The Emerging Role of Artificial Intelligence in Dermatology: A Systematic Review of Its Clinical Applications
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
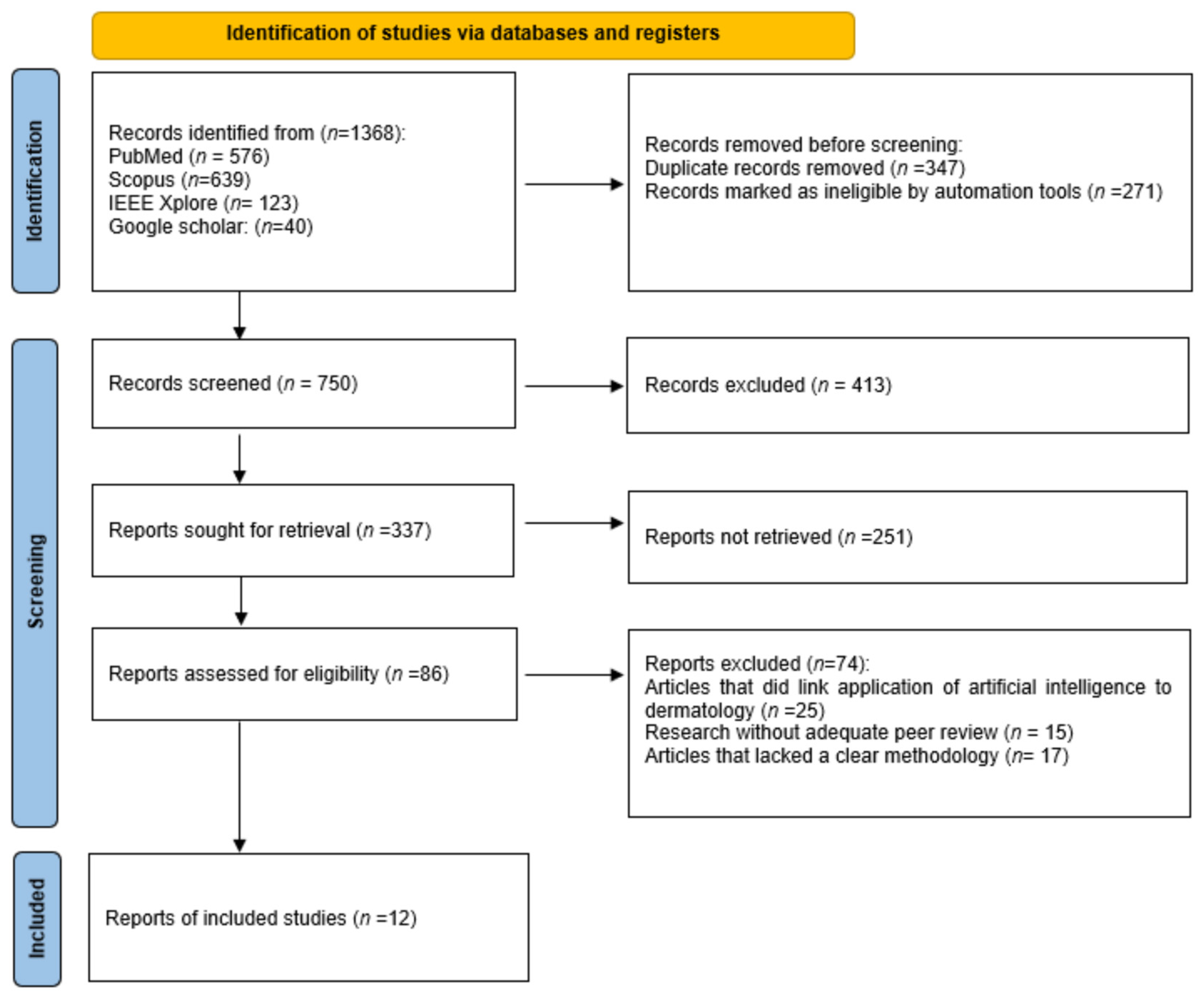
2.4. Study Selection Process
2.5. Data Collection Process and Data Items
2.6. Certainty of Evidence and Reporting Bias
2.7. Risk of Bias Assessment
3. Results
3.1. Included Studies
3.2. Analysis and Discussion
4. Discussion
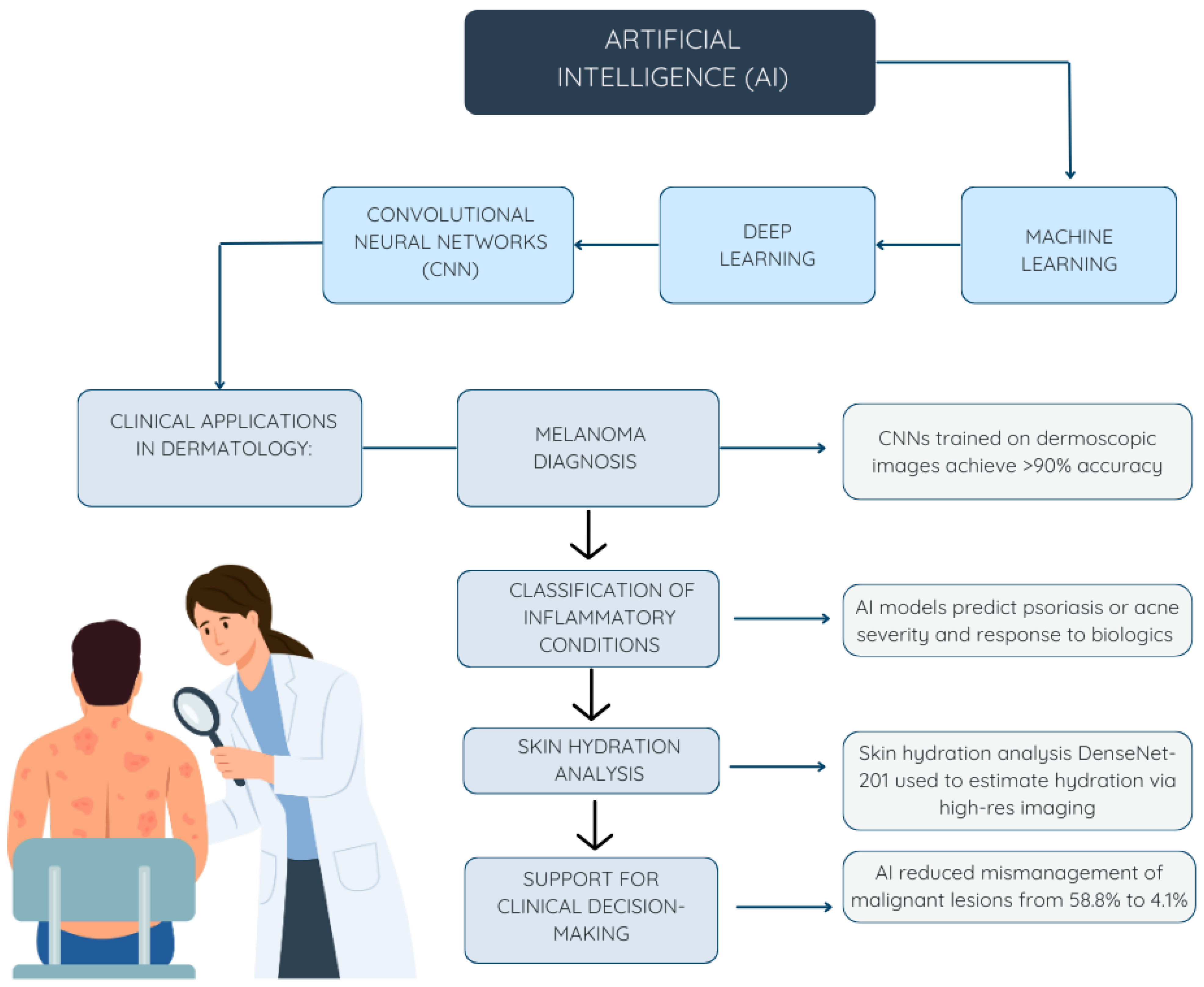
4.1. AI in Dermatology
4.1.1. Identification of Malignancies
4.1.2. Inflammatory Skin Diseases
4.1.3. Uses in Cosmetic Dermatology
4.2. Emerging Technologies
4.3. Application of AI in Real Cases
4.4. Possible Bioethical Problems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CNN | Convolutional Neural Network |
References
- Fateeva, A.; Eddy, K.; Chen, S. Current State of Melanoma Therapy and Next Steps: Battling Therapeutic Resistance. Cancers 2024, 16, 1571. [Google Scholar] [CrossRef] [PubMed]
- Liopyris, K.; Gregoriou, S.; Dias, J.; Stratigos, A.J. Artificial Intelligence in Dermatology: Challenges and Perspectives. Dermatol. Ther. 2022, 12, 2637–2651. [Google Scholar] [CrossRef]
- Aksoy, S.; Demircioglu, P.; Bogrekci, I. Advanced Artificial Intelligence Techniques for Comprehensive Dermatological Image Analysis and Diagnosis. Dermato 2024, 4, 173–186. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; de Sousa, R.M.; Paciello, V.Z.d.A.; Vitor, W.G.; Okita, A.L.; Prôa, R.; Severino, G.L.D.S.; Schinaid, A.A.; Espírito Santo, R.; Machado, B.S. Implementation of Artificial Intelligence Algorithms for Melanoma Screening in a Primary Care Setting. PLoS ONE 2021, 16, e0257006. [Google Scholar] [CrossRef]
- Cerminara, S.E.; Cheng, P.; Kostner, L.; Huber, S.; Kunz, M.; Maul, J.-T.; Böhm, J.S.; Dettwiler, C.F.; Geser, A.; Jakopović, C.; et al. Diagnostic Performance of Augmented Intelligence with 2D and 3D Total Body Photography and Convolutional Neural Networks in a High-Risk Population for Melanoma under Real-World Conditions: A New Era of Skin Cancer Screening? Eur. J. Cancer 2023, 190, 112954. [Google Scholar] [CrossRef] [PubMed]
- Madarkar, M.S.; Koti, V.R. FotoFinder Dermoscopy Analysis and Histopathological Correlation in Primary Localized Cutaneous Amyloidosis. Dermatol. Pract. Concept. 2021, 11, e2021057. [Google Scholar] [CrossRef]
- Li, Z.; Koban, K.C.; Schenck, T.L.; Giunta, R.E.; Li, Q.; Sun, Y. Artificial Intelligence in Dermatology Image Analysis: Current Developments and Future Trends. J. Clin. Med. 2022, 11, 6826. [Google Scholar] [CrossRef] [PubMed]
- Sies, K.; Winkler, J.K.; Fink, C.; Bardehle, F.; Toberer, F.; Buhl, T.; Enk, A.; Blum, A.; Stolz, W.; Rosenberger, A.; et al. Does Sex Matter? Analysis of Sex-Related Differences in the Diagnostic Performance of a Market-Approved Convolutional Neural Network for Skin Cancer Detection. Eur. J. Cancer 2022, 164, 88–94. [Google Scholar] [CrossRef]
- Winkler, J.K.; Kommoss, K.S.; Vollmer, A.S.; Blum, A.; Stolz, W.; Kränke, T.; Hofmann-Wellenhof, R.; Enk, A.; Toberer, F.; Haenssle, H.A. Computerizing the First Step of the Two-Step Algorithm in Dermoscopy: A Convolutional Neural Network for Differentiating Melanocytic from Non-Melanocytic Skin Lesions. Eur. J. Cancer 2024, 210, 114297. [Google Scholar] [CrossRef]
- Veeramani, N.; Jayaraman, P. A Promising AI Based Super Resolution Image Reconstruction Technique for Early Diagnosis of Skin Cancer. Sci. Rep. 2025, 15, 5084. [Google Scholar] [CrossRef]
- Pham, T.-C.; Luong, C.-M.; Hoang, V.-D.; Doucet, A. AI Outperformed Every Dermatologist in Dermoscopic Melanoma Diagnosis, Using an Optimized Deep-CNN Architecture with Custom Mini-Batch Logic and Loss Function. Sci. Rep. 2021, 11, 17485. [Google Scholar] [CrossRef] [PubMed]
- Useini, V.; Tanadini-Lang, S.; Lohmeyer, Q.; Meboldt, M.; Andratschke, N.; Braun, R.P.; Barranco García, J. Automatized Self-Supervised Learning for Skin Lesion Screening. Sci. Rep. 2024, 14, 12697. [Google Scholar] [CrossRef]
- Winkler, J.K.; Blum, A.; Kommoss, K.; Enk, A.; Toberer, F.; Rosenberger, A.; Haenssle, H.A. Assessment of Diagnostic Performance of Dermatologists Cooperating with a Convolutional Neural Network in a Prospective Clinical Study: Human with Machine. JAMA Dermatol. 2023, 159, 621–627. [Google Scholar] [CrossRef]
- Crawford, M.E.; Kamali, K.; Dorey, R.A.; MacIntyre, O.C.; Cleminson, K.; MacGillivary, M.L.; Green, P.J.; Langley, R.G.; Purdy, K.S.; DeCoste, R.C.; et al. Using Artificial Intelligence as a Melanoma Screening Tool in Self-Referred Patients. J. Cutan. Med. Surg. 2024, 28, 37–43. [Google Scholar] [CrossRef]
- Kommoss, K.S.; Winkler, J.K.; Mueller-Christmann, C.; Bardehle, F.; Toberer, F.; Stolz, W.; Kraenke, T.; Hofmann-Wellenhof, R.; Blum, A.; Enk, A.; et al. Observational Study Investigating the Level of Support from a Convolutional Neural Network in Face and Scalp Lesions Deemed Diagnostically ‘Unclear’ by Dermatologists. Eur. J. Cancer 2023, 185, 53–60. [Google Scholar] [CrossRef]
- Anqi, S.; Xiukun, S.; Ai’e, X. Quantitative Evaluation of Sensitive Skin by ANTERA 3D® Combined with GPSkin Barrier®. Ski. Res. Technol. 2022, 28, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Omiye, J.A.; Gui, H.; Daneshjou, R.; Cai, Z.R.; Muralidharan, V. Principles, Applications, and Future of Artificial Intelligence in Dermatology. Front. Med. 2023, 10, 1278232. [Google Scholar] [CrossRef]
- Koka, S.S.-A.; Burkhart, C.G. Inteligencia artificial en dermatología: Usos actuales, deficiencias y posibles oportunidades para una mayor implementación en el diagnóstico y la atención. Open Dermatol. J. 2023, 17, e187437222304140. [Google Scholar] [CrossRef]
- Kania, B.; Montecinos, K.; Goldberg, D.J. Artificial Intelligence in Cosmetic Dermatology. J. Cosmet. Dermatol. 2024, 23, 3305–3311. [Google Scholar] [CrossRef]
- Salim, F.; Saeed, F.; Basurra, S.; Qasem, S.N.; Al-Hadhrami, T. DenseNet-201 and Xception Pre-Trained Deep Learning Models for Fruit Recognition. Electronics 2023, 12, 3132. [Google Scholar] [CrossRef]
- Paluri, K.V.; Gupta, N.; Mishra, A.K.; Gupta, A.; Nain, G. An Explainable AI-Based Automated Acne Diagnosis Using Transfer Learning and DenseNet121. In Proceedings of the 2024 10th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 14–15 March 2024; pp. 1086–1091. [Google Scholar] [CrossRef]
- Vatiwutipong, P.; Vachmanus, S.; Noraset, T.; Tuarob, S. Artificial Intelligence in Cosmetic Dermatology: A Systematic Literature Review. IEEE Access 2023, 11, 71407–71425. [Google Scholar] [CrossRef]
- Morihisa, Y.; Rikimaru-Nishi, Y.; Ohmaru, Y.; Ino, K.; Rikimaru, H.; Kiyokawa, K. Scientific Validation of Clinical Visual Scales and Antera 3DTM Consistency with Derived Measurements in the Assessment of Infantile Haemangioma after Laser Therapy. J. Plast. Reconstr. Aesthetic Surg. 2024, 91, 47–55. [Google Scholar] [CrossRef]
- Horsham, C.; O’Hara, M.; Sanjida, S.; Ma, S.; Jayasinghe, D.; Green, A.C.; Schaider, H.; Aitken, J.F.; Sturm, R.A.; Prow, T.; et al. The Experience of 3D Total-Body Photography to Monitor Nevi: Results From an Australian General Population-Based Cohort Study. JMIR Dermatol. 2022, 5, e37034. [Google Scholar] [CrossRef] [PubMed]
- Humans.txt MDR Certificate for FotoFinder. Available online: https://www.fotofinder.de/es/compania/news/mdr-certificate-for-fotofinder (accessed on 30 April 2025).
- Ning, Y.; Teixayavong, S.; Shang, Y.; Savulescu, J.; Nagaraj, V.; Miao, D.; Mertens, M.; Ting, D.S.W.; Ong, J.C.L.; Liu, M.; et al. Generative Artificial Intelligence and Ethical Considerations in Health Care: A Scoping Review and Ethics Checklist. Lancet Digit. Health 2024, 6, e848–e856. [Google Scholar] [CrossRef]
- Patil, R.S.; Kulkarni, S.B.; Gaikwad, V.L. Artificial Intelligence in Pharmaceutical Regulatory Affairs. Drug Discov. Today 2023, 28, 103700. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Hanna, M.; Pantanowitz, J.; Lennerz, J.; Henricks, W.H.; Shen, P.; Quinn, B.; Bennet, S.; Rashidi, H.H. Regulatory Aspects of Artificial Intelligence and Machine Learning. Mod. Pathol. 2024, 37, 100609. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
| Database | Keywords | Search Strategy | Filters Applied | Number of Possible Items Selected |
|---|---|---|---|---|
| PubMed | Artificial intelligence, machine learning, Deep learning, neural networks, dermatology, skin desease, skin cancer, melanoma | (“Artificial Intelligence” [MeSH] OR “Machine Learning” [MeSH] OR “Deep Learning” OR “Neural Networks, Computer” [MeSH]) AND (“Dermatology” [MeSH] OR “Skin Diseases” [MeSH] OR “Skin Neoplasms” [MeSH] OR “Skin Cancer”) | Full texts available. Clinical trials, observational studies, systematic reviews. Published between 2020–2025. | 576 |
| Scopus | Artificial intelligence, machine learning, Deep learning, neural networks, dermatology, skin desease, skin cancer, melanoma | “Artificial intelligence” OR “machine learning” OR “deep learning” OR “neural networks”) AND (“dermatology” OR “skin diseases” OR “skin cancer” OR “cutaneous”) | Original articles and reviews, published in Spanish or English between 2020–2025. | 639 |
| IEEE Xplore | Artificial intelligence, machine learning, Deep learning, neural networks, dermatology, skin desease, skin cancer, melanoma | (“All Metadata”:”artificial intelligence” OR “machine learning” OR “deep learning” OR “neural networks”) AND (“All Metadata”:”dermatology” OR “skin diseases” OR “skin cancer”) | Journals published in English between 2020–2025. | 132 |
| Google Scholar | Artificial intelligence, machine learning, Deep learning, neural networks, dermatology, skin desease, skin cancer, melanoma | “Artificial Intelligence” OR “Machine Learning” OR “Deep Learning” OR “Neural Networks” AND “Dermatology” OR “Skin Diseases” OR “Skin Cancer” | Peer-reviewed or indexed scientific articles published in Spanish or English between 2020–2025. | 40 |
| Paper | Report Type | Pathology/Application | Sofware Used | Main Findings |
|---|---|---|---|---|
| Sies et al. (2022) [8] | Retrospective, non-interventional observational study | Patients at high risk for melanoma | Moleanalyzer Pro (FotoFinder Systems GmbH, Bad Birnbach, Bavaria, Germany), a market-approved convolutional neural network (CNN) developed by FotoFinder Systems | Despite gender imbalance in the training data (60% males vs. 40% females), the evaluated CNN showed no significant differences in sensitivity, specificity, or AUC when stratified by sex. It achieved high accuracy for skin cancer classification regardless of gender. |
| Cerminara et al. (2023) [5]. | Prospective, observational, multi-center study | Skin cancer detection and dermoscopic screening | Vectra WB360 (v4.7.1) for 3D total body photography-FotoFinder ATBM Master (v3.3.1.0) for 2D total body photography-Medicam 1000 and VISIOMED D200evo for manual lesion photography | The 3D CNN showed higher sensitivity and consistency than the 2D approach for melanoma detection, with performance comparable to dermatologists. However, both systems overestimated nevus counts compared to manual counting. |
| Winkler et al. (2024) [9] | Cross-sectional study | Classification of melanocytic vs. non-melanocytic lesions | SPSS version 29 for statistical analysis and a modified neural network architecture | The CNN achieved a 91% accuracy (AUC 0.981) in distinguishing melanocytic from non-melanocytic lesions, outperforming the majority of dermatologists. External validation was conducted on over 1100 lesions. |
| Giavina-Bianchi et al. (2021) [4] | Retrospective, single-center observational study | Melanoma screening and malignant skin lesion triage in primary care | EfficientNetB6 ensemble model with synthetic data generated via StyleGAN2, trained using ISIC2019 and PH2 datasets | The AI-assisted computer-aided diagnosis (CAD) tool reached a precision of 89.3% using dermoscopic images and 84.7% with clinical images, providing reliable management recommendations for the detection of skin cancer in primary care settings. |
| Veeramani and Jayaraman (2025) [10] | Experimental study with technical validation | Image reconstruction of intermediate skin lesions (melanoma) | MELIIGAN, a CNN-based reconstruction system using residual attention | MELIIGAN improved the reconstruction quality of skin lesion images, achieving PSNR > 40 and SSIM > 0.94. It enhanced fine detail visibility and reduced classification errors in intermediate melanoma lesions. |
| Pham et al. (2021) [11] | Experimental computational study | Melanoma diagnosis using dermoscopic images | DenseNet169 CNN was implemented in Python 3.8 using TensorFlow 2.10.0 (Google LLC, Mountain View, CA, USA) and PyTorch 1.12.1 (Meta Platforms, Inc., Menlo Park, CA, USA) | The AI system outperformed 157 dermatologists (MCass-D), reaching a sensitivity of 90% and specificity of 93.8%, with performance superior to the current state of the art. |
| Useini et al. (2023) [12] | Prospective, single-center observational study | AI-based full-body detection tool with self-supervised learning | Deep learning framework PyTorch, combined with the Lightly library for self-supervised learning models | The total-body screening tool achieved 82% precision in detecting the 10 most suspicious lesions and reached an average sensitivity of 95% for expert-confirmed cases, improving diagnostic agreement and supporting broader generalization in clinical practice. |
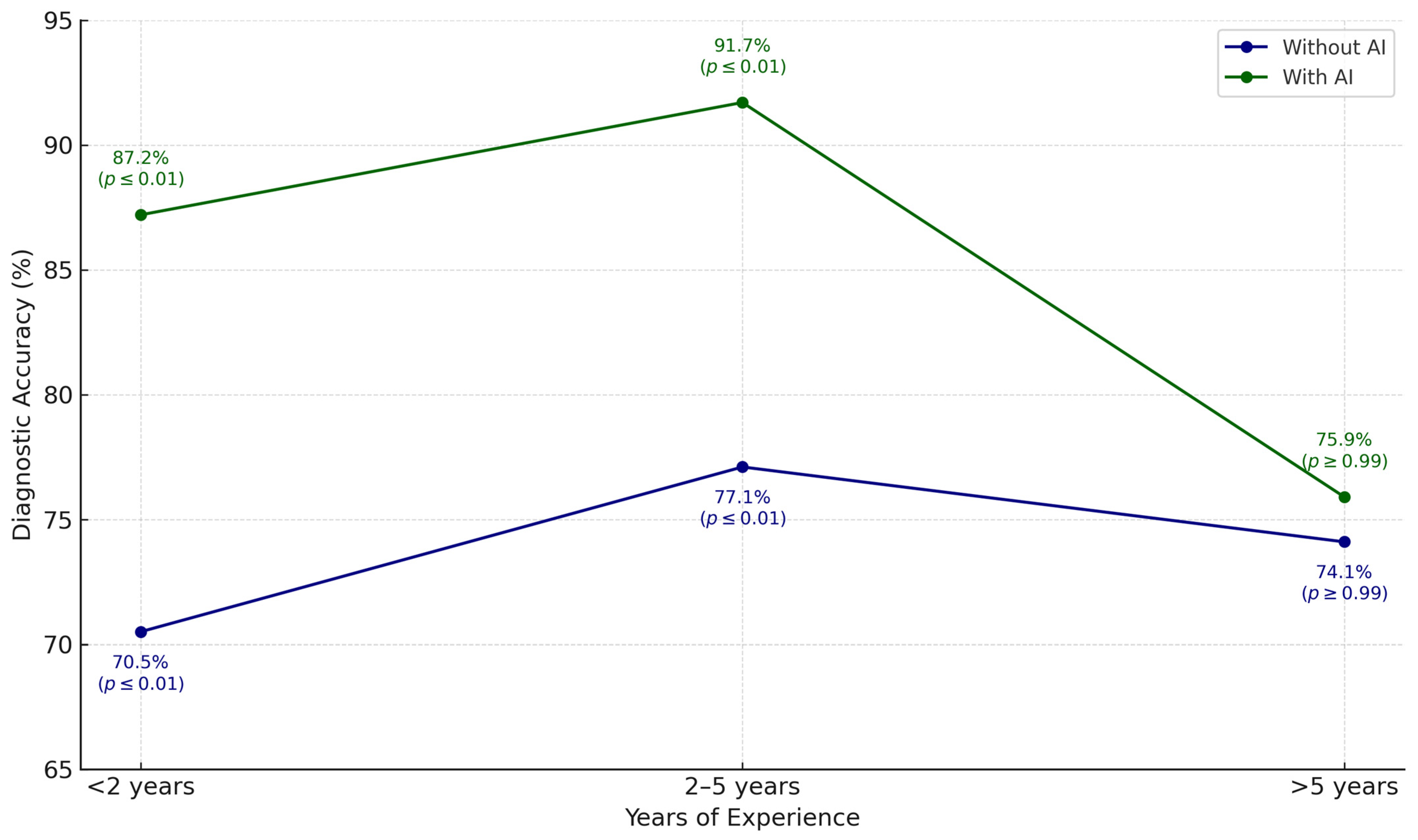
| Winkler et al. (2023) [13] | Prospective, multi-center clinical study | Diagnosis of melanocytic lesions | Commercial CNN (Moleanalyzer Pro, FotoFinder Systems) | The cooperation between dermatologists and CNN significantly improved their sensitivity, specificity, and diagnostic accuracy, while also reducing unnecessary excisions. The benefit was most evident among professionals with less clinical experience. |
| Hull et al. (2023) [14]. | Prospective, single-center observational study | Screening for malignant skin lesions | Commercial CNN (Moleanalyzer Pro, FotoFinder Systems) | The AI system demonstrated sensitivity and specificity comparable to expert dermatologists in detecting malignant skin lesions, although it missed 6 in situ melanomas. It is proposed as a useful tool for populations with limited access to dermatology specialists. |
| Kommoss et al. (2023) [15]. | Multi-center, retrospective observational study | Diagnosis of facial and scalp skin lesions with unclear clinical presentation | CNN (Moleanalyzer Pro, FotoFinder Systems) | The AI model reduced incorrect clinical management decisions in diagnostically unclear cases, lowering the error rate for malignant lesions from 58.8% to 4.1% and for benign lesions from 43.9% to 31.7%. The model demonstrated superior performance compared to dermatologists, even when they had full access to patient clinical data. |
| Anqi et al. (2022) [16]. | Observational clinical study | Objective evaluation of sensitive skin and sensitive skin syndrome | ANTERA 3D® (Miravex Ltd., Dublin, Ireland) combined with GPSkin Barrier® (GPOWER Inc., Seoul, Republic of Korea) | In patients with sensitive skin, ANTERA 3D® detected significant increases in hemoglobin texture and affected area, while GPSkin Barrier® revealed higher transepidermal water loss (TEWL) and lower skin hydration. The combined use of both tools provided objective and quantifiable measures to support the clinical characterization of sensitive skin. |
| System | FDA Approval | CE Approval (MDR) | Countries Where It Is Approved | Type of Approval |
|---|---|---|---|---|
| FotoFinder | No | Yes | European Union countries | Class IIa medical device under Regulation (EU) 2017/745 (MDR) [23]. |
| Canfield Vectra WBS360 | No | Unavailable | Unavailable | No public information is available on its specific regulatory approval. |
| Antera 3D | Unavailable | Unavailable | Unavailable | No public information is available on its regulatory approval status. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Vargas, E.; Mora-Jiménez, J.; Arguedas-Chacón, S.; Hernández-López, J.; Zavaleta-Monestel, E. The Emerging Role of Artificial Intelligence in Dermatology: A Systematic Review of Its Clinical Applications. Dermato 2025, 5, 9. https://doi.org/10.3390/dermato5020009
Martínez-Vargas E, Mora-Jiménez J, Arguedas-Chacón S, Hernández-López J, Zavaleta-Monestel E. The Emerging Role of Artificial Intelligence in Dermatology: A Systematic Review of Its Clinical Applications. Dermato. 2025; 5(2):9. https://doi.org/10.3390/dermato5020009
Chicago/Turabian StyleMartínez-Vargas, Ernesto, Jeaustin Mora-Jiménez, Sebastian Arguedas-Chacón, Josephine Hernández-López, and Esteban Zavaleta-Monestel. 2025. "The Emerging Role of Artificial Intelligence in Dermatology: A Systematic Review of Its Clinical Applications" Dermato 5, no. 2: 9. https://doi.org/10.3390/dermato5020009
APA StyleMartínez-Vargas, E., Mora-Jiménez, J., Arguedas-Chacón, S., Hernández-López, J., & Zavaleta-Monestel, E. (2025). The Emerging Role of Artificial Intelligence in Dermatology: A Systematic Review of Its Clinical Applications. Dermato, 5(2), 9. https://doi.org/10.3390/dermato5020009








