Sex-Dependent Skin Aging and Rejuvenation Strategies
Abstract
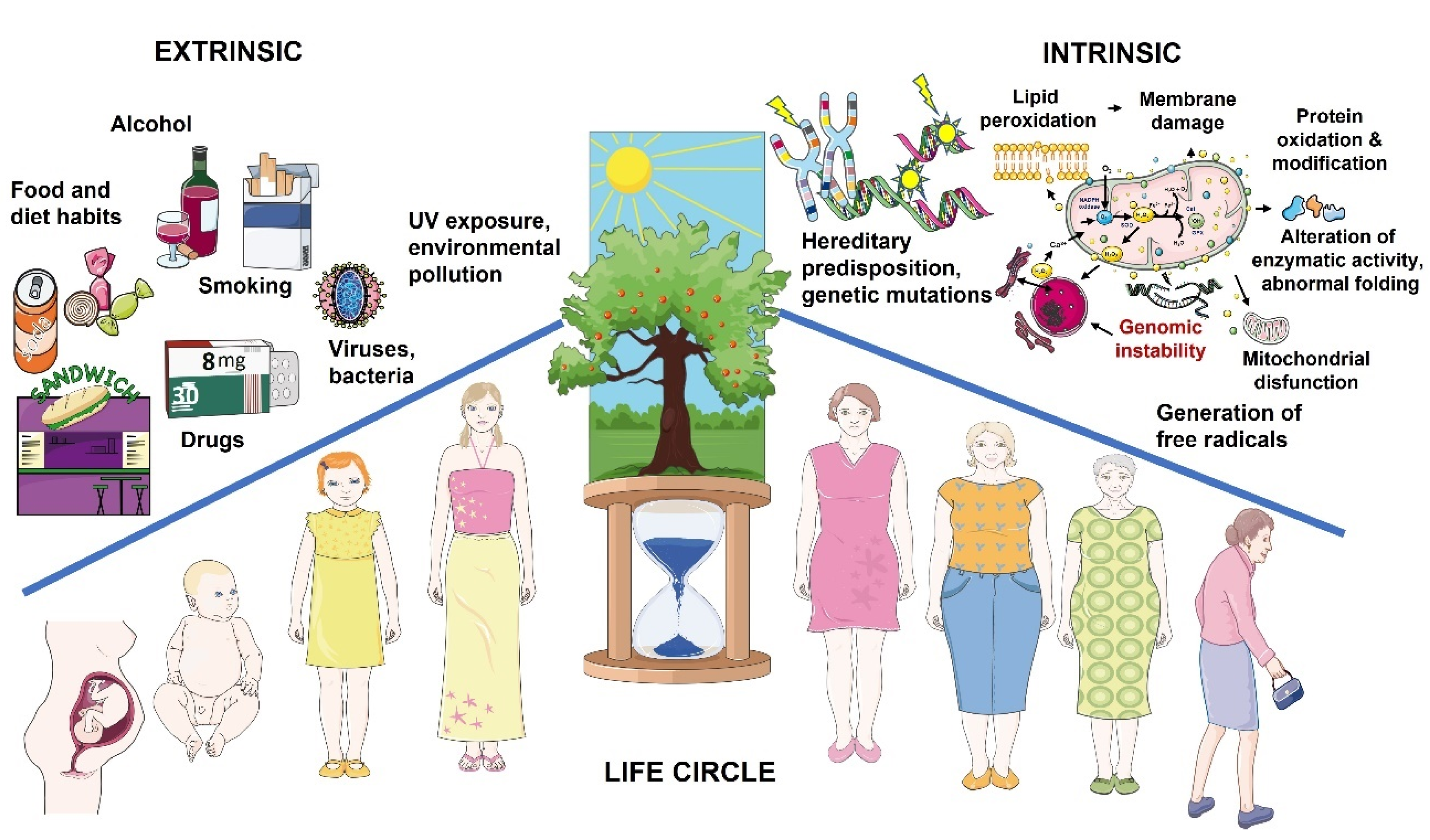
1. Introduction
2. Main Hallmarks of Cutaneous Aging
3. Skin Functions and Structure
3.1. Epidermis
| Skin Properties | Newborn/Toddlers | Puberty Group/Teenagers | 18–30 Years Old | 30–50 Years Old | 50–70 Years Old | 70 Years Old and Older |
|---|---|---|---|---|---|---|
| Hydration | A, B, ♀ = ♂ [7] | F, FA, ♀ = ♂ [8] | F*, C, N* A*, ♀ < ♂; H*, ♀ > ♂ [9]; F*, FA, ♀ < ♂ [8]; FA, ♀ < ♂ [25] | F, N*, A*, ♀ < ♂; C, H*, ♀ > ♂ [9]; F, FA, ♀ < ♂ [8] | F*, C, A, H, ♀ > ♂; N, ♀ < ♂ [9]; F*, FA, ♀ < ♂ [8]; A, ♀ > ♂ (baseline); ♀ < ♂* (after mineral water therapy) [26] | F*, C*, N, A, H*, ♀ > ♂ [9]; F, ♀ = ♂ FA, ♀ > ♂ [8] |
| TEWL | A, B, ♀ = ♂ [7] | F, ♀ > ♂; C*, A*, N*, H, ♀ < ♂ [9]; FA, ♀ < ♂ [25] | F*, C, N*, A*, ♀ > ♂; H ♀ < ♂ [9] | F, C, N, ♀ < ♂; A*, H, ♀ > ♂ [9]; A, ♀ < ♂* (baseline and after mineral water therapy) [26] | F, ♀ < ♂; C, N, A*, H*, ♀ > ♂ [9] | |
| pH | A, B, ♀ = ♂ [7] | F, FA, ♀ > ♂ [8] | F* N*, A*, C*, H*, ♀ > ♂ [9]; F*, FA, ♀ > ♂ [8]; FA*, ♀ > ♂ [25] | F*, C*, N*, A*, H*, ♀ > ♂ [9]; F*, FA*, ♀ > ♂ [8] | F*, N*, A*, C*, H*, ♀ > ♂ [9]; F, FA, ♀ > ♂ [8] | F, C*, N*, A*, H*, ♀ < ♂ [9]; F, FA, ♀ < ♂ [8] |
| Elasticity | F, CN, H, ♀ = ♂ [6] | F, CN, H, ♀ = ♂ [6]; A, ♀ = ♂ [27] | ♀ = ♂ [22]; A, ♀ = ♂ [27]; | A, ♀ = ♂ [27] | A*, ♀ < ♂ (baseline and after mineral water therapy) [26]; A, ♀ = ♂ [27] | F, CN, H, ♀ = ♂ [6]; A, ♀ = ♂ [27]; ♀ = ♂ [22] |
| Thickness | ♀ = ♂ [22] | A, ♀ < ♂ [27]; ♀ < ♂ [22] | A, ♀ < ♂ [27]; ♀ < ♂ [22] | A, ♀ < ♂ [27]; ♀ < ♂ [22] | A, ♀ < ♂ [27]; ♀ < ♂ [22] | A, ♀ < ♂ [27]; ♀ < ♂ [22] |
| Sebum | N/A | F, ♀ > ♂, FA, ♀ > ♂ [8] | F, C*, ♀ < ♂ [9]; F, ♀ < ♂*, FA, ♀ < ♂* [8], FA, ♀ < ♂ [25] | F, C*, ♀ < ♂ [9]; F, ♀ < ♂*, FA, ♀ > ♂ [8] | F*, C*, ♀ < ♂ [9]; F*, FA, ♀ < ♂ [8] | F*, C*, ♀ < ♂ [9]; F*, FA, ♀ < ♂ [8] |
| Wrinkles | N/A | N/A | C*, F*, EC*, G*, UE, LE*, NG, MC*, ♀ < ♂ [28] | C, F*, EC*, G, UE, LE*, NG*, MC*, ♀ < ♂ [28] | C, F*, EC*, G, UE, LE*, NG*, MC*, ♀ < ♂ [28] | C, F*, LE, MC*, ♀ < ♂, G*, UE, EC, NG, ♀ > ♂ [28] |
| Wrinkle roughness | N/A | N/A | F*, EC*, LE*, NG, MC*, ♀ < ♂ [28] | F*, EC*, LE*, NG*, MC*, ♀ < ♂ [28] | F*, EC*, LE*, NG*, MC*, ♀ < ♂ [28] | F*, EC*, LE*, NG, MC*, ♀ < ♂ [28] |
3.2. Differences between Male and Female Epidermal Structure and Functions
3.3. Dermis
3.4. Differences between Male and Female Dermal Structure and Functions
3.5. Hypodermis
3.6. Hormones and Physiopathological Aspects of Gender-Related Cutaneous Aging
4. Treatments That Improve Cutaneous Appearance
4.1. Invasive Procedures
4.2. Gender Dominance in Anti-Aging and Rejuvenation Surgical Services
4.3. Micro-Invasive Procedures
4.4. Energy-Based Dermal Rejuvenation
4.4.1. Intense Pulse Light Devices
4.4.2. Laser Treatment
4.4.3. Radiofrequency Microneedling
| Procedure/Compounds | Properties | Benefits | Limitation | Adverse Effects |
|---|---|---|---|---|
| Energy-based dermal rejuvenation | ||||
| Lasers and radiofrequency (RF) [64] | RF is a non-ablative technology that uses an electric current to target photodamage, unlike lasers that use a light source | RF and lasers can be applied to multiple skin colors and types. It is minimally invasive or non-invasive and can focus high energy to treat various skin conditions | Therapeutic standards and operation specifications for RF therapy have not been unified | Erythema, edema and mild to moderate pain during treatment |
| Injectable dermal rejuvenation techniques | ||||
| Dermal fillers (collagen, hyaluronic acid (HA), autologous fat) [65,66,67] | HA is a GAG naturally found in the extracellular matrix. HA is the most commonly used filler | Removes wrinkles and restores facial volume to create a rejuvenated look | Patients requiring a large volume of fillers (advanced skin laxity, deep rhytids) may have to deal with costly procedures | Intravascular occlusion, Nicolau syndrome, vision loss [65,66], hematomas, asymmetry, hypersensitivity, hyperpigmentation, infection, extrusion, or migration of the implant, neuropraxia, glabellar or perioral skin necrosis [67] |
| Botulinum neurotoxin (Botox) [68] | Botox blocks acetylcholine release resulting in paralysis of muscles. Following Botox injection, paralysis occurs within 24 h to 2 weeks and lasts 3–6 months | Improves wrinkles and nasolabial folds; treats hyperhidrosis, lichen simplex, and dyshidrotic eczema | It has little to no effect on dry skin, pigmentation disorders, and vascular abnormalities; has no effect on skin texture; and cannot discontinue the skin aging process | Bleeding, swelling, erythema, and pain at injection sites. Over time, the effect of Botox gradually resolves, resulting in reduced muscle paralysis |
| Chemical peeling | ||||
| Light (e.g., alpha hydroxyl and salicylic acid peel), medium (e.g., TCA 30%), and deep peels (e.g., phenol) [4,57] | Peels remove the uppermost layers of the skin, depending on the depth of the peel | Instant result—smooth, radiant, and young-looking skin; removal of dead skin cells; cleaning up clogged pores and reduction of their size; decrease in facial wrinkles and fine lines; improvement of cutaneous texture and tone; boosting collagen production and promotion of healthy cell growth | Increased sun sensitivity; carbolic acid (phenol) can damage kidneys, liver, and heart muscle, causing arrhythmias, which limits its application in patients with corresponding pathologies | Hyperpigmentation, solar lentigines, risk of post-operative infections, and especially herpetic ones [4] |
4.5. Injectable Cutaneous Rejuvenation Options and Dermal Fillers
4.5.1. Dermal Fillers
4.5.2. Neurotoxins
4.5.3. Chemical Peeling
- Light peels: These peels exfoliate the epidermal layers without extending beyond the basal layer. Examples of substances in this category include α-hydroxy acids (such as glycolic acid at concentrations of 20–70%, lactic acid, pyruvic acid, malic acid, and tartaric acid), β-hydroxy acids (salicylic acid at concentrations of 10–30%), carbon dioxide snow, Jessner’s solution, lipo-hydroxy acids, resorcinol, retinoic acid, Unna paste, 5-fluorouracil, retinoic acid, and trichloroacetic acid (TCA) at concentrations of 10–30%;
- Medium peels: These peels reach a depth of approximately 0.45 mm into the upper reticular dermis. Examples of peeling agents in this category include TCA at concentrations of 35–50% combined with glycolic acid at 70% or Jessner’s solution, as well as TCA at 35% combined with solid CO2;
- Deep peels: These peels penetrate the lower reticular dermis, reaching the basal layer where melanocytes, which synthesize and store melanin pigment, are located. Melanin is stored in melanosomes, which are membrane-bound organelles. Stimulated melanocytes release melanin to the surrounding keratinocytes, helping to maintain balanced dermal pigmentation. Deep peels that expose or remove the papillary or reticular layer of the dermis effectively reduce wrinkles (rhytids). Deep peels typically contain TCA concentrations exceeding 50% and may involve combinations of croton oil and phenol, such as the Baker–Gordon formula.
4.5.4. Non-Invasive Aesthetic Strategies
| Treatment | Benefits | Limitation | Adverse Effects |
|---|---|---|---|
| Antioxidants for topical use | |||
| Topical retinoids (vitamin A, tretinoin, and tazarotene) [4,12,57,82] | Improve mottled hyperpigmentation, fine wrinkles, roughness, and lentigines via compaction of the stratum corneum and epidermal thickening; improve synthesis and deposition of GAGs; induce collagen biosynthesis; and reduce the expression of MMP 1 (collagenase 1) [4] | Beneficial effects of topical retinoids are only evident over a long period of time, which often leads to early discontinuation [82] | Skin irritation, erythema, dermatitis, pruritus, peeling, stinging, or burning, and sensitivity |
| Ascorbic acid (AA) [4,83,84] | Catalyzes the hydroxylation reaction in collagen synthesis, thereby increasing collagen synthesis and reducing degradation. AA reduces pigmentation by decreasing melanin formation; daily topical application of 3% vitamin C over four months led to a significant increase in the density of dermal papillae 831; 5–15% was proven to have an anti-aging skin effect by inducing the production of collagens 1 and 3 and modulating MMP1 [4] | L-ascorbic acid is an unstable molecule, hydrophilic and charged, that reduceS penetration into the skin; albeit, lowering the pH to <3.5 improves penetration [84]. Exposed to light and heat, AA inactivates, while air oxidize extremely fast to dehydroascorbic acid | Allergy |
| Topical and systemic endocrinological therapies | |||
| Vital hormones (DHEA, DHEAS, testosterone, estrogen, TSH, T3, T4) [79,85,86,87] | Improves cutaneous hydration and reduces skin atrophy [79,85]; improves sebum production and cutaneous hydration with less progressive skin thinning in older patients [86]. 50 mg of DHEA daily for a year reduced bone mineral density loss in 140 postmenopausal women between the age of 60–79 years [87]; estrogen face creams contained 0.3% estriol (slightly superior results) or 0.01% estradiol for 6 months showed improvement in cutaneous vascularization, firmness and elasticity, hydration, and reduction in wrinkle depth and pore size in perimenopausal women [79] | Use of hormonal therapy more than 5 years is associated with increased thromboembolic risk, breast, ovarian and endometrial cancers [86] | Increases thromboembolic risks; breast, ovarian, endometrial cancers |
| Botanicals | |||
| Green tea polyphenols [4,88] | An increase in the minimal erythema dose decreases the number of Langerhans cells and reduces DNA damage in the skin before UV exposure [4] | Reported inhibitory effects on metabolizing enzymes; hepatotoxicity [88] | Erythema and papular lesions [88] |
| Rosmarinus officinalis [89,90] | Antimicrobial, antimycotic, antiviral, anti-inflammatory, anti-mutagen, and antioxidative effects; inhibition of UV-induced MMP-1 [89]; treatment of cellulitis [90] | Biodegradation of active components depends on cosmetic product m (oil vs. methanol/ethanol extracts) [90] | Allergic contact dermatitis |
| Mushroom treatments | |||
| Mushroom extracts (ethanolic extracts prepared from Agaricus bisporus, Pleurotus ostreatus, and Lentinula edodes [80,81] | Anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial | Lack of evidence-based clinical trials | Allergy |
4.6. Herbal and Myco-Based Remedies in Dermatology and Cosmetology
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boraldi, F.; Annovi, G.; Tiozzo, R.; Sommer, P.; Quaglino, D. Comparison of ex vivo and in vitro human fibroblast ageing models. Mech. Ageing Dev. 2010, 131, 625–635. [Google Scholar] [CrossRef] [PubMed]
- del Río, C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The role of microRNAs in organismal and skin aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Xin, S.; Man, W.; Fluhr, J.W.; Song, S.; Elias, P.M.; Man, M.-Q. Cutaneous resonance running time varies with age, body site and gender in a normal Chinese population. Ski. Res. Technol. 2010, 16, 413–421. [Google Scholar] [CrossRef]
- Giusti, F.; Martella, A.; Bertoni, L.; Seidenari, S. Skin barrier, hydration, and pH of the skin of infants under 2 years of age. Pediatr. Dermatol. 2001, 18, 93–96. [Google Scholar] [CrossRef]
- Man, M.; Xin, S.; Song, S.; Cho, S.; Zhang, X.; Tu, C.; Feingold, K.; Elias, P. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large chinese population. Ski. Pharmacol. Physiol. 2009, 22, 190–199. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin physiology in men and women: In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef]
- Rahrovan, S.; Fanian, F.; Mehryan, P.; Humbert, P.; Firooz, A. Male versus female skin: What dermatologists and cosmeticians should know. Int. J. Womens Dermatol. 2018, 4, 122–130. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the senescence-associated phenotype in human skin fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Coutière, P.; Woillez, M.; Mérillon, J.-M.; Vitrac, X. DNA macroarray study of skin aging-related genes expression modulation by antioxidant plant extracts on a replicative senescence model of human dermal fibroblasts. Phytother. Res. 2011, 25, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Medve-König, K.; Pfahlberg, A.; Gers-Barlag, H.; Gefeller, O.; Krutmann, J. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J. Investig. Dermatol. 2004, 122, 1277–1283. [Google Scholar] [CrossRef]
- Kang, S.M.; Han, S.; Oh, J.-H.; Lee, Y.M.; Park, C.-H.; Shin, C.-Y.; Lee, D.H.; Chung, J.H. A synthetic peptide blocking TRPV1 activation inhibits UV-induced skin responses. J. Dermatol. Sci. 2017, 88, 126–133. [Google Scholar] [CrossRef][Green Version]
- Cavinato, M.; Koziel, R.; Romani, N.; Weinmüllner, R.; Jenewein, B.; Hermann, M.; Dubrac, S.; Ratzinger, G.; Grillari, J.; Schmuth, M.; et al. UVB-induced senescence of human dermal fibroblasts involves impairment of proteasome and enhanced autophagic activity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 632–639. [Google Scholar] [CrossRef][Green Version]
- Kang, S. Fitzpatrick’s Dermatology; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II keratin intermediate filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef]
- Fuchs, E. Keratins and the skin. Annu. Rev. Cell Dev. Biol. 1995, 11, 123–153. [Google Scholar] [CrossRef]
- Kalla, A.K.; Tiwari, S.C. Sex differences in skin colour in man. Acta Genet. Med. Gemellol. 1970, 19, 472–476. [Google Scholar] [CrossRef][Green Version]
- Tur, E. Physiology of the skin-differences between women and men. Clin. Dermatol. 1997, 15, 5–16. [Google Scholar] [PubMed]
- Roh, K.-Y.; Kim, D.; Ha, S.-J.; Ro, Y.-J.; Kim, J.-W.; Lee, H.-J. Pigmentation in Koreans: Study of the differences from caucasians in age, gender and seasonal variations. Br. J. Dermatol. 2001, 144, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The transdermal delivery of therapeutic cannabinoids. Pharmaceutics 2022, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Gautier, J.; Sterry, W.; Lademann, J. Gender-related differences in the physiology of the stratum corneum. Dermatology 2005, 211, 312–317. [Google Scholar] [CrossRef]
- Mac-Mary, S.; Creidi, P.; Marsaut, D.; Courderot-Masuyer, C.; Cochet, V.; Gharbi, T.; Guidicelli-Arranz, D.; Tondu, F.; Humbert, P. Assessment of effects of an additional dietary natural mineral water uptake on skin hydration in healthy subjects by dynamic barrier function measurements and clinic scoring. Ski. Res. Technol. 2006, 12, 199–205. [Google Scholar]
- Escoffier, C.; de Rigal, J.; Rochefort, A.; Vasselet, R.; Lévêque, J.L.; Agache, P.G. Age-related mechanical properties of human skin: An in vivo study. J. Investig. Dermatol. 1989, 93, 353–357. [Google Scholar] [CrossRef]
- Tsukahara, K.; Hotta, M.; Osanai, O.; Kawada, H.; Kitahara, T.; Takema, Y. Gender-dependent differences in degree of facial wrinkles. Ski. Res. Technol. 2013, 19, e65–e71. [Google Scholar] [CrossRef]
- Liu, Z.; Song, S.; Luo, W.; Elias, P.M.; Man, M.Q. Sun-induced changes of stratum corneum hydration vary with age and gender in a normal Chinese population. Ski. Res. Technol. 2012, 18, 22–28. [Google Scholar] [CrossRef]
- Denda, M.; Horii, I.; Takahashi, M.; Hara, M.; Tagami, H. Age-and sex-dependent change in stratum corneum sphingolipids. Arch. Dermatol. Res. 1993, 285, 415–417. [Google Scholar]
- Kiistala, U. Dermal-epidermal separation. I. The influence of age, sex and body region on suction blister formation in human skin. Ann. Clin. Res. 1972, 4, 10–22. [Google Scholar]
- Hatje, L.K.; Richter, C.; Blume-Peytavi, U.; Kottner, J. Blistering time as a parameter for the strength of dermoepidermal adhesion: A systematic review and meta-analysis. Br. J. Dermatol. 2015, 172, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Savvas, M.; Bishop, J. Type III collagen content in the skin of postmenopausal women receiving oestradiol and testosterone implants. Br. J. Obs. Gynaecol. 1993, 100, 154–156. [Google Scholar] [CrossRef]
- Brincat, M.; Moniz, C.F.; Studd, J.W.; Darby, A.J.; Magos, A.; Cooper, D. Sex hormones and skin collagen content in postmenopausal women. Proc. R. Soc. Med. 1983, 287, 989–993. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Mechanical properties of human skin in vivo: A comparative evaluation in 300 men and women. Ski. Res. Technol. 2014, 20, 127–135. [Google Scholar] [CrossRef]
- Tur, E.; Maibach, H.I. Gender and Dermatology; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Sadick, N.S. The pathophysiology of the male aging face and body. Dermatol. Clin. 2018, 36, 1–4. [Google Scholar]
- Fleg, J.L.; Morrell, C.H.; Bos, A.G.; Brant, L.J.; Talbot, L.A.; Wright, J.G.; Lakatta, E.G. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005, 112, 674–682. [Google Scholar] [CrossRef]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Womens Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Quan, T. Molecular Mechanisms of Skin Aging and Age-Related Diseases; CRC Press: Abingdon, UK, 2016. [Google Scholar] [CrossRef]
- Marques, E.; Chen, T.M. Actinic Keratosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557401/ (accessed on 14 January 2023).
- Kapur, S.; Watson, W.; Carr, S. Atopic dermatitis. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 5. [Google Scholar] [CrossRef]
- Chen, W.; Mempel, M.; Schober, W.; Behrendt, H.; Ring, J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. J. R. Coll. Physicians Lond. 2021, 21, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.C.; Daniel, B.S. Cutaneous lupus erythematosus: A review of the literature. Int. J. Womens Dermatol. 2019, 5, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sinha, J.; Ghosh, S.; Raghunath, M. Progeria: A rare genetic premature ageing disorder. Indian J. Med. Res. 2014, 139, 667–674. [Google Scholar]
- Gerasymchuk, M. Genomic instability and aging: Causes and consequences. In Genome Stability; Academic Press: Cambridge, MA, USA, 2021; pp. 533–553. [Google Scholar] [CrossRef]
- Croteau, D.L.; Popuri, V.; Opresko, P.L.; Bohr, V.A. Human RecQ helicases in DNA repair, recombination, and replication. Annu. Rev. Biochem. 2014, 83, 519–552. [Google Scholar] [CrossRef]
- Lehmann, A.R. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie 2003, 85, 1101–1111. [Google Scholar] [CrossRef]
- Bensenouci, S.; Louhibi, L.; de Verneuil, H.; Mahmoudi, K.; Saidi-Mehtar, N. Diagnosis of Xeroderma pigmentosum groups A and C by detection of two prevalent mutations in west algerian population: A rapid genotyping tool for the frequent XPC mutation c.1643-1644delTG. Biomed. Res. Int. 2016, 2016, 2180946. [Google Scholar] [CrossRef]
- The Aesthetic Society. Aesthetic Plastic Surgery National Databank for 2020–2021. 2021. Available online: https://cdn.theaestheticsociety.org/media/statistics/2021-TheAestheticSocietyStatistics.pdf (accessed on 14 January 2023).
- Santosa, K.B.; Oliver, J.D.; Thompson, G.; Beil, R.J. Perioperative management of the facelift patient. Clin. Plast. Surg. 2019, 46, 625–639. [Google Scholar] [CrossRef]
- Charafeddine, A.H.; Drake, R.; McBride, J.; Zins, J.E. Facelift: History and anatomy. Clin. Plast. Surg. 2019, 46, 505–513. [Google Scholar] [PubMed]
- Goldberg, D.J. Current trends in intense pulsed light. J. Clin. Aesthet. Dermatol. 2012, 5, 45–53. [Google Scholar]
- Beigvand, H.H.; Razzaghi, M.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Safari, S.; Rezaei-Tavirani, M.; Mansouri, V.; Heidari, M.H. Assessment of laser effects on skin rejuvenation. J. Lasers Med. Sci. 2020, 11, 212–219. [Google Scholar]
- Sator, P.G. Skin treatments and dermatological procedures to promote youthful skin. Clin. Interv. Aging 2006, 1, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kesty, K.; Goldberg, D.J. 650 usec 1064nm Nd:YAG laser treatment of acne: A double-blind randomized control study. J. Cosmet. Dermatol. 2020, 19, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Manturova, N.; Kruglova, L.; Ikonnikova, E. Treatment of moderate to severe acne and scars with a 650-microsecond 1064-nm laser and isotretinoin. J. Drugs Dermatol. 2020, 19, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, M.G.; Kothare, A.; Karavitis, M. A novel 1726-nm laser system for safe and effective treatment of acne vulgaris. Lasers Med. Sci. 2022, 37, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Preissig, J.; Hamilton, K.; Markus, R. Current laser resurfacing technologies: A review that delves beneath the surface. Semin. Plast. Surg. 2012, 26, 109–116. [Google Scholar] [CrossRef]
- Chandrashekar, B.; Sriram, R.; Mysore, R.; Bhaskar, S.; Shetty, A. Evaluation of microneedling fractional radiofrequency device for treatment of acne scars. J. Cutan. Aesthet. Surg. 2014, 7, 93–97. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Alavi, S.; Heidari-Kharaji, M.; Hanifnia, A.R.; Mahmoudbeyk, M.; Karimi, Z.; Kahe, F. Biometric changes of skin parameters in using of microneedling fractional radiofrequency for skin tightening and rejuvenation facial. Ski. Res. Technol. 2020, 26, 859–866. [Google Scholar]
- Lyu, J.J.; Liu, S.X. Radiofrequency in facial rejuvenation. Int. J. Dermatol. Venereol. 2022, 5, 94–100. [Google Scholar] [CrossRef]
- Andre, P.; Haneke, E. Nicolau syndrome due to hyaluronic acid injections. J. Cosmet. Laser Ther. 2016, 18, 239–244. [Google Scholar]
- Akinbiyi, T.; Othman, S.B.; Familusi, O.; Calvert, C.; Card, E.B.B.; Percec, I. Better results in facial rejuvenation with fillers. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2763. [Google Scholar] [CrossRef]
- Murray, C.A.; Zloty, D.; Warshawski, L. The evolution of soft tissue fillers in clinical practice. Dermatol. Clin. 2005, 23, 343–363. [Google Scholar] [PubMed]
- Satriyasa, B.K. Botulinum toxin (Botox) a for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Daniels, M.A.; Roth, M.Z. Mesotherapy, microneedling, and chemical peels. Clin. Plast. Surg. 2016, 43, 583–595. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA-Approved Dermal Fillers. 2020. Available online: https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/fda-approved-dermal-fillers (accessed on 14 January 2023).
- Jacovella, P.F. Calcium hydroxylapatite facial filler (RadiesseTM): Indications, technique, and results. Clin. Plast. Surg. 2006, 33, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Javadi, P.; Daines, S.M.; Williams, E.F. Quantitative assessment of the longevity of poly-L-lactic acid as a volumizing filler using 3-dimensional photography. JAMA Facial Plast. Surg. 2015, 17, 39–43. [Google Scholar] [CrossRef]
- Lewandowski, M.; Świerczewska, Z.; Barańska-rybak, W. Off-label use of Botulinum toxin in dermatology—Current state of the art. Molecules 2022, 27, 3143. [Google Scholar]
- Iranmanesh, B.; Khalili, M.; Mohammadi, S.; Amiri, R.; Aflatoonian, M. Employing microbotox technique for facial rejuvenation and face-lift. J. Cosmet. Dermatol. 2022, 21, 4160–4170. [Google Scholar] [CrossRef]
- Lee, K.C.; Wambier, C.G.; Soon, S.L.; Sterling, J.B.; Landau, M.; Rullan, P.; Brody, H.J. Basic chemical peeling: Superficial and medium-depth peels. J. Am. Acad. Dermatol. 2019, 81, 313–324. [Google Scholar] [CrossRef]
- Brody, H.J.; Monheit, G.D.; Resnik, S.S.; Alt, T.H. A history of chemical peeling. Dermatol. Surg. 2000, 26, 405–409. [Google Scholar] [CrossRef]
- O’Connor, A.A.; Lowe, P.M.; Shumack, S.; Lim, A.C. Chemical peels: A review of current practice. Australas. J. Dermatol. 2018, 59, 171–181. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. The effects of nutrient signaling regulators in combination with phytocannabinoids on the senescence-associated phenotype in human dermal fibroblasts. Int. J. Mol. Sci. 2022, 23, 8804. [Google Scholar]
- Schmidt, J.; Binder, M.; Macheiner, W.; Kainz, C.; Gitsch, G.; Bieglmayer, C. Treatment of skin ageing symptoms in perimenopausal females with estrogen compounds. A pilot study. Maturitas 1994, 20, 25–30. [Google Scholar]
- Schmidt, J.; Binder, M.; Macheiner, W.; Kainz, C.; Gitsch, G.; Bieglmayer, C. Development of mushroom-based cosmeceutical formulations with anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial properties. Molecules 2016, 21, 1372. [Google Scholar]
- Yan, Z.F.; Yang, Y.; Tian, F.H.; Mao, X.X.; Li, Y.; Li, C.T. Inhibitory and acceleratory effects of Inonotus obliquus on tyrosinase activity and melanin formation in b16 melanoma cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 259836. [Google Scholar]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar]
- Al-Niaimi, F.; Yi Zhen Chiang, N. Topical vitamin C and the skin: Mechanisms of action and clinical applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar] [PubMed]
- Pinnell, S.R.; Yang, H.; Omar, M.; Riviere, N.M.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar]
- Borda, L.J.; Wong, L.L.; Tosti, A. Bioidentical hormone therapy in menopause: Relevance in dermatology. Dermatol. Online J. 2019, 25, 1. [Google Scholar]
- Baulieu, E.-E.; Thomas, G.; Legrain, S.; Lahlou, N.; Roger, M.; Debuire, B.; Faucounau, V.; Girard, L.; Hervy, M.-P.; Latour, F.; et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. Proc. Natl. Acad. Sci. USA 2000, 97, 4279–4284. [Google Scholar]
- Daniell, H.W. Oral dehydroepiandrosterone might prevent frequent tears in atrophic skin: A case report. JAAD Case Rep. 2017, 3, 534–535. [Google Scholar]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [PubMed]
- Cronin, H.; Draelos, Z.D. Top 10 botanical ingredients in 2010 anti-aging creams. J. Cosmet. Dermatol. 2010, 9, 218–225. [Google Scholar] [PubMed]
- de Macedo, L.M.; Santos, É.M.D.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and its topical applications: A review. Plants 2020, 9, 651. [Google Scholar]
- McKeown, D.J.; Payne, J. Significant improvement in body contour with multiple cycles of CoolSculpting: Results of a prospective study. Dermatol. Ther. 2021, 34, e14850. [Google Scholar]
- Thring, T.S.; Hili, P.A.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar]
- Izzo, A.A.; Camilleri, M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut 2008, 57, 1140–1155. [Google Scholar]
- Pacher, P.; Mukhopadhyay, P.; Mohanraj, R.; Godlewski, G.; Bátkai, S.; Kunos, G. Modulation of the endocannabinoid system in cardiovascular disease: Therapeutic potential and limitations. Hypertension 2008, 52, 601–607. [Google Scholar]
- Scherer, T.; Buettner, C. The dysregulation of the endocannabinoid system in diabesity-a tricky problem. J. Mol. Med. 2009, 87, 663–668. [Google Scholar] [CrossRef]
- Xing, C.; Zhuang, Y.; Xu, T.-H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM structure of the human cannabinoid receptor CB2-Gi signaling complex. Cell 2020, 180, 645–654.e13. [Google Scholar]
- Pacher, P.; Haskó, G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2008, 153, 252–262. [Google Scholar]
- Husni, A.S.; McCurdy, C.R.; Radwan, M.M.; Ahmed, S.A.; Slade, D.; Ross, S.A.; ElSohly, M.A.; Cutler, S.J. Evaluation of phytocannabinoids from high-potency Cannabis sativa using in vitro bioassays to determine structure-activity relationships for cannabinoid receptor 1 and cannabinoid receptor 2. Med. Chem. Res. 2014, 23, 4295–4300. [Google Scholar]
- Sivesind, T.E.; Maghfour, J.; Rietcheck, H.; Kamel, K.; Malik, A.S.; Dellavalle, R.P. Cannabinoids for the treatment of dermatologic conditions. JID Innov. 2022, 2, 100095. [Google Scholar]
- Lim, M.; Kirchhof, M. Dermatology-related uses of medical cannabis promoted by dispensaries in Canada, Europe, and the United States. J. Cutan. Med. Surg. 2018, 23, 178–184. [Google Scholar] [PubMed]
- Ramot, Y.; Sugawara, K.; Zákány, N.; Toth, B.I.; Bíró, T.; Paus, R. A novel control of human keratin expression: Cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ 2013, 1, e40. [Google Scholar] [PubMed]
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.C.; Bíró, T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar]
- Kemény, L.; Bíró, T.; Abels, C.; Gertsch, J.; Nicolussi, S.; Tubak, V.; Soeberdt, M.; Ambrus, L.; Oláh, A. Inhibition of fatty acid amide hydrolase exerts cutaneous anti-inflammatory effects both in vitro and in vivo. Exp. Dermatol. 2016, 25, 328–330. [Google Scholar]
- Yuan, C.; Wang, X.-M.; Guichard, A.; Tan, Y.-M.; Qian, C.-Y.; Yang, L.-J.; Humbert, P. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: Results of a randomized, double-blind, controlled study in 60 patients. Clin. Interv. Aging 2014, 9, 1163–1169. [Google Scholar]
- Maghfour, J.; Rundle, C.W.; Rietcheck, H.R.; Dercon, S.; Lio, P.; Mamo, A.; Runion, T.M.; Fernandez, J.; Kahn, J.; Dellavalle, R.P.; et al. Assessing the effects of topical cannabidiol in patients with atopic dermatitis. Dermatol. Online J. 2021, 27, 15. [Google Scholar]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar]
- Chalmers, S.; Garcia, S.; Draganski, A.; Doerner, J.; Friedman, A.; Friedman, J.; Putterman, C. Topical endocannabinoid ad-ministration protects MRL-Lpr/Lpr mice from cutaneous lupus erythematosus. In Proceedings of the 2018 ACR/ARHP Annual Meeting, Chicago, IL, USA, 19–24 October 2018; Volume 70. [Google Scholar]
- Ardigò, M.; Franceschini, C.; Campione, E.; Cosio, T.; Lanna, C.; Bianchi, L.; Milani, M. Efficacy of a topical product containing purified omental lipids and three anti-itching compounds in the treatment of chronic pruritus/prurigo nodularis in elderly subjects: A prospective, assessor-blinded, 4-week trial with transepidermal water loss and optical coherence tomography assessments. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1051–1058. [Google Scholar] [PubMed]
- Eberlein, B.; Eicke, C.; Reinhardt, H.W.; Ring, J. Adjuvant treatment of atopic eczema: Assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 73–82. [Google Scholar] [PubMed]
- Robinson, E.S.; Alves, P.; Bashir, M.M.; Zeidi, M.; Feng, R.; Werth, V.P. Cannabinoid reduces inflammatory cytokines, tumor necrosis factor-α, and type I interferons in dermatomyositis in vitro. J. Investig. Dermatol. 2017, 137, 2445–2447. [Google Scholar] [CrossRef]
- Chelliah, M.P.; Zinn, Z.; Khuu, P.; Teng JM, C. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr. Dermatol. 2018, 35, e224–e227. [Google Scholar]
- Luca, T.; Di Benedetto, G.; Scuderi, M.R.; Palumbo, M.; Clementi, S.; Bernardini, R.; Cantarella, G. The CB1/CB2 receptor agonist WIN-55,212-2 reduces viability of human Kaposi’s sarcoma cells in vitro. Eur. J. Pharmacol. 2009, 616, 16–21. [Google Scholar] [CrossRef]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Anagnostou, M.E.; Babatunde, F.; Corazzari, M.; Redfern, C.P.F.; et al. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar]
- Gęgotek, A.; Atalay, S.; Rogowska-Wrzesińska, A.; Skrzydlewska, E. The effect of cannabidiol on UV-induced changes in intracellular signaling of 3D-cultured skin keratinocytes. Int. J. Mol. Sci. 2021, 22, 1501. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Sand, J.M.; Bauer, S.J.; Hafeez, B.B.; Meske, L.; Verma, A.K. Topically applied Hsp90 inhibitor 17AAG inhibits UVR-induced cutaneous squamous cell carcinomas. J. Investig. Dermatol. 2015, 135, 1098–1107. [Google Scholar] [CrossRef]
- Friedman, A.J.; Momeni, K.; Kogan, M. Topical cannabinoids for the management of Psoriasis Vulgaris: Report of a case and review of the literature. J. Drugs Dermatol. 2020, 19, 795. [Google Scholar]
- Vincenzi, C.; Tosti, A. Efficacy and tolerability of a shampoo containing broad-spectrum cannabidiol in the treatment of scalp inflammation in patients with mild to moderate scalp psoriasis or seborrheic dermatitis. Ski. Appendage Disord. 2020, 6, 355–361. [Google Scholar]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults With Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R.; Kim, S.W. Dronabinol, a cannabinoid agonist, reduces hair pulling in trichotillomania: A pilot study. Psychopharmacology 2011, 218, 493–502. [Google Scholar]
- Gerasymchuk, M.; Robinson, G.I.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Kovalchuk, O.; Kovalchuk, I. Phytocannabinoids stimulate rejuvenation and prevent cellular senescence in human dermal fibroblasts. Cells 2022, 11, 3939. [Google Scholar]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive effect of Cannabis sativa L. Herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules 2021, 26, 802. [Google Scholar]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’agli, M.; Piazza, S.; Sangiovanni, E. Cannabis sativa and skin health: Dissecting the role of phytocannabinoids. Planta Med. 2022, 88, 492–506. [Google Scholar] [CrossRef]
- Eleleemy, M.; Amin, B.; Nasr, M.; Sammour, O. A succinct review on the therapeutic potential and delivery systems of Eugenol. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 290–311. [Google Scholar] [CrossRef]
- El-Far, A.H.; Mohamed, H.H.; Elsabagh, D.A.; Mohamed, S.A.; Noreldin, A.E.; Al Jaouni, S.K.; Alsenosy, A.A. Eugenol and carvacrol attenuate brain d-galactose-induced aging-related oxidative alterations in rats. Environ. Sci. Pollut. Res. 2022, 29, 47436–47447. [Google Scholar]
- Hwang, E.; Lin, P.; Ngo HT, T.; Yi, T.H. Clove attenuates UVB-induced photodamage and repairs skin barrier function in hairless mice. Food Funct. 2018, 9, 4936–4947. [Google Scholar] [CrossRef]
- Zanikov, T.; Gerasymchuk, M.; Gojani, E.G.; Robinson, G.I.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cameron, M.; et al. The effect of combined treatment of psilocybin and eugenol on lipopolysaccharide-induced brain inflammation in mice. Molecules 2023, 28, 2624. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The therapeutic potential of psilocybin. Molecules 2021, 26, 2948. [Google Scholar]
- Halberstadt, A.L. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015, 277, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-L.; Liang, C.-S.; Yang, F.-C.; Tu, Y.-K.; Hsu, C.-W.; Carvalho, A.F.; Stubbs, B.; Thompson, T.; Tsai, C.-K.; Yeh, T.-C.; et al. Trajectory of antidepressant effects after single-or two-dose administration of psilocybin: A systematic review and multivariate meta-analysis. J. Clin. Med. 2022, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pham, M.; Panenka, W.J.; Honer, W.G.; Barr, A.M. Chronic treatment with Psilocybin decreases changes in body weight in a rodent model of obesity. Front. Psychiatry 2022, 13, 891512. [Google Scholar] [CrossRef] [PubMed]
- Germann, C.B. The Psilocybin-Telomere Hypothesis: An empirically falsifiable prediction concerning the beneficial neuropsychopharmacological effects of psilocybin on genetic aging. Med. Hypotheses 2020, 134, 109406. [Google Scholar] [CrossRef]
- Park, H.-J.; Ahn, S.; Lee, H.; Hahm, D.; Kim, K.; Yeom, M. Acupuncture ameliorates not only atopic dermatitis-like skin inflammation but also acute and chronic serotonergic itch possibly through blockade of 5-HT2 and 5-HT7 receptors in mice. Brain Behav. Immun. 2021, 93, 399–408. [Google Scholar] [CrossRef]


| Disease | Etiology | Gender Predisposition | Age | Pathogenesis | Cutaneous Manifestations | Management | Adverse Effects |
|---|---|---|---|---|---|---|---|
| Skin diseases with gender predisposition | |||||||
| Acne Vulgaris [39] | Cutibacterium acnes (formerly known as Propionibacterium) | ♀ > ♂ | Adolescent | Increased androgen production during puberty; genetics, diets, drugs—predisposing factors [39] | Papules, pustules, cysts, nodules, comedones | Topical: salicylic acid, benzoyl peroxide, retinoids, glycolic acid; antibiotics: Doxycycline | Scarring, major depressive disorder, poor self-image |
| Actinic (solar) keratosis [40,41] | Sun exposure—UV radiation accumulated over time | ♂ | Elderly—as a result of cumulative lifetime exposure | The intraepidermal proliferation of dysplastic keratinocytes | Hyperkeratotic scaly clusters of papules and patches | Cryotherapy, shave excision, 5-Fluorouracil cream, Imiquimod | Often precancerous conditions evolve into squamous cell carcinoma |
| Atopic Dermatitis (eczema) [42,43] | Multifactorial—immune dysregulation (IgE) and environmental factors | Adult ♀; preschool children show insignificant gender difference [43] | Approximately 45% of all cases begin within the first 6 months of life, 60%—the first year of life, 85%—before 5 years of age | Filaggrin gene mutation, which leads to TEWL and pH alteration | Xerosis, itchy and lichenified (thickened) skin | Topical corticosteroid, calcineurin inhibitors | Lichenification of the skin, increased cutaneous markings |
| Bullous Pemphigoid (BP) [18,40] | Autoantibodies against hemidesmosomes—BP antigen 180 (BP180 or type XVII collagen) and BP230 [40] | ♀ > ♂ | >60 years | Triggered by trauma, UV irradiation, burns and drugs | Itchy papules, patches, vesicles, or bullae | Oral and topical corticosteroids, Tacrolimus, Doxycycline, Azathioprine | Bacterial superinfection |
| Ichthyosis Vulgaris [18] | Loss of function in the Filaggrin gene | ♀ > ♂ | Neonates | Filaggrin mutation leads to impaired epidermal hydration, decreased hydrolysis of DHEA and DHEAS | Reduced skin moisturization, scaly skin, xerosis (skin dryness), lichenification | Long baths to remove scales, keratolytics (e.g., urea, alpha-hydroxy acid, salicylic acid | Skin allergies, bacterial super infection |
| Psoriasis [44,45] | Immune-mediated | ♀ = ♂, but earlier onset in ♀ | Bimodal distribution: 30–39 years and 60–69 years; 10 years earlier in women [44] | Often multifactorial, but genetics is a primary contributor in early-onset (<40 years) plaque appearance | Pink plaques, pustules, and silvery-white scales affecting the extensor surfaces (elbows and knees), trunk and scalp | Topical corticosteroids, vitamin D analog, phototherapy, psoralen and UV A radiation; Methotrexate, Cyclosporine, Dimethyl fumarate and Apremilast [45] | Psoriatic arthropathy, cardiovascular and hepatic diseases |
| Senile Purpura [40] | Trauma to blood vessels in the skin | ♀ = ♂ | Elderly | Increased vessel fragility due to atrophy of the dermis | Dark purple well-demarcated macules | Sunscreen, vitamin K, topical retinoids | Resolves spontaneously |
| Systemic Lupus Erythematosus (SLE) [46] | Autoantibodies directed against ANA, dsDNA | ♀ | Reproductive age (middle-aged women) | Immune dysregulation results in increased cytokine activity, T and B-cells activation, and immune complex deposition, which leads to cell death and accumulation of cellular debris [46] | Malar rash, erythematous macules and papules typically in sun-exposed areas with nasolabial sparing | Sunscreens, topical corticosteroids, hydroxychloroquine, dapsone, oral vitamin A derivatives, Methotrexate, Mycophenolate mofetil, Azathioprine | Skin scarring, arthropathy, renal failure, cardiovascular complications |
| Premature aging diseases | |||||||
| Hutchinson-Gilford Progeria syndrome (HGPS) [47] | Mutation in LMNA and ZMPSTE24 genes that results in impaired Lamin A | ♀ = ♂ | Infants | De novo point mutations. These children look normal at birth but develop symptoms within a year | Atrophied, wrinkled and dry skin; alopecia | Anticancer medications—Farnesyltransferase inhibitors (FTIs), associated with increased prelamin A [47] | Atherosclerosis, osteoporosis, decreased life expectancy (≈13 years) |
| Rothmund-Thomson syndrome (RTS) or poikiloderma congenitale [48,49] | Mutation in a RecQ-like helicase RECQL4 and ANAPC1 | ♀ = ♂ | Infants (3–6 months) | RTS is an autosomal recessive with a defect of the DNA repair [49] | Growth deficiency, gray hair, cataracts, atrophic skin areas with hypopigmentation/hyperpigmentation changes and telangiectasias (poikiloderma), osteosarcomas, skin cancers | Laser treatment of telangiectasias; surgical cure of cataracts; complementary cancer therapy; growth hormone; sunscreens. | Osteosarcoma, basal cell carcinoma, squamous cell carcinoma, and intraepidermal carcinoma (Bowen disease) cataracts, bone fractures |
| Werner syndrome (WS) [48,49] | WRN mutation | ♀ = ♂ | The early third decade of life | WS is an autosomal recessive disorder with a defected telomere maintenance, DNA recombination and repair | Atrophic skin, loss of cutaneous firmness, wrinkles, thin gray hair, hair loss all over the body, osteoporosis, type II diabetes, autoimmunity, skin and muscle atrophy, poor wound healing, cataracts, atherosclerosis, hypogonadism, cancer [49] | Vitamin C, management of symptoms and complications | Skin ulcers, dental pathologies, diabetes, infertility, cancer nd myocardial infarction; life expectancy (≈40–50) |
| Xeroderma pigmentosum (XP) [48,50,51] | XPA and XPC genes mutation | ♀ = ♂ | Early infancy, around 1–2 years | XP is an autosomal recessive disorder accompanied by defected NER pathway, which enables them to repair DNA damage caused by UV light due to poor CPD repair [50,51] | Hypersensitivity to UV exposure, pigment alterations, high incidence of skin and eye cancer and neurological abnormalities. Other skin manifestations including poikiloderma, skin atrophy, telangiectasia, actinic keratoses, angiomas, and keratoacanthomas | Vitamin D, sunscreen with an SPF 50 and higher, UV-blocking sunglasses, avoidance of cigarette smoke; surgical treatment (excision) of precancerous and cancerous lesions | Increased incidence in childhood melanoma—10,000-fold |
| Surgical Procedures | Total Procedures | Surgical Procedures | Total Procedures |
|---|---|---|---|
| Women | Men | ||
| Liposuction | 458,628 | Liposuction | 30,806 |
| Breast augmentation: | 362,346 | Gynecomastia (male breast reduction) | 22,467 |
| Abdominoplasty (tummy tuck) | 234,696 | Blepharoplasty | 18,688 |
| Mastopexy (breast lift) | 165,968 | Rhinoplasty (nose) | 10,487 |
| Removal replacement of breast implants | 146,731 | Abdominoplasty (tummy tuck) | 7335 |
| Blepharoplasty | 130,489 | Facelift | 5061 |
| Top surgical—by age | |||
| Surgical procedures | Count | Age | % of surgical procedures |
| Breast augmentation | 162,700 | 17–35 | 25% |
| Liposuction | 251,500 | 36–50 | 41% |
| Liposuction | 108,100 | 51–70 | 31% |
| Blepharoplasty | 15,000 | 71+ | 3% |
| Non-Surgical Procedures | Total Procedures | Non-Surgical Procedures | Total Procedures |
|---|---|---|---|
| Women | Men | ||
| Neurotoxins | 3,474,160 | Neurotoxins | 155,882 |
| Dermal fillers | 1,777,989 | Dermal fillers | 69,450 |
| Skin treatment (hydrofacials, chemical peels, etc.) | 1,323,811 | Skin treatment (hydrofacials, chemical peels, etc.) | 47,999 |
| Hair removal | 423,861 | Skin treatment (Combination lasers) | 28,824 |
| Skin treatment (Combination lasers) | 400,255 | Hair removal | 26,430 |
| Skin tightening | 374,030 | Fat reduction | 22,513 |
| Percentage of non-surgical—by age | |||
| Age | Percentage of procedures | ||
| 17–35 | 17% | ||
| 36–50 | 41% | ||
| 51–70 | 37% | ||
| 71+ | 5% | ||
| Disease or Pathologic Condition | Target Receptor(s)/Components of ECS | Treatment | Tested Object/Type of Study |
|---|---|---|---|
| Acne vulgaris | TRPV4 | CBD | In vitro (human immortalized SZ95 sebocytes) [24,103] |
| Allergic contact dermatitis | CB1, CB2, FAAH | WOBE440, WOBE479 | In vitro (primary normal human epidermal keratinocytes and immortalized (HPV-KER) human epidermal keratinocytes); in vivo (NC/Tnd mice) [104] |
| Asteatotic eczema (dermatitis) | PPAR-α | PEA/AEA | Clinical trial (double-blind, randomized study—66 participants) [105] |
| Atopic dermatitis | CB1, CB2 | Ec. Extract, CBD | In vitro (HaCaT keratinocytes); Clinical trial (pre-post observational study) [106,107] |
| Cutaneous Lupus Erythematosus | CB1, CB2 | AEA-np | In vivo (MRL-Lpr/Lpr mice) [108] |
| Chronic pruritus | PPAR-α | PEA | Clinical trials: (1) open application observation—22 participants; (2) multinational, multicentre, observational, non-controlled, prospective cohort study—2456 participants [109,110] |
| Dermatomyositi | CB2 | Ajulemic acid/lenabasum, a CB2R agonist | In vitro (peripheral blood mononuclear cells) [111] |
| Epidermolysis bullos | CB2, TRPV1 | Cannabidiol oil | 3 clinical case reports [112] |
| Kaposi Sarcoma | CB1, CB2 | CB1/CB2 agonist WIN-55,212–2 | In vitro (human Kaposi’s sarcoma cell line KS-IMM) [113] |
| Melanoma | CB1, CB2 | Sativex (1:1 ratio of THC and CBD) | In vitro (melanoma cell lines CHL-1, A375, and SK-MEL-28) [114] |
| Photodamage | CB1, CB2, HSP90 | CBD, 17AAG | In vitro (human epidermal keratinocytes, CDD 1102 KERTr); In vivo (SKH-1 hairless mice) [115,116] |
| Psoriasis | CB1, CB2 | THC distillate, CBD shampoo | Clinical case report of a 33-year-old male patient; Clinical trial (50—participants) [117,118] |
| Systemic sclerosis | CB2 | Oral lenabasum (an agonist of CB2) | Clinical trial (randomized, double-blind, placebo-controlled, phase II study—42 participants) [119] |
| Trichotillomania | CB1, CB2 | Dronabinol 2.5–15 mg/day | Clinical trial (pilot study—14 subjects) [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasymchuk, M.; Robinson, G.I.; Vardinska, N.; Ayedun, S.A.; Alozie, S.C.; Robinson, J.W.; Kovalchuk, O.; Kovalchuk, I. Sex-Dependent Skin Aging and Rejuvenation Strategies. Dermato 2023, 3, 196-223. https://doi.org/10.3390/dermato3030016
Gerasymchuk M, Robinson GI, Vardinska N, Ayedun SA, Alozie SC, Robinson JW, Kovalchuk O, Kovalchuk I. Sex-Dependent Skin Aging and Rejuvenation Strategies. Dermato. 2023; 3(3):196-223. https://doi.org/10.3390/dermato3030016
Chicago/Turabian StyleGerasymchuk, Marta, Gregory Ian Robinson, Nataliia Vardinska, Samuel Abiola Ayedun, Sandra Chinwe Alozie, John Wesley Robinson, Olga Kovalchuk, and Igor Kovalchuk. 2023. "Sex-Dependent Skin Aging and Rejuvenation Strategies" Dermato 3, no. 3: 196-223. https://doi.org/10.3390/dermato3030016
APA StyleGerasymchuk, M., Robinson, G. I., Vardinska, N., Ayedun, S. A., Alozie, S. C., Robinson, J. W., Kovalchuk, O., & Kovalchuk, I. (2023). Sex-Dependent Skin Aging and Rejuvenation Strategies. Dermato, 3(3), 196-223. https://doi.org/10.3390/dermato3030016









