Stimulator of InterferoN Genes (STING)-Associated Vasculopathy with Onset in Infancy Syndrome (SAVI) Associated with Disseminated Molluscum Contagiosum Under Baricitinib Treatment
Abstract
1. Introduction
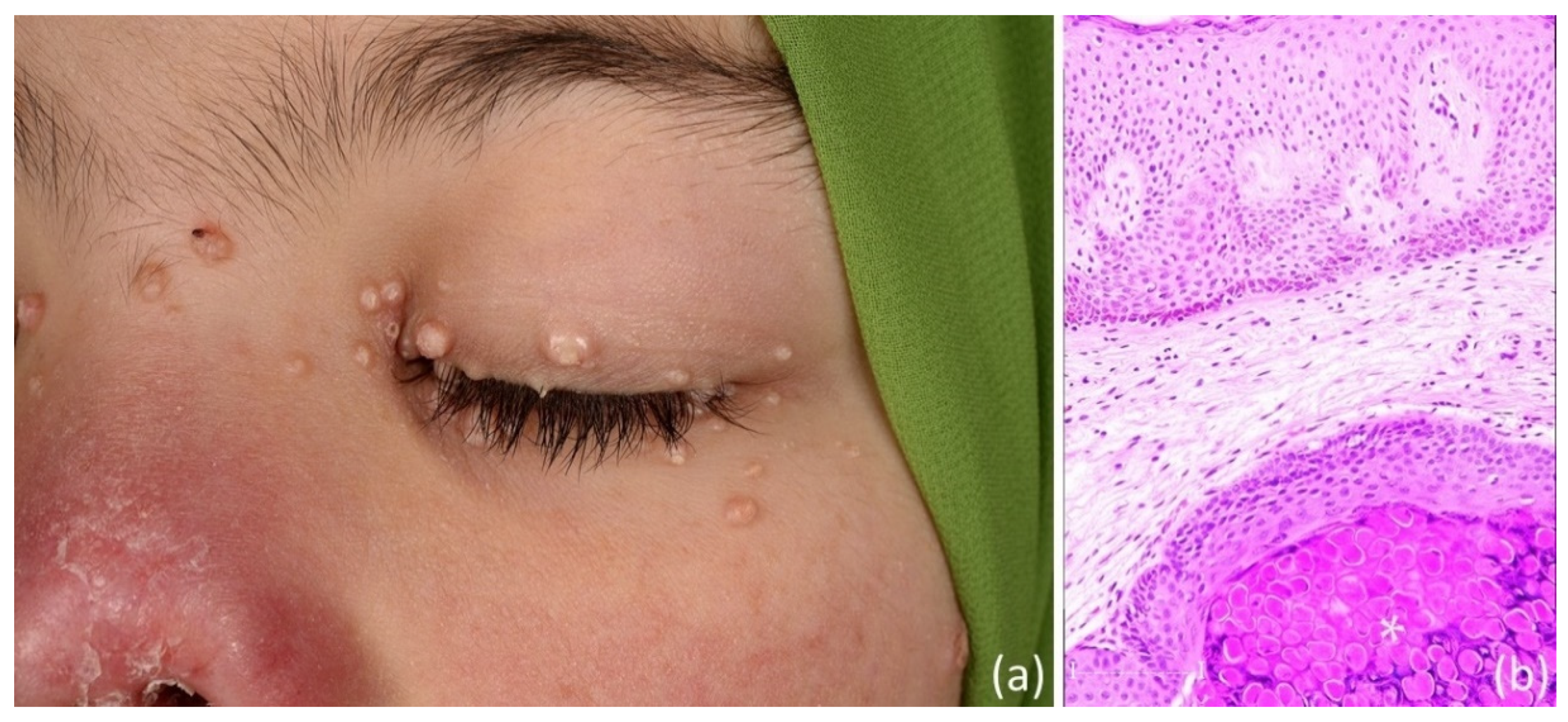
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Sanchez, G.A.M.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [PubMed]
- Volpi, S.; Insalaco, A.; Caorsi, R.; Santori, E.; Messia, V.; Sacco, O.; Terheggen-Lagro, S.; Cardinale, F.; Scarselli, A.; Pastorino, C.; et al. Efficacy and Adverse Events During Janus Kinase Inhibitor Treatment of SAVI Syndrome. J. Clin. Immunol. 2019, 39, 476–485. [Google Scholar] [PubMed]
- Dai, Y.; Liu, X.; Zhao, Z.; He, J.; Yin, Q. Stimulator of Interferon Genes-Associated Vasculopathy with Onset in Infancy: A Systematic Review of Case Reports. Front. Pediatr. 2020, 8, 577918. [Google Scholar]
- Mendonça, L.O.; Frémond, M.L. Interferonopathies: From concept to clinical practice. Best Pract. Res. Clin. Rheumatol. 2024, 38, 101975. [Google Scholar] [PubMed]
- Kretzschmar, G.; Páez, L.P.; Tan, Z.; Wang, J.; Gonzalez, L.; Mugabo, C.H.; Johnsson, A.; Chen, Y.; Mikeš, J.; Lakshmikanth, T.; et al. Normalized Interferon Signatures and Clinical Improvements by IFNAR1 Blocking Antibody (Anifrolumab) in Patients with Type I Interferonopathies. J. Clin. Immunol. 2024, 45, 31. [Google Scholar] [CrossRef] [PubMed]
- Frémond, M.L.; Rodero, M.P.; Jeremiah, N.; Belot, A.; Jeziorski, E.; Duffy, D.; Bessis, D.; Cros, G.; Rice, G.I.; Charbit, B.; et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J. Allergy Clin. Immunol. 2016, 138, 1752–1755. [Google Scholar] [PubMed]
- Sanchez, G.A.M.; Reinhardt, A.; Ramsey, S.; Wittkowski, H.; Hashkes, P.J.; Berkun, Y.; Schalm, S.; Murias, S.; Dare, J.A.; Brown, D.; et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J. Clin. Investig. 2018, 128, 3041–3052. [Google Scholar] [PubMed]
- Sadjadian, P.; Wille, K.; Griesshammer, M. Ruxolitinib-Associated Infections in Polycythemia Vera: Review of the Literature, Clinical Significance, and Recommendations. Cancers 2020, 12, 3132. [Google Scholar] [CrossRef] [PubMed]
- Hebert, A.A.; Bhatia, N.; Del Rosso, J.Q. Molluscum Contagiosum: Epidemiology, Considerations, Treatment Options, and Therapeutic Gaps. J. Clin. Aesthet. Dermatol. 2023, 16 (Suppl. S1), S4–S11. [Google Scholar] [PubMed]
- Meza-Romero, R.; Navarrete-Dechent, C.; Downey, C. Molluscum contagiosum: An update and review of new perspectives in etiology, diagnosis, and treatment. Clin. Cosmet. Investig. Dermatol. 2019, 12, 373–381. [Google Scholar] [PubMed]
- Reiss, B.T.; Bouza, L.; Thomas, S.; Suarez, C.D.; Hill, E.R.; Nichols, D.B. The MC160 protein of the molluscum contagiosum virus dampens cGAS/STING-induced interferon-β activation. Exp. Mol. Pathol. 2023, 134, 104876. [Google Scholar] [CrossRef] [PubMed]
- Isufi, D.; Jensen, M.B.; Loft, N.; Skov, L.; Elberling, J.; Alinaghi, F. Risk of infections during treatment with oral Janus kinase inhibitors in randomized placebo-controlled trials: A systematic review and meta-analysis. JAAD Int. 2024, 18, 106–116. [Google Scholar] [PubMed]
- Abu Rached, N.; Gambichler, T.; Ocker, L.; Schultheis, B.; Susok, L.; Schmidt, W.; Bechara, F.G. Upadacitinib treatment associated with varicella zoster infection complicated by haemophagocytic lymphohistiocytosis in a patient with severe hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e139–e141. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.N.; Lee, S.; Foley, P. A case of widespread molluscum contagiosum caused by baricitinib, a Janus kinase inhibitor. Australas. J. Dermatol. 2019, 60, e334–e335. [Google Scholar] [PubMed]
- De Luca, E.; Gori, N.; Chiricozzi, A.; Di Stefani, A.; Peris, K. Periocular molluscum contagiosum in an atopic dermatitis patient treated with upadacitinib. J. Dermatolog Treat. 2022, 33, 3068–3069. [Google Scholar] [PubMed]
- Kawano, N.; Shiratori, T.; Kawada, A. Case of molluscum contagiosum of an aged female in association with the use of upadacitinib. J. Dermatol. 2022, 49, e171–e172. [Google Scholar] [PubMed]
- Kinoshita, M.; Ogawa, Y.; Kawamura, T.; Kirito, K.; Shimada, S. Case of disseminated molluscum contagiosum caused by ruxolitinib, a Janus kinase 1 and 2 inhibitor. J. Dermatol. 2016, 43, 1387–1388. [Google Scholar] [PubMed]
- Lamberg, O.; Pandher, K.; Troost, J.P.; Lim, H.W. Long-term adverse event risks of oral JAK inhibitors versus immunomodulators: A literature review. Arch. Dermatol. Res. 2024, 317, 109. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Reuther, J.; Scheel, C.H.; Becker, J.C. On the use of immune checkpoint inhibitors in patients with viral infections including COVID-19. J. Immunother. Cancer 2020, 8, e001145, Erratum in J. Immunother. Cancer 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Devrim, Y.; Susok, L. Stimulator of InterferoN Genes (STING)-Associated Vasculopathy with Onset in Infancy Syndrome (SAVI) Associated with Disseminated Molluscum Contagiosum Under Baricitinib Treatment. Dermato 2025, 5, 6. https://doi.org/10.3390/dermato5020006
Gambichler T, Devrim Y, Susok L. Stimulator of InterferoN Genes (STING)-Associated Vasculopathy with Onset in Infancy Syndrome (SAVI) Associated with Disseminated Molluscum Contagiosum Under Baricitinib Treatment. Dermato. 2025; 5(2):6. https://doi.org/10.3390/dermato5020006
Chicago/Turabian StyleGambichler, Thilo, Yusa Devrim, and Laura Susok. 2025. "Stimulator of InterferoN Genes (STING)-Associated Vasculopathy with Onset in Infancy Syndrome (SAVI) Associated with Disseminated Molluscum Contagiosum Under Baricitinib Treatment" Dermato 5, no. 2: 6. https://doi.org/10.3390/dermato5020006
APA StyleGambichler, T., Devrim, Y., & Susok, L. (2025). Stimulator of InterferoN Genes (STING)-Associated Vasculopathy with Onset in Infancy Syndrome (SAVI) Associated with Disseminated Molluscum Contagiosum Under Baricitinib Treatment. Dermato, 5(2), 6. https://doi.org/10.3390/dermato5020006







