Abstract
Background/objectives: According to the Chapel Hill Consensus Conference nomenclature, single-organ cutaneous small vessel vasculitis (SOCV) is defined histopathologically by immune complex-mediated vasculitis of the dermal capillaries/venules without systemic involvement. There is a lack of investigations studying predictors of SOCV outcomes. This multicenter retrospective study aimed to assess whether baseline serum liver scores could predict SOCV recurrence. Methods: Data from 204 inpatients with histopathologically confirmed idiopathic SOCV treated between 2000 and 2022 were analyzed. All patients had baseline blood tests for platelets and liver parameters; those with systemic diseases were excluded. The study evaluated the AST to Platelet Ratio Index (APRI), Fibrosis-4 (FIB-4) index, and De Ritis ratio (DRR). Results: Recurrence was observed in 17.6% of patients. Univariable analysis identified hospital stay length, DRR, cutaneous extent, and cardiovascular comorbidities as predictors, but logistic regression confirmed only cutaneous extent and DRR as independent predictors of disease recurrence. Conclusions: A higher DRR (cut-off > 1.13) may indicate necrosis from reduced blood flow, making it a potential predictor of SOCV recurrence.
1. Introduction
Single-organ cutaneous small vessel vasculitis (SOCV), previously referred to as cutaneous leukocytoclastic vasculitis or hypersensitivity angiitis, is a distinct clinical entity recognized within the Chapel Hill Consensus Conference (CHCC) 2012 nomenclature for vasculitides [,]. This classification underscores the importance of differentiating vasculitic processes confined solely to the skin from those with systemic involvement. Hence, SOCV is characterized by inflammation predominantly affecting the small vessels in the skin, typically the postcapillary venules, without any evidence of systemic disease. The nomenclature revision emphasizes the necessity of a thorough evaluation to exclude extracutaneous involvement before establishing the diagnosis of a single-organ vasculitis [,]. Outcomes in patients with SOCV are generally favorable, but data on long-term outcomes and recurrence rates are somewhat limited and vary between studies. In many cases, the condition resolves with appropriate management—often within a few weeks—and the overall prognosis is excellent when systemic involvement is excluded. However, recurrence is not uncommon, with some studies on cutaneous small vessel vasculitis reporting recurrence rates of about 20% [,,]. The variability in recurrence rates highlights the need for individualized patient management and long-term follow-up. Continued research and more focused longitudinal studies on SOCV defined strictly by the CHCC 2012 criteria are necessary to further clarify these outcomes and to guide optimal management strategies [,,]. We aimed to assess whether baseline serum liver scores can help to predict recurrence of SOCV.
2. Materials and Methods
2.1. Patients and Study Setting
In this multicenter, retrospective study, we analyzed clinical and laboratory data from 204 inpatients treated for histopathologically confirmed idiopathic SOCV at our dermatology departments between 2000 and 2022. Patients with SOCV linked to systemic conditions, such as granulomatosis with polyangiitis or autoimmune disorders including systemic lupus erythematosus, were not included in the subsequent analyses. Data retrieved from the patients’ records encompassed comorbidities, the extent of cutaneous SOCV involvement (categorized as 0 for involvement limited to the lower legs and 1 for additional skin sites beyond the lower legs, such as the trunk and/or upper extremities), duration of hospitalization in days, and disease recurrence—defined as the occurrence of at least one SOCV relapse after a minimum two-week remission period. Treatment involved supportive measures like the use of compression stockings and a tapered regimen of intravenous corticosteroids administered over 2–4 weeks; in the event of SOCV recurrence, patients received immunosuppressive, steroid-sparing agents such as methotrexate or azathioprine. Patient characteristics are presented in Table 1. This investigation was performed in compliance with the Declaration of Helsinki and received approval from the Ethical Review Board (#4088-11).

Table 1.
Clinical details and comorbidities in inpatients with single-organ cutaneous small vessel vasculitis (SOCV, n = 204).
2.2. Laboratory Investigations and Scores
All patients underwent a baseline blood collection that included platelets and liver parameters. We determined the AST to Platelet Ratio Index (APRI): aspartate aminotransferase (AST) to platelet ratio; the Fibrosis-4 (FIB-4) index [Age (years) × AST (U/L)/[PLT(109/L) × alanine aminotransferase (ALT) 1/2 (U/L)]; and De Ritis ratio (DRR) [AST to ALT ratio] [].
2.3. Statistics
MedCalc software (Ostend, Belgium, version 23.0.2) was utilized for the analysis. Univariable analyses comprised the Chi2 test, Spearman’s rank correlation, and ROC curve evaluations. For multivariable analysis, a logistic regression model was employed, with statistical significance defined as p < 0.05.
3. Results
As shown in Table 1, we studied 204 inpatients [median (range) age: 68 (16–98); 104 males, 100 females] with SOCV. All patients had palpable purpura on the lower extremities, whereas 89 of 204 (43.6%) also had lesions on the upper extremities and other skin sites (e.g., upper legs, arms). The median length of hospitalization was 8 days (range: 2–37 days). Recurrence of SOCV was observed in 36 of 204 patients (17.6%).
On univariable analysis, significant predictors of SOCV recurrence were length of hospital stay (p = 0.023), DRR [AUC 0.75 (95% CI 0.68 to 0.81), associated criterion: > 1.13, p < 0.0001; Figure 1], cutaneous extent of SOCV (p = 0.0060), and cardiovascular comorbidities (p = 0.044). ROC analysis of APRI [AUC 0.53 (95% CI 0.45 to 0.60), associated criterion: > 0.2; p = 0.65] and FIB-4 [AUC 0.58 (95% CI 0.50 to 0.65), associated criterion: > 1.23; p = 0.15] revealed an insignificant association between these scores and SOCV recurrence. However, logistic regression analysis identified only cutaneous extent of SOCV (OR 2.65, 95% CI 1.18–5.93; p = 0.018) and DRR (OR 3.02, 95% CI 1.67–5.46; p = 0.0003) as independent predictors, with DRR showing a much stronger predictive performance (Youden index of 0.54, 95% CI 0.38–0.64). DRR significantly correlated with age (r = 0.22, p = 0.0015). However, age was not significantly associated with SOCV recurrence (AUC 0.55, associated criterion > 77; p = 0.39).
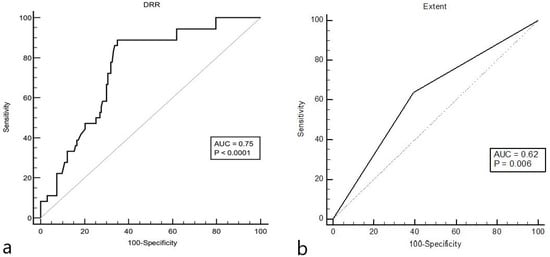
Figure 1.
The receiver operating curve for the relationship between the De Ritis ratio (DRR, associated criterion: > 1.13) and relapse of single-organ cutaneous small vessel vasculitis (SOCV, (a)). The Youden index of 0.54 (95% CI 0.38 to 0.64) indicated a good prognostic performance of the model. Compared to DRR, the cutaneous extent of SOCV (associated criterion: > 0) showed a poorer performance as indicated by a Youden index of only 0.25 (b).
4. Discussion
As demonstrated in this work, SOCV recurrence is observed in less than 20% of patients. Nevertheless, systemic immunosuppressants are often necessary in those who experience a relapse to prevent further recurrences [,,]. We showed that both the extent of cutaneous involvement and a higher DRR independently predict SOCV recurrence. Skin involvement beyond the lower extremities reflects a more widespread and severe disease, which may increase the risk of SOCV recurrence. In a different patient cohort, however, disease severity was not significantly associated with SOCV relapse []. It is difficult to explain why relapses in SOCV patients are associated with increased DRR. The latter has been shown to be linked to outcomes in a variety of other conditions, including malignancies and COVID-19 []. Moreover, a link between liver scores and ANCA-associated vasculitides, including granulomatosis with polyangiitis and microscopic polyangiitis, has previously been reported [,].
In general clinical practice, a DRR close to 1.0 is considered normal. Values above 1.0—often starting around 1.0 to 1.1—can prompt further evaluation, as they may indicate conditions such as advanced fibrosis, cirrhosis, or other forms of liver injury. For instance, in alcoholic liver disease, the ratio can be even higher (often >2), while in nonalcoholic liver diseases, modest elevations slightly above 1 may be observed. Hence, the cut-off 1.13 of the DRR for recurrence prognosis lies at the order of abnormal DRR. In older patients, an elevated DRR may indicate age-associated declines in hepatic mitochondrial function, often correlating with more advanced fibrosis or cirrhosis. Age-dependent liver enzyme variations mean that the same cut-off might have different prognostic implications compared to younger cohorts. Although we observed a positive correlation between DRR and age, the latter was not significantly associated with SOCV recurrence, excluding age as a potential confounder. When interpreted alongside other biomarkers and imaging findings, the DRR cut-off may contribute to risk stratification in patients with liver steatosis or nonalcoholic fatty liver disease, providing a quick, cost-effective initial assessment that needs confirmation with more specific diagnostics [,,].
In SOCV and other vasculitides, histologic markers, such as the intensity of neutrophilic infiltration, the degree of leukocytoclasis, the extent of fibrinoid necrosis, and the pattern of immune complex deposition, reliably correlate with disease activity and potential systemic involvement [,,]. These classical features inform clinicians about the severity of vascular injury and can guide both prognostication and therapeutic strategies. Comparatively, while emerging clinical or laboratory markers might offer additional insights, they generally serve as complementary tools rather than replacing histopathology []. For instance, circulating biomarkers, including lactate dehydrogenase, C-reactive protein, and serum C3, have been explored to predict proteinuria and disease recurrence, respectively [,]. Probably, an integrative approach that combines both histopathological features and ancillary clinical data appears to enhance the overall prognostic assessment in SOCV.
What are possible explanations for the association between DRR and SOCV recurrence? ALT is usually observed in the cytosol of hepatocytes, while AST can be found in both the cytoplasm and mitochondria. ALT activity in the liver is approximately 10-fold higher than in the heart and skeletal muscles, underscoring its role in indicating parenchymal liver disease or injury []. Meanwhile, AST is most active in the liver, cardiac, and skeletal muscle, but is also present in other tissues such as the kidneys, lungs, brain, pancreas, red blood cells, and white blood cells. Therefore, an increased DRR is likely driven primarily by AST from various organs. Additionally, AST is mainly located in the mitochondria, while ALT is predominantly cytosolic; reduced blood flow in SOCV may quickly damage mitochondria, resulting in increased AST release []. DRR is also elevated in skeletal muscle damage, which may occur in severe SOCV due to disturbed blood flow in the small skin vessels []. Overall, an increased DRR could indicate necrosis, leading to more frequent recurrences. However, our hypotheses remain speculative and require further prospective investigation. Hence, this study includes several limitations, such as retrospective design potentially linked to selection bias, incomplete data, and patient and treatment variability. Moreover, diagnostic procedures, including ultrasound and elastography, were not performed. Moreover, we have to discuss potential confounding by pre-existing liver diseases (e.g., non-alcoholic fatty liver disease, liver cirrhosis) or comorbidities that may have independently contributed to increased DRR. Hence, stratified analyses, including the above-mentioned confounders, should be performed in future studies.
5. Conclusions
We demonstrated for the first time that DRR is a potential independent predictor of disease recurrence in patients with SOCV.
Author Contributions
T.G. and N.A.R. contributed to the study conception and design. Material preparation, data collection, analysis, and interpretation were predominantly performed by, L.A., B.D., L.S. and T.G. The first draft of the manuscript was written by T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This investigation was approved by Institutional Review Board at the Ruhr-University Bochum (IRB Study #4088-11).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no competing interests.
References
- Loricera, J.; Blanco, R.; Ortiz-Sanjuán, F.; Hernández, J.L.; Pina, T.; González-Vela, M.C.; Calvo-Río, V.; Rueda-Gotor, J.; Alvarez, L.; González-López, M.A.; et al. Single-organ cutaneous small-vessel vasculitis according to the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides: A study of 60 patients from a series of 766 cutaneous vasculitis cases. Rheumatology 2015, 54, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Baigrie, D.; Goyal, A.; Crane, J.S. Leukocytoclastic Vasculitis. 2023 Aug 8. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Gambichler, T.; Ardabili, L.; Domin, B.; Susok, L.; Abu Rached, N. Predictors of disease severity, length of hospitalization, and recurrence in inpatients with single-organ cutaneous small vessel vasculitis. Eur. J. Dermatol. 2024, 34, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Abu Rached, N.; Reis, M.M.D.S.; Stockfleth, E.; Käpynen, R.; Gambichler, T. Analysis of Calculated Liver Scores for Long-Term Outcome in 423 Cutaneous Melanoma Patients. Cancers 2024, 16, 3217. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Becker, J.C.; Susok, L.; Käpynen, R.; Abu Rached, N. Model for End-Stage Liver Disease Correlates with Disease Relapse and Death of Patients with Merkel Cell Carcinoma. Cancers 2023, 15, 3195. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, D.Y.; Ahn, S.H.; Park, Y.B.; Han, K.H.; Park, J.Y. Subclinical but significant liver fibrosis in patients with ANCA-associated vasculitis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 117), 26–31. [Google Scholar] [PubMed]
- Park, H.J.; Park, J.Y.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Fibrosis-4 index at diagnosis is associated with all-cause mortality in patients with microscopic polyangiitis and granulomatosis with polyangiitis. BMC Gastroenterol. 2019, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.; Botta, F.; Fasoli, A.; Cerioni, F.; Risso, D. Liver enzyme alteration: A guide for clinicians. Clevel. Clin. J. Med. 2005, 72, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.S.; Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Halpern, Z.; Oren, R.; Kessler, N.; Webb, M. The role of the De Ritis ratio in predicting nonalcoholic fatty liver disease severity. J. Hepatol. 2008, 49, 810–817. [Google Scholar]
- Sunderkötter, C.; Sindrilaru, A.; Mrowietz, U. Histopathological findings in leukocytoclastic vasculitis—Lessons learned from direct immunofluorescence. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 511–517. [Google Scholar]
- Pagnoux, C.; Hogan, S.; Jennette, J.C. Prognostic markers in systemic vasculitides: Lessons from histopathologic evaluation. Semin. Arthritis Rheum. 2007, 37, 348–354. [Google Scholar]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Watts, R.A. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Bui, D.; Domin, B.; Ardabili, L.; Devrim, Y.; Abu Rached, N.; Susok, L. Comparison of clinical and laboratory data of adult patients with cutaneous IgA vasculitis and non-IgA vasculitis. Clin. Exp. Dermatol. 2024, 49, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Glinghammar, B.; Rafter, I.; Lindström, A.K.; Hedberg, J.J.; Andersson, H.B.; Lindblom, P.; Berg, A.L.; Cotgreave, I. Detection of the mitochondrial and catalytically active alanine aminotransferase in human tissues and plasma. Int. J. Mol. Med. 2009, 23, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K. Abnormal liver function tests associated with severe rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).