwara, K.; Shirakawa, M.; Yamatoya, K. Gelation of tamarind seed polysaccharide xyloglucan in the presence of ethanol. Food Hydrocoll. 2000, 14, 125–128. [Google Scholar] [CrossRef]
- Bergström, E.M.; Salmen, L.; Kochumalayil, J.; Berglund, L. Plasticized xyloglucan for improved toughness—Thermal and mechanical behaviour. Carbohydr. Polym. 2012, 87, 2532–2537. [Google Scholar] [CrossRef]
- Kittle, J.D.; Qian, C.; Edgar, E.; Roman, M.; Esker, A. Adsorption of Xyloglucan onto Thin Films of Cellulose Nanocrystals and Amorphous Cellulose: Film Thickness Effects. ACS Omega 2018, 3, 14004–14012. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Deshmukh, S.R. Development and evaluation of gel-forming ocular films based on xyloglucan. Carbohydr. Polym. 2015, 122, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kochumalayil, J.J.; Zhou, Q.; Kasai, W.; Berglund, L.A. Regioselective modification of a xyloglucan hemicellulose for high-performance biopolymer barrier films. Carbohydr. Polym. 2013, 93, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kochumalayil, J.J.; Berglund, L.A. Water-soluble hemicelluloses for high humidity applications—Enzymatic modification of xyloglucan for mechanical and oxygen barrier properties. Green Chem. 2013, 16, 1904–1910. [Google Scholar] [CrossRef]
- Simi, C.K.; Abraham, T.E. Biodegradable biocompatible xyloglucan films for various applications. Colloid Polym. Sci. 2009, 288, 297–306. [Google Scholar] [CrossRef]
- Ajovalasit, A.; Sabatino, M.A.; Todaro, S.; Alessi, S.; Giacomazza, D.; Picone, P.; Di Carlo, M.; Dispenza, C. Xyloglucan-based hydrogel films for wound dressing: Structure-property relationships. Carbohydr. Polym. 2018, 179, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Endo, Y. Effects of Glutaraldehyde Exposure on Human Health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef]
- Avachat, A.M.; Gujar, K.N.; Wagh, K.V. Development and evaluation of tamarind seed xyloglucan-based mucoadhesive buccal films of rizatriptan benzoate. Carbohydr. Polym. 2013, 91, 537–542. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Cook, M.T.; Khutoryanskiy, V.V. Mucoadhesion and mucosa-mimetic materials—A mini-review. Int. J. Pharm. 2015, 495, 991–998. [Google Scholar] [CrossRef]
- Wu, S. Formation of adhesive bond. In Polymer Interface and Adhesion; Marcel Dekker Inc.: New York, NY, USA, 1982; pp. 359–447. [Google Scholar]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive Delivery Systems. I. Evaluation of Mucoadhesive Polymers for Buccal Tablet Formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Schmidt, S.A.; Lee, E.; Samprasit, W.; Opanasopit, P.; Khutoryanskiy, V.V. Synthesis of mucoadhesive thiol-bearing microgels from 2-(acetylthio)ethylacrylate and 2-hydroxyethylmethacrylate: Novel drug delivery systems for chemotherapeutic agents to the bladder. J. Mater. Chem. B 2015, 3, 6599–6604. [Google Scholar] [CrossRef]
- Sakuma, S.; Sudo, R.; Suzuki, N.; Kikuchi, H.; Akashi, M.; Hayashi, M. Mucoadhesion of polystyrene nanoparticles having surface hydrophilic polymeric chains in the gastrointestinal tractle. Int. J. Pharm. 1999, 177, 161–172. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Hansson, G.C. Membrane mucins of the intestine at a glance. J. Cell Sci. 2020, 133, 240929. [Google Scholar] [CrossRef]
- Piqué, N.; Gómez-Guillén, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef]
- da Silva, J.B.; Ferreira, S.B.S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef]
- Hassan, E.E.; Gallo, J.M. A Simple Rheological Method for the in Vitro Assessment of Mucin-Polymer Bioadhesive Bond Strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef]
- Caramella, C.; Bonferoni, M.C.; Rossi, S.; Ferrari, F. Rheological and tensile tests for the assessment of polymer-mucin interactions. Eur. J. Pharm. Biopharm. 1994, 40, 213–217. [Google Scholar]
- Pecora, T.M.; Ragazzo, B.; Bertin, W.; Ragonese, A.; Mascagni, M.; Maffei, P.; Pignatello, R. Rheological behavior of a new mucoadhesive oral formulation based on sodium chondroitin sulfate, xyloglucan and glycerol. J. Funct. Biomater. 2021, 12, 28. [Google Scholar] [CrossRef]
- Fraile, B.; Alcover, J.; Royuela, M.; Rodríguez, D.; Chaves, C.; Palacios, R.; Piqué, N. Xyloglucan, hibiscus and propolis for the prevention of urinary tract infections: Results of in vitro studies. Future Microbiol. 2017, 12, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Zaman, M.; Qureshi, S. Thiomers: A Blessing to Evaluating Era of Pharmaceuticals. Int. J. Polym. Sci. 2015, 2015, 146329. [Google Scholar] [CrossRef]
- Madgulkar, A.R.; Bhalekar, M.R.; Asgaonkar, K.D.; Dikpati, A.A. Synthesis and characterization of a novel mucoadhesive derivative of xyloglucan. Carbohydr. Polym. 2016, 135, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Lanza, M.; Filippone, A.; Paterniti, I.; Casili, G.; Scuderi, S.A.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E. Evaluation of a Product Containing Xyloglucan and Pea Protein on Skin Barrier Permeability. Ski. Pharmacol. Physiol. 2020, 33, 231–236. [Google Scholar] [CrossRef]
- De Servi, B.; Ranzini, F.; Piqué, N. Protective barrier properties of Rhinosectan® spray (containing xyloglucan) on an organotypic 3D airway tissue model (MucilAir): Results of an in vitro study. Allergy, Asthma Clin. Immunol. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Constant, S.; Perek, N.; Boudard, D.; Delavenne, X. Pharmacological characterization of the 3D MucilAir™ nasal model. Eur. J. Pharm. Biopharm. 2019, 139, 186–196. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kulkarni, A.D.; Belgamwar, V.S.; Surana, S.J. Xyloglucan for drug delivery applications. In Fundamental Biomaterials: Polymers; Woodhead Publishing: Amsterdam, The Netherlands, 2018; pp. 143–169. [Google Scholar] [CrossRef]
- Suisha, F.; Kawasaki, N.; Miyazaki, S.; Shirakawa, M.; Yamatoya, K.; Sasaki, M.; Attwood, D. Xyloglucan gels as sustained release vehicles for the intraperitoneal administration of mitomycin C. Int. J. Pharm. 1998, 172, 27–32. [Google Scholar] [CrossRef]
- Miyazaki, S.; Suisha, F.; Kawasaki, N.; Shirakawa, M.; Yamatoya, K.; Attwood, D. Thermally reversible xyloglucan gels as vehicles for rectal drug delivery. J. Control. Release 1998, 56, 75–83. [Google Scholar] [CrossRef]
- Kumar, A.; Garg, T.; Sarma, G.S.; Rath, G.; Goyal, A.K. Optimization of combinational intranasal drug delivery system for the management of migraine by using statistical design. Eur. J. Pharm. Sci. 2015, 70, 140–151. [Google Scholar] [CrossRef]
- Bozkurt, H.S.; Kara, B. A new treatment for ulcerative colitis: Intracolonic Bifidobacterium and xyloglucan application. Eur. J. Inflamm. 2020, 18, 2058739220942626. [Google Scholar] [CrossRef]
- Bullock, A.; Pickavance, P.; Haddow, D.; Rimmer, S.; MacNeil, S. Development of a calcium-chelating hydrogel for treatment of superficial burns and scalds. Regen. Med. 2010, 5, 55–64. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Hrynyk, M.; Martins-Green, M.; Barron, A.E.; Neufeld, R.J. Alginate-PEG Sponge Architecture and Role in the Design of Insulin Release Dressings. Biomacromolecules 2012, 13, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sabatino, M.A.; Ajovalasit, A.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Biocompatibility, hemocompatibility and antimicrobial properties of xyloglucan-based hydrogel film for wound healing application. Int. J. Biol. Macromol. 2018, 121, 784–795. [Google Scholar] [CrossRef]
- Picone, P.; Sabatino, M.A.; Ajovalasit, A.; Brancato, O.R.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Data concerning the protein absorption and retention properties of xyloglucan-based hydrogel film. Data Brief 2018, 21, 1950–1953. [Google Scholar] [CrossRef]
- Hirose, K.; Sasatsu, M.; Toraishi, T.; Onishi, H. Novel xyloglucan sheet for the treatment of deep wounds: Preparation, physicochemical characteristics, and in vivo healing effects. Biol. Pharm. Bull. 2019, 42, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Aprea, G.; Surfaro, G.; Amato, M.; Giuliani, A.; Paccone, M.; Salzano, A.; Russo, A.; Tafuri, D.; Amato, B. Prevention and treatment of peritoneal adhesions in patients affected by vascular diseases following surgery: A review of the literature. Open Med. 2016, 11, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Şahin, H.; Toman, H.; Kiraz, H.A.; Şimşek, T.; Erbaş, M.; Özkul, F.; Arık, M.K.; Hancı, V. Effects of sugammadex on the prevention of postoperative peritoneal adhesions. Kaohsiung J. Med. Sci. 2015, 31, 463–467. [Google Scholar] [CrossRef]
- Zhang, E.; Li, J.; Zhou, Y.; Che, P.; Ren, B.; Qin, Z.; Ma, L.; Cui, J.; Sun, H.; Yao, F. Biodegradable and injectable thermoreversible xyloglucan based hydrogel for prevention of postoperative adhesion. Acta Biomater. 2017, 55, 420–433. [Google Scholar] [CrossRef]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañas, L. Urinary tract infections in the elderly: A review of disease characteristics and current treatment options. Drugs Context 2020, 9, 2020-4-13. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Beaufrand, C.; Harkat, C.; Theodorou, V. The role of mucoprotectants in the management of gastrointestinal disorders. Expert Rev. Gastroenterol. Hepatol. 2017, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Burta, O.; Iacobescu, C.; Mateescu, R.B.; Nicolaie, T.; Tiuca, N.; Pop, C.S. Efficacy and safety of APT036 versus simethicone in the treatment of functional bloating: A multicentre, randomised, double-blind, parallel group, clinical study. Transl. Gastroenterol. Hepatol. 2018, 3, 72. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Bacarea, A.; Marusteri, M.; Bacarea, V.; Constantin, M.; Manolache, M. Efficacy and safety of APT198K for the treatment of infantile colic: A pilot study. J. Comp. Eff. Res. 2017, 6, 137–144. [Google Scholar] [CrossRef]
- Spanish Agency for Medicines and Medical Devices. Retirada del Mercado de la Suspensión Oral de uso Pediátrico Aprotecol, Fabricada por Noventure S.L. 2018. Available online: https://www.aemps.gob.es/informa/notasInformativas/productosSanitarios/seguridad/2018/docs/NI-PS_10-2018-Aprotecol-Noventure.pdf (accessed on 6 August 2022).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Donovan, E.J. A diagnostic approach to chronic diarrhea. Dis. Colon Rectum 1969, 12, 364–370. [Google Scholar] [CrossRef]
- Serban, E.D.; Manolache, M. Gelatin tannate versus other antidiarrheal medication in children with acute gastroenteritis: A retrospective, observational study. J. Comp. Eff. Res. 2019, 8, 187–194. [Google Scholar] [CrossRef]
- Condratovici, C.P.; Bacarea, V.; Piqué, N. Xyloglucan for the treatment of acute gastroenteritis in children: Results of a randomized, controlled, clinical trial. Gastroenterol. Res. Pract. 2016, 2016, 6874207. [Google Scholar] [CrossRef]
- Santos, J.; Musta, V.; Luca, C.M.; Belei, O.A.; Cambrea, S.C. Randomized, placebo-controlled trial of xyloglucan and gelose for the treatment of acute diarrhea in children. Expert Rev. Gastroenterol. Hepatol. 2020, 15, 325–331. [Google Scholar] [CrossRef]
- de Servi, B.; Ranzini, F.; Piqué, N. Effect of Utipro® (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Futur. Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef]
- Trifan, A.; Burta, O.; Tiuca, N.; Petrisor, D.C.; Lenghel, A.; Santos, J. Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial. United Eur. Gastroenterol. J. 2019, 7, 1093–1101. [Google Scholar] [CrossRef]
- Colomier, E.; Algera, J.; Melchior, C. Pharmacological Therapies and Their Clinical Targets in Irritable Bowel Syndrome with Diarrhea. Front. Pharmacol. 2021, 11, 629026. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, P.; Kindt, S. Diagnostic approach to chronic diarrhoea and recent insights in treatment of functional diarrhoea including irritable bowel syndrome. Acta Gastro-Enterol. Belg. 2020, 83, 461–474. [Google Scholar]
- Periasamy, S.; Lin, C.-H.; Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R.; Liu, M.-Y. Mucoadhesive role of tamarind xyloglucan on inflammation attenuates ulcerative colitis. J. Funct. Foods 2018, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Tamanini, I.; Cocci, A.; Di Maida, F.; Caciagli, P.; Migno, S.; Mereu, L.; Tateo, S.; Malossini, G.; Palmieri, A.; et al. Xyloglucan, hibiscus and propolis to reduce symptoms and antibiotics use in recurrent UTIs: A prospective study. Futur. Microbiol. 2019, 14, 1013–1021. [Google Scholar] [CrossRef]
- Cai, T.; Anceschi, U.; Tamanini, I.; Migno, S.; Rizzo, M.; Liguori, G.; Garcia-Larrosa, A.; Palmieri, A.; Verze, P.; Mirone, V.; et al. Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 11, 14. [Google Scholar] [CrossRef]
- Costache, R.C.; Novac, B.; Bardan, T.R.; Agapie, D.N.; Edu, A. Xyloglucan + Gelose Combination versus Placebo as Adjuvant Therapy to First-Line Antimicrobials for Uncomplicated Urinary Tract Infection in Adults. Urol. Int. 2019, 102, 468–475. [Google Scholar] [CrossRef]
- Piqué, N.; De Servi, B. Rhinosectan® spray (containing xyloglucan) on the ciliary function of the nasal respiratory epithelium; results of an in vitro study. Allergy, Asthma Clin. Immunol. 2018, 14, 41. [Google Scholar] [CrossRef]
- Allegrini, A.; Pavone, D.; Carluccio, F. A randomized controlled trial comparing a xyloglucan-based nasal spray with saline in adults with symptoms of rhinosinusitis. Curr. Med. Res. Opin. 2017, 34, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Silbert, J.A.; Bitton, E.; Bhagat, K. Advances in Diagnosis and Management of Dry Eye Disease. Adv. Ophthalmol. Optom. 2019, 4, 13–38. [Google Scholar] [CrossRef]
- Craig, J.P.; Muntz, A.; Wang, M.T.; Luensmann, D.; Tan, J.; Huarte, S.T.; Xue, A.L.; Jones, L.; Willcox, M.D.; Wolffsohn, J.S. Developing evidence-based guidance for the treatment of dry eye disease with artificial tear supplements: A six-month multicentre, double-masked randomised controlled trial. Ocul. Surf. 2021, 20, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Valente, C. Establishing the tolerability and performance of tamarind seed polysaccharide (TSP) in treating dry eye syndrome: Results of a clinical study. BMC Ophthalmol. 2007, 7, 5. [Google Scholar] [CrossRef]
- Barabino, S.; Rolando, M.; Nardi, M.; Bonini, S.; Aragona, P.; Traverso, C.E. The Effect of an Artificial Tear Combining Hyaluronic Acid and Tamarind Seeds Polysaccharide in Patients with Moderate Dry Eye Syndrome: A New Treatment for Dry Eye. Eur. J. Ophthalmol. 2014, 24, 173–178. [Google Scholar] [CrossRef]
- Sánchez-González, J.M.; De-Hita-Cantalejo, C.; Sánchez-González, M.C. Crosslinked hyaluronic acid with liposomes and crocin for management symptoms of dry eye disease caused by moderate meibomian gland dysfunction. Int. J. Ophthalmol. 2020, 13, 1368–1373. [Google Scholar] [CrossRef]
- Rahić, O.; Tucak, A.; Omerović, N.; Sirbubalo, M.; Hindija, L.; Hadžiabdić, J.; Vranić, E. Novel Drug Delivery Systems Fighting Glaucoma: Formulation Obstacles and Solutions. Pharmaceutics 2020, 13, 28. [Google Scholar] [CrossRef]
- Radcliffe, N. The impact of timolol maleate on the ocular tolerability of fixed-combination glaucoma therapies. Clin. Ophthalmol. 2014, 8, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Burgalassi, S.; Chetoni, P.; Panichi, L.; Boldrini, E.; Saettone, M. Xyloglucan as a Novel Vehicle for Timolol: Pharmacokinetics and Pressure Lowering Activity in Rabbits. J. Ocul. Pharmacol. Ther. 2000, 16, 497–509. [Google Scholar] [CrossRef] [PubMed]
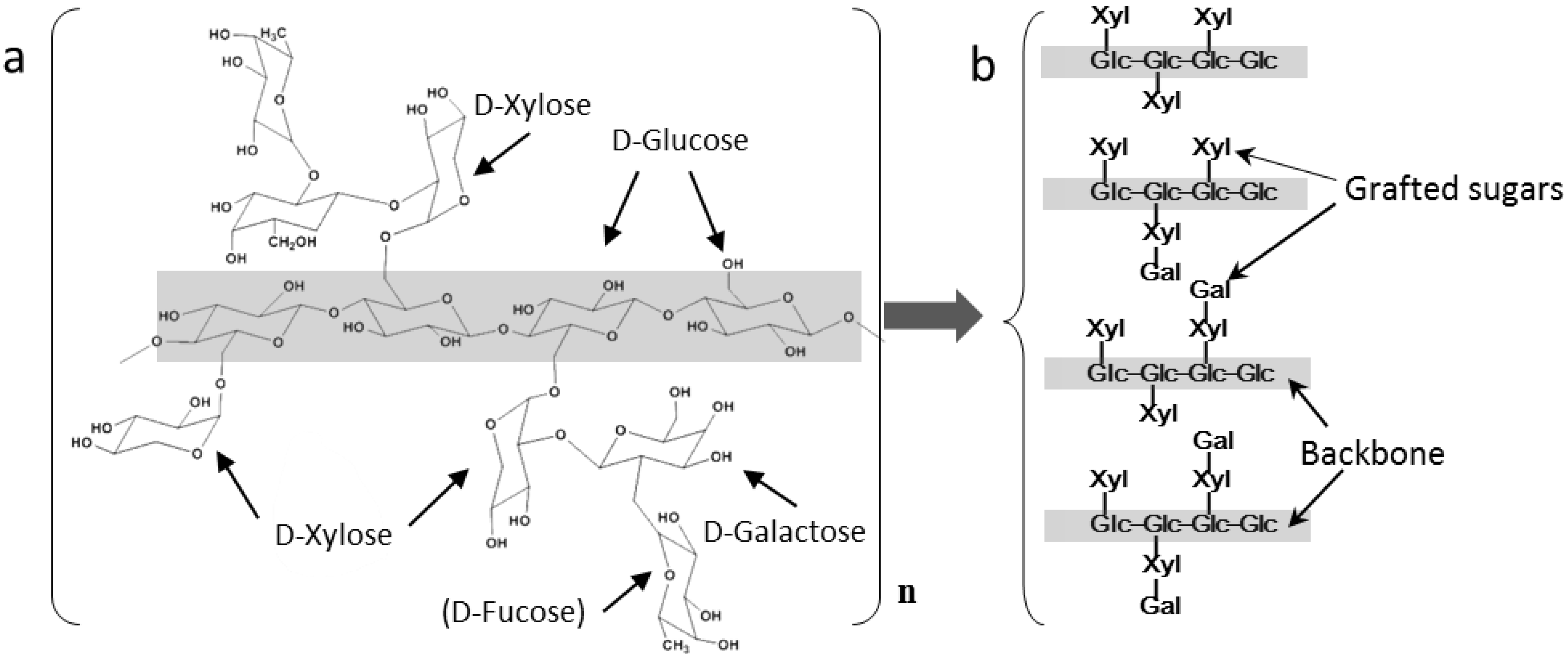

| D-Glucose | D-Xylose | D-Galactose | |
|---|---|---|---|
| Afzelia africana | 1 | 0.80 | 0.41 |
| Detarium microcarpum | 1 | 0.81 | 0.37 |
| Hymenaea courbaril | 1 | 0.73 | 0.25 |
| Tamarindus indica | 1 | 0.75 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquena-Moret, J. A Review of Xyloglucan: Self-Aggregation, Hydrogel Formation, Mucoadhesion and Uses in Medical Devices. Macromol 2022, 2, 562-590. https://doi.org/10.3390/macromol2040037
Esquena-Moret J. A Review of Xyloglucan: Self-Aggregation, Hydrogel Formation, Mucoadhesion and Uses in Medical Devices. Macromol. 2022; 2(4):562-590. https://doi.org/10.3390/macromol2040037
Chicago/Turabian StyleEsquena-Moret, J. 2022. "A Review of Xyloglucan: Self-Aggregation, Hydrogel Formation, Mucoadhesion and Uses in Medical Devices" Macromol 2, no. 4: 562-590. https://doi.org/10.3390/macromol2040037
APA StyleEsquena-Moret, J. (2022). A Review of Xyloglucan: Self-Aggregation, Hydrogel Formation, Mucoadhesion and Uses in Medical Devices. Macromol, 2(4), 562-590. https://doi.org/10.3390/macromol2040037