Abstract
Background: The lipid components of the skin barrier have the strongest structure when arranged in an orthorhombic packing. This structure can be influenced by the external supply of lipophilic ingredients. While the benefits of ceramide supplementation are well-documented, the effects of the cosmetic formulation’s oil-based ingredients have been less explored. Methods: The packing structures of commonly used oil and wax ingredients in cosmetics were analyzed using FT-IR. These components were then combined to formulate a cosmetic composition with an orthorhombic packing structure. The strength of the skin barrier was assessed by measuring transepidermal water loss (TEWL), and the lipid packing of the porcine skin was analyzed using FT-IR. Results: In cosmetic oil ingredients, structurally simple oils such as mineral oil and squalane exhibited orthorhombic lipid packing, while more complex oils like isopropyl myristate (IPM) and isononyl isononanoate (ININ) showed hexagonal packing. Based on these structural characteristics, cosmetic formulations were designed by selectively combining oils, waxes, and emulsifiers to achieve a desired packing structure. Formulations incorporating orthorhombically packed oils successfully resulted in orthorhombic overall structures, whereas those including hexagonally packed oils tended to form hexagonal packing. The orthorhombic oils and formulation effectively maintained the structure and function of the porcine skin lipid barrier without disruption. Conclusions: This study demonstrated that orthorhombic oils and emulsions with orthorhombic packing effectively maintained skin barrier integrity, unlike hexagonal structures.
1. Introduction
The skin barrier plays a crucial role in maintaining homeostasis by regulating water loss and protecting against external irritants [1]. The outermost layer of the skin, the stratum corneum, is composed of a highly organized lipid matrix primarily consisting of ceramides, free fatty acids, and cholesterol [2,3]. These intercellular lipids exist in distinct structural arrangements, such as orthorhombic and hexagonal packing [4], which directly influence barrier integrity and function [4]. The stability of the lipid organization is critical for maintaining the protective function of the skin, and disruptions in this structure can lead to increased transepidermal water loss (TEWL) and vulnerability to external stressors [5].
Understanding lipid packing arrangements and their impact on barrier function is essential for developing effective cosmetic and dermatological formulations. Fourier Transform Infrared Spectroscopy (FT-IR) has been widely utilized to analyze lipid structural organization within the skin [6,7]. The vibration of a substance can be analyzed by detecting the absorption of infrared light. Unlike the method that analyzes the wavelengths absorbed after passing through the material, the ATR (Attenuated Total Reflectance) mode determines absorption by irradiating light onto the surface and analyzing the reflected light [8]. This method allows the infrared absorption spectrum of not only liquid substances but also substances in liquid form to be analyzed effectively. This method can be used to analyze the lipid packing state of the skin or formulations. In the hydrocarbon chains of lipids, which are key structural components, hydrogen atoms in CH2 groups exhibit a scissoring bending motion around the central carbon. This motion typically absorbs infrared light around 1470 cm−1. The energy required for this bending varies depending on the lipid packing state, leading to shifts in the absorption wavenumber. In the orthorhombic packing state, where lipids are more tightly packed than in the hexagonal state, one dimension is more compressed than the others. Unlike the symmetrical hexagonal packing, this asymmetry results in two distinct absorption peaks, corresponding to the two different energy levels required for vibration. This splitting of absorption peaks serves as a key indicator for distinguishing between the orthorhombic and hexagonal packing states [9]. FT-IR spectroscopy allows for the identification of specific vibrational modes associated with hydrocarbon chain stretching, scissoring, making it a powerful tool for assessing the impact of external agents on lipid integrity (Figure 1). In particular, the symmetric CH2 stretching peak at approximately 2848 cm−1 and the scissoring bands at 1471 cm−1 and 1463 cm−1 serve as key markers for distinguishing orthorhombic and hexagonal lipid arrangements [10]. Changes in these spectral features provide insights into how oils and emulsions influence the lipid organization of the skin.
Traditionally, X-ray diffraction (XRD) has been used to analyze lattice spacing in materials, making it an essential tool for determining molecular packing [11]. However, XRD cannot differentiate between lipid and protein fiber packing [12], requiring lipids to be extracted and reconstructed separately for analysis in skin studies [13].
In contrast, FT-IR spectroscopy provides a non-invasive method for analyzing lipid packing in the skin. Since the amide bands of proteins appear at different wavenumbers than lipid-specific bands, FT-IR enables selective lipid analysis without interference from proteins. Due to this advantage, FT-IR has been widely applied in various studies for characterizing lipid organization in biological systems.
Despite the extensive research on ceramides [14,15,16] and other bioactive components in skin barrier function [17], studies on the impact of oil-based ingredients in cosmetic formulations remain limited [18]. Additionally, research analyzing the packing structures of oil-based components is lacking. The molecular structure of oils is expected to play a crucial role in their effects on the skin barrier. Components such as linoleic acid have also been found to weaken lipid packing [10]. Oils can be broadly classified into hydrocarbons, ester oils [19], and triglycerides based on their chemical composition and molecular configuration. Hydrocarbon oils, such as mineral oil and squalane [20], are composed solely of carbon and hydrogen and tend to exhibit stable, non-polar characteristics. Triglycerides, which consist of three fatty acid chains attached to a glycerol backbone, include natural oils such as coconut oil and shea butter, providing enhanced emollient properties and lipid replenishment [21]. Ester-based oils, which contain ester bonds linking fatty acid chains, include ingredients like isopropyl myristate and isononyl isononanoate. These are synthetically derived oils not found in nature. Compared to long-chain triglycerides, which often provide a greasy or slippery feel, ester oils offer a lighter sensory texture and are therefore commonly used in cosmetic formulations.
Waxes, categorized as solid lipid materials, consist of long-chain fatty acids esterified with long-chain alcohols. They participate in the formation of the lipid matrix and influence its physical stability and occlusivity. In natural systems, waxes such as beeswax and carnauba wax are primarily composed of ester compounds formed between fatty acids and fatty alcohols, along with minor components like hydrocarbons and free acids. These natural waxes contribute to the lamellar structure and surface cohesion of skin lipids. In contrast, paraffin waxes, which are derived from petroleum, consist of saturated straight-chain hydrocarbons with relatively extended chain lengths. In cosmetic formulations, waxes are also commonly used to modulate the hardness and structural integrity of the product, enabling the transformation of fluid formulations, such as lotions, into thicker, cream-like textures.
Meanwhile, ceramides with a sphingosine backbone possess two hydrocarbon chains and are endogenously generated during keratinocyte differentiation. These molecules are typically observed in the stratum corneum, where they play a key role in maintaining skin barrier integrity. Owing to their physiological relevance, ceramides are often incorporated into cosmetic formulations to reinforce the barrier function [22].
Upon topical application, such lipophilic components can exhibit an occlusive effect on the skin surface or be absorbed into the intercellular lipid matrix. However, there has been a lack of research regarding how these oily components in formulations influence the packing structure of skin lipids. This study aims to investigate the lipid packing states of various cosmetic oils and emulsions to determine their potential to maintain or disrupt the skin barrier. By elucidating the relationship between lipid structural organization and barrier function, this research provides valuable insights into the formulation of skin-friendly cosmetic products that can effectively mimic or support the natural lipid architecture of the skin.
Various excised tissues with structural similarity to human skin have been utilized in dermatological and transdermal research. Among them, porcine skin has been widely adopted due to its close resemblance to human skin. In this study, porcine dorsal skin was selected as the model system. While ear skin is also frequently used, dorsal skin offers practical advantages, including a broader surface area and the ability to obtain large, uniformly structured sections. Owing to its comparable stratum corneum thickness and epidermal architecture, porcine dorsal skin is commonly employed as a substitute for human skin in studies evaluating skin permeability and barrier function [23].
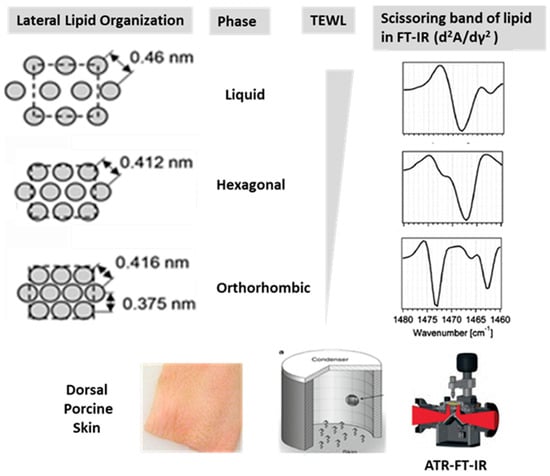
Figure 1.
Analysis of Lipid Packing State in Porcine Skin Model Using ATR-FT-IR and TEWL meter [10,24]. The lipid packing structure of the skin surface was analyzed using a non-invasive method. In the case of the orthorhombic phase, which represents the most tightly packed state, the asymmetric packing results in two absorption wavenumbers for CH2 scissoring vibrations.
2. Materials and Methods
2.1. Materials
Squalane (Synthetic, derived from fish oil)
- INCI name: Squalane. Emollient
- Liquid. It is a branched hydrocarbon with the raw formula C30H62. It is a hydrogenated version of fish squalene (Kishimoto, Osaka, Japan)
Mineral oil (Natural, mineral origin)
- INCI name: Paraffinum Liquidum, Emollient
- Liquid, Mixture of hydrocarbons (typically ranging from C15 to C40, obtained from petroleum (Michang, Ulsan, Republic of Korea)
Isononyl Isononanoate (ININ) (Synthetic),
- INCI name: Isononyl Isononanoate. Emollient
- Liquid. It is a branched hydrocarbon with the raw formula C30H62. It is a hydrogenated version of fish squalene (BASF, Ludwigshafen, Germany)
Isopropyl myristate (IPM) (Synthetic)
- INCI name: Isopropyl myristate. Emollient
- Liquid. Isopropyl myristate is a liquid ester synthesized from isopropyl alcohol and myristic acid (C17H34O2). The ester formed from isopropyl alcohol and myristic acid (Acid Chem, Penang, Malaysia)
n-Paraffin wax (Synthetic, mineral origin)
- INCI name: Paraffin wax. Viscosity controlling
- A solid mixture of hydrocarbons (C51–59), produced by catalytic conversion of low-molecular-weight gaseous hydrocarbons. Melting point 70 °C (MDS, Bintulu, Sarawak, Malaysia),
Beeswax (Natural, animal origin)
- INCI: Beeswax, Viscosity controlling
- A solid consist esters of free fatty acid (C24–C30) and various long-chain alcohol (C24–C36) (Dain, Seoul, Republic of Korea),
Ceramide 3B (Synthetic)
- INCI: Ceramide NP
- Solid, Ceramide 3B is composed of a saturated sphingosine base with 14 carbon atoms, conjugated via an amide bond to oleic acid, which contains one double bond (Doosan, Seoul, Republic of Korea)
Montanov68 (Synthetic, natural origin)
- INCI: Cetearyl Alcohol and Cetearyl Glucoside
- Solid, Montanov 68 is a nonionic emulsifier composed of fatty alcohol (Cetearyl Alcohol) and glucose derivative (Cetearyl Glucoside), providing stable oil-in-water emulsification (Seppic, La Garenne-Colombes, France).
1,2-Hexanediol (Teakyung, Ansan, Gyeonggi-do, Republic of Korea), Glycerin (Goldenbell, Selangor, Malaysia), Stearic acid (Willmar, Gresik, Indonesia), SDS (Sodium Dodecyl sulfate, Sigma, St. Louis, MO, USA), PBS (Phosphate-Buffered Saline, pH 7.4, Thermo Fisher, Waltham, MA, USA), Porcine Dorsal skin (1 mm thick) from a 6-month-old MICROPIG (~20 kg, APURES, Hwaseong, Gyeonggi-do, Republic of Korea) were used.
ATR-FT-IR (Jasco 4200; ATR PRO450-S, JASCO, Tokyo, Japan) and TEWL meter (Barrier Pro II, GP Skin, Seongnam-si, Gyeonggi-do, Republic of Korea) were used.
2.2. Preparation of the Emulsion for Structural Analysis
The emulsion contained 76% water phase, which included 5% glycerin and 2% 1,2-hexanediol. The oil phase accounted for 24% of the total formulation, consisting of 16% oil, 4% wax, and 4% Montanov 68 emulsifier (Table 1). Each phase was heated to 60 °C, followed by mixing using a homogenizer. The sample was cooled at room temperature. All experiments were conducted starting at least one week after the emulsion was prepared and completed within six months. The emulsion was either applied to porcine skin or used for structural analysis via ATR-FT-IR.

Table 1.
Composition of Emulsion Formulation.
2.3. Sample Treatment for Analyzing the Impact on Skin Barrier
A 1 cm × 1 cm piece of porcine dorsal skin was used in the experiment. The surface was washed with distilled water and wiped with Kimwipes before being mounted onto a Franz diffusion cell (Diameter: 0.8 cm) for sample treatment. PBS was used as the receiver medium in the Franz diffusion cell. In the donor chamber, 100 µL of each test solution was applied, including PBS (control), 5% and 10% SDS solutions diluted in PBS, oils, and formulated emulsions. All samples were incubated at 32 °C for 16 h. Squalane, Mineral oil, ININ, and IPM oil were applied as 100% raw materials. To prevent evaporation and maintain the liquid state, the Franz cell was sealed with a lid during the experiment. After incubation, the skin was washed three times with 0.01% SDS solution, followed by a final wash with distilled water. After removal from the Franz diffusion cell, the surface moisture of the porcine skin was gently blotted using Kimwipes. A single round of tape stripping was then performed to remove any residual oil-based components. Subsequently, a piece of gauze was placed at the bottom of a 6-well plate, and 500 µL of PBS was added to moisten it. The skin sample was mounted on top of the gauze with the stratum corneum facing upward. TEWL was measured under this setup, followed by ATR-FT-IR analysis.
2.4. ATR-FT-IR Measurement
ATR-FT-IR measurements were performed at five different points using ATR PRO450-S accessory (JASCO, Tokyo, Japan) on an ATR-FT-IR spectrometer. A drop of each liquid was carefully placed on the ZnSe crystal for ATR-FTIR analysis. In the case of skin samples, the stratum corneum was oriented toward the crystal. Spectra were acquired at room temperature with 24 scans over the spectral range of 3000–400 cm−1, with a resolution of 4 cm−1.
2.5. IR Data Analysis
In this study, a second derivative transformation was applied to the absorbance spectra to enhance peak visibility by resolving overlapping bands. This technique improves the detectability of minor spectral features and facilitates the identification of peak splitting. As peak intensity decreases, the corresponding peak height in the second derivative spectrum also diminishes. For the CH2 scissoring band, a second derivative graph [25] was generated within the range of 1460–1480 cm−1. In the orthorhombic lipid phase, this region typically exhibits two well-resolved CH2 scissoring peaks. A reduction in the height of either peak suggests a weakened lipid packing structure. In particular, as the orthorhombic tendency diminishes, the peak at 1463 cm−1 becomes less prominent compared to the one at 1471 cm−1. When these two peaks merge into a single broad peak centered around 1466 cm−1, the lipid structure is considered to have shifted to a hexagonal phase. All samples were analyzed by FT-IR in five replicates, and a representative spectrum was selected for presentation.
2.6. Statistical Processing
For TEWL analysis, all samples were measured with five replicates, followed by the mean and standard deviation. Only the significant difference between the two groups was analyzed, and * p < 0.05 was indicated after the statistical verification of the Student’s t-test (Microsoft EXCEL 2021, Redmond, WA, USA).
3. Results
3.1. ATR-FT-IR Analysis of Molecular Packing in Skin Barrier Lipids
We first analyzed the lipid packing structure of porcine dorsal skin, a commonly used model for human skin. We also examined the structural features of representative barrier lipids—ceramides and free fatty acid (FFA). In preparation for subsequent experiments involving barrier disruption, the lipid organization of sodium dodecyl sulfate (SDS) was additionally assessed. As a result, porcine skin exhibited two distinct peaks at 1463 and 1471 cm−1, indicating an orthorhombic packing structure (Figure 2E). In contrast, ceramide NP showed a single peak at 1466 cm−1, characteristic of hexagonal packing (Figure 2C). This is presumed to result from the limited packing density caused not only by the bent geometry of the two hydrocarbon chains linked to the sphingosine backbone, but also by the presence of an unsaturated fatty acid (Figure 2A). On the other hand, stearic acid, a saturated free fatty acid, exhibited an orthorhombic packing structure (Figure 2D). This suggests that a single saturated hydrocarbon chain attached to a carboxylic acid group (Figure 2A) typically forms an orthorhombic phase. Ceramide itself is unable to form an orthorhombic packing structure, combination of these lipid components in the skin likely results in an overall orthorhombic structure, which is further stabilized by the addition of cholesterol, as supported by previous findings [26]. However, sodium dodecyl sulfate (SDS), a known skin barrier-disrupting agent, exhibited hexagonal packing despite possessing a single hydrocarbon chain (Figure 2F). This may be attributed to the bulky, strongly anionic headgroup of SDS (Figure 2B), which interferes with tight molecular packing. Overall, while the skin lipid matrix consists of components capable of forming various packing structures, the predominant organization appears to be orthorhombic, and this harmonized structure may be disrupted by the penetration of external substances (Figure 2G).
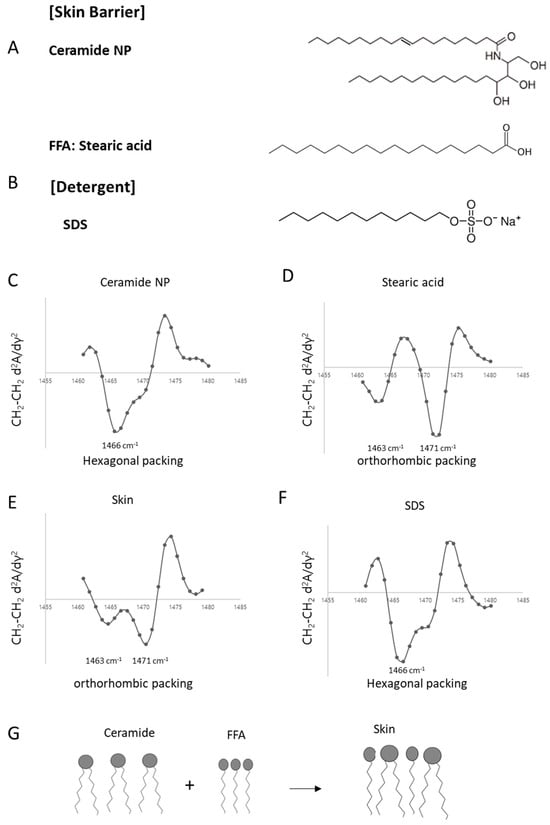
Figure 2.
Analysis of lipid packing structures in skin lipid components and porcine skin using second derivative ATR-FT-IR spectroscopy. Representative molecular structures (A,B) and second derivative ATR-FT-IR spectra (middle) of the CH2 scissoring band (1460–1480 cm−1) are shown for ceramide NP (C), stearic acid (FFA, Free Fatty Acid) (D), SDS (F), and porcine skin (E). Schematic illustrations (G) depict the packing behavior of individual components and their combination in skin lipids.
3.2. Analysis of Skin Barrier Function and Lipid Packing Structure in Porcine Skin Following SDS Treatment
We aimed to investigate how the skin model responds to treatment with sodium dodecyl sulfate (SDS), a surfactant traditionally used to disrupt the skin barrier. Porcine skin was treated with 5% SDS, 10% SDS or PBS (control) for 16 h, followed by surface washing, and the skin barrier function was analyzed using a TEWL device. In the control group treated with PBS, the transepidermal water loss (TEWL) was measured at 14.90 ± 2.18 g/m2/h, whereas the SDS 5%-treated group exhibited significantly increased TEWL at 31.75 ± 3.40 g/m2/h, confirming skin barrier disruption Notably, treatment with 10% SDS further exacerbated this effect, with TEWL reaching 42.90 ± 1.66 g/m2/h, confirming a dose-dependent impairment of barrier function (Figure 3B).
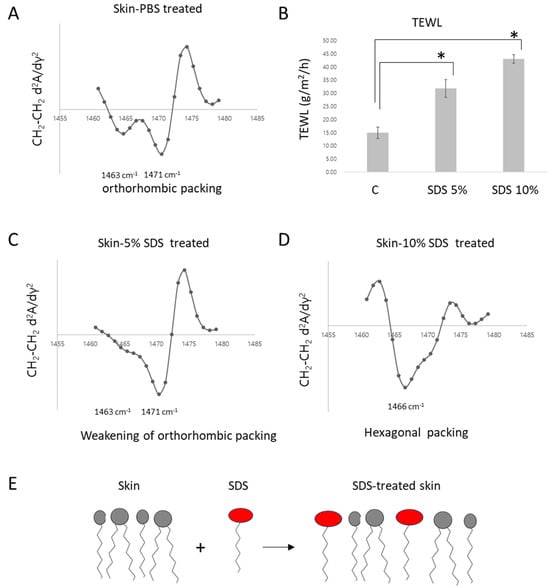
Figure 3.
Relationship Between Lipid Packing State and Barrier Function in Porcine Skin Damage Analysis Induced by SDS. FT-IR second derivative spectra of the CH2 scissoring band in PBS-treated skin (A), SDS 5% treated skin (C), and 10% SDS treated skin (D). Transepidermal water loss (TEWL) increased significantly following SDS treatment in a concentration-dependent manner (* p < 0.05) (B). Schematic illustration of the proposed mechanism: SDS molecule (E).
To further investigate the lipid packing state, Fourier Transform Infrared Spectroscopy (FT-IR) analysis was performed. In the SDS 5%-treated group, the characteristic 1463 cm−1 band of the orthorhombic phase disappeared (Figure 3C), indicating a disruption and weakening of the ordered orthorhombic lipid packing. Furthermore, when the SDS concentration was increased to 10%, the split bands characteristic of the orthorhombic phase were completely lost, and a single peak emerged at 1466 cm−1, corresponding to the hexagonal phase (Figure 3D), suggesting a phase transition to a more disordered lipid arrangement.
The hexagonal packing observed in SDS is presumed to result from electrostatic repulsion associated with its high charge density and large molecular volume. Upon absorption into the skin, these properties are likely to exert comparable disruptive effects on the lamellar lipid structure, leading to a weakening of the barrier. This mechanism is consistent with the classical understanding of SDS-induced skin barrier disruption (Figure 3E).
3.3. Analysis of Lipid Packing Structure in Cosmetic Oils
Importantly, not only surfactants like SDS but also various cosmetic oils possess inherent packing tendencies based on their chemical structures, which may affect the lipid architecture of the stratum corneum upon topical application. Thus, understanding the packing characteristics of ingredients such as squalane, ININ, IPM, and mineral oil is critical for predicting their interactions with the skin barrier.
Squalane, a single-chain hydrocarbon, exhibited characteristic orthorhombic peaks at 1471 cm−1 and 1463 cm−1, (Figure 4C) indicating a tightly packed hydrocarbon structure. Similarly, mineral oil, another linear hydrocarbon mixture, also displayed orthorhombic packing characteristics (Figure 4E). In contrast, ININ, an ester-based oil with a branched structure, displayed a peak near 1466 cm−1 (Figure 4D), characteristic of a hexagonal packing arrangement. A similar trend was observed for ester oils such as IPM (Figure 4F). The simple linear structure of squalane allows efficient hydrocarbon packing, resulting in an orthorhombic phase (Figure 4A). Conversely, the ester bond in ININ creates a branched molecular structure (Figure 4B), making tight packing more challenging and leading to a hexagonal phase.
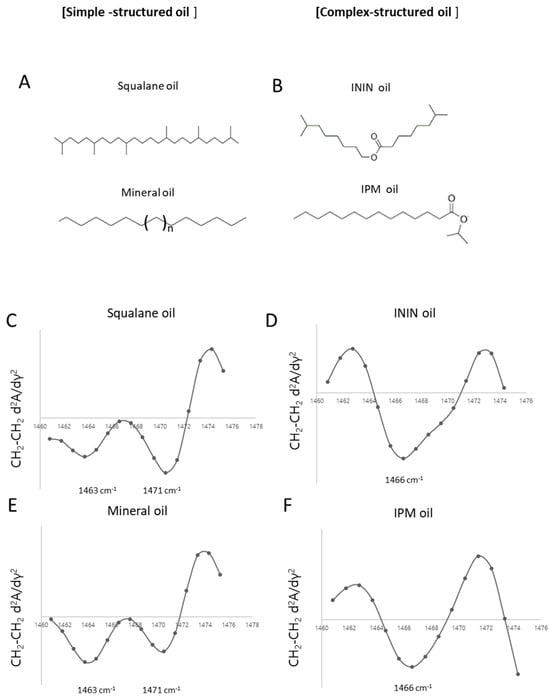
Figure 4.
Comparison of lipid packing structures in cosmetic oils with different molecular architectures. (A,B) Molecular structures of representative simple-structured oils (squalane, mineral oil) and complex-structured oils (isononyl isononanoate [ININ], isopropyl myristate [IPM]). (C,E) ATR-FT-IR second derivative spectra of squalane and mineral oil show characteristic orthorhombic packing with split peaks at 1463 cm−1 and 1471 cm−1. (D,F) In contrast, ININ and IPM display a single peak at 1466 cm−1, indicative of hexagonal packing.
3.4. Impact of Oil Packing Structures on Skin Barrier Function
To investigate the effects of oil components with different molecular structures on the skin barrier, four representative cosmetic oils—squalane, mineral oil, ININ, and IPM were selected for analysis. These substances were applied to porcine skin for 16 h, followed by surface washing, and their impact on skin barrier function was evaluated through transepidermal water loss (TEWL) and lipid packing analysis.
The PBS-treated control group showed a TEWL of 11.21 ± 3.39 g/m2/h (Figure 5B). Squalane treatment significantly decreased TEWL to 5.58 ± 1.36 g/m2/h, indicating enhanced barrier function. Similarly, mineral oil reduced TEWL to 6.57 ± 2.29 g/m2/h, also suggesting a protective effect on the barrier. In contrast, ININ treatment increased TEWL to 19.44 ± 4.88 g/m2/h, while IPM treatment resulted in a TEWL of 17.35 ± 3.88 g/m2/h, both indicating a deterioration of barrier integrity.
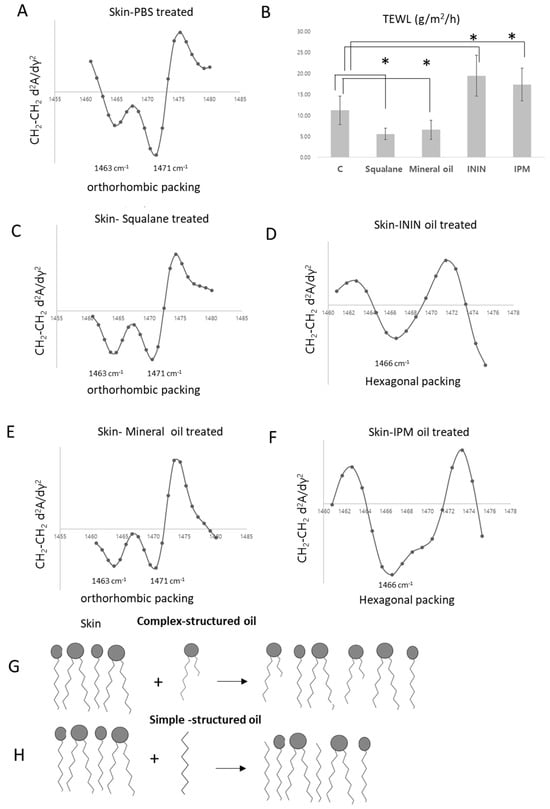
Figure 5.
Structural and Functional Changes in the Skin Barrier Induced by Application of Oils with Different Packing Structures. (A,C–F) show the second derivative curves of the scissoring band for porcine skin after treatment with PBS (A), Squalane (C), ININ (D), Mineral oil (E), and IPM (F), respectively. (B) presents the TEWL data corresponding to these conditions (* p < 0.05). (G) illustrates the interaction of complex-structured oils with the orthorhombically packed skin lipid domains, suggesting enhanced structural compatibility. (H) depicts the looser alignment of simple-structured oils, indicating weakening of orthorhombic lipid packing.
To explore whether these functional differences were associated with alterations in lipid packing structures, ATR-FT-IR analysis was conducted. The PBS-treated group exhibited characteristic split bands at 1463 cm−1 and 1471 cm−1 (Figure 5A), consistent with an orthorhombic packing structure. In the squalane-treated group, the intensity of the 1463 cm−1 peak was enhanced (Figure 5C), suggesting reinforcement of orthorhombic order. Likewise, the mineral oil-treated skin maintained distinct peaks at 1463 cm−1 and 1471 cm−1, confirming preservation of the orthorhombic phase (Figure 5E).
Conversely, the ININ-treated skin showed loss of the characteristic orthorhombic bands, replaced by a single peak near 1466 cm−1 (Figure 5D), indicating a transition to hexagonal packing. A similar shift to hexagonal structure was observed in the IPM-treated group, which also lacked the orthorhombic features (Figure 5F).
These findings suggest that topically applied oils penetrate into the intercellular lipid domains and modulate the structural organization of the skin barrier. Maintaining or reinforcing orthorhombic lipid packing—observed with squalane and mineral oil—appears advantageous for preserving barrier integrity (Figure 5H), while a shift toward hexagonal packing, as seen with ININ and IPM, may compromise barrier function (Figure 5G).
3.5. Formulation of Products Exhibiting Orthorhombic and Hexagonal Lipid Packing Structures
Most cosmetic formulations are designed as oil-in-water (O/W) emulsions rather than using oil alone. Therefore, it is important to evaluate the effects of emulsified systems on the skin. To this end, an emulsion with orthorhombic lipid packing characteristics was formulated. Emulsifiers were incorporated to stabilize the system, and in addition to liquid oils, solid waxes were included as key structural components. The lipid packing structures of these individual ingredients were analyzed to understand their inherent organization and to guide the formulation strategy. By combining components with known packing tendencies, the final emulsion structure was tuned to achieve a desired lipid arrangement.
Packing analysis was conducted on normal paraffin, a solid single-chain hydrocarbon (Figure 6C) with a melting point of approximately 70 °C. It exhibited an orthorhombic structure, as indicated by its peaks at 1472 cm−1 and 1463 cm−1 (Figure 6G). Beeswax, composed of ester-bonded long-chain hydrocarbons (Figure 6D), also showed similar orthorhombic features with the same characteristic peak (Figure 6H). Compared to ester oils (Figure 6B), the longer hydrocarbon chains in beeswax likely result in a more tightly packed structure. Interestingly, despite its simple linear structure, normal paraffin exhibited even stronger packing interactions than beeswax. To further assess how these components behave in a complete formulation, O/W emulsions were prepared using Montanov 68 as the emulsifier. The formulations contained 76% water, 16% oil, 4% wax, and 4% emulsifier. The structural analysis revealed that formulations composed of squalane (Figure 6A) and normal paraffin maintained an orthorhombic packing arrangement (Figure 6I), whereas those containing ININ and beeswax exhibited a hexagonal phase (Figure 6J). This study demonstrates that even without the presence of ceramides, free fatty acids, and cholesterol—key components of the skin lipid barrier—an orthorhombic structure can still be formed. This suggests that formulations containing simple, unbranched hydrocarbon-based oils can effectively mimic the tightly packed structures found in the stratum corneum.
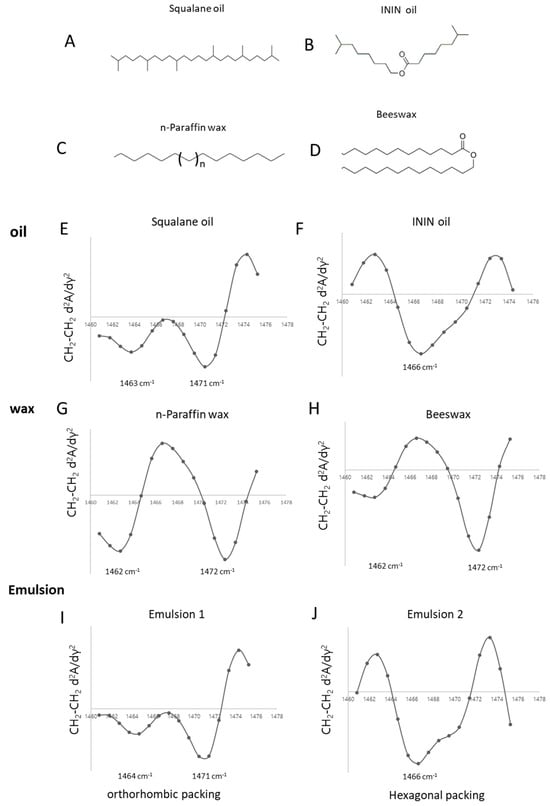
Figure 6.
Lipid packing characteristics of cosmetic raw materials and formulated emulsions analyzed by ATR-FT-IR spectroscopy. (A–D) Molecular structures of oils and waxes used in formulation: squalane (linear hydrocarbon), ININ (branched ester), n-paraffin wax (long-chain saturated hydrocarbon), and beeswax (natural ester-based wax). (E–J) show the second derivative curves of lipid and emulsion, Squalane(E), ININ (F), n-paraffin wax (G), Beeswax (H), Emulsion 1 (I), and Emulsion 2 (J), respectively.
3.6. Effects of Emulsion Formulations on Skin Barrier Function: A Comparative Analysis of Orthorhombic and Hexagonal Lipid Packing Structures
Finally, the effects of emulsions with different lipid packing states on the skin barrier were evaluated. In the control group, the transepidermal water loss (TEWL) was measured at 8.42 ± 1.70 g/m2/h. The formulation exhibiting orthorhombic packing showed a TEWL of 8.23 ± 2.21 g/m2/h (Figure 7B), indicating a similar level of barrier function to the control. In contrast, the formulation with a hexagonal lipid packing structure resulted in a significantly increased TEWL of 13.28 ± 4.26 g/m2/h (Figure 7B), suggesting compromised barrier integrity. Furthermore, lipid packing structure analysis revealed that skin treated with the hexagonal packing formulation showed the disappearance of the 1463 cm−1 peak (Figure 7D), indicating a substantial alteration of the orthorhombic structure. Meanwhile, the formulation with orthorhombic packing also maintained the lipid barrier structure (Figure 7A,C). These findings suggest that even in O/W emulsions, an orthorhombic lipid packing structure is more beneficial for preserving skin barrier integrity compared to a hexagonal packing structure.
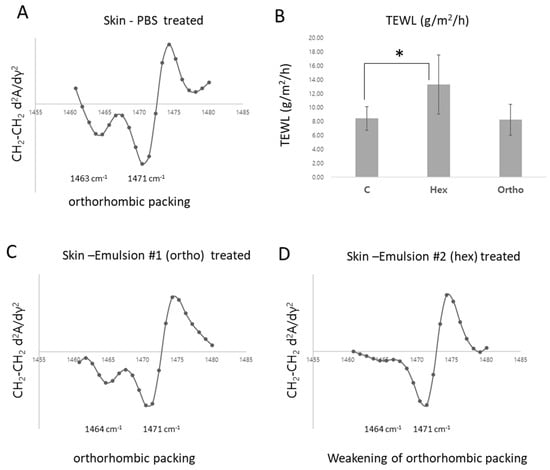
Figure 7.
Structural and Functional Changes in the Skin Barrier of Pigskin Induced by the Application of Emulsions with Different Packing Structure. (A) represents the second derivative curves of the scissoring band for porcine skin after treatment with PBS, orthorhombic emulsion, and hexagonal emulsion. (B) presents the TEWL analysis results corresponding to these conditions (* p < 0.05). (C) displays the second-derivative spectrum for skin treated with the orthorhombic emulsion, indicating the preservation of orthorhombic lipid packing. (D) shows the second-derivative spectrum for skin treated with the hexagonal emulsion, illustrating the weakening of orthorhombic packing.
4. Discussion
This study investigated the effects of lipid components in cosmetic formulations, which constitute a significant portion of formulations and are expected to influence the lipid barrier when applied to the skin. Since an orthorhombic lipid structure provides the most stable barrier, we analyzed the packing structures of individual lipid components and examined their impact on skin barrier integrity. Squalane and normal paraffin, both of which have simple linear structures [20], exhibited orthorhombic packing, whereas ester oils with ester bonds forming branched structures did not. However, despite having ester bonds, beeswax, which has a longer molecular structure, formed an orthorhombic packing structure. In general, waxes that exist as solid-state materials demonstrated a more pronounced orthorhombic structure compared to squalane. The peak interval between the second derivatives of scissoring was larger [6], and the local height was also greater. Interestingly, porcine skin exhibited a weaker orthorhombic structure than waxes. For intercellular lipids composed of ceramides, fatty acids, and cholesterol, an appropriate balance of these components is necessary to maintain an orthorhombic packing structure [21]. The study confirmed that disruptive agents such as SDS (sodium dodecyl sulfate) could disturb this structure. However, simple oils like squalane and mineral oil did not compromise the barrier function; rather, they enhanced it. In contrast, hexagonal-structured ININ and IPM disrupted the skin barrier. Unlike SDS, which disturbs the lamellar structure due to its strong hydrophilic charge, hexagonal-structured ININ, which features looser lipid packing, also influenced the skin’s lipid organization. The findings are consistent with previous studies demonstrating that IPM functions as a penetration enhancer by integrating into intercellular lipids and inducing structural perturbations in the skin barrier [27]. Similarly, mineral oil has been reported to strengthen the skin barrier more effectively than many vegetable oils [28].
Furthermore, when formulating emulsions using a combination of oils and waxes with simple molecular structures, orthorhombic-structured formulations were successfully developed (Figure 8) and no significant impact on the skin barrier function was observed based on TEWL measurements. On the other hand, formulations with a hexagonal packing structure had a detrimental effect on both skin barrier function and lipid organization. Future research should explore whether stronger orthorhombic structures can be designed through different lipid combinations and whether such formulations could enhance barrier function or aid in the recovery of a damaged skin barrier. Additionally, further studies should investigate whether formulations with non-orthorhombic lipid components can still establish an orthorhombic structure, as seen in natural skin lipid organization. This study holds significant implications as it confirms that lipid packing structure can directly influence skin barrier function.
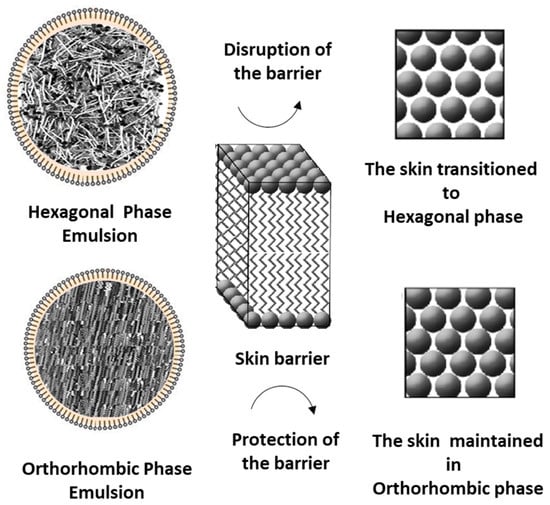
Figure 8.
A Model of Skin Barrier Structural Changes Based on Lipid Packing States.
The oil components of the formulation are absorbed into the intercellular lipids of the skin barrier, influencing its structure. Components that can be densely arranged in an orthorhombic packing are expected to maintain the orthorhombic structure of the barrier even after being absorbed into the skin
A limitation of this study is that the employed ATR-FTIR technique primarily provides information on the lateral packing of lipid chains, such as orthorhombic or hexagonal arrangements. However, it does not allow direct assessment of lamellar organization or interlamellar repeat distances along the vertical axis. Therefore, potential alterations in lamellar stacking or long periodicity phases [29], which may also influence barrier properties, could not be evaluated in this study. Furthermore, it has been shown that changes in barrier function may arise independently of shifts in the lateral packing phase, suggesting that other structural factors such as lamellar organization or lipid composition may also play a critical role [30].
Author Contributions
Conceptualization, Methodology, Supervision; S.-H.L. Validation, Investigation, Data curation; Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This thesis was supported by the Dongduk Women’s University Grant [2023-06237].
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to laboratory confidentiality.
Conflicts of Interest
The authors state no conflicts of interest to declare.
References
- Bouwstra, J.A.; Ponec, M. The Skin Barrier in Healthy and Diseased State. Biochim. Biophys. Acta Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef]
- Coderch, L.; Lopez, O.; de la Maza, A.; Parra, J.L. Ceramides and Skin Function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- Vietri Rudan, M.; Watt, F.M. Mammalian Epidermis: A Compendium of Lipid Functionality. Front. Physiol. 2022, 12, 804824. [Google Scholar] [CrossRef]
- Damien, F.; Boncheva, M. The Extent of Orthorhombic Lipid Phases in the Stratum Corneum Determines the Barrier Efficiency of Human Skin In Vivo. J. Investig. Dermatol. 2010, 130, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Alsamad, F.; Stamatas, G.N. Directional Assessment of the Skin Barrier Function in Vivo. Skin Res. Technol. 2023, 29, e13346. [Google Scholar] [CrossRef] [PubMed]
- Pensack, R.D.; Michniak, B.B.; Moore, D.J.; Mendelsohn, R. Infrared Kinetic/Structural Studies of Barrier Reformation in Intact Stratum Corneum Following Thermal Perturbation. Appl. Spectrosc. 2006, 60, 1399–1404. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, S.H.; Yeom, J.; Park, S.G.; Lee, C.K.; Kang, N.G. Optical Clearing Agent Reduces Scattering of Light by the Stratum Corneum and Modulates the Physical Properties of Coenocytes via Hydration. Skin Res. Technol. 2018, 24, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yarovoy, Y.; Drutis, D.M.; Hancewicz, T.M.; Garczarek, U.; Ananthapadmanabhan, K.P.; Misra, M. Quantification of Lipid Phase Order of In Vivo Human Skin Using Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy and Multivariate Curve Resolution Analysis. Appl. Spectrosc. 2019, 73, 182–194. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Nădăban, A.; Bras, W.; McCabe, C.; Bunge, A.; Gooris, G.S. The Skin Barrier: An Extraordinary Interface with an Exceptional Lipid Organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef]
- Boncheva, M.; Damien, F.; Normand, V. Molecular Organization of the Lipid Matrix in Intact Stratum Corneum Using ATR-FTIR Spectroscopy. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1344–1355. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Gooris, G.S.; Dubbelaar, F.E.R.; Ponec, M. Phase Behavior of Stratum Corneum Lipid Mixtures Based on Human Ceramides: The Role of Natural and Synthetic Ceramide 1. J. Investig. Dermatol. 2002, 118, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.H.; Lee, S.H. Arginine-Fructose-Glucose from Red Ginseng Extract Reduces Stiffness of Keratin Fiber in Corneocyte of Skin. Skin Res. Technol. 2023, 29, e13217. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Berkers, T.; Visscher, D.; Gooris, G.S.; Bouwstra, J.A. Topically Applied Ceramides Interact with the Stratum Corneum Lipid Matrix in Compromised Ex Vivo Skin. Pharm. Res. 2018, 35, 48. [Google Scholar] [CrossRef]
- Kucharekova, M.; Schalkwijk, J.; Van De Kerkhof, P.C.M.; Van De Valk, P.G.M. Effect of a Lipid-Rich Emollient Containing Ceramide 3 in Experimentally Induced Skin Barrier Dysfunction. Contact Dermat. 2002, 46, 331–338. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, W.R.; Kim, J.H.; Cho, E.C.; An, E.J.; Kim, J.-W.; Oh, S.-G. Fabrication of Pseudo-Ceramide-Based Lipid Microparticles for Recovery of Skin Barrier Function. Colloids Surf. B Biointerfaces 2012, 94, 236–241. [Google Scholar] [CrossRef]
- Hashizume, E.; Nakano, T.; Kamimura, A.; Morishita, K. Topical Effects of N-Acetyl-L-Hydroxyproline on Ceramide Synthesis and Alleviation of Pruritus. Clin. Cosmet. Investig. Dermatol. 2013, 6, 43–49. [Google Scholar] [CrossRef]
- Nisbet, S.; Mahalingam, H.; Gfeller, C.F.; Biggs, E.; Lucas, S.; Thompson, M.; Cargill, M.R.; Moore, D.; Bielfeldt, S. Cosmetic Benefit of a Biomimetic Lamellar Cream Formulation on Barrier Function or the Appearance of Fine Lines and Wrinkles in Randomized Proof-of-Concept Clinical Studies. Int. J. Cosmet. Sci. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Demski, K.; Ding, B.-J.; Wang, H.-L.; Tran, T.N.T.; Durrett, T.P.; Lager, I.; Löfstedt, C.; Hofvander, P. Manufacturing Specialized Wax Esters in Plants. Metab. Eng. 2022, 72, 391–402. [Google Scholar] [CrossRef]
- Soni, V.K.; Sharma, R.K. Palladium-Nanoparticles-Intercalated Montmorillonite Clay: A Green Catalyst for the Solvent-Free Chemoselective Hydrogenation of Squalene. ChemCatChem 2016, 8, 1763–1768. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of Medium-Chain Fatty Acids: A Concise Review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef] [PubMed]
- Supe, S.; Takudage, P. Methods for Evaluating Penetration of Drug into the Skin: A Review. Skin Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Pilgram, G.S.K.; Vissers, D.C.J.; Van Der Meulen, H.; Pavel, S.; Lavrijsen, S.P.M.; Bouwstra, J.A.; Koerten, H.K. Aberrant Lipid Organization in Stratum Corneum of Patients with Atopic Dermatitis and Lamellar Ichthyosis. J. Investig. Dermatol. 2001, 117, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of Second Derivative Spectroscopy for Increasing Molecular Specificity of Fourier Transform Infrared Spectroscopic Imaging of Articular Cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Gooris, G.S.; Bouwstra, J.A. Phase Behavior of Skin Lipid Mixtures: The Effect of Cholesterol on Lipid Organization. Soft Matter 2015, 11, 4326–4336. [Google Scholar] [CrossRef]
- Engelbrecht, T.N.; Demé, B.; Dobner, B.; Neubert, R.H.H. Study of the Influence of the Penetration Enhancer Isopropyl Myristate on the Nanostructure of Stratum Corneum Lipid Model Membranes Using Neutron Diffraction and Deuterium Labelling. Skin Pharmacol. Physiol. 2012, 25, 200–207. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Lombard, K.J. A Review on the Extensive Skin Benefits of Mineral Oil. Int. J. Cosmet. Sci. 2012, 34, 511–518. [Google Scholar] [CrossRef]
- Uche, L.E.; Gooris, G.S.; Beddoes, C.M.; Bouwstra, J.A. New Insight into Phase Behavior and Permeability of Skin Lipid Models Based on Sphingosine and Phytosphingosine Ceramides. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1317–1328. [Google Scholar] [CrossRef]
- Groen, D.; Poole, D.S.; Gooris, G.S.; Bouwstra, J.A. Is an Orthorhombic Lateral Packing and a Proper Lamellar Organization Important for the Skin Barrier Function? Biochim. Biophys. Acta Biomembr. 2011, 1808, 1529–1537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).