Abstract
Advanced cutaneous squamous cell carcinoma (cSCC) can be a life-threatening disease for which effective and safe treatment in advanced stages is very limited. GP-2250 has been recently proven to have—in vitro and in vivo—antineoplastic effects on cancer cells. This study aims to investigate the potential anti-neoplastic effects of GP-2250 on the cSCC cell lines SCC13 and A431 through dose finding assessments, MTT cytotoxicity assays, cell migration assays, BrdU proliferation assays and FCM analysis. Our preliminary results have shown for the first time evidence for anti-neoplastic effects of GP-2250 on cSCC cells, enhancing cytotoxicity, attenuating cancer cell proliferation, inducing apoptosis and reducing tumour cell migration. Further investigations evaluating the modes of action of GP-2250 on cSCC cell lines are warranted in order to justify the use in vivo studies.
1. Introduction
Non-melanoma skin cancer (NMSC) represents the most common diagnosed cancer in Caucasian populations. Cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma (BCC) are the most frequent NMSCs, representing about 20% and 80%, respectively, in Western countries [1]. In the past decades, the prevalence of cSCC showed a rapidly increasing trend with an increased incidence by 50% and up to 200% [2]. The incidence of cSCC is estimated to keep rising until the year 2040 [1,2]. In the United States, the incidence of cSCC is estimated to be 15–35/100.000 per year with an expected increase of 2–4% every year [1]. Similar increasing incidence rates of cSCC were reported in several European countries [3,4,5]. In New Zealand, the age-standardized incidence of cSCC even reached 305/100.000 patient years [6]. The actual cSCC burden is expected to be largely underestimated, due to its data omission from many cancer registries, recording of the primary tumour only or even due to absence of legal cSCC report obligation [3,4].
In contrast to BCC, a predominantly locally destructive cancer that rarely metastasises, cSCC poses a higher threat with its ability to evolve into a life-threatening disease. The larger the expansion and thickness, the higher the risk of a primary cSCC to metastasise. A total of up to 5% of all cSCC can invade regional lymph nodes and subsequently metastasise to distant tissue, leading to a very poor prognosis with a median survival of <2 years [1,2,7,8,9,10]. Although achieving an excellent overall prognosis in most patients with treatment by complete surgical excision, cSCC in advanced stages is usually impossible to treat by surgical excision alone [11]. In such situations, therapeutic options are still limited. Relatively unspecific cytotoxic agents, mainly including cisplatin, 5-fluorouracil and radiotherapy, are commonly used in this setting, alone or in combination [12,13]. These therapies, however, are associated with numerous serious side effects. Patients are diagnosed with advanced cSCC at a mean age of 70 years and are often affected with a variety of significant comorbidities [14,15]. Their general condition often precludes thus the implementation of any aggressive chemotherapy [1,16]. Previous standard therapies, including platin-based regimens, usually result in very poor outcomes with high morbidity, poor pregression-free survival rates, and low overall survival rates [1]. The epidermal growth factor receptor (EGFR) represents a more specific therapeutic target since it is often overexpressed and/or abnormally activated in several epithelial malignancies including cSCC [16]. However, the response rates of EGFR-inhibitors are rather disappointing in patients with cSCC. Recently, immunotherapies such as programmed cell death protein (PD-1)-blockade were approved by the US Food and Drug Administration (FDA) and European Medicine Agency (EMA) for the treatment of metastatic or locally advanced inoperable cSCC. Sustained response rates were observed in cSCC patients treated with PD-1 inhibitors, whereas therapy-induced long-term response is seen in after all about half of patients [10,11,17].
Novel promising non-toxic and effective agents are needed to enhance the therapeutic spectrum for patients with advanced cSCC. The new substance GP-2250 (Figure 1), a 1.4.5-oxathiazan-dioxid-4.4, is an aliphatic cyclic sulphonamide [18]. The classical, aromatic sulfonamides constitute a class of drugs, with several types of pharmacological agents unfolding antibacterial, hypoglycaemic, diuretic, and more recently also anti-neoplastic activities [19]. The novel substance GP-2250 forms a part of the 1.4.5-Oxathiazin derivatives, which are almost unexplored, whereas derivatives of 1.2.3-Oxathiazin are already identified, mainly as artificial sweeteners [18,20].
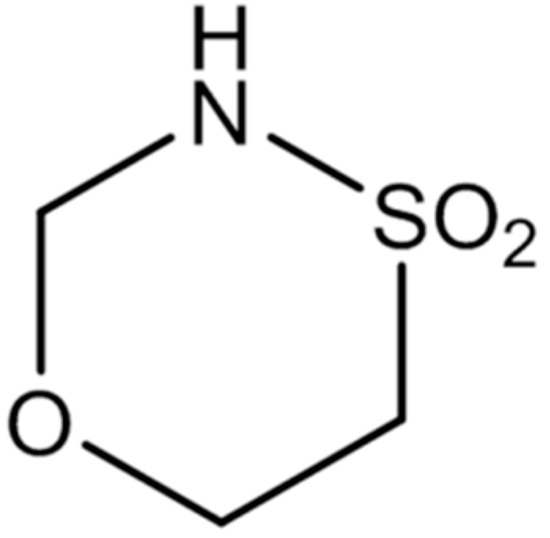
Figure 1.
Molecular structure of the oxathiazine derivative substance GP-2250 (1.4.5-oxathiazan-dioxid-4.4).
Two studies have been published so far, showing promising anti-neoplastic effects of the substance GP-2250 on malignant pancreatic carcinoma cells. GP-2250 inhibits pancreatic tumour cell proliferation, reduces tumour growth in vitro and in vivo, and induces apoptotic cell death [18,21]. To the best of our knowledge, this is the first study to provide an analysis of antineoplastic effects of the innovative substance GP-2250 on cSCC.
2. Materials and Methods
2.1. Cell Lines and Culture Methods
For all assays, two cSCC cell lines were used, namely SCC13 and A431. SCC13 are human, epidermal, tumorigenic, p16- and p53-deficient squamous carcinoma cells [22]. A431 are epidermoid, tumorigenic, p53-deficient squamous carcinoma cells, showing high EGFR expression [23]. The cSCC cell lines A431 and SCC13 were cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with the antibiotics’ penicillin (100 U/mL), streptomycin (100 U/mL) and 2 mM L-Glutamine. Cells were grown in cell culture dishes (diameter 100 mm, surface 60 cm2) in humidified 5% CO2 atmosphere at 37 °C and grown as monolayer for at least 72 h.
2.2. Substance
The ultrapure powder of GP-2250 (kindly provided by Geistlich Pharma AG, Wolhusen, Switzerland) was dissolved in double distilled water (ddH2O), set to a physiological pH and underwent sterile filtration. The substance was freshly prepared and controlled in functionality and reproducibility once per week.
2.3. Dose Finding Trials and Cell Characteristics
The different cell lines were incubated in a sub-confluent state with different concentrations of the substance, compared to an untreated control for 6, 12, 24 and 48 h, in order to determine the dose-effect-relation and to establish the most effective single concentration. Dose finding trials were performed using the MTT cytotoxicity assay, as described here below.
SCC13 are rapidly growing cSCC cells, which show a high proliferation rate. The most effective concentrations for all carried out SCC13 assays were for substance GP-2250 increasing dosages of 100, 150, 200, 300, 400 μmol/L. The A431 cells are slower proliferating cells than SCC13 cells. The most effective concentrations for all carried out A431 assays were therefore increasing dosages of 50, 100, 125, 150, 200 μmol/L.
2.4. MTT Assay
In order to analyse and quantify the anti-neoplastic effect of substance GP-2250 on cell viability and to determine its dose–response effect, colorimetric MTT assays were performed with both cell lines. Cells were seeded to a density of 5 × 103 cells/well in 96-well plates and incubated for 24 h in order to obtain a sub-confluent monolayer. The medium was then removed and cells were incubated for 6, 12, 24 and 48 h with new medium (100 µL/well) containing increasing concentrations of substance GP-2250 and ddH2O as negative control. The most effective dose–responses for SCC13 cells were 100, 150, 200, 300, 400 μmol/L of substance GP-2250 and for A431 cells 50, 100, 125, 150, 200 μmol/L. Two hours before measurement, the yellow coloured MTT reagent 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (5 mg/mL) was administrated. It is reduced through mitochondrial dehydrogenase of metabolic viable cells into violet coloured formazan crystals, which can be measured by photometry [24]. The test media were removed and 100 μL DMSO (Dimethyl sulfoxide) was applied. After an incubation time of 10 min the viability of cells was analysed by using a microplate absorbance reader (ASYS, UVM340, Anthos Mikrosystheme GmbH, Friesoythe, Germany), measuring the optical density. The amount of violet formazan crystals was directly proportional to the amount of viable cells [25]. For every cell line, six independent assays were performed with consecutive cell passages.
2.5. Cell Migration Assay
Cell motility of cSCC cells A431 and SCC13 was analysed using cell migration assays. Cells were seeded in 6-well culture plates to a density of 6 × 105 and grown to 100% confluence in approximately 24 h, depending on the cell line. An artificial gap of approximately 1100 μm wide was then generated using a p200 pipet tip and cells were incubated separately with substance GP-2250 and ddH2O for negative control. Duplicate testing was performed for all concentrations. For each cell line, the two closest concentrations to the IC50 (half maximal inhibitory concentration) were chosen: 100 and 200 μmol/L GP-2250 for cell line A431 and 100 and 150 μmol/L for the SCC13 cell line. Cells at the gap edge polarised and migrated into the gap space. The cell migration (gap closure) was recorded using differential interference contrast (DIC) microscopy in order to not only observe and measure the cell progression, but also to visualise the change in shape, size and behaviour of the incubated cells. Pictures were taken at 0, 6, 12 and 24 h.
2.6. BrdU Proliferation Assay, ELISA
In order to analyse and quantify the anti-proliferative effect of substance GP-2250, BrdU (5-bromo-2-deoxyuridine) proliferation assays, ELISA (Roche Applied Science, Mannheim, Germany), were performed with all cell lines, according to the manufacturer’s instructions (Version 16, content version: January 2013, Cat. No. 11 647 229 001). This proliferation assay was a non-isotopic colorimetric immunoassay for quantification of BrdU incorporation into newly synthesised DNA of actively proliferating cells.
Cells were seeded to a density of 8 × 103 cells/well in 96-well plates and incubated for 24 h in order to obtain a sub-confluent monolayer. The medium was then removed and cells were incubated for 6 h and 24 h with new medium (100 µL/well) containing increasing separate concentrations of substance GP-2250 and ddH2O as negative control. The most effective dose–responses for SCC13 cells were 100, 150, 200, 300, 400 μmol/L and for A431 cells 50, 100, 125, 150, 200 μmol/L. After the 6 or 24 h incubation period the BrdU reagent was added for additional 2 h, before cells were introduced to the BrdU proliferation assay (Roche Applied Science, Mannheim, Germany), as described by the manufacturer’s instructions. BrdU is a nucleoside analogue of (3H)-thymidine, which is incorporated into new strands of synthesised chromosomal DNA during the S-phase of the cell cycle [26]. The amount of cell proliferation was detected and measured via optical density, using a microplate absorbance reader (ASYS, UVM340, Anthos Mikrosystheme GmbH, Germany). All BrdU assays were performed with eight replicates of three independent experiments with consecutive passages. The incubation time of 6 h has been shown to be the most appropriate for the BrdU proliferation assays.
2.7. Flow Cytometry (FCM)
Cells were seeded to a density of 2 × 105 cells/well in 6-well plates and incubated for 24 h in order to obtain a sub-confluent monolayer. The medium was then removed and cells were incubated for 24 and 48 h with new medium containing increasing separate concentrations of substance GP-2250 and ddH2O as negative control. The most effective dose–responses for SCC13 cells were 100, 150, 200, 300, 400 μmol/L and for A431 cells 50, 100, 125, 150, 200 μmol/L.
Cell numbers were determined individually from each dose-dependent suspension, then fixed in 200 μL binding buffer (Bender MedSystems, Vienna, Austria) and 5 μL Annexin V-FITC (BD Biosciences, Heidelberg, Germany) was added. After 15 min incubation at room temperature and light deprivation 10 μL Propidium iodide (PI) (Bender MedSystems, Vienna, Austria) was added. Cells were immediately analysed for Annexin V-FITC and PI binding using a flow cytometer (FACS Calibur BD Biosciences, Heidelberg, Germany) and quantified through dot plots histograms analysed by CellQuest Pro software (BD Biosciences, Heidelberg, Germany). Viability was defined by Annexin V-FITC and PI negative, apoptosis by Annexin V-FITC positive and PI negative and necrosis by Annexin V-FITC negative and PI positive cells.
2.8. Statistical Analysis
The data of the anti-neoplastic effects of all MTT assays (percentage of viable cells), BrdU proliferation assays (percentage of proliferating cells) and FCM-analysis (percentage of viable, apoptotic and necrotic cells) were statistically analysed with the data processing GraphPad Prism software (version 8.0). The results are expressed as mean value (%) and its standard deviation (±SD%). For the statistical comparison between the experimental groups, considering normal distribution, a one-way ANOVA was performed, followed by a Turkey’s post hoc test. p-values less than 0.05 were considered statistically significant. Significance levels were categorised and indicated in the figures as follows: * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.001 and n.s. = not significant.
3. Results
3.1. Substance GP-2250 Shows a Significant Cytotoxic Effect on Both cSCC Cell Lines
MTT assays were conducted with SCC13 and A431 cell lines in order to analyse the effects of substance GP-2250 on cell viability. As indicated in Figure 2, both cell lines had been incubated for 24 h with different increasing concentrations of GP-2250: SCC13 cells were separately incubated with 100, 150, 200, 300 and 400 μmol/L; A431 cells with 50, 100, 125, 150 and 200 μmol/L. Controls were set to 100% and defined as baseline.
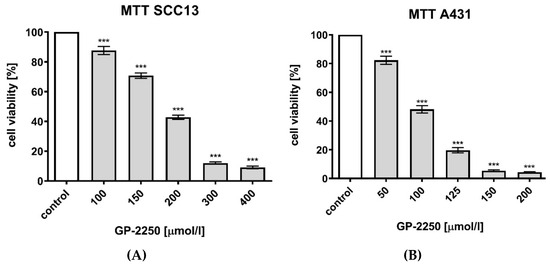
Figure 2.
Effects of Substance GP-2250 in different cSCC cell lines, measured by MTT assay. (A) SCC13 cells were incubated with GP-2250 (100, 150, 200, 300 and 400 μmol/L) for 24 h. (B) A431 cells were incubated with GP-2250 (50, 100, 125, 150 and 200 μmol/L) for 24 h. Values are expressed as mean value ± SD of 8 independent experiments. The asterisk symbols indicate significant differences between controls.
Compared to untreated controls (ddH2O), GP-2250 lead to a significant dose-dependent reduction of cell viability, as revealed by optical density measurement. The greater the concentration, the greater the cytotoxic effect of GP-2250 in both cell lines. Viability reduction of 50% or greater compared to the untreated control (100%) was obtained for SCC13 cells with a concentration of 150 μmol/L GP-2250 showing 42.74% (±1.43%) cell viability and for A431 cells with a concentration of 100 μmol/L showing 48.13% (±2.51%) (p < 0.001) cell viability.
The maximum dose of GP-2250 led to an intense cytotoxic effect in all cell lines, leading with a concentration of 400 μmol/L in SCC13 cells to a viability of 9.09% (±0.85%) and, respectively, with a concentration of 200 μmol/L in A431 cells to 4.20% (±0.47%) cell viability (Table 1). Even though concentrations were different in both cell lines, the dose-dependent effect of each substance on viable cells was comparable and proportional in both MTT groups. The IC50 value was determined by the dose of GP-2250 which caused a 50% reduction in cell numbers compared to controls. The IC50 for GP-2250 varied between the two cell lines. IC50 was obtained in SCC13 cells with a concentration of 190 μmol/L for substance GP-2250 and in A431 cells with concentrations of 96 μmol/L, respectively.

Table 1.
Results * of MTT and BrdU assays of GP-2250 in both cSCC cell lines (SCC13 and A431).
3.2. Substance GP-2250 Significantly Inhibits Proliferation in All cSCC Cell Lines
BrdU assays were conducted with both cSCC cell lines in order to analyse the effects of substance GP-2250 on cell proliferation. As depicted in Figure 3, both cell lines were incubated for 6 h with different increasing concentrations of GP-2250: SCC13 cells were incubated with 100, 150, 200, 300 and 400 μmol/L; A431 cells with 50, 100, 125, 150 and 200 μmol/L. Controls were set to 100% and defined as baseline.
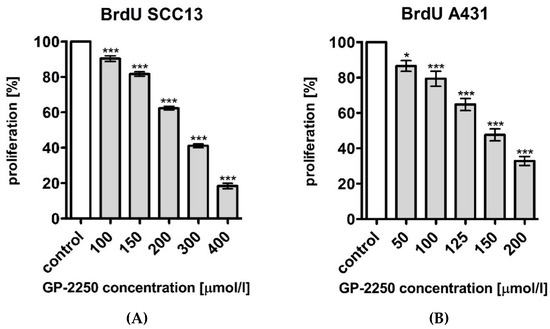
Figure 3.
Effects of Substance GP-2250 in different cSCC cell lines, measured by BrdU assay. (A) SCC13 cells were incubated with GP-2250 (100, 150, 200, 300 and 400 μmol/L) for 6 h. (B) A431 cells were incubated with GP-2250 (50, 100, 125, 150 and 200 μmol/L) for 6h. Values are expressed as mean value ± SD of three independent experiments. The asterisk symbols indicate significant differences between controls.
Compared to untreated controls (ddH2O), GP-2250 led to a significant dose-dependent reduction of cell proliferation, as revealed by optical density measurement. Lowest dosages of GP-2250 showed already a significant reduction of proliferated cells compared to the untreated control (Figure 3). The greater the concentration, the fewer the amount of proliferating cells. The highest dosages of GP-2250 showed a highly significant reduction of proliferated cells of more than 75% compared to the control in SCC13 cells, leading for a dosage of 400 μmol to 18.41% (±1.50%); a dosage of 200 μmol lead to 32.85% (±2.52) of proliferation in A431 cells. The dose-related effect of substance GP-2250 can be characterised as comparable and proportional in both cSCC cell lines.
3.3. Substance GP-2250 Significantly Induces Apoptotic Cell Death in Both cSCC Cell Lines
FCM analysis was conducted to evaluate the impact of substance GP-2250 on inducing apoptosis. SCC13 and A431 cells had been incubated for 24 h with substance GP-2250 in different concentrations, as summarised in Figure 4, and evaluated by FCM analysis, resulting in a significant viable cell reduction and increase in apoptotic and necrotic cells in comparison to controls (with ddH2O).
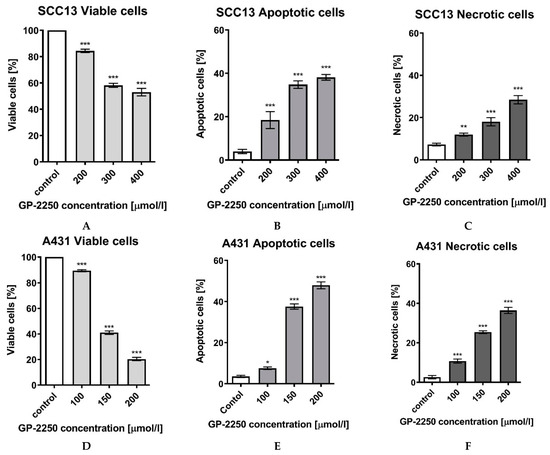
Figure 4.
Effects of Substance GP-2250 in different cSCC cell lines, measured by FCM-analysis. SCC13 cells were incubated with GP-2250 (200, 300 and 400 μmol/L) and A431 cells were incubated with GP-2250 (100, 150 and 200 μmol/L), both for 24h. The percentages of viable (A), apoptotic (B) and necrotic SCC13 cells (C) as well as viable (D), apoptotic (E) and necrotic A431 cells (F) were determined by FCM-analysis with Annexin V-FITC and Propidium iodide. Values are expressed as mean value ± SD of 6 independent experiments. The asterisk symbols indicate significant differences between controls.
Compared to the respective negative controls, both cell lines showed a significant total reduction of cell viability, being coherent to a significant increase in apoptotic cells in all cell lines (Figure 4). In SCC13 cells, a significant reduction of cell viability was observed at every dosage, reaching from 84.35% (±1.3%) at lowest dosage of 200 μmol/L GP-2250 to a cell viability of 52.89% (±2.9%) at a dosage of 400 μmol/L GP-2250.
In A431 cells, a stronger reduction of cell viability was observed, with a cell viability reduction to 20.35% (±1.2%) at highest dosage of 200 μmol/L GP-2250. Similar significant cell line dependent patterns were observed comparing the apoptotic and necrotic effect of substance GP-2250, showing a greater contribution of apoptosis on cell death than the one of necrosis in both cell lines. In the SCC13 cell line, the highest used dosages of GP-2250 lead to 38.15% (±1.3%) apoptotic cells and to 20.45% (±0.5%) of necrotic cells; in A431 cells 47.95% (±1.6%) and 36.46% (±1.6%), respectively (Figure 4).
3.4. Substance GP-2250 Reduces Tumor Cell Migration in Both cSCC Cell Lines
Cell migration assays were conducted with both cell lines in order to analyse and visualise the effects of substance GP-2250 on cell migration. Both cell lines were separately incubated with 100 and 150 μmol/L GP-2250 for the SCC13 cell line and 100 and 200 μmol/L, respectively, for the A431 cell line. Duplicate testing was performed for all chosen concentrations. Cell migration was recorded using DIC microscopy. Compared to untreated controls, rising concentrations of GP-2250 led to significant increasing anti-migratory effects (Figure 5). While the gaps of the control groups measured after 24 h 0.07% (±0.3%) in the SCC13 cells and in 6.36% (±0.3) in A431 cells, the percentages of gap closure were significantly higher with applied GP-2250 in both cell lines leading to 47.26% (±0.8%) gap closure at highest dosages in SCC13 cells and 73.03% (±1.0%) in A431 cells, respectively (Figure 5). Morphological changes in shape and size were observed after 24 h of incubation with substance GP-2250. Both cSCC cell lines exhibited cytoplasmic shrinkage and vacuolization on one hand and cytoplasmic swelling on the other hand.
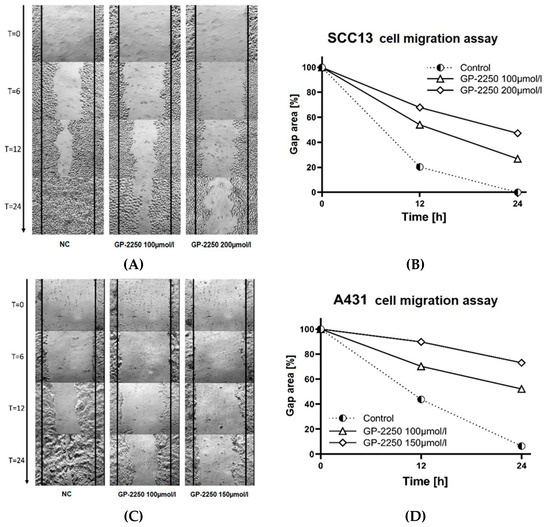
Figure 5.
The substance GP-2250 reduces cutaneous squamous carcinoma cell motility in the cell migration assay. Representative images of the SCC13 cells treated with GP-2250 or negative control at 0 h, 6 h, 12 h and 24 h after the scratches were made (×10 magnification) (A) and related percentage of total gap closure at 0 h, 12 h and 24 h (B) Representative images of the A431 cells transfected with GP-2250 or negative control at 0 h, 6 h, 12 h and 24 h after the scratches were made at the same starting point (×10 magnification) (C) and related percentage of total gap closure at 0 h, 12 h and 24 h (D).
Cells either detached from each other or floated in the medium. These findings suggest substance-induced apoptosis and, respectively, necrosis.
4. Discussion
The present pilot study was conducted in order to analyse and quantify for the first-time antineoplastic activities of substance GP-2250 in cSCC cell lines. Our experiments were based on the hypothesis that the substance GP-2250 could inhibit cell growth and proliferation of cSCC cells in vitro, as well as induce cell death. Furthermore, this study was designed to elucidate the treatment related effectiveness of GP-2250. Therefore, in the first part of this study, we determined dose–response characterization of substance GP-2250 regarding cell viability reduction. Dose-effects of GP-2250 were homogenous and proportional among both cell lines. As expected, comparing GP-2250 to untreated control groups, the substance revealed antineoplastic effects. Rising concentrations of substance GP-2250 led to significant and subsequently increasing antineoplastic effects compared to the untreated control groups in all carried out assays after 24 h: the maximal achieved viability reduction in A431 cells was 95.80% (±0.47) and in SCC13 cells 91.18% (±0.99) (Figure 2).
We then investigated the further antineoplastic effects of substance GP-2250 analysing related proliferation inhibition, cell motility reduction, as well as the relative contribution of apoptosis and necrosis during GP-2250-induced cell death. The same proportional dose-effect pattern of substance GP-2250 was detected after 24 h in anti-proliferative effects of BrdU analysis (Figure 3), FCM analysis (Figure 4) and observed in anti-migratory effects in cell migration assays, during 24 h with intermittent measurements (Figure 5). These proportional dose-effect patterns of substance GP-2250 are consistent with the ones in one recent GP-2250 study [18]. All the highest tested concentrations of GP-2250 (400 μmol/L for SCC13 and 200 μmol/L for A431 cells) lead to pronounced antineoplastic, anti-proliferative and apoptotic effects in all assays, significantly greater than lower administrated concentrations and compared to untreated controls. Minimum concentrations of 100 μmol/L GP-2250 in SCC13 cells and 50 μmol/L GP-2250 in A431 were observed to be the lowest doses capable of significantly reducing cell viability and inhibiting proliferation rates in both cell lines. Comparing these dosages of GP-2250 to the ones found in the study of Buchholz at al. [18] with comparable assays, similar study design and analogue handling of the assays (i.e., cell density, incubation time, measurements), we noticed that the effect-related concentrations used in this study were much lower. Buchholz at al. [18] showed comparable effects analysing the effect of substance GP-2250 on five different human pancreatic cancer cell lines (AsPC-1, BxPC-3, MiaPaca-2, Panc-1, Panc-TuI) with a higher concentration range from 100 to 2000 μmol/L. At a concentration of 1000 μmol/L, more than 50% reduction of viable cells was monitored in four out of five cell lines [18].
We observed that SCC13 cells required higher concentrations of GP-2250 than A431 cells in order to achieve comparable antineoplastic, anti-proliferative and apoptotic effects among the assays of the two cSCC cell lines (Table 1). Similar results were found in studies comparing A431 with SCC13 cell lines [27,28]. Analysing both cell lines offers a possible explanation to the divergence in cell responses, elucidating small differences between their origins and characteristics. Both cell lines are human, epidermal squamous carcinoma cells. While the A431 cells were originally derived from a 85-year-old female patient and characterised by p53-deficientcy [23], the SCC13 cells were derived from a primary facial cSCC tumour of a 56-year-old female patient and were p53- also p16-deficient, probably enhancing a quicker tumour cell replication, hence tumour proliferation [22,29,30,31]. Differences in dose-related effectiveness between SCC13 and A431 may further be due to inherent differences in cell turnover, unequal expression of EGFR and sensitivity to reactive oxygen species (ROS) [32,33,34]. Excessive ROS production during mitochondrial oxidative metabolism can lead to oxidative stress and macromolecular damage in cancer [35].
FCM analysis displayed in all cSCC cell lines a dose–response correlation concerning relative distributions of viable, apoptotic and necrotic cells. Rising concentrations of GP-2250 caused a decrease in viable cells and an increase in apoptosis and necrosis, with a predominant contribution of apoptosis to cell death. This dose-depending ratio of viable, apoptotic and necrotic cells correlates with the findings of Buchholz at al. [18]. Apoptosis leads to a caspase-dependent cell destruction with DNA fragmentation and cell shrinkage or to caspase-independent cell destruction with subsequent phagocytosis of the apoptotic cells. Considered the lack of phagocytosis in a cell culture setting due to absence of inflammatory cells, a secondary necrosis follows, an autolytic process of cell disintegration, characterised by the same appearance of primary necrosis. The necrosis marker (PI) used in this study could ultimately not differentiate between a substance-induced primary necrosis or secondary necrosis-due to the lack of phagocytosis. The third type of programmed cell death, next to autophagy and apoptosis, which is amongst others activated through ROS, is programmed necrosis, causing cell swelling and membrane rupture [36].
Previous studies have not only shown that the generation of ROS plays an important role in substance GP-2250-induced programmed cell death (PCD) [18], but also revealed that ROS expression under one and the same treatment differs in intensity in the same cancer type depending on whether tumour tissues were extracted from metastatic or non-metastatic cancer [37]. These insights could explain our previous findings, that one and the same concentration of substance GP-2250 had stronger antineoplastic effects on A431 than on SCC13 cells. Similar results are reflected in a couple of other studies, conducting amongst others comparative assays with the SCC13 and A431 cell lines [32,37].
Notably, this pilot study providing only preliminary results included some limitations that have to be addressed in future investigations in this field. Most importantly, additional control cell lines (e.g., keratinocytes) and mechanistic investigations have to be included in future studies. With respect to the possible issue of drug resistance development, consecutive rounds of GP-2250 applications should be carried out on surviving cSCC cells. Furthermore, in vitro cell invasion assays should be considered in future investigations in order to exclude that GP-2250 causes a paradoxical enhancement of cell invasion in the cSCC cell lines employed.
5. Conclusions
This is the first study evaluating the antineoplastic effects of substance GP-2250 on cSCC cell lines. We provide preliminary evidence for antineoplastic effects of GP-2250 against cSCC cells, enhancing cytotoxicity, attenuating cancer cell proliferation, inducing apoptosis and necrosis, as well as reducing tumour cell migration. Both cell lines were susceptible to substance GP-2250-induced cell death. Investigation into further effects of substance GP-2250 on the hallmarks of cancer, including precise mechanisms of action and pathways inducing cell death, as well as gene expression analysis in cSCC cell lines, appear to be warranted [38,39].
Author Contributions
M.B. (Milan Barras), T.G., C.B., M.S., M.B. (Marie Buchholz) and T.M. (Thomas Müller) contributed to the study conception and design. Material preparation, data collection, analysis, and interpretation were predominantly performed by M.B. (Milan Barras), C.B., L.S., W.U., M.S., M.B. (Marie Buchholz), T.M. (Thomas Meyer), E.S., J.C.B. and T.G. The first draft of the manuscript was written by M.B. (Milan Barras). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The cells were kindly provided by Dr. Ben Novak from the Institute of Animal Physiology, Ruhr-University Bochum, Germany.
Informed Consent Statement
Not applicable.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on reasonable request.
Acknowledgments
This work is part of the doctoral thesis of Milan Barras. The substance GP-2250 was kindly provided by Geistlich Pharma AG (Wolhusen, Switzerland).
Conflicts of Interest
The authors declare that they have no competing interest. T.M. (Thomas Müller) is employed by Geistlich Pharma AG, Wolhusen, Switzerland.
Correction Statement
This article has been republished with a minor correction of the information included in the Institutional Review Board Statement. This change does not affect the scientific content of the article.
References
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Eigentler, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Garbe, C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Investig. Dermatol. 2017, 137, 1860–1867. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Schnoor, M.; Eisemann, N.; Katalinic, A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. JDDG J. Dtsch. Dermatol. Ges. 2015, 13, 788–797. [Google Scholar] [CrossRef]
- Elliott, B.; Douglass, B.R.; McConnell, D.; Johnson, B.; Harmston, C. Incidence, demographics and surgical outcomes of cutaneous squamous cell carcinoma diagnosed in Northland, New Zealand. N. Z. Med. J. 2018, 131, 61–68. [Google Scholar]
- Samarasinghe, V.; Madan, V.; Lear, J.T. Management of high-risk squamous cell carcinoma of the skin. Expert Rev. Anticancer Ther. 2011, 11, 763–769. [Google Scholar] [CrossRef]
- Weinberg, A.S.; Ogle, C.A.; Shim, E.K. Metastatic Cutaneous Squamous Cell Carcinoma: An Update. Dermatol. Surg. 2007, 33, 885–899. [Google Scholar] [CrossRef]
- Pastuszek, A.; Hanson, M.; Grigg, R. Squamous cell carcinoma of the lip: Depth of invasion, local recurrence and regional metastases. Experience of a rural multidisciplinary head and neck unit. J. Laryngol. Otol. 2015, 130, S32–S37. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur. J. Cancer 2020, 128, 83–102. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Potenza, C.; Bernardini, N.; Balduzzi, V.; Losco, L.; Mambrin, A.; Marchesiello, A.; Tolino, E.; Zuber, S.; Skroza, N.; Proietti, I. A Review of the Literature of Surgical and Nonsurgical Treatments of Invasive Squamous Cells Carcinoma. BioMed Res. Int. 2018, 2018, 94891. [Google Scholar] [CrossRef]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef]
- Han, R.; Yang, Y.M.; Dietrich, J.; Luebke, A.; Mayer-Pröschel, M.; Noble, M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J. Biol. 2008, 7, 12. [Google Scholar] [CrossRef]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Krause-Bergmann, A.; Schulze, H.-J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns—Results of a non-interventional study of the DeCOG. Eur. J. Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.F.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Buchholz, M.; Majchrzak-Stiller, B.; Hahn, S.; Vangala, D.; Pfirrmann, R.W.; Uhl, W.; Braumann, C.; Chromik, A.M. Innovative substance 2250 as a highly promising anti-neoplastic agent in malignant pancreatic carcinoma—In vitro and in vivo. BMC Cancer 2017, 17, 216. [Google Scholar] [CrossRef]
- Casini, A.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C. Sulfonamides and Sulfonylated Derivatives as Anticancer Agents. Curr. Cancer Drug Targets 2002, 2, 55–75. [Google Scholar] [CrossRef]
- Lipinski, G.-W.V.R.; Clauß, K.; Lück, E. Acetosulfam, ein neuer Süßstoff. Eur. Food Res. Technol. 1976, 162, 37–40. [Google Scholar] [CrossRef]
- Braumann, C.; Buchholz, M.; Stiller, B.M.; Hahn, S.; Uhl, W.; Kasi, A.; Mueller, T. Metabolism-based GP-2250 in combination with gemcitabine as a novel approach to pancreatic cancer: A mouse xenograft study. J. Clin. Oncol. 2020, 38, e16750. [Google Scholar] [CrossRef]
- Degen, M.; Natarajan, E.; Barron, P.; Widlund, H.R.; Rheinwald, J.G. MAPK/ERK-Dependent Translation Factor Hyperactivation and Dysregulated Laminin γ2 Expression in Oral Dysplasia and Squamous Cell Carcinoma. Am. J. Pathol. 2012, 180, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Somers, K.D.; Merrick, M.A.; Lopez, M.E.; Incognito, L.S.; Schechter, G.L.; Casey, G. Frequent p53 mutations in head and neck cancer. Cancer Res. 1992, 52, 5997–6000. [Google Scholar] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Van Merloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Zhao, P.; Fu, J.L.; Yao, B.Y.; Jia, Y.R.; Zhou, Z.C. S phase cell percentage normalized BrdU incorporation rate, a new parameter for determining S arrest. Biomed. Environ. Sci. 2014, 27, 215–219. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P.; Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Adhikary, G.; Grun, D.; Kerr, C.; Balasubramanian, S.; Rorke, E.A.; Vemuri, M.; Boucher, S.; Bickenbach, J.R.; Hornyak, T.; Xu, W.; et al. Identification of a Population of Epidermal Squamous Cell Carcinoma Cells with Enhanced Potential for Tumor Formation. PLoS ONE 2013, 8, e84324. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar]
- Popp, S.; Waltering, S.; Herbst, C.; Moll, I.; Boukamp, P. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int. J. Cancer 2002, 99, 352–360. [Google Scholar] [CrossRef]
- Natarajan, E.; Saeb, M.; Crum, C.P.; Woo, S.B.; McKee, P.H.; Rheinwald, J.G. Co-Expression of p16INK4A and Laminin 5 γ2 by Microinvasive and Superficial Squamous Cell Carcinomas in Vivo and by Migrating Wound and Senescent Keratinocytes in Culture. Am. J. Pathol. 2003, 163, 477–491. [Google Scholar] [CrossRef]
- Austin, E.; Koo, E.; Jagdeo, J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci. Rep. 2018, 8, 12599. [Google Scholar] [CrossRef]
- Canueto, J.; Cardenoso, E.; Garcia, J.L.; Santos-Briz, A.; Castellanos-Martin, A.; Fernandez-Lopez, E.; Blanco Gomez, A.; Perez-Losada, J.; Roman-Curto, C. Epidermal growth factor receptor expression is associated with poor outcome in cutaneous squamous cell carcinoma. Br. J. Dermatol. 2017, 176, 1279–1287. [Google Scholar] [CrossRef]
- Grudinkin, P.S.; Zenin, V.V.; Kropotov, A.V.; Dorosh, V.N.; Nikolsky, N.N. EGF-induced apoptosis in A431 cells is dependent on STAT1, but not on STAT3. Eur. J. Cell Biol. 2007, 86, 591–603. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Vanlangenakker, N.; Parthoens, E.; Deckers, W.; Devos, M.; Festjens, N.; Guerin, C.J.; Brunk, U.T.; Declercq, W.; Vandenabeele, P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010, 17, 922–930. [Google Scholar] [CrossRef]
- Qin, X.; Kuang, H.; Chen, L.; Wei, S.; Yu, D.; Liang, F. Coexpression of growth differentiation factor 11 and reactive oxygen species in metastatic oral cancer and its role in inducing the epithelial to mesenchymal transition. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 697–706. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sasahira, T.; Kirita, T. Hallmarks of Cancer-Related Newly Prognostic Factors of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).