Abstract
Image-guided superficial radiation therapy (IGSRT) combines superficial radiation therapy (SRT) with full dermal visualization (FDV) via high-resolution dermal ultrasound (HRDUS) for the treatment of non-melanoma skin cancer (NMSC). The gold standard for IGSRT delivery includes a comprehensive cancer care model with support for dermatologists from a multidisciplinary team. When delivered in this model, IGSRT can achieve cure rates of 99% for treatment of NMSC. This paper focuses on the benefits of HRDUS used in conjunction with SRT for NMSC. Medical records from 7 dermatology clinics of 883 patients with 1507 cases of NMSC treated with IGSRT between 2017 and 2018 were retrospectively reviewed. In total, 92% of the NMSC lesions showed daily depth fluctuations, 60.32% of lesions did not require changes during therapy, and nearly 40% of lesions required at least one compensatory change during therapy. In total, 83% of NMSC lesions were labeled as high risk based on the 2024 NCCN guidelines. Increasing and decreasing tumor depth measurements during IGSRT inform dermatologists when adaptive changes in energy (kV), TDF, and dose will result in more efficacy and less toxicity, respectively.
1. Introduction
The concept of seeing what you treat is extremely strong [1]. Image-guided superficial radiation therapy (IGSRT) combines superficial radiation therapy (SRT) with full dermal visualization (FDV) via high-resolution dermal ultrasound (HRDUS). IGSRT utilizes the SRT-100 Vision, a medical device that was FDA-cleared for the treatment of non-melanoma skin cancer (NMSC) and keloids in 2013.
The gold standard for delivery of IGSRT includes a comprehensive cancer care model that supports dermatology practices with an organized multidisciplinary team. This team is composed of radiation therapists, medical physicists, radiation oncologists, and dermatologists, all of whom have extensive experience in the safe and effective delivery of IGSRT. A multidisciplinary team of experts, combined with the ability to visualize the entire dermis offers a multitude of benefits before, during, and after IGSRT.
These improvements have allowed dermatologists using IGSRT to achieve NMSC cure rates that exceed 99% across multiple retrospective studies [2,3,4,5]. One meta-analysis showed a statistically significant improvement in NMSC 2-year recurrence probability when NMSC lesions were treated with IGSRT compared to Mohs micrographic surgery (MMS) [6].
IGSRT evolved from its predecessor, image-guided radiation therapy (IGRT) (Table 1). IGSRT utilizes low-energy superficial radiation (SRT) and is primarily used by dermatologists for treating skin cancer. Radiation delivered by IGSRT is primarily absorbed in the dermis, where skin cancers grow. IGSRT utilizes ultrasound imaging, which has the advantage of quickly and easily gathering images without exposing the patient to any additional radiation. In contrast, IGRT utilizes a more penetrating form of radiation called external beam (XRT), which requires a linear accelerator. IGRT is primarily used by radiation oncologists to treat cancers deep inside the body, i.e., breast, lung, colon, or prostate cancer, due to its ability to penetrate deep tissues. IGRT utilizes imaging methods including computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) scans, which may expose the patient to a higher dose of radiation via CT or PET scans. Both IGSRT and IGRT are associated with an improved therapeutic index, which means less toxicity and improved tumor control.

Table 1.
A comparison of image-guided superficial radiation therapy (IGSRT) and image-guided radiation therapy (IGRT).
Our aim was to evaluate the benefits of IGSRT during treatment of NMSC tumors, which include the following:
- Visual confirmation of changing tumor depth before each radiation dose is delivered.
- Adaptive radiotherapy: adapting to tumor depth changes by adjusting treatment parameters like energy (kV), TDF (time, dose, fractionation), and dose/boost.
- Visual confirmation of radiobiologic response: replacement of cancerous cells (solid black, hypoechoic) with healthy normal tissue (speckled green/hyperechoic).
- Patient visualizes tumor response, improving compliance and patient outcomes.
- Ability to treat NMSC tumors classified as high risk according to NCCN guidelines.
2. Materials and Methods
Medical records from 7 dermatology clinics of 546 male and 337 female patients (mean age 73.3 [SD ± 10.86]) with 1507 cases of NMSC treated with IGSRT between 2017 and 2018 were retrospectively reviewed. The data were analyzed to determine the following endpoints:
- Percentage of HRDUS images that measured a change in the NMSC’s depth of invasion (DOI) compared to that of the previous image.
- How often HRDUS identifies the need for compensatory changes in treatment parameters like kV, TDF, dose and boost doses. All compensatory changes were documented as a percentage of the total number of cases.
- Percentage of HRDUS images that confirmed a biologically effective dose (BED) was delivered as evidenced by a consistently uniform pattern of the tumor kill/repopulation cycle. A grading system of 1+ (mild), 2+ (moderate), 3+ (complete) indicates the degree of repopulation seen on HRDUS imaging.
- Ability to treat NMSC tumors classified as high risk according to 2024 NCCN guidelines.
Demographic and baseline characteristics, original lesion depth, tumor staging, and Radiation Therapy Oncology Group (RTOG) toxicity levels were summarized descriptively. Categorical outcomes were summarized with frequency and percentages. Chi-squared tests were performed to evaluate significant differences between cancer types. Statistical significance was set at p < 0.05.
3. Results
In this study, 1507 NMSC lesions treated with IGSRT between 2017 and 2018, consisting of 633 basal cell carcinoma (BCC), 459 squamous cell carcinoma (SCC), 411 squamous cell carcinoma in situ (SCCIS), 2 mixed SCC/BCC, and 2 without a reported type, were retrospectively reviewed (Table 2). Of the 1494 lesions with the tumor stage reported, 27.3% (408/1494) were Tis, 62.4% (933/1494) were T1, and 10.2% (153/1494) were T2. The tumor stage was not reported on 13 lesions. Among 284 lesions with RTOG toxicity grade data available, 61.6% (178/284) were reported as grade 1, 37% (105/284) as grade 2, 0.7% (2/284) as grade 3, and 1.4% (4/285) as grade 4. Five lesions were reported as RTOG grade 1 and 2 and were included in the percentage calculation for both grades.

Table 2.
Cancer types and initial lesion ultrasound depth.
The number of HRDUS images collected, the daily depth fluctuations, adaptive replanning requirements, and treatment of high-risk lesions per the NCCN guidelines were evaluated in a total of 883 patients. Patients were primarily men (61.8%). At the time of their first IGSRT treatment, the mean age of patients was 73.3 (SD ± 10.86) with the range from 32.6 to 104.5. Patient records from seven dermatology clinics were reviewed. Each clinic followed the same treatment protocol and used the same model of treatment machine. Differences between the study sites include location, providers, and radiation therapists.
In total, 26,975 HRDUS images were collected, with an average of 17.9 HRDUS images taken per NMSC lesion. A total of 92% of NMSC lesions (1386 out of 1507) displayed daily depth fluctuations with 93.8% of BCC, 92.4% of SCC, and 89.1% of SCCIS lesions exhibiting daily fluctuations in depth (Table 3). The difference in the number of lesions with daily depth fluctuations between BCC and SCC lesions (p = 0.342) and between SCC and SCCIS (p = 0.090) was not statistically significant, whereas it was statistically significant (p < 0.05) between BCC and SCCIS. This is significant because when these fluctuations increase tumor depth, adaptive replanning can be used to improve efficacy. Conversely, when these fluctuations decrease tumor depth, adaptive replanning can be used to reduce toxicity according to the ALARA principle of radiation safety.

Table 3.
Daily depth fluctuations by cancer type.
Of the NMSC lesions, 40% (598 out of 1507) required at least 1 adaptive replanning during therapy, while 60% (909 out of 1507) of lesions required no changes during therapy. In total, 42.2% (267/633) of BCC, 39.9% (183/459) of SCC, and 20.7% (85/411) of SCCIS lesions required changes to the treatment dose (Table 4). This is important because it demonstrates the need for constant imaging so that the high frequency of changes can be detected so that adaptive replanning can be successful. The number of dose changes between BCC and SCC was not statistically significant (p = 0.444), while the difference in dose changes between SCCIS vs. BCC and SCCIS vs. SCC were both statistically significant (p = 0.001).

Table 4.
Number of dose changes by cancer type.
Of the NMSC lesions, 83% (1250 out of 1507) were considered high risk per the 2024 NCCN guidelines. This is significant because SRT without imaging was generally limited to low-risk NMSC due to lack of visualization. The 100% visualization possible with IGSRT allows this new technology to treat both low- and high-risk NMSC.
4. Discussion
4.1. Full Dermal Visualization (FDV)
The high cure rates of IGSRT are also attributed to its ability to visualize the entire dermis before, during, and after therapy. Before therapy, FDV through the use of IGSRT imaging allows dermatologists to clearly define the location of the tumor to avoid a geographic miss in the absence of surface erythema (Figure 1). FDV allows dermatologists and radiation therapists to accurately measure and document frequent fluctuations in depth and monitor tumor response prior to each fraction of radiation delivered. Full dermal visualization of the constantly changing NMSC tumor depth throughout therapy allows the dermatologist and radiation therapist to make compensatory adjustments (in kV, TDF and dose) in real time prior to every dose delivered. This marks the first time in history that dermatologists and radiation therapists have been able to visualize the reactive changes that BCC and SCC undergo during radiation therapy. The two most significant patterns of reactive change that have emerged from the FDV analysis conducted prior to each fraction are fluctuating tumor depth (aka “the moving target”) and progressive tumor replacement (aka “repopulation”). Both of these visual patterns are dynamic and unpredictable, and are therefore difficult to accurately assess without serial imaging.
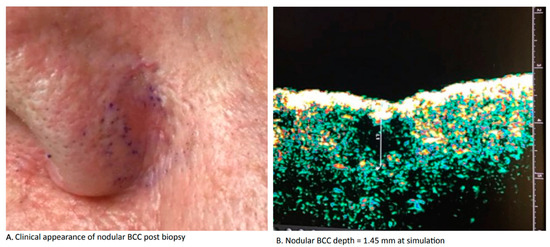
Figure 1.
HRDUS imaging confirms tumor location when clinical presentation of nodular basal cell carcinoma is subtle or challenging.
4.2. NMSC Measured Depth Fluctuations
While the reasons why NMSC tumors expand and contract repeatedly during IGSRT (Figure 2) are not fully understood, the changes in size make NMSCs treated with radiation moving targets. This “moving target” or “constantly changing depth” is significant because of the percentage depth dose (PDD), which is the percentage of the original radiation dose that is delivered at a given measured depth. As radiation passes into the skin, there is a reduction in effective dose for deeper layers. PDD relays what percentage of the prescribed radiation dose actually reaches a particular depth in the dermis, most notably the depth at the bottom of the tumor. For thicker tumors, this translates into possible undertreatment of the tumor base. Thus, performing HRDUS prior to each fraction allows for continuous measurement of the effect dose at the depth most at risk for recurrence.
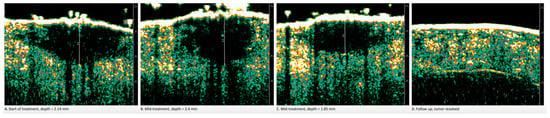
Figure 2.
Lesion depth fluctuations during IGSRT mid-treatment, in moderately differentiated SCC.
4.3. Rapidly Growing Tumors
Per fraction HRDUS is also beneficial for the early detection of rapidly growing well-differentiated SCC tumors, such as keratoacanthoma. In these scenarios, the danger is that these tumors can reach depths that are inappropriate for IGSRT much more quickly than IGSRT can shrink these tumors. Such tumors would require electrodessication and curettage before continuing IGSRT, or conversion of the treatment modality to surgery.
4.4. Repopulation
Repopulation is a visual pattern that confirms biologically effective dosing (BED) during IGSRT, and can be used to monitor the treatment response. BED is confirmed by a gradual and uniform reduction in (hypoechoic or black) tumor cell density, while treatment response is confirmed by a gradual and uniform infiltration of healthy dermal tissue (green speckling) within the tumor. It is theorized that healthy, normal tissue infiltrates into areas where the tumor has undergone damage/destruction. This monitoring is achieved by viewing serial HRDUS images of the NMSC tumor and the surrounding normal dermis during therapy. Prior to radiation, the clearly defined solid tumor, regardless of morphology, is predominantly hypoechoic (black). The dense green speckled pattern that surrounds the tumor indicates its infiltration into healthy dermis (Figure 3).

Figure 3.
Increasing repopulation during IGSRT, nodular basal cell carcinoma.
A uniformly distributed pattern of cancer cell necrosis within the NMSC tumor indicates that the biologic effect of the radiation is optimized for complete tumor clearance.
Repopulation can confirm the biologic effectiveness of IGSRT by confirming the progressive emergence of healthy dermis (green speckling) within the tumor over the course of therapy. Green speckling is associated with healthy normal dermis, and healthy cells cannot migrate into a solid malignant tumor unless the tumor itself is damaged enough to present an opportunity, such as wounds or cavities formed via cancer cell necrosis. Repopulation patterns in which HRDUS images reveal a uniform and evenly spaced pattern of healthy normal tissue (green speckling) within the tumor indicates a uniform and evenly spaced pattern of cancer cell death, and the absence of radioresistance.
Radiation causes necrosis and death of cancer cells via a combination of traumatic breaks in double-stranded DNA and a complex immune response [7]. Macrophages then remove necrotic cancer cells via phagocytosis and this cycle repeats with each dose of radiation creating small defects or “wounds” within the tumor. Those wounds then activate fibroblasts, which migrate into the wound cavity and begin creating new extracellular matrix and collagen (healthy dermis, green speckling) until the wound or cavity is completely repopulated with healthy skin.
Absence or diminution of the expected repopulation pattern on HRDUS alerts the dermatologist to the possibility of a tumor with greater radioresistance and the subsequent possibility of residual pockets of tumor at the end of therapy. Within the NMSC tumor, the uniformity of the green speckling pattern in repopulating areas within the cancer mass confirms that the radiation is eradicating cancer cells effectively at all tumor depths.
In cases where HRDUS identifies a residual tumor 3–6 weeks after the final fraction, the radiation oncologist can assist in determining the most appropriate boost doses, known as salvage therapy. In addition, tiny foci of radioresistant tumor can be accurately localized using HRDUS so that targeted injections with 5-FU can be delivered to assist in tumor clearance.
Dermatologists and radiation therapists can also share HRDUS images of repopulation with patients so that they can visually confirm that the radiation therapy is working effectively. Theoretically, when patients can see their cancer being steadily killed off and replaced by healthy normal tissue at each visit, patient compliance with therapy may increase and they may be less likely to miss appointments. In this manner, repopulation images can theoretically be utilized to improve patient outcomes.
4.5. Biologic Effectiveness
A dermatologist using IGSRT imaging can confirm that a biologically effective dose is being delivered by identifying a uniform decrease in tumor cell density and an increase in healthy dermal tissue infiltrating into the tumor.
The reasons for tumor depth fluctuations in BCC and SCC during IGSRT are not fully understood but are most likely related to the inflammatory and biologic effects of radiation therapy. Radiation creates traumatic DNA double-strand breaks causing cell death in highly replicating tumor cells. The death of those cancer cells triggers an immunological reaction that contributes to eradicating the tumor via antigen presentation and subsequent T-cell activation [8]. Cancer cell death and the immunological reaction it triggers both manifest histologically as acute radiation dermatitis (ARD) during IGSRT.
Histologically, ARD is a vacuolar interface dermatitis characterized by epidermal edema, vacuolization of the basal cell layer, and a lymphocytic infiltrate in the papillary dermis. Individual keratinocytes, predominantly in the basal layer, are necrotic, manifesting as colloid or Civatte bodies. Dermal changes include dermal and endothelial cell edema, vasodilation, erythrocyte extravasation, and fibrin thrombi in vessels. The papillary dermis may include an accumulation of melanophages. A lymphocytic inflammatory infiltrate is noted throughout the dermis [9,10,11,12]. More studies are necessary to better understand ARD, as well as the precise mechanisms of NMSC tumor depth fluctuations and repopulation. This is challenging because ARD is rarely biopsied, mostly due to concerns about wound healing during RT (radiation therapy) [13].
4.6. Clinical Example
An 83-year-old male presents with a NMSC being treated with a 5 cm cone at an energy of 70 kV. The PDD at the epidermal surface (depth = 0.0 mm) is 100%. This means that 100% of the dose is delivered on the skin surface of the NMSC. The dose is 100% because it has not yet entered the skin. The deepest depth of the NMSC is measured at a thickness (depth) of 2.0 mm. PDD tables show that at the deepest tumor depth of 2.0 mm the dose has been diminished to 88%. Based on this, adaptive changes can be made to compensate for the decrease in dose to the bottom of the tumor.
Additional reasons to perform HRDUS prior to each fraction are to confirm the correct anatomic location of the tumor prior to delivering each radiation dose (patient safety to avoid a geographic miss) and to monitor the effectiveness by observing the tumor’s response to therapy, known as repopulation (decreased tumor cell density and uniformity of replacement of cancer cells with healthy normal tissue).
However, once IGSRT has begun, the depth of the BCC is constantly changing, in an unpredictable fashion. Thus, our BCC, which was previously only 2.0 mm deep, has now expanded to a deepest depth measurement of 3.0 mm, at which point the PDD has dropped to 83%, meaning the cancer cells at the base of the tumor receive only 83% of the originally prescribed tumoricidal dose.
When the tumor depth reaches 3.0 mm for any reason, adaptive changes, such as switching the energy from 70 kV to 100 kV can be made mid-treatment to temporarily boost the dose delivered to the base of the tumor from a PDD of 83% to 86%, until the tumor depth changes again. This change in energy does not change the total dose. However, if the increase in PDD from 83% to 86% is not enough to adequately dose the tumor, we can always increase the time dose fractionation (TDF), a mathematical expression used in radiotherapy to calculate the biological effect of a given radiation dose. Increasing the TDF increases the biological effectiveness of a radiation treatment and increases the total dose. The primary goal of IGSRT is to reach a high enough TDF to eradicate the NMSC (biologic effect), while the secondary goal is to keep the total dose low enough to avoid too many side effects. Performing HRDUS prior to every fraction allows this delicate balance between efficacy and tolerability to be optimized based on objective measurements.
The delivery of SRT without full dermal visualization via HDRUS imaging prior to each fraction (treatment, dose given) prevents clinicians from monitoring NMSC tumor depth changes. Tumor depth is constantly changing. This pattern of constant change is not predictable, so depth measurements must be repeated prior to each fraction of radiation given. In the absence of the ability to monitor depth changes prior to each fraction (treatment, dose given), the clinician cannot gauge when the tumor has reached a maximal depth that requires a compensatory adjustment in energy (kV) or TDF (or both), which could put the patient at risk for incomplete tumor clearance and recurrence. Without IGSRT, the ability to achieve a 99% cure rate for the BCC or SCC is lost.
4.7. Rationale for Daily Imaging during IGSRT
4.7.1. Tumor Expansion: Adaptive Changes Optimize Tumor Control
Non-melanoma skin cancer (NMSC) tumors expand multiple times during IGSRT therapy. On these occasions, the increased tumor depth triggers an adaptive increase in kV or dose, especially with regard to the cancer cells at the bottom of the tumor. The mathematical model that quantifies the increase in radiation dose as tumor depth increases is known as percentage depth dose. Without HRDUS imaging prior to delivery of every dose, radiation therapists and dermatologists would not know when it is appropriate to compensate for deeper tumor depths by increasing energy (kV), time dose fractionation (TDF), or dose.
Image-driven, real-time adaptations to tumor expansion explain why IGSRT consistently achieves 99% cure rates in multiple retrospective studies [2,3,4,5]. This is because HRDUS imaging collects precise tumor measurements prior to each radiation dose delivery. These measurements inform the provider when to increase energy, TDF, and dose to ensure better tumor coverage. Without HRDUS imaging prior to delivery of every dose, these tumor depth increases could never be known or addressed, resulting in reduced treatment efficacy.
A meta-analysis compared the cure rates of radiotherapy modalities with (IGSRT) and without (XRT and SRT) image guidance. IGSRT’s real-time adaptive use imaging prior to every dose led to superior cure rates (local control) when compared to XRT and SRT without imaging [13]. This study demonstrates the connection between image guidance and high rates of efficacy. Using IGSRT without adaptive radiotherapy could pose a higher risk of NMSC recurrence.
Furthermore, one of the IGSRT cure rate studies demonstrated that 29% of NMSC tumors treated with IGSRT required an adaptive energy change during therapy based on image-driven measurements [4].
4.7.2. Tumor Shrinkage: Adaptive Changes Minimize Toxicity
NMSC tumors shrink multiple times during IGSRT therapy. On these occasions, the decreased tumor depth causes an unnecessary increase in radiation dose and toxicity. The mathematical model that quantifies the increase in radiation dose as tumor depth decreases is known as the percentage depth dose. Without pre-fraction HRDUS images, radiation therapists and dermatologists would not know when surges in radiation toxicity could be avoided by decreasing energy (kV), time dose fractionation (TDF), or dose.
Image-driven, real-time adaptations to tumor shrinkage explain why IGSRT consistently reduces toxicity as evidenced by RTOG scores in multiple retrospective studies [2,3,4,5]. This is because HRDUS imaging collects precise tumor measurements prior to each radiation delivery. These measurements inform the provider when to decrease energy, TDF, and dose to ensure lower toxicity scores.
Minimizing radiation toxicity is highly significant and medically necessary, according to the ALARA principle. In an effort to maximize radiation protection, the International Commission on Radiological Protection (ICRP) introduced the “as low as reasonably achievable” (ALARA) principle in the 1970s [14]. Assuming that an increase in radiation increases the cancer risk at any dose, the goal of ALARA is to achieve the lowest radiation dose possible to the population, taking into account societal factors and costs [14]. ALARA is particularly important in radiation therapy for cancer treatment, as the physician must balance the beneficial effects of radiation while simultaneously working to minimize the harmful radiation effects [15]. This plays into the physician’s duty to uphold the principle of primum non nocere, as many of the major cancers and diseases in the Western world are thought to be preventable by reducing exposure to disease-causing carcinogens [14,15].
4.7.3. IGSRT’s Imaging/Adaptive Changes: Optimizing the Fundamentals of Radiotherapy
The main point is that the imaging component of IGSRT allows better coverage of the tumor and minimizes unnecessary normal tissue dose/complications, in a real-time adaptive manner. Achieving an optimal balance between tumor control and minimizing normal tissue toxicity is a fundamental goal in radiotherapy planning. Ideally, we would want 100% tumor control with 0% normal tissue complications, but achieving this balance is challenging. The decision to perform image guidance prior to every radiation dose delivered during IGSRT provides real-time data that provide dermatologists and radiation therapists with a clear pathway to achieving the optimal balance between tumor control and minimizing toxicity, as evidenced by IGSRT’s high cure rates and low toxicity [2,3,4,5]. The importance of achieving this optimal balance is even more relevant and significant in a vulnerable patient population of frail elderly patients with co-morbidities.
5. Conclusions
In summary, this study demonstrates that 92% of NMSC tumors undergoing IGSRT exhibit measurable changes in the depth of invasion compared to that of the previous image. Because these measurements are collected immediately prior to treatment, they allow the opportunity for adaptive changes in treatment parameters, such as kV, TDF, dose and boost. These adaptive changes are necessary in nearly 40% of cases, directly benefiting patients by maximizing efficacy and minimizing toxicity.
Any measured increase in NMSC depth is clinically significant during IGSRT because it lowers the percent of the prescription dose received by the tumor cells as a function of their depth (PDD). A lower PDD means significantly less radiation is delivered to the deepest tumor cells, diminishing therapeutic efficacy, potentially allowing the deepest cells to receive significantly less than required for cure. Thus, by performing HRDUS depth measurements prior to every fraction, dermatologists and RTTs know exactly when to make adaptive increases in kV, TDF and dose/boost. This ensures that every NMSC tumor is adequately and uniformly radiated to achieve 99% cure rates [2,3,4,5].
Any measured decrease in NMSC depth is clinically significant during IGSRT because it is an opportunity to decrease unnecessary radiation, minimizing toxicity. The guiding principle of radiation safety is ALARA, which stands for “as low as reasonably achievable”. ALARA means avoiding exposure to radiation that does not have a direct benefit to the patient, even if the dose is small. Thus, by performing HRDUS depth measurements prior to every fraction, dermatologists and RTTs know exactly when to make adaptive decreases in kV, TDF and dose/boost. This ensures that the safety of every NMSC patient is optimized by reducing radiation toxicity whenever possible.
This study also discussed the vital nature of repopulation, a visual pattern that confirms biologically effective dosing (BED) during IGSRT and allows for monitoring of treatment response. HRDUS imaging before each treatment session allows dermatologists and radiation therapists to monitor the gradual repopulation of a tumor and gauge the biological effectiveness of IGSRT therapy. Additionally, this visualization allows clinicians to monitor for radioresistance within a tumor, which is a possibility in the absence of the normal, expected repopulation pattern on HRDUS. Further studies are needed to better understand the precise nature of depth fluctuations and repopulation.
Author Contributions
Conceptualization, J.B.S.; writing—original draft preparation, J.B.S. and P.M.H.; writing—review and editing, J.B.S., J.H., A.S.F. and P.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Jeffrey Stricker and Janine Hopkins have no conflicts of interest to disclose. Aaron Farberg is an advisor for Castle Biosciences, Inc., Novartis, Sun Pharma, Regeneron. Peyton Harris has no conflicts of interest to disclose.
References
- Gregoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Menard, C.; Potter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.; Kaczmarksi, P.; Zheng, S.; Malik, A.; Ladd, D.; Serure, D.; Yu, L. Updated Results of 3,050 Non-melanoma Skin Cancer (NMSC) Lesions in 1725 Patients Treated with High Resolution Dermal Ultrasound-Guided Superficial Radiotherapy, A Multi-institutional Study. J. Investig. Dermatol. 2022, 142. [Google Scholar] [CrossRef]
- Tran, A.; Moloney, M.; Kaczmarski, P.; Zheng, S.; Desai, A.; Desai, T.; Yu, L. Analysis of image-guided superficial radiation therapy (IGSRT) on the treatment of early-stage non-melanoma skin cancer (NMSC) in the outpatient dermatology setting. J. Cancer Res. Clin. Oncol. 2023, 149, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Oh, C.; Shea, C.R. The Treatment of Non-Melanoma Skin Cancer with Image-Guided Superficial Radiation Therapy: An Analysis of 2917 Invasive and In Situ Keratinocytic Carcinoma Lesions. Oncol. Ther. 2021, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Moloney, M.; Beers, R.; Serure, D. Enhancing Cosmesis While Acheiving High Cure-Rates for Early-Stage Non-Melanoma Skin Cancer In The Outpatient Dermatology Clinic Using Novel Non-Invasive Modality. Am. J. Biomed. Sci. Res. 2021, 12, 525–532. [Google Scholar] [CrossRef]
- McClure, E.M.; Sedor, G.; Jin, Y.; Kattan, M.W. Image-guided superficial radiation therapy has superior 2-year recurrence probability to Mohs micrographic surgery. Clin. Transl. Radiat. Oncol. 2023, 43, 100678. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Bok, S.; Hong, B.J.; Choi, H.S.; Ahn, G.O. Radiation-induced immune responses: Mechanisms and therapeutic perspectives. Blood Res. 2016, 51, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gomez, V.; Mustapha, R.; Ng, K.; Ng, T. Radiation therapy and the innate immune response: Clinical implications for immunotherapy approaches. Br. J. Clin. Pharmacol. 2020, 86, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, M. Vacuolar Interface Dermatitis. Available online: https://patholines.org/Vacuolar_interface_dermatitis (accessed on 5 March 2024).
- Horn, T.D.; Junkins-Hopkins, J.M. Interface Dermatitis. In Barnhill’s Dermatopathology, 4th ed.; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- Bolognia, J.; Jorizzo, J.L.; Rapini, R.P. Dermatology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2. [Google Scholar]
- Junkins-Hopkin, J. Disorders associated with physical agents: Heat, cold, radiation, and trauma. In Lever’s Histopathology of the Skin, 10th ed.; Wolters Kluwer-Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Kisonas, J.; Venius, J.; Grybauskas, M.; Dabkeviciene, D.; Burneckis, A.; Rotomskis, R. Acute Radiation Dermatitis Evaluation with Reflectance Confocal Microscopy: A Prospective Study. Diagnostics 2021, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Goldman, L. Regulation. In Cancer Epidemiology and Prevention; Thun, M., Linet, M.S., Cerhan, J.R., Haiman, C.A., Schottenfeld, D., Eds.; Oxford University Press: Oxford, UK, 2017; pp. 1239–1254. [Google Scholar]
- Siegel, E. Primum non nocere: A call for a re-evaluation of radiation doses used in CT. Appl. Radiol. 2006. Available online: https://appliedradiology.com/articles/guest-editorial-primum-non-nocere-a-call-for-a-re-evaluation-of-radiation-doses-used-in-ct# (accessed on 5 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).