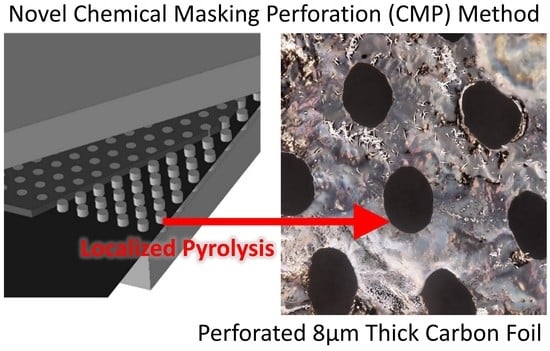
Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Sulfornation
2.2. Carbonization
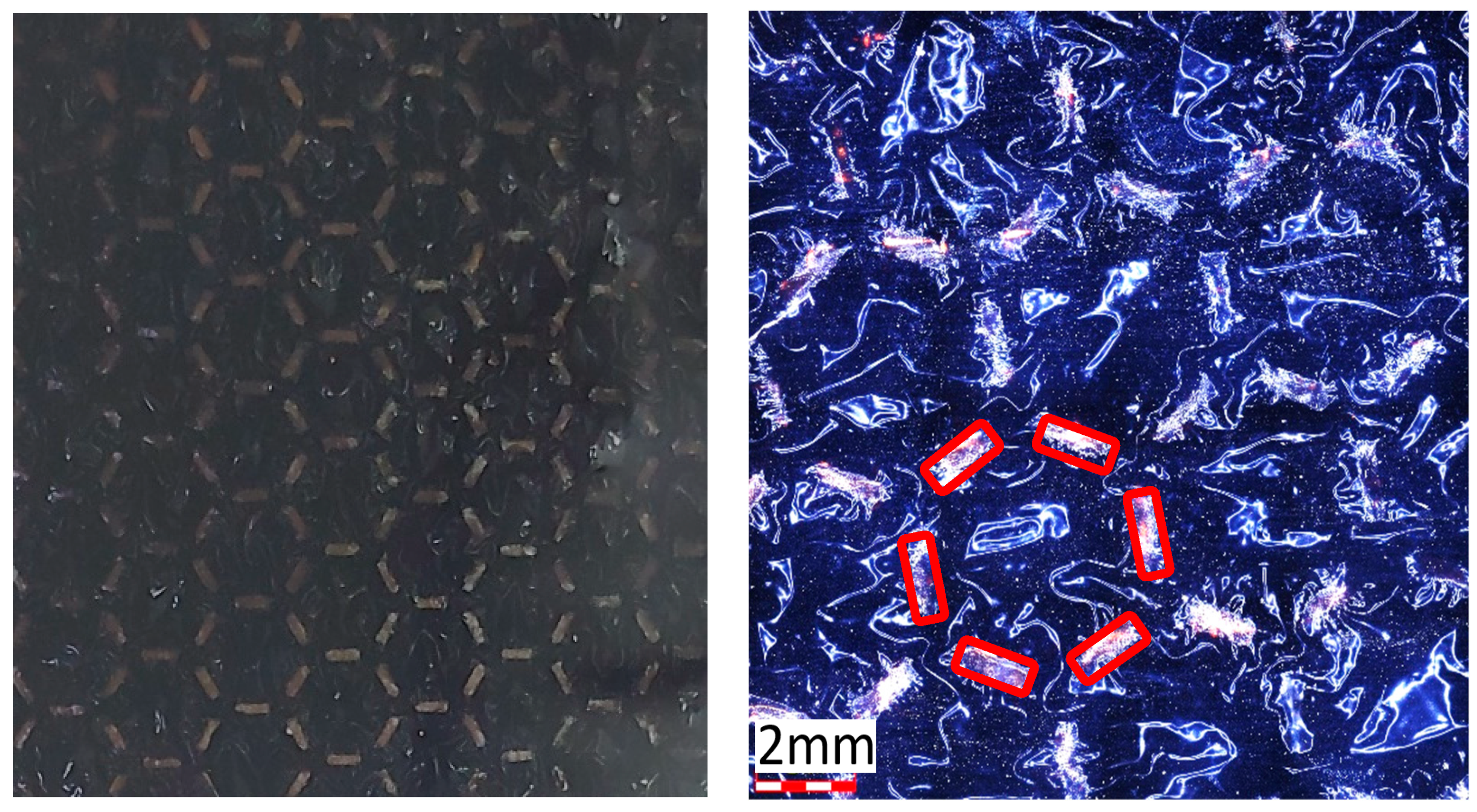
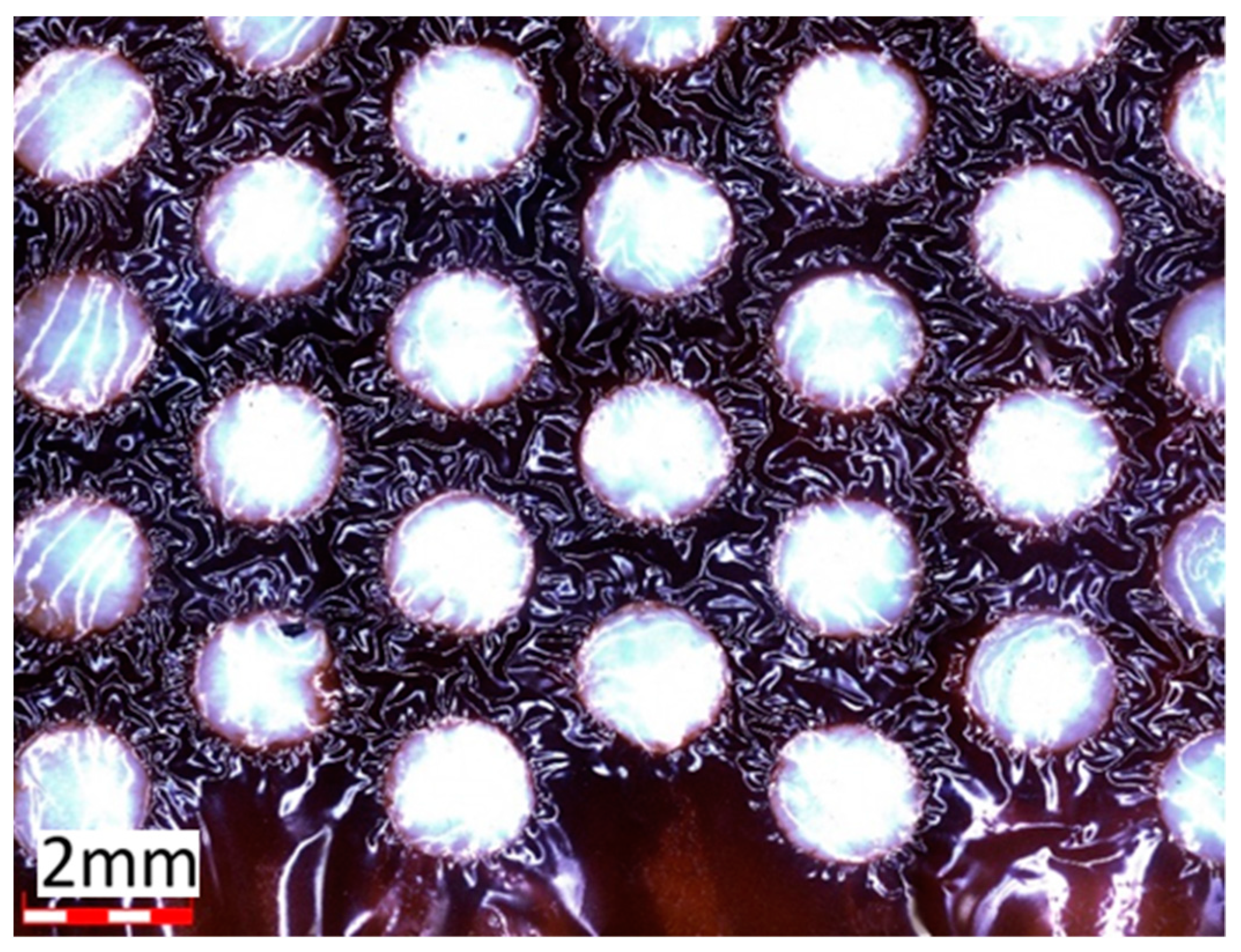
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roch, T.; Beyer, E.; Lasagni, A. Surface modification of thin tetrahedral amorphous carbon films by means of UV direct laser interference patterning. Diam. Relat. Mater. 2010, 19, 1472–1477. [Google Scholar] [CrossRef]
- Lünsdorf, H.; Spiess, E. A rapid method of preparing perforated supporting foils for the thin carbon films used in high resolution transmission electron microscopy. J. Microsc. 1986, 144, 211–213. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, S.; Wu, M.; Wilde, G. Surface patterning using templates: Concept, properties and device applications. Chem. Soc. Rev. 2011, 40, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, J.H.; Hong, N.; Bae, J.; Yang, C.-S.; Shin, K. Ultrathin carbon film from carbonization of spin-cast polyacrylonitrile film. J. Ind. Eng. Chem. 2013, 19, 1631–1637. [Google Scholar] [CrossRef]
- Hasebe, H.; Okuno, H.; Kuboki, H.; Ryuto, H.; Fukunishi, N.; Kamigaito, O.; Goto, A.; Kase, M.; Yano, Y. Development of long-life carbon stripper foils for uranium ion beams. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 613, 453–456. [Google Scholar] [CrossRef]
- Wei, L.; Nitta, N.; Yushin, G. Lithographically patterned thin activated carbon films as a new technology platform for on-chip devices. ACS Nano 2013, 7, 6498–6506. [Google Scholar] [CrossRef]
- Schueller, O.J.A.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by pyrolysis of microfabricated polymeric precursors. Adv. Mater. 1997, 9, 477–480. [Google Scholar] [CrossRef]
- Schueller, O.J.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by soft lithography. Sens. Actuators A Phys. 1999, 72, 125–139. [Google Scholar] [CrossRef]
- Fuchs, A.N.; Schoeberl, M.; Tremmer, J.; Zaeh, M.F. Laser Cutting of Carbon Fiber Fabrics. Phys. Procedia 2013, 41, 372–380. [Google Scholar] [CrossRef]
- Sakhavand, N. Mechanics of Platelet-Matrix Composites across Scales: Theory, Multiscale Modeling, and 3D Fabrication. Ph.D. Dissertation, Rice University, Houston, TX, USA, 2015. [Google Scholar]
- Sakhavand, N.; Shahsavari, R. Universal composition-structure-property maps for natural and biomimetic platelet-matrix composites and stacked heterostructures. Nat. Commun. 2015, 6, 6523. [Google Scholar] [CrossRef]
- Rouhana, R.; Stommel, M. Modelling and Experimental Investigation of Hexagonal Nacre-Like Structure Stiffness. J. Compos. Sci. 2020, 4, 91. [Google Scholar] [CrossRef]
- Behr, S.; Köllner, A.; Schneider, G.A. Tailoring Toughness and Mechanical Reliability by Controlled Defects: Nature-Inspired Composite Laminates of Laser-Perforated Yttria-Stabilized Zirconia. Adv. Eng. Mater. 2016, 18, 1877–1883. [Google Scholar] [CrossRef]
- Behr, S.; Vainio, U.; Müller, M.; Schreyer, A.; Schneider, G.A. Large-scale parallel alignment of platelet-shaped particles through gravitational sedimentation. Sci. Rep. 2015, 5, 9984. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Dastjerdi, A.K.; Barthelat, F. Overcoming the brittleness of glass through bio-inspiration and micro-architecture. Nat. Commun. 2014, 5, 3166. [Google Scholar] [CrossRef]
- Lipperman, F.; Ryvkin, M.; Fuchs, M.B. Crack arresting low-density porous materials with periodic microstructure. Int. J. Eng. Sci. 2008, 46, 572–584. [Google Scholar] [CrossRef]
- Marshall, A.L.; Karandikar, P.G.; Givens, B.P.; Liszkiewicz, A.F.; Segura, R.; Klier, E.M.; Doherty, K.J. Geometrical Effect on Damage in Reaction Bonded Ceramic Composites having Experienced High Strain Rate Impact. Ceram. Eng. Sci. Proc. 2014, 41–52. [Google Scholar] [CrossRef]
- Chan, K.S.; He, M.Y.; Hutchinson, J.W. Cracking and stress redistribution in ceramic layered composites. Mater. Sci. Eng. A 1993, 167, 57–64. [Google Scholar] [CrossRef]
- Choi, D.; Yeo, J.-S.; Joh, H.-I.; Lee, S. Carbon Nanosheet from Polyethylene Thin Film as a Transparent Conducting Film: “Upcycling” of Waste to Organic Photovoltaics Application. ACS Sustain. Chem. Eng. 2018, 6, 12463–12470. [Google Scholar] [CrossRef]
- Ali, Z.; Gao, Y.; Tang, B.; Wu, X.; Wang, Y.; Li, M.; Hou, X.; Li, L.; Jiang, N.; Yu, J. Preparation, Properties and Mechanisms of Carbon Fiber/Polymer Composites for Thermal Management Applications. Polymers 2021, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, J.S. Preparation of carbon fibers from linear low density polyethylene. Carbon 2015, 94, 524–530. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, H.-M.; An, J.-H.; Kim, B.-S.; Min, B.-G.; Kang, S.-J.; An, K.-H.; Kim, B.-J. Effects of cross-linking methods for polyethylene-based carbon fibers: Review. Carbon Lett. 2015, 16, 147–170. [Google Scholar] [CrossRef]
- Morris, E.A.; Weisenberger, M.C.; Bradley, S.B.; Abdallah, M.G.; Mecham, S.J.; Pisipati, P.; McGrath, J.E. Synthesis, spinning, and properties of very high molecular weight poly(acrylonitrile-co-methyl acrylate) for high performance precursors for carbon fiber. Polymer 2014, 55, 6471–6482. [Google Scholar] [CrossRef]
- Ball, L.E. Process for Preparing Biaxially Oriented Acrylonitrile Polymer Film. U.S. Patent US3418406, 24 December 1968. [Google Scholar]
- Karacan, I.; Benli, H. Use of sulfonation procedure for the development of thermally stabilized isotactic polypropylene fibers prior to carbonization. J. Appl. Polym. Sci. 2012, 123, 234–245. [Google Scholar] [CrossRef]
- Singh Gill, A.; Visotsky, D.; Mears, L.; Summers, J.D. Cost Estimation Model for Polyacrylonitrile-Based Carbon Fiber Manufacturing Process. J. Manuf. Sci. Eng. 2017, 139, 268. [Google Scholar] [CrossRef]
- Pereña, J.; Lorenzo, V.; Zamfirova, G.; Dimitrova, A. Microhardness of polyethylene surface modified by chlorosulphonic acid. Polym. Test. 2000, 19, 231–236. [Google Scholar] [CrossRef]
- Carl Roth GmbH + Co. KG. Clear Cling Film LLDPE. 2022. Available online: https://www.carlroth.com (accessed on 5 August 2022).
- Sigma-Aldrich Chemie GmbH. Chlorosulphonic Acid 571024-1KG. 2022. Available online: https://www.sigmaaldrich.com (accessed on 13 October 2022).
- Postema, A.R.; de Groot, H.; Pennings, A.J. Amorphous carbon fibres from linear low density polyethylene. J. Mater. Sci. 1990, 25, 4216–4222. [Google Scholar] [CrossRef]
- Palmenaer, A.; Wortberg, G.; Drissen, F.; Seide, G.; Gries, T. Production of Polyethylene Based Carbon Fibres. Chem. Eng. Trans. 2015, 1699–1704. [Google Scholar] [CrossRef]
- Gordon, J.L.; Jones, D.P.; Banas, D.; Hutula, D.N. A Collapse Surface for a Perforated Plate With an Equilateral Triangular Array of Penetrations. J. Press. Vessel Technol. 2002, 124, 201–206. [Google Scholar] [CrossRef]
- Jones, D.P.; Gordon, J.L. Elasto-Plastic Analysis of Perforated Plates Containing Triangular Penetration Patterns of 10 Percent Ligament Efficiency. J. Press. Vessel Technol. 1979, 101, 210–215. [Google Scholar] [CrossRef]
- Penning, J.P.; Lagcher, R.; Pennings, A.J. The effect of diameter on the mechanical properties of amorphous carbon fibres from linear low density polyethylene. Polym. Bull. 1991, 25, 405–412. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouhana, R.; Stommel, M.; Stanko, M.; Muth, M. Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film. Macromol 2022, 2, 554-561. https://doi.org/10.3390/macromol2040036
Rouhana R, Stommel M, Stanko M, Muth M. Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film. Macromol. 2022; 2(4):554-561. https://doi.org/10.3390/macromol2040036
Chicago/Turabian StyleRouhana, Rami, Markus Stommel, Michael Stanko, and Markus Muth. 2022. "Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film" Macromol 2, no. 4: 554-561. https://doi.org/10.3390/macromol2040036
APA StyleRouhana, R., Stommel, M., Stanko, M., & Muth, M. (2022). Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film. Macromol, 2(4), 554-561. https://doi.org/10.3390/macromol2040036