The Sleep–Skin Axis: Clinical Insights and Therapeutic Approaches for Inflammatory Dermatologic Conditions
Abstract
1. Introduction
2. Skin Conditions
2.1. Atopic Dermatitis
2.2. Psoriasis
2.3. Acne
2.4. Rosacea
2.5. Hidradenitis Suppurativa
3. Therapeutic Interventions
3.1. Melatonin
3.2. L-Theanine
3.3. Magnesium-L-Threonate
3.4. Inositol
3.5. Cinnamomi Cortex
3.6. Nervous System Regulation
3.7. Proper Sleep Hygiene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hettwer, S.; Besic Gyenge, E.; Obermayer, B. Influence of cosmetic formulations on the skin’s circadian clock. Int. J. Cosmet. Sci. 2020, 42, 313–319. [Google Scholar] [CrossRef]
- Salazar, A.; von Hagen, J. Circadian Oscillations in Skin and Their Interconnection with the Cycle of Life. Int. J. Mol. Sci. 2023, 24, 5635. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Vollmers, C.; de la Cruz, D.; Chaix, A.; Ramos, R.; Panda, S.; Chuong, C.M. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc. Natl. Acad. Sci. USA 2013, 110, E2106–E2115. [Google Scholar] [CrossRef]
- Al-Nuaimi, Y.; Hardman, J.A.; Bíró, T.; Haslam, I.S.; Philpott, M.P.; Tóth, B.I.; Farjo, N.; Farjo, B.; Baier, G.; Watson, R.E.B.; et al. A meeting of two chronobiological systems: Circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J. Investig. Dermatol. 2014, 134, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Van Spyk, E.N.; Pham, K.; Geyfman, M.; Kumar, V.; Takahashi, J.S.; Andersen, B. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythm. 2015, 30, 163–182. [Google Scholar] [CrossRef]
- Zanello, S.B.; Jackson, D.M.; Holick, M.F. Expression of the circadian clock genes clock and period1 in human skin. J. Investig. Dermatol. 2000, 115, 757–760. [Google Scholar] [CrossRef]
- Lyons, A.B.; Moy, L.; Moy, R.; Tung, R. Circadian Rhythm and the Skin: A Review of the Literature. J. Clin. Aesthetic Dermatol. 2019, 12, 42–45. [Google Scholar]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K. Sleep-wake disorders and dermatology. Clin. Dermatol. 2013, 31, 118–126. [Google Scholar] [CrossRef]
- Altemus, M.; Rao, B.; Dhabhar, F.S.; Ding, W.; Granstein, R.D. Stress-induced changes in skin barrier function in healthy women. J. Investig. Dermatol. 2001, 117, 309–317. [Google Scholar] [CrossRef]
- Xerfan, E.M.S.; Andersen, M.L.; Facina, A.S.; Tufik, S.; Tomimori, J. Sleep loss and the skin: Possible effects of this stressful state on cutaneous regeneration during nocturnal dermatological treatment and related pathways. Dermatol. Ther. 2022, 35, e15226. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Lyu, F.; Wu, T.; Bian, Y.; Zhu, K.; Xu, J.; Li, F. Stress and its impairment of skin barrier function. Int. J. Dermatol. 2023, 62, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.; Cirelli, C.; Dijk, D.J.; Van Cauter, E.; Schwartz, S.; Chee, M.W. Disrupted sleep: From molecules to cognition. J. Neurosci. 2015, 35, 13889–13895. [Google Scholar] [CrossRef]
- Schmitt, K.; Grimm, A.; Dallmann, R.; Oettinghaus, B.; Restelli, L.M.; Witzig, M.; Ishihara, N.; Mihara, K.; Ripperger, J.A.; Albrecht, U.; et al. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 2018, 27, 657–666e5. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef]
- Passeron, T.; Zouboulis, C.C.; Tan, J.; Andersen, M.L.; Katta, R.; Lyu, X.; Aguilar, L.; Kerob, D.; Morita, A.; Krutmann, J.; et al. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1963–1975. [Google Scholar] [CrossRef]
- Kahan, V.; Ribeiro, D.A.; Egydio, F.; Barros, L.A.; Tomimori, J.; Tufik, S.; Andersen, M.L. Is lack of sleep capable of inducing DNA damage in aged skin? Skin. Pharmacol. Physiol. 2014, 27, 127–131. [Google Scholar] [CrossRef]
- Inoue, M.; Shiozawa, K.; Yoshihara, R.; Yamane, T.; Shima, Y.; Hirano, T.; Makimoto, K. Predictors of Poor Sleep Quality in Patients With Systemic Lupus Erythematosus. Clin. Rheumatol. 2017, 36, 1053–1062. [Google Scholar] [CrossRef]
- Li, T.; Cui, C.; Li, Y.; Wang, L. The Impacts of Resilience on the Association Between Illness Uncertainty and Sleep Quality Among Chinese Women With Systemic Lupus Erythematosus. Clin. Rheumatol. 2020, 39, 1609–1616. [Google Scholar] [CrossRef]
- Palagini, L.; Tani, C.; Mauri, M.; Carli, L.; Vagnani, S.; Bombardieri, S.; Gemignani, A.; Mosca, M. Sleep Disorders and Systemic Lupus Erythematosus. Lupus 2014, 23, 115–123. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 1 in 3 Adults Don’t Get Enough Sleep. CDC. Available online: https://archive.cdc.gov/www_cdc_gov/media/releases/2016/p0215-enough-sleep.html (accessed on 18 December 2024).
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef]
- National Institute of Allergy and Infectious Diseases. Eczema (Atopic Dermatitis). National Institutes of Health. Available online: https://www.niaid.nih.gov/diseases-conditions/eczema-atopic-dermatitis (accessed on 13 December 2024).
- Arndt, J.; Smith, N.; Tausk, F. Stress and atopic dermatitis. Curr. Allergy Asthma Rep. 2008, 8, 312–317. [Google Scholar] [CrossRef]
- Buske-Kirschbaum, A.; Geiben, A.; Höllig, H.; Morschhäuser, E.; Hellhammer, D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J. Clin. Endocrinol. Metab. 2002, 87, 4245–4251. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef]
- Estefan, J.; Ferreira, D.C.; Cavalcante, F.S.; Dos Santos, K.R.N.; Ribeiro, M. Investigation of possible relationship between atopic dermatitis and salivary biomarkers, stress, and sleep disorders. World J. Clin. Cases 2023, 11, 3958–3966. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chiang, B.L. Mechanism of Sleep Disturbance in Children with Atopic Dermatitis and the Role of the Circadian Rhythm and Melatonin. Int. J. Mol. Sci. 2016, 17, 462. [Google Scholar] [CrossRef]
- Stefanovic, N.; Irvine, A.D.; Flohr, C. The Role of the Environment and Exposome in Atopic Dermatitis. Curr. Treat. Options Allergy 2021, 8, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Talamonti, M.; Galluzzo, M.; Silvaggio, D.; Lombardo, P.; Tartaglia, C.; Bianchi, L. Quality of Life and Psychological Impact in Patients with Atopic Dermatitis. J. Clin. Med. 2021, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Donnelly, A.; Broderick, C.; Arichi, T.; Bartsch, U.; Dazzan, P.; Elberling, J.; Godfrey, E.; Gringras, P.; Heathcote, L.C.; et al. Mind and skin: Exploring the links between inflammation, sleep disturbance and neurocognitive function in patients with atopic dermatitis. Allergy 2024, 79, 26–36. [Google Scholar] [CrossRef]
- Ramirez, F.D.; Chen, S.; Langan, S.M.; Prather, A.A.; McCulloch, C.E.; Kidd, S.A.; Cabana, M.D.; Chren, M.M.; Abuabara, K. Association of Atopic Dermatitis With Sleep Quality in Children. JAMA Pediatr. 2019, 173, e190025. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Mueller, K.; Kruse, L.; Boor, P.; Sheldon, S.; Zee, P.; Paller, A.S. Sleep disturbance in children with moderate/severe atopic dermatitis: A case-control study. J. Am. Acad. Dermatol. 2018, 78, 336–341. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chou, Y.T.; Lee, J.H.; Lee, P.L.; Dai, Y.S.; Sun, C.; Lin, Y.T.; Wang, L.C.; Yu, H.H.; Yang, Y.H.; et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 2014, 134, e397–e405. [Google Scholar] [CrossRef]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Nocturnal itch: Why do we itch at night? Acta Derm. Venereol. 2007, 87, 295–298. [Google Scholar] [CrossRef]
- Meštrović-Štefekov, J.; Novak-Bilić, G.; Kuna, M.; Pap, N.; Lugović-Mihić, L. Psychological Stress in Patients with Atopic Dermatitis. Acta Dermatovenerol. Croat. 2018, 26, 297–303. [Google Scholar]
- Nutan; Kanwar, A.J.; Bhansali, A.; Parsad, D. Evaluation of hypothalamic-pituitary-adrenal axis in patients with atopic dermatitis. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E. Psoriasis--epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Musumeci, M.L.; Nasca, M.R.; Boscaglia, S.; Micali, G. The role of lifestyle and nutrition in psoriasis: Current status of knowledge and interventions. Dermatol. Ther. 2022, 35, e15685. [Google Scholar] [CrossRef]
- Choudhary, S.; Pradhan, D.; Pandey, A.; Khan, M.K.; Lall, R.; Ramesh, V.; Puri, P.; Jain, A.K.; Thomas, G. The association of metabolic syndrome and psoriasis: A systematic review and meta-analysis of observational study. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Gelfant, S.; Ozawa, A.; Chalker, D.K.; Smith, J.G., Jr. Circadian rhythms and differences in epidermal and in dermal cel proliferation in uninvolved and involved psoriatic skin in vivo. J. Investig. Dermatol. 1982, 78, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Mozzanica, N.; Tadini, G.; Radaelli, A.; Negri, M.; Pigatto, P.; Morelli, M.; Frigerio, U.; Finzi, A.; Esposti, G.; Rossi, D. Plasma melatonin levels in psoriasis. Acta Derm. Venereol. 1988, 68, 312–316. [Google Scholar]
- Hirotsu, C.; Rydlewski, M.; Araújo, M.S.; Tufik, S.; Andersen, M.L. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS ONE 2012, 7, e51183. [Google Scholar] [CrossRef]
- Li, W.Q.; Qureshi, A.A.; Schernhammer, E.S.; Han, J. Rotating night-shift work and risk of psoriasis in US women. J. Investig. Dermatol. 2013, 133, 565–567. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- James, F.O.; Cermakian, N.; Boivin, D.B. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep 2007, 30, 1427–1436. [Google Scholar] [CrossRef]
- Shutty, B.G.; West, C.; Huang, K.E.; Landis, E.; Dabade, T.; Browder, B.; O’Neill, J.; Kinney, M.A.; Feneran, A.N.; Taylor, S.; et al. Sleep disturbances in psoriasis. Dermatol. Online J. 2013, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Goon, A.; Wee, J.; Chan, Y.H.; Goh, C.L. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br. J. Dermatol. 2000, 143, 969–973. [Google Scholar] [CrossRef]
- Ferguson, F.J.; Lada, G.; Hunter, H.J.A.; Bundy, C.; Henry, A.L.; Griffiths, C.E.M.; Kleyn, C.E. Diurnal and seasonal variation in psoriasis symptoms. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e45–e47. [Google Scholar] [CrossRef]
- Sahin, E.; Hawro, M.; Weller, K.; Sabat, R.; Philipp, S.; Kokolakis, G.; Christou, D.; Metz, M.; Maurer, M.; Hawro, T. Prevalence and factors associated with sleep disturbance in adult patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Kang, J.H.; Lin, H.C. Increased risk of psoriasis following obstructive sleep apnea: A longitudinal population based study. Sleep Med. 2012, 13, 285–289. [Google Scholar] [CrossRef]
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2013, 134, 1527–1534. [Google Scholar] [CrossRef]
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R.P. Acne vulgaris: Pathogenesis, treatment, and needs assessment. Dermatol. Clin. 2012, 30, 99–106. [Google Scholar] [CrossRef]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef]
- González-Mondragón, E.A.; Ganoza-Granados, L.D.C.; Toledo-Bahena, M.E.; Valencia-Herrera, A.M.; Duarte-Abdala, M.R.; Camargo-Sánchez, K.A.; Mena-Cedillos, C.A. Acne and diet: A review of pathogenic mechanisms. Bol. Med. Hosp. Infant. Mex. 2022, 79, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Besra, L.; Kar, H.K. Evaluation of Risk Factors Associated With Adult-Onset Acne in Patients Attending a Tertiary Care Center in East India: A Case-Control Study. Cureus 2024, 16, e53296. [Google Scholar] [CrossRef]
- Khormi, G.; Aldubayyan, N.; Hakami, M.; Daghriri, S.; Aqeel, S. Impact of Lifestyle and Dietary Habits on the Prevalence of Acne Vulgaris: A Cross-Sectional Study From Saudi Arabia. Cureus 2024, 16, e57200. [Google Scholar] [CrossRef]
- Bilgiç, Ö.; Bilgiç, A.; Altinyazar, H.C. Relationship between sleep quality and facial sebum levels in women with acne vulgaris. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 313–314. [Google Scholar] [CrossRef]
- Misery, L.; Wolkenstein, P.; Amici, J.M.; Maghia, R.; Brenaut, E.; Cazeau, C.; Voisard, J.J.; Taïeb, C. Consequences of acne on stress, fatigue, sleep disorders and sexual activity: A population-based study. Acta Derm. Venereol. 2015, 95, 485–488. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Rossi, A.B.; Thouvenin, M.D.; Peraud, C.; Mengeaud, V.; Bacquey, A.; Saint Aroman, M. Fragility of epidermis: Acne and post-procedure lesional skin. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Suggs, A.; Ezaldein, H.H.; Ya, J.; Fu, P.; Jamora, J.; Verallo-Rowel, V.; Baron, E.D. The Effect of Shift Work and Poor Sleep on Self-Reported Skin Conditions: A Survey of Call Center Agents in the Philippines. Clocks Sleep 2019, 1, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: Transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Investig. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S.; Pelle, E.; Dong, K.; Pernodet, N. Biological Rhythms in the Skin. Int. J. Mol. Sci. 2016, 17, 801. [Google Scholar] [CrossRef]
- Xerfan, E.M.S.; Andersen, M.L.; Facina, A.S.; Tufik, S.; Tomimori, J. Rosacea, poor sleep quality, and obstructive sleep apnea: A commentary on potential interconnected features. J. Cosmet. Dermatol. 2022, 21, 4234–4236. [Google Scholar] [CrossRef]
- Chae, K.; Cho, M.; Kim, S.; Woo, Y.R. Increased risk of sleep disturbances in patients with rosacea: A nationwide population-based cohort study. J. Dermatol. 2024, 51, 70–75. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, H.; Gong, Y.; Ouyang, Y.; Deng, F.; Tang, Y.; Li, J. Relationship between rosacea and sleep. J. Dermatol. 2020, 47, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Zager, A.; Andersen, M.L.; Ruiz, F.S.; Antunes, I.B.; Tufik, S. Effects of acute and chronic sleep loss on immune modulation of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R504–R509. [Google Scholar] [CrossRef]
- Rico-Rosillo, M.G.; Vega-Robledo, G.B. Sueño y sistema immune [Sleep and immune system]. Rev. Alerg. Mex. 2018, 65, 160–170. [Google Scholar] [CrossRef]
- Jemec, G.B. Clinical practice. Hidradenitis suppurativa. N. Engl. J. Med. 2012, 366, 158–164. [Google Scholar] [CrossRef]
- Yeroushalmi, S.; Ildardashty, A.; Elhage, K.G.; Chung, M.; Bartholomew, E.; Hakimi, M.; Tahir, P.; Naik, H.B.; Bhutani, T.; Liao, W. Hidradenitis suppurativa and sleep: A systematic review. Arch. Dermatol. Res. 2023, 315, 1409–1415. [Google Scholar] [CrossRef]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Influence of Itch and Pain on Sleep Quality in Patients with Hidradenitis Suppurativa. Acta Derm. Venereol. 2018, 98, 757–761. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; Schoenmakers, A.; van Straalen, K.R.; Prens, E.P.; van der Zee, H.H. Assessing Pruritus in Hidradenitis Suppurativa: A Cross-Sectional Study. Am. J. Clin. Dermatol. 2017, 18, 687–695. [Google Scholar] [CrossRef]
- Matusiak, Ł.; Szczęch, J.; Kaaz, K.; Lelonek, E.; Szepietowski, J.C. Clinical Characteristics of Pruritus and Pain in Patients with Hidradenitis Suppurativa. Acta Derm. Venereol. 2018, 98, 191–194. [Google Scholar] [CrossRef]
- Kimball, A.B.; Sundaram, M.; Gauthier, G.; Guérin, A.; Pivneva, I.; Singh, R.; Ganguli, A. The Comorbidity Burden of Hidradenitis Suppurativa in the United States: A Claims Data Analysis. Dermatol. Ther. 2018, 8, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Lin, M.H.; Lee, J.H.; Lee, P.L.; Dai, Y.S.; Chu, K.H.; Sun, C.; Lin, Y.T.; Wang, L.C.; Yu, H.H.; et al. Melatonin Supplementation for Children With Atopic Dermatitis and Sleep Disturbance: A Randomized Clinical Trial. JAMA Pediatr. 2016, 170, 35–42. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Díaz-Castro, J.; Kajarabille, N.; García, C.; Guisado, I.M.; De Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011, 51, 373–380. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin improves skin barrier damage caused by sleep restriction through gut microbiota. J. Pineal Res. 2023, 75, e12874. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, J.; Hu, J.; Zou, Z.; Zhao, Y.; Lai, L.; Xu, P.; Song, Y.; Cheng, H. L-Theanine Alleviates IMQ-Induced Psoriasis Like Skin Inflammation by Downregulating the Production of IL-23 and Chemokines. Front. Pharmacol. 2021, 12, 719842. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D. A randomized, double-blind, placebo-controlled, comparator trial evaluating Magtein® magnesium supplement on quality of life as related to levels of stress, anxiety, fear and other indicators. EC Nutr. 2022, 17, 7–14. [Google Scholar]
- Hausenblas, H.A.; Lynch, T.; Hooper, S.; Shrestha, A.; Rosendale, D.; Gu, J. Magnesium-L-threonate improves sleep quality and daytime functioning in adults with self-reported sleep problems: A randomized controlled trial. Sleep Med. X 2024, 8, 100121. [Google Scholar] [CrossRef]
- Mashayekh-Amiri, S.; Delavar, M.A.; Bakouei, F.; Faramarzi, M.; Esmaeilzadeh, S. The impact of myo-inositol supplementation on sleep quality in pregnant women: A randomized, double-blind, placebo-controlled study. J. Matern. Fetal Neonatal Med. 2022, 35, 3415–3423. [Google Scholar] [CrossRef]
- Pezza, M.; Carlomagno, V.; Sammarco, E.; Trischitta, A.; Ceddia, C.; Vitiello, A.; Baj, G.; Citi, V.; Colletti, A. Association of Myo-Inositol and Microlipodispersed Magnesium in Androgen-Dependent Dermatological Diseases: A Retrospective Study. Pharmaceuticals 2025, 18, 251. [Google Scholar] [CrossRef]
- Ramanan, E.A.; Ravi, S.; Anbu, K.R.R.; Michael, M. Efficacy and Safety of Tracnil™ Administration in Patients with Dermatological Manifestations of PCOS: An Open-Label Single-Arm Study. Dermatol. Res. Pract. 2020, 2020, 7019126. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.; Lee, S.H.; Jung, E.M.; Lee, K.H. Modulatory effects of cinnamomi cortex and its components epicatechin and linalool on skin circadian rhythms. Sci. Rep. 2025, 15, 4480. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Ganguli-Indra, G.; Athar, M.; Indra, A.K.; Reiter, R.J.; Kleszczyński, K. Melatonin and the Skin: Current Progress and Perspectives for Human Health. J. Investig. Dermatol. 2025, 145, 1345–1360.e2. [Google Scholar] [CrossRef]
- Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int. J. Mol. Sci. 2020, 21, 326. [Google Scholar] [CrossRef]
- Chan, V.; Lo, K. Efficacy of dietary supplements on improving sleep quality: A systematic review and meta-analysis. Postgrad. Med. J. 2022, 98, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef]
- Zisapel, N.; Tarrasch, R.; Laudon, M. The relationship between melatonin and cortisol rhythms: Clinical implications of melatonin therapy. Drug Dev. Res. 2005, 65, 119–125. [Google Scholar] [CrossRef]
- Graubard, R.; Perez-Sanchez, A.; Katta, R. Stress and Skin: An Overview of Mind Body Therapies as a Treatment Strategy in Dermatology. Dermatol. Pract. Concept. 2021, 11, e2021091. [Google Scholar] [CrossRef]
- Bae, B.G.; Oh, S.H.; Park, C.O.; Noh, S.; Noh, J.Y.; Kim, K.R.; Lee, K.H. Progressive muscle relaxation therapy for atopic dermatitis: Objective assessment of efficacy. Acta Derm. Venereol. 2012, 92, 57–61. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep Med. 2015, 11, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Léger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, N.; Yu, P.K.; Siegel, N.S. Sleep physiology, pathophysiology, and sleep hygiene. Prog. Cardiovasc. Dis. 2023, 77, 59–69. [Google Scholar] [CrossRef]
- Potter, G.D.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef]
- Clark, I.; Landolt, H.P. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M.M.; Sharma, R.; Sahota, P. Alcohol disrupts sleep homeostasis. Alcohol 2015, 49, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Grummon, A.H.; Sokol, R.L.; Lytle, L.A. Is late bedtime an overlooked sleep behaviour? Investigating associations between sleep timing, sleep duration and eating behaviours in adolescence and adulthood. Public Health Nutr. 2021, 24, 1671–1677. [Google Scholar] [CrossRef]
- Kinsey, A.W.; Ormsbee, M.J. The health impact of nighttime eating: Old and new perspectives. Nutrients 2015, 7, 2648–2662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, F.; Zheng, W.; Zhang, L.; Ng, C.H.; Ungvari, G.S.; Xiang, Y.T. Mindfulness-Based Interventions for Insomnia: A Meta-Analysis of Randomized Controlled Trials. Behav. Sleep Med. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Togo, F.; Aizawa, S.; Arai, J.; Yoshikawa, S.; Ishiwata, T.; Shephard, R.J.; Aoyagi, Y. Influence on human sleep patterns of lowering and delaying the minimum core body temperature by slow changes in the thermal environment. Sleep 2007, 30, 797–802. [Google Scholar] [CrossRef]
- Menczel Schrire, Z.; Phillips, C.L.; Chapman, J.L.; Duffy, S.L.; Wong, G.; D’Rozario, A.L.; Comas, M.; Raisin, I.; Saini, B.; Gordon, C.J.; et al. Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 2022, 72, e12782. [Google Scholar] [CrossRef]
- Cruz-Sanabria, F.; Bruno, S.; Crippa, A.; Frumento, P.; Scarselli, M.; Skene, D.J.; Faraguna, U. Optimizing the Time and Dose of Melatonin as a Sleep-Promoting Drug: A Systematic Review of Randomized Controlled Trials and Dose-Response Meta-Analysis. J. Pineal Res. 2024, 76, e12985. [Google Scholar] [CrossRef]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.P.; Ozeki, M.; Juneja, L.R. In Search of a Safe Natural Sleep Aid. J. Am. Coll. Nutr. 2015, 34, 436–447. [Google Scholar] [CrossRef] [PubMed]
| Skin Condition | Key Findings | References |
|---|---|---|
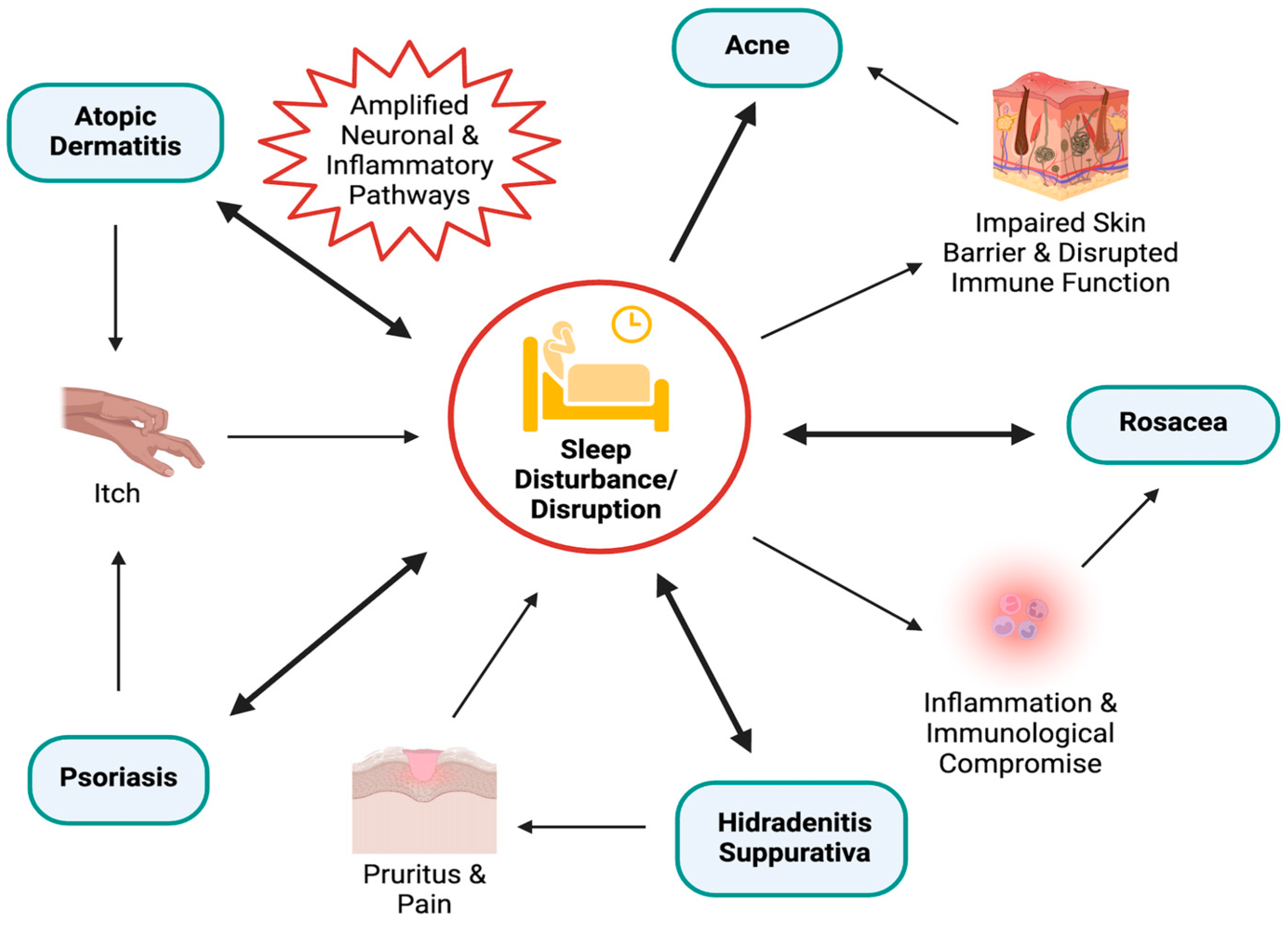
| Atopic Dermatitis (AD) | Multidirectional relationship between AD, psychological stress, and sleep disruption, with sleep disorders observed in up to 60% of patients with AD. | [30,31] |
| Intense pruritus in patients with AD disrupts sleep, this sleep disruption likely amplifies neuronal and inflammatory pathways. | [32,33] | |
| Impaired sleep quality in children with AD independent of symptom severity. | [34] | |
| Sleep alterations were identified in approximately 60% of children with AD. | [35] | |
| Children with AD have significantly longer sleep latency, increased sleep disruptions, and decreased sleep efficiency. | [36] | |
| Nocturnal pruritus may be mediated by circadian rhythm regulation of cortisol. | [37] | |
| Psoriasis | Circadian dysregulation contributes to psoriasis pathophysiology | [47,48,49] |
| An increased risk of psoriasis has been observed among night-shift workers, suggesting chronic circadian misalignment may be implicated in pathogenesis. | [50,51,52] | |
| Psoriatic flares, particularly pruritus, follow a diurnal pattern, often leading to sleep disturbances in patients with psoriasis. | [53,54,55] | |
| Pruritus is a key predictor of sleep disturbance, individuals with psoriasis slept one hour less than controls. | [56] | |
| Comorbid sleep disorders, such as obstructive sleep apnea (OSA) have been associated with nearly double the risk of psoriasis. | [57] | |
| Acne | There is a correlation between the prevalence of acne and poor sleep quality. | [64,65] |
| Psychosocial stress is associated with morning fatigue and occurrence of acne | [66] | |
| Impaired skin barriers due to poor sleep can weaken defenses against external stimuli, and induce a state of disrupted immune function, increasing susceptibility to conditions like acne. | [67] | |
| Poor sleep quality in night-shift workers with circadian disruption show a higher prevalence and severity of skin diseases, including acne. | [68] | |
| Sebaceous gland activity follows a circadian rhythm, peaking midday and declining overnight, suggesting rhythmic endocrine regulation may contribute to acne pathogenesis. | [68,69,70] | |
| Rosacea | Individuals with rosacea were found to have a significantly higher prevalence of sleep disorders compared to controls. | [72] |
| Sleep deprivation aggravates the rosacea-like phenotype in mice, evidenced by increased pro-inflammatory substrate expression. | [73] | |
| Sleep disturbances disrupt immune regulation, leading to increased release of inflammatory cytokines (TNF-α, IL-1, and IL-6) potentially playing a role in the development and worsening of rosacea. | [71,74,75] | |
| The classic symptom of a burning sensation in patients with rosacea may be exacerbated by poor sleep, as sleep plays a key role in temperature regulation and catecholamine release. | [74,75] | |
| Poor sleep may result in temperature dysregulation and vasodilation of the face, further aggravating rosacea. | [71] | |
| Hidradenitis Suppurativa (HS) | Patients with HS experience worse sleep quality as a consequence of disease-associated pruritus and pain. | [77,78,79,80] |
| Sleep–wake disorders including insomnia and hypersomnia were found to be 1.5 times more prevalent among patients with HS. | [81] | |
| The comorbidity between OSA and HS highlights the strong connection between sleep and the development of HS. | [77] |
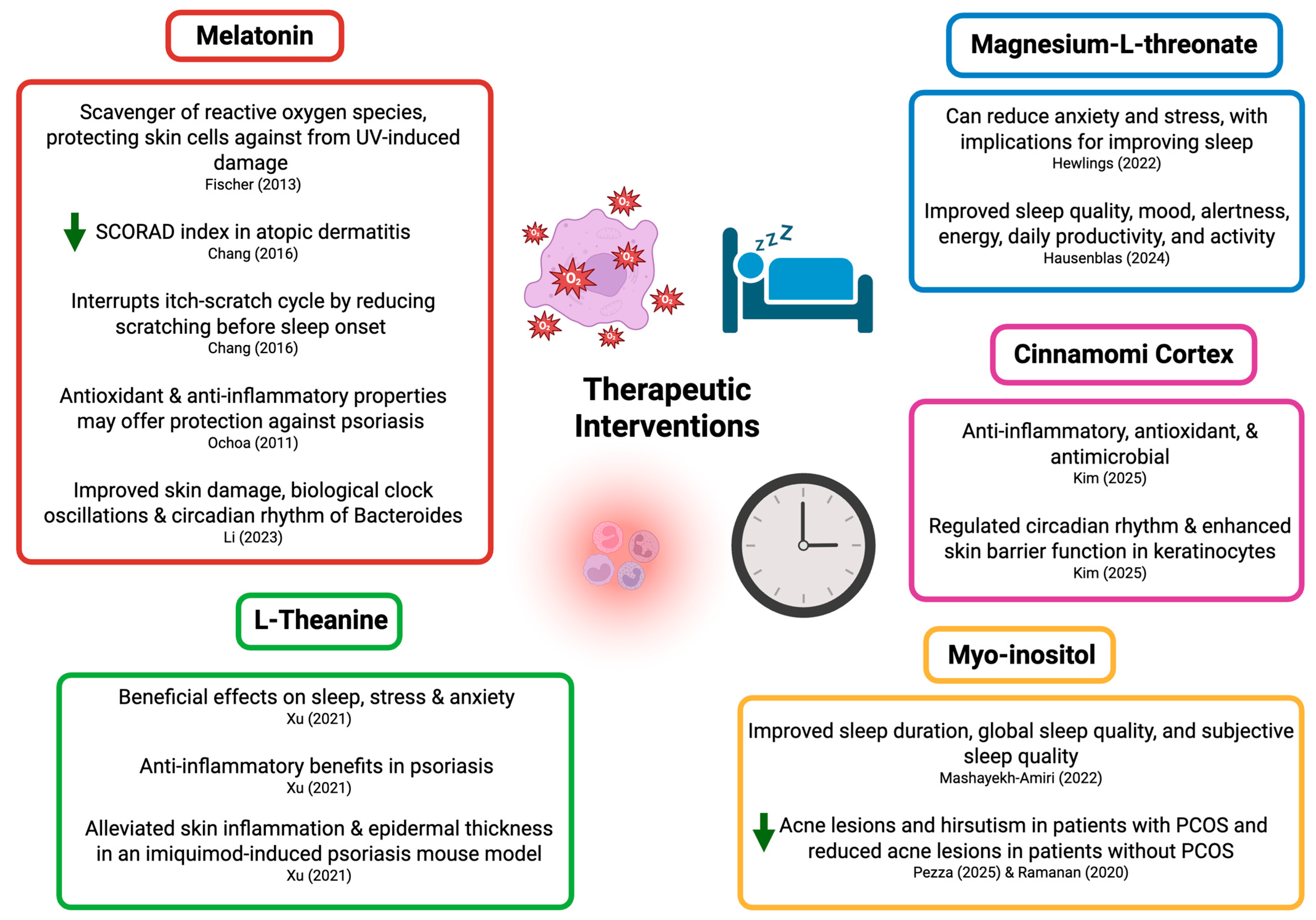
| Intervention | Recommendations | Clinical Context |
|---|---|---|
| Melatonin | 0.5–5 milligrams (mg) melatonin to improve sleep [111] 4 mg of melatonin taken 3 hours before sleep to improve total sleep time and reduce sleep onset latency in adults [112] 3 mg of melatonin to shorten sleep latency and improve SCORAD index in pediatric patients with AD [83] | General adult population with subjective sleep disturbances Adults with insomnia Pediatric patients with AD |
| L-Theanine (L-THE) | 200 mg to reduce sleep latency and disturbance [113] and improve sleep quality [114] | Healthy adults, demonstrated efficacy in preclinical models of psoriasis |
| Magnesium-L-threonate (MgT) | 1 g/day of MgT to improve sleep quality, particularly deep/REM sleep stages [88] | Adults with subjective sleep disturbances and mood symptoms |
| Myo-inositol | 2000 mg of myo-inositol to improve global sleep quality, subjective sleep quality, and sleep duration [89]. | Pregnant women, potential benefit in PCOS-associated acne and sleep disruption |
| Cinnamomi Cortex | Further research is necessary to understand the potential sleep-related benefits of cinnamomi cortex and appropriate dosage. | Theoretical relevance to circadian regulation and skin barrier function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadur, A.; Joerg, L.; Van Doren, A.S.; Lee, E.T.; Shah, D.; Asees, A.K.; Choudhary, S. The Sleep–Skin Axis: Clinical Insights and Therapeutic Approaches for Inflammatory Dermatologic Conditions. Dermato 2025, 5, 13. https://doi.org/10.3390/dermato5030013
Sadur A, Joerg L, Van Doren AS, Lee ET, Shah D, Asees AK, Choudhary S. The Sleep–Skin Axis: Clinical Insights and Therapeutic Approaches for Inflammatory Dermatologic Conditions. Dermato. 2025; 5(3):13. https://doi.org/10.3390/dermato5030013
Chicago/Turabian StyleSadur, Alana, Lucie Joerg, Amelia Stapleton Van Doren, Ellen T. Lee, Dia Shah, Aniket K. Asees, and Sonal Choudhary. 2025. "The Sleep–Skin Axis: Clinical Insights and Therapeutic Approaches for Inflammatory Dermatologic Conditions" Dermato 5, no. 3: 13. https://doi.org/10.3390/dermato5030013
APA StyleSadur, A., Joerg, L., Van Doren, A. S., Lee, E. T., Shah, D., Asees, A. K., & Choudhary, S. (2025). The Sleep–Skin Axis: Clinical Insights and Therapeutic Approaches for Inflammatory Dermatologic Conditions. Dermato, 5(3), 13. https://doi.org/10.3390/dermato5030013






