Violaceous Lesions on the Leg: What Else Apart from Kaposi Sarcoma? Differential Diagnosis with a Narrative Review of the Literature
Abstract
1. Introduction
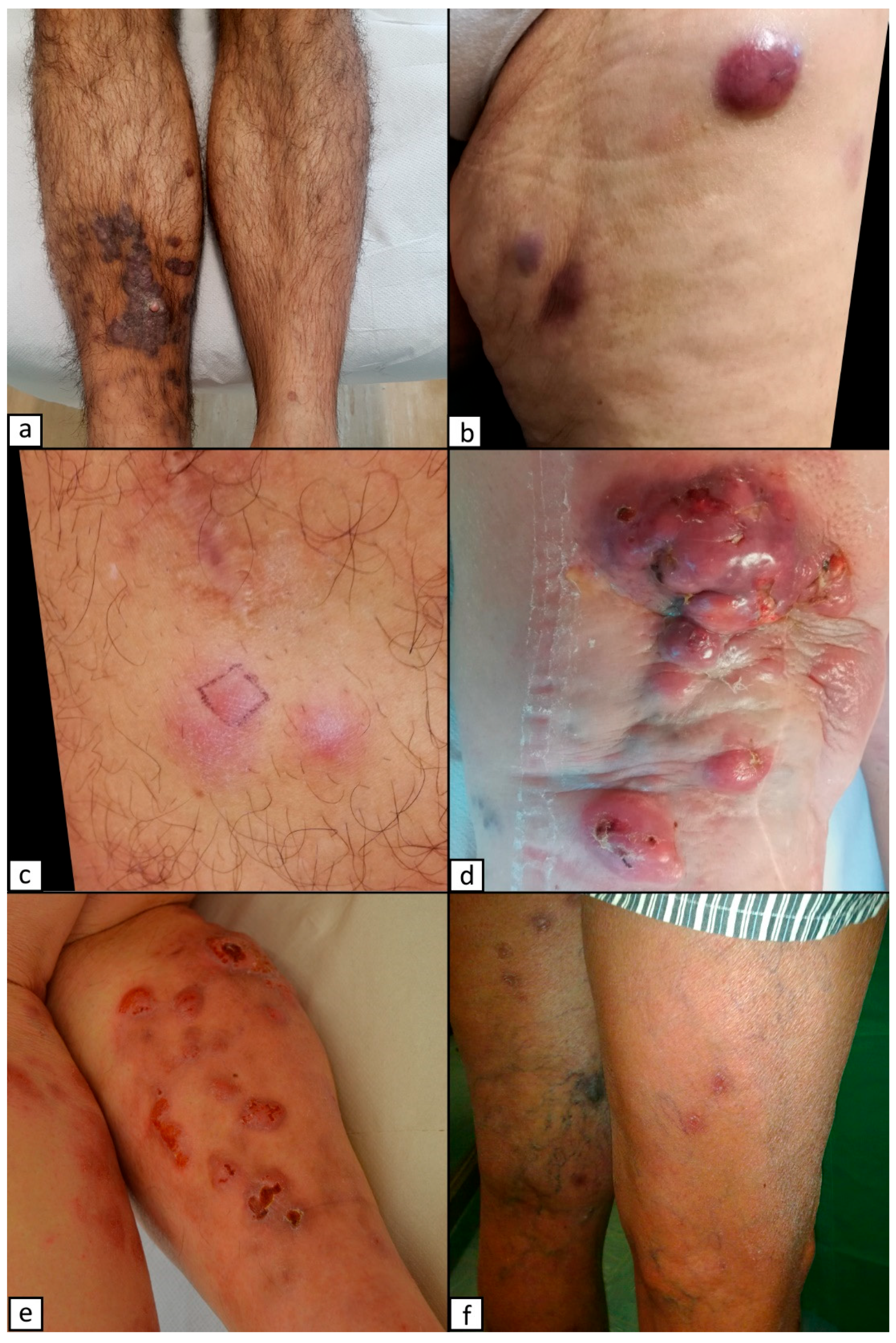
2. Kaposi Sarcoma
3. Pyogenic Granuloma
4. Amelanotic Melanoma
5. Pigmented Basal Cell Carcinoma (SCC)
6. Squamous Cell Carcinoma (SCC)
7. Lymphomatoid Papulosis (LyP)
8. Anaplastic Large Cell Lymphoma (ALCL)
9. Primary Cutaneous Marginal Zone Lymphoma
10. Primary Cutaneous Follicle Centre Lymphoma
11. Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type
12. Mycosis Fungoides
13. Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcoval, J.; Bonfill-Ortí, M.; Martínez-Molina, L.; Valentí-Medina, F.; Penín, R.M.; Servitje, O. Evolution of Kaposi Sarcoma in the Past 30 Years in a Tertiary Hospital of the European Mediterranean Basin. Clin. Exp. Dermatol. 2019, 44, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Etemad, S.A.; Dewan, A.K. Kaposi Sarcoma Updates. Dermatol. Clin. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Lodi, S.; Guiguet, M.; Costagliola, D.; Fisher, M.; de Luca, A.; Porter, K.; the CASCADE Collaboration. Kaposi Sarcoma Incidence and Survival Among HIV-Infected Homosexual Men After HIV Seroconversion. JNCI J. Natl. Cancer Inst. 2010, 102, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.K.; Linet, M.S.; Clarke, C.A.; Pawlish, K.S.; Engels, E.A.; Pfeiffer, R.M. Risk of Kaposi Sarcoma after Solid Organ Transplantation in the United States: Kaposi Sarcoma in Transplant Recipients. Int. J. Cancer 2018, 143, 2741–2748. [Google Scholar] [CrossRef]
- Parkin, D.M.; Sitas, F.; Chirenje, M.; Stein, L.; Abratt, R.; Wabinga, H. Part I: Cancer in Indigenous Africans—Burden, Distribution, and Trends. Lancet Oncol. 2008, 9, 683–692. [Google Scholar] [CrossRef]
- Brambilla, L.; Genovese, G.; Berti, E.; Peris, K.; Rongioletti, F.; Micali, G.; Ayala, F.; Della Bella, S.; Mancuso, R.; Calzavara Pinton, P.; et al. Diagnosis and Treatment of Classic and Iatrogenic Kaposi’s Sarcoma: Italian Recommendations. Ital J Dermatol Venerol 2021, 156, 356–365. [Google Scholar] [CrossRef]
- Silva, L.; Azurara, L.; Monteiro, A.F.; Miroux-Catarino, A.; Amaro, C.; Viana, I. Pediatric Kaposi’s Sarcoma Associated with Immune Reconstitution Inflammatory Syndrome. Pediatr. Dermatol. 2020, 37, 239–240. [Google Scholar] [CrossRef]
- El-Mallawany, N.K.; McAtee, C.L.; Campbell, L.R.; Kazembe, P.N. Pediatric Kaposi Sarcoma in Context of the HIV Epidemic in Sub-Saharan Africa: Current Perspectives. PHMT 2018, 9, 35–46. [Google Scholar] [CrossRef]
- Micali, G.; Nasca, M.R.; De Pasquale, R.; Innocenzi, D. Primary Classic Kaposi’s Sarcoma of the Penis: Report of a Case and Review. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 320–323. [Google Scholar] [CrossRef]
- Requena, C.; Alsina, M.; Morgado-Carrasco, D.; Cruz, J.; Sanmartín, O.; Serra-Guillén, C.; Llombart, B. Sarcoma de Kaposi y angiosarcoma cutáneo: Directrices para el diagnóstico y tratamiento. Actas Dermo-Sifiliográficas 2018, 109, 878–887. [Google Scholar] [CrossRef]
- Naimo, E.; Zischke, J.; Schulz, T.F. Recent Advances in Developing Treatments of Kaposi’s Sarcoma Herpesvirus-Related Diseases. Viruses 2021, 13, 1797. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Poizot-Martin, I.; Lions, C.; Cheret, A.; Rey, D.; Duvivier, C.; Jacomet, C.; Allavena, C.; Huleux, T.; Bani-Sadr, F.; Obry-Roguet, V.; et al. Kaposi Sarcoma in People Living with HIV: Incidence and Associated Factors in a French Cohort between 2010 and 2015. AIDS 2020, 34, 569–577. [Google Scholar] [CrossRef]
- Plachouri, K.-M.; Georgiou, S. Therapeutic Approaches to Pyogenic Granuloma: An Updated Review. Int. J. Dermatol. 2019, 58, 642–648. [Google Scholar] [CrossRef]
- Requena, L.; Sangueza, O.P. Cutaneous Vascular Proliferation. Part II. Hyperplasias and Benign Neoplasms. J. Am. Acad. Dermatol. 1997, 37, 887–919; quiz 920–922. [Google Scholar] [CrossRef]
- Megaly, M.; Boshra, N. Pyogenic Granuloma-like Kaposi’s Sarcoma. Lancet 2022, 399, e38. [Google Scholar] [CrossRef]
- Jha, A.K.; Sonthalia, S.; Khopkar, U. Dermoscopy of Pyogenic Granuloma. Indian Dermatol. Online J. 2017, 8, 523–524. [Google Scholar] [CrossRef]
- Kissou, A.; Hassam, B.E. Dermoscopy of Pyogenic Granuloma. Pan Afr. Med. J. 2017, 27, 110. [Google Scholar] [CrossRef]
- Zaballos, P.; Carulla, M.; Ozdemir, F.; Zalaudek, I.; Bañuls, J.; Llambrich, A.; Puig, S.; Argenziano, G.; Malvehy, J. Dermoscopy of Pyogenic Granuloma: A Morphological Study. Br. J. Dermatol. 2010, 163, 1229–1237. [Google Scholar] [CrossRef]
- Stojkovic-Filipovic, J.; Kittler, H. Dermatoscopy of Amelanotic and Hypomelanotic Melanoma. J. Dtsch. Dermatol. Ges. 2014, 12, 467–472. [Google Scholar] [CrossRef]
- Gong, H.-Z.; Zheng, H.-Y.; Li, J. Amelanotic Melanoma. Melanoma Res. 2019, 29, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.; Recule, F. Unusual Clinical Presentations of Malignant Melanoma: A Review of Clinical and Histologic Features with Special Emphasis on Dermatoscopic Findings. Am. J. Clin. Dermatol. 2018, 19 (Suppl. 1), 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.W.; Chamberlain, A.J.; Staples, M.P.; McAvoy, B. Nodular Melanoma. No Longer as Simple as ABC. Aust. Fam. Physician 2003, 32, 706–709. [Google Scholar] [PubMed]
- Sbano, P.; Nami, N.; Grimaldi, L.; Rubegni, P. True Amelanotic Melanoma: The Great Masquerader. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, e307–e308. [Google Scholar] [CrossRef]
- Dika, E.; Scarfì, F.; Ferracin, M.; Broseghini, E.; Marcelli, E.; Bortolani, B.; Campione, E.; Riefolo, M.; Ricci, C.; Lambertini, M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5572. [Google Scholar] [CrossRef]
- Naik, P.P.; Desai, M.B. Basal Cell Carcinoma: A Narrative Review on Contemporary Diagnosis and Management. Oncol. Ther. 2022, 10, 317–335. [Google Scholar] [CrossRef]
- Di Matteo, E.; Pampena, R.; Pizzichetta, M.A.; Cinotti, E.; Chester, J.; Kaleci, S.; Manfredini, M.; Guida, S.; Dika, E.; Moscarella, E.; et al. Unusual Dermoscopic Patterns of Basal Cell Carcinoma Mimicking Melanoma. Exp. Dermatol. 2022, 31, 890–898. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Karia, P.S.; Han, J.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Estimated Incidence of Disease, Nodal Metastasis, and Deaths from Disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef]
- Waldman, A.; Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous Cell Carcinoma of the Skin: Epidemiology, Classification, Management, and Novel Trends. Int. J. Dermatol. 2015, 54, 130–140. [Google Scholar] [CrossRef]
- Kim, C.; Ko, C.J.; Leffell, D.J. Cutaneous Squamous Cell Carcinomas of the Lower Extremity: A Distinct Subset of Squamous Cell Carcinomas. J. Am. Acad. Dermatol. 2014, 70, 70–74. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and P53 in the Onset of Skin Cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Paolino, G.; Donati, M.; Didona, D.; Mercuri, S.R.; Cantisani, C. Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines 2017, 5, 71. [Google Scholar] [CrossRef]
- Toumi, A.; Fazal, S.; Litaiem, N. Lymphomatoid Papulosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fauconneau, A.; Pham-Ledard, A.; Cappellen, D.; Frison, E.; Prochazkova-Carlotti, M.; Parrens, M.; Dalle, S.; Joly, P.; Viraben, R.; Franck, F.; et al. Assessment of Diagnostic Criteria between Primary Cutaneous Anaplastic Large-Cell Lymphoma and CD30-Rich Transformed Mycosis Fungoides; a Study of 66 Cases. Br. J. Dermatol. 2015, 172, 1547–1554. [Google Scholar] [CrossRef]
- El Shabrawi-Caelen, L.; Kerl, H.; Cerroni, L. Lymphomatoid Papulosis: Reappraisal of Clinicopathologic Presentation and Classification into Subtypes A, B, and C. Arch. Dermatol. 2004, 140, 441–447. [Google Scholar] [CrossRef]
- Tsuyama, N.; Sakamoto, K.; Sakata, S.; Dobashi, A.; Takeuchi, K. Anaplastic Large Cell Lymphoma: Pathology, Genetics, and Clinical Aspects. J. Clin. Exp. Hematop. 2017, 57, 120–142. [Google Scholar] [CrossRef]
- Kaseb, H.; Mukkamalla, S.K.R.; Rajasurya, V. Anaplastic Large Cell Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Grandi, V.; Alberti Violetti, S.; La Selva, R.; Cicchelli, S.; Delfino, C.; Fava, P.; Fierro, M.T.; Pileri, A.; Pimpinelli, N.; Quaglino, P.; et al. Primary Cutaneous B-Cell Lymphoma: Narrative Review of the Literature. G. Ital. Dermatol. Venereol. 2019, 154, 466–479. [Google Scholar] [CrossRef]
- Goyal, A.; LeBlanc, R.E.; Carter, J.B. Cutaneous B-Cell Lymphoma. Hematol./Oncol. Clin. N. Am. 2019, 33, 149–161. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Jaffe, E.S. Navigating the Cutaneous B-Cell Lymphomas: Avoiding the Rocky Shoals. Mod. Pathol. 2020, 33 (Suppl. 1), 96–106. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Patrizi, A.; Agostinelli, C.; Neri, I.; Sabattini, E.; Bacci, F.; Piccaluga, P.P.; Pimpinelli, N.; Pileri, S.A. Primary Cutaneous Lymphomas: A Reprisal. Semin. Diagn. Pathol. 2011, 28, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Battistella, M.; Ram-Wolff, C.; Bagot, M.; de Masson, A. Diagnosis and Treatment of Primary Cutaneous B-Cell Lymphomas: State of the Art and Perspectives. Cancers 2020, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 Update of the WHO-EORTC Classification for Primary Cutaneous Lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Vitiello, P.; Sica, A.; Ronchi, A.; Caccavale, S.; Franco, R.; Argenziano, G. Primary Cutaneous B-Cell Lymphomas: An Update. Front. Oncol. 2020, 10, 651. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, H.-T.; Lee, D.-Y.; Lee, J.-H.; Yang, J.-M.; Jang, K.-T.; Ko, Y.-H. World Health Organization–European Organization for Research and Treatment of Cancer Classification of Cutaneous Lymphoma in Korea: A Retrospective Study at a Single Tertiary Institution. J. Am. Acad. Dermatol. 2012, 67, 1200–1209. [Google Scholar] [CrossRef]
- Malachowski, S.J.; Sun, J.; Chen, P.-L.; Seminario-Vidal, L. Diagnosis and Management of Cutaneous B-Cell Lymphomas. Dermatol. Clin. 2019, 37, 443–454. [Google Scholar] [CrossRef]
- Bradford, P.T.; Devesa, S.S.; Anderson, W.F.; Toro, J.R. Cutaneous Lymphoma Incidence Patterns in the United States: A Population-Based Study of 3884 Cases. Blood 2009, 113, 5064–5073. [Google Scholar] [CrossRef]
- Markova, A.; Weinstock, M.A. Trends in Cutaneous Lymphoma Epidemiology. Clin. Lymphoma Myeloma Leuk. 2010, 10, S63–S66. [Google Scholar] [CrossRef]
- Hamada, T.; Iwatsuki, K. Cutaneous Lymphoma in Japan: A Nationwide Study of 1733 Patients. J. Dermatol. 2014, 41, 3–10. [Google Scholar] [CrossRef]
- Korgavkar, K.; Weinstock, M.A. Changing Incidence Trends of Cutaneous B-Cell Lymphoma. J. Investig. Dermatol. 2014, 134, 840–842. [Google Scholar] [CrossRef]
- Servitje, O.; Climent, F.; Colomo, L.; Ruiz, N.; García-Herrera, A.; Gallardo, F.; Mercadal, S.; Pomares, H.; Muniesa, C.; Martin-Callizo, C.; et al. Primary Cutaneous vs Secondary Cutaneous Follicular Lymphomas: A Comparative Study Focused on BCL2, CD10, and t(14;18) Expression. J. Cutan. Pathol. 2019, 46, 182–189. [Google Scholar] [CrossRef]
- Suárez, A.L.; Pulitzer, M.; Horwitz, S.; Moskowitz, A.; Querfeld, C.; Myskowski, P.L. Primary Cutaneous B-Cell Lymphomas. J. Am. Acad. Dermatol. 2013, 69, e1–e329. [Google Scholar] [CrossRef]
- Chen, S.T.; Barnes, J.; Duncan, L. Primary Cutaneous B-Cell Lymphomas— Clinical and Histopathologic Features, Differential Diagnosis, and Treatment. Sem. Cutan. Med. Surg. 2018, 37, 49–55. [Google Scholar] [CrossRef]
- Hope, C.B.; Pincus, L.B. Primary Cutaneous B-Cell Lymphomas with Large Cell Predominance–Primary Cutaneous Follicle Center Lymphoma, Diffuse Large B-Cell Lymphoma, Leg Type and Intravascular Large B-Cell Lymphoma. Semin. Diagn. Pathol. 2017, 34, 85–98. [Google Scholar] [CrossRef]
- Pileri, A.; Agostinelli, C.; Bertuzzi, C.; Grandi, V.; Maio, V.; Lastrucci, I.; Santucci, M.; Pimpinelli, N. BCL-2 Expression in Primary Cutaneous Follicle Center B-Cell Lymphoma and Its Prognostic Role. Front. Oncol. 2020, 10, 662. [Google Scholar] [CrossRef]
- Pileri, A.; Guglielmo, A.; Fuligni, F.; Lastrucci, I.; Patrizi, A.; Pimpinelli, N.; Italian Lymphoma Foundation (FIL)-Cutaneous Lymphoma Task Force. Second Neoplasm in Cutaneous T-Cell Lymphoma Patients: A Marker of Worse Prognosis? Ital. J. Dermatol. Venerol. 2021, 156, 484–488. [Google Scholar] [CrossRef]
- Szablewski, V.; Ingen-Housz-Oro, S.; Baia, M.; Delfau-Larue, M.-H.; Copie-Bergman, C.; Ortonne, N. Primary Cutaneous Follicle Center Lymphomas Expressing BCL2 Protein Frequently Harbor BCL2 Gene Break and May Present 1p36 Deletion: A Study of 20 Cases. Am. J. Surg. Pathol. 2016, 40, 127–136. [Google Scholar] [CrossRef]
- Kim, B.K.; Surti, U.; Pandya, A.; Cohen, J.; Rabkin, M.S.; Swerdlow, S.H. Clinicopathologic, Immunophenotypic, and Molecular Cytogenetic Fluorescence In Situ Hybridization Analysis of Primary and Secondary Cutaneous Follicular Lymphomas. Am. J. Surg. Pathol. 2005, 29, 69–82. [Google Scholar] [CrossRef]
- Lucioni, M.; Berti, E.; Arcaini, L.; Croci, G.A.; Maffi, A.; Klersy, C.; Goteri, G.; Tomasini, C.; Quaglino, P.; Riboni, R.; et al. Primary Cutaneous B-Cell Lymphoma Other than Marginal Zone: Clinicopathologic Analysis of 161 Cases: Comparison with Current Classification and Definition of Prognostic Markers. Cancer Med. 2016, 5, 2740–2755. [Google Scholar] [CrossRef]
- de Leval, L.; Harris, N.L.; Longtine, J.; Ferry, J.A.; Duncan, L.M. Cutaneous B-Cell Lymphomas of Follicular and Marginal Zone Types: Use of Bcl-6, CD10, Bcl-2, and CD21 in Differential Diagnosis and Classification. Am. J. Surg. Pathol. 2001, 25, 732–741. [Google Scholar] [CrossRef]
- Pham-Ledard, A.; Cowppli-Bony, A.; Doussau, A.; Prochazkova-Carlotti, M.; Laharanne, E.; Jouary, T.; Belaud-Rotureau, M.-A.; Vergier, B.; Merlio, J.-P.; Beylot-Barry, M. Diagnostic and Prognostic Value of BCL2 Rearrangement in 53 Patients With Follicular Lymphoma Presenting as Primary Skin Lesions. Am. J. Clin. Pathol. 2015, 143, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahab, A.; Tang, S.-Y.; Robson, A.; Morris, S.; Agar, N.; Wain, E.M.; Child, F.; Scarisbrick, J.; Neat, M.; Whittaker, S. Chromosomal Anomalies in Primary Cutaneous Follicle Center Cell Lymphoma Do Not Portend a Poor Prognosis. J. Am. Acad. Dermatol. 2014, 70, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Gulia, A.; Saggini, A.; Wiesner, T.; Fink-Puches, R.; Argenyi, Z.; Ferrara, G.; Müller, C.S.L.; Vale, E.; Cerroni, L. Clinicopathologic Features of Early Lesions of Primary Cutaneous Follicle Center Lymphoma, Diffuse Type: Implications for Early Diagnosis and Treatment. J. Am. Acad. Dermatol. 2011, 65, 991–1000.e7. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Massone, C.; Chott, A.; Metze, D.; Kerl, H.; Cerroni, L. Primary Cutaneous Large B-Cell Lymphomas: Clinicopathologic Features, Classification, and Prognostic Factors in a Large Series of Patients. Blood 2005, 106, 2491–2497. [Google Scholar] [CrossRef]
- Cerroni, L.; Volkenandt, M.; Rieger, E.; Soyer, H.P.; Kerl, H. Bcl-2 Protein Expression and Correlation with the Interchromosomal 14;18 Translocation in Cutaneous Lymphomas and Pseudolymphomas. J. Investig. Dermatol. 1994, 102, 231–235. [Google Scholar] [CrossRef]
- Cyrenne, B.M.; Lewis, J.M.; Weed, J.G.; Carlson, K.R.; Mirza, F.N.; Foss, F.M.; Girardi, M. Synergy of BCL2 and Histone Deacetylase Inhibition against Leukemic Cells from Cutaneous T-Cell Lymphoma Patients. Blood 2017, 130, 2073–2083. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Quaglino, P.; Pimpinelli, N.; Berti, E.; Baliva, G.; Rupoli, S.; Martelli, M.; Alaibac, M.; Borroni, G.; Chimenti, S.; et al. Prognostic Factors in Primary Cutaneous B-Cell Lymphoma: The Italian Study Group for Cutaneous Lymphomas. JCO 2006, 24, 1376–1382. [Google Scholar] [CrossRef]
- Peñate, Y.; Hernández-Machín, B.; Pérez-Méndez, L.I.; Santiago, F.; Rosales, B.; Servitje, O.; Estrach, T.; Fernández-Guarino, M.; Calzado, L.; Acebo, E.; et al. Intralesional Rituximab in the Treatment of Indolent Primary Cutaneous B-Cell Lymphomas: An Epidemiological Observational Multicentre Study. The Spanish Working Group on Cutaneous Lymphoma: Intralesional Rituximab for Indolent PCBL. Br. J. Dermatol. 2012, 167, 174–179. [Google Scholar] [CrossRef]
- Perry, A.; Vincent, B.J.; Parker, S.R.S. Intralesional Corticosteroid Therapy for Primary Cutaneous B-Cell Lymphoma: Correspondence. Br. J. Dermatol. 2010, 163, 223–225. [Google Scholar] [CrossRef]
- Coors, E.A.; Schuler, G.; Von Den Driesch, P. Topical Imiquimod as Treatment for Different Kinds of Cutaneous Lymphoma. Eur. J. Dermatol. 2006, 16, 391–393. [Google Scholar]
- Paulli, M.; Viglio, A.; Vivenza, D.; Capello, D.; Rossi, D.; Riboni, R.; Lucioni, M.; Incardona, P.; Boveri, E.; Bellosta, M.; et al. Primary Cutaneous Large B-Cell Lymphoma of the Leg: Histogenetic Analysis of a Controversial Clinicopathologic Entity. Hum. Pathol. 2002, 33, 937–943. [Google Scholar] [CrossRef]
- Patrizi, A.; Raone, B.; Sabattini, E.; Gurioli, C.; Pileri, A., Jr.; D’Acunto, C. Primary Cutaneous Large B-Cell Lymphoma, Leg Type, Localized on the Dorsum. Case Rep. Dermatol. 2009, 1, 87–92. [Google Scholar] [CrossRef]
- Grange, F.; Beylot-Barry, M.; Courville, P.; Maubec, E.; Bagot, M.; Vergier, B.; Souteyrand, P.; Machet, L.; Dalac, S.; Esteve, E.; et al. Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type: Clinicopathologic Features and Prognostic Analysis in 60 Cases. Arch. Dermatol. 2007, 143, 1144–1150. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Prince, H.M.; Vermeer, M.H.; Quaglino, P.; Horwitz, S.; Porcu, P.; Stadler, R.; Wood, G.S.; Beylot-Barry, M.; Pham-Ledard, A.; et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. JCO 2015, 33, 3766–3773. [Google Scholar] [CrossRef]
- Quaglino, P.; Fava, P.; Pileri, A.; Grandi, V.; Sanlorenzo, M.; Panasiti, V.; Guglielmo, A.; Alberti-Violetti, S.; Novelli, M.; Astrua, C.; et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Dermatol. 2021, 141, 484–495. [Google Scholar] [CrossRef]
- Travaglino, A.; Russo, D.; Varricchio, S.; Pignatiello, S.; Baldo, A.; Picardi, M.; Pane, F.; Mascolo, M. Prognostic Significance of CD30 in Transformed Mycosis Fungoides. Am. J. Clin. Pathol. 2021, 156, 350–355. [Google Scholar] [CrossRef]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer Consensus Recommendations for the Treatment of Mycosis Fungoides/Sézary Syndrome–Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef]
- Grandi, V.; Baldo, A.; Berti, E.; Quaglino, P.; Rupoli, S.; Alaibac, M.; Alberti-Violetti, S.; Amerio, P.; Brazzelli, V.; Bruni, P.L.; et al. Italian Expert-based Recommendations on the Use of Photo(Chemo)Therapy in the Management of Mycosis Fungoides: Results of an E-Delphi Consensus. Photodermatol. Photoimmunol. Photomed. 2021, 37, 334–342. [Google Scholar] [CrossRef]
- Ramcke, T.; Enk, A.; Gholam, P. Folliculotropic Mycosis Fungoides in the Tumour Stage Mimics Venous Leg Ulcers. Acta Derm. Venereol. 2021, 101, adv00537. [Google Scholar] [CrossRef]
- Sacchelli, L.; Patrizi, A.; Neri, I.; Sechi, A.; Sabattini, E.; Bertuzzi, C.; Pileri, A. Brownish Asymptomatic Lesions on the Arms and Legs. JDDG J. Der Dtsch. Dermatol. Ges. 2019, 17, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Santamarina-Albertos, A.; Muñoz-Martínez, R.; Alvarez-Gago, T.; Miranda-Romero, A. Micosis fungoide papular en las piernas, a propósito de un caso. Actas Dermo-Sifiliográficas 2014, 105, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Delfino, C.; Grandi, V.; Agostinelli, C.; Pileri, S.A.; Pimpinelli, N. Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN): The Cutaneous Sanctuary. G. Ital. Dermatol. Venereol. 2012, 147, 603–608. [Google Scholar] [PubMed]
- Sapienza, M.R.; Abate, F.; Melle, F.; Orecchioni, S.; Fuligni, F.; Etebari, M.; Tabanelli, V.; Laginestra, M.A.; Pileri, A.; Motta, G.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm: Genomics Mark Epigenetic Dysregulation as a Primary Therapeutic Target. Haematologica 2019, 104, 729–737. [Google Scholar] [CrossRef]
- For the AIRC 5xMille Consortium ‘Genetics-Driven Targeted Management of Lymphoid Malignancies’ and the Italian Registry on Blastic Plasmacytoid Dendritic Cell Neoplasm; Sapienza, M.R.; Fuligni, F.; Agostinelli, C.; Tripodo, C.; Righi, S.; Laginestra, M.A.; Pileri, A.; Mancini, M.; Rossi, M.; et al. Molecular Profiling of Blastic Plasmacytoid Dendritic Cell Neoplasm Reveals a Unique Pattern and Suggests Selective Sensitivity to NF-KB Pathway Inhibition. Leukemia 2014, 28, 1606–1616. [Google Scholar] [CrossRef]
- Nizza, D.; Simoneaux, S.F. Blastic Plasmacytoid Dendritic Cell Neoplasm Presenting as a Subcutaneous Mass in an 8-Year-Old Boy. Pediatr. Radiol. 2010, 40 (Suppl. 1), 40–42. [Google Scholar] [CrossRef]
- Facchetti, F.; Cigognetti, M.; Fisogni, S.; Rossi, G.; Lonardi, S.; Vermi, W. Neoplasms Derived from Plasmacytoid Dendritic Cells. Mod. Pathol. 2016, 29, 98–111. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Stuart, L.; Skupsky, H.; Lee, Y.-S.; Tsuchiya, A.; Cassarino, D.S. Blastic Plasmacytoid Dendritic Cell Neoplasm in the Pediatric Population: A Case Series and Review of the Literature. Am. J. Dermatopathol. 2015, 37, 924–928. [Google Scholar] [CrossRef]
- Kempf, W.; Kazakov, D.V.; Belousova, I.E.; Mitteldorf, C.; Kerl, K. Paediatric Cutaneous Lymphomas: A Review and Comparison with Adult Counterparts. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1696–1709. [Google Scholar] [CrossRef]
- Petrella, T.; Meijer, C.J.L.M.; Dalac, S.; Willemze, R.; Maynadié, M.; Machet, L.; Casasnovas, O.; Vergier, B.; Teitell, M.A. TCL1 and CLA Expression in Agranular CD4/CD56 Hematodermic Neoplasms (Blastic NK-Cell Lymphomas) and Leukemia Cutis. Am. J. Clin. Pathol. 2004, 122, 307–313. [Google Scholar] [CrossRef]
- Cota, C.; Vale, E.; Viana, I.; Requena, L.; Ferrara, G.; Anemona, L.; Metze, D.; Fink-Puches, R.; Wiesner, T.; Cerroni, L. Cutaneous Manifestations of Blastic Plasmacytoid Dendritic Cell Neoplasm—Morphologic and Phenotypic Variability in a Series of 33 Patients. Am. J. Surg. Pathol. 2010, 34, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Julia, F.; Petrella, T.; Beylot-Barry, M.; Bagot, M.; Lipsker, D.; Machet, L.; Joly, P.; Dereure, O.; Wetterwald, M.; d’Incan, M.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm: Clinical Features in 90 Patients. Br. J. Dermatol. 2013, 169, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Rahman, F.; Good, D.; Silverman, J.; Brennan, M.K.; Dimov, N.; Liesveld, J.; Ryan, D.H.; Richard Burack, W.; Bennett, J.M. Blastic Plasmacytoid Dendritic Cell Neoplasm with Leukemic Presentation, Lacking Cutaneous Involvement: Case Series and Literature Review. Leuk. Res. 2012, 36, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, J.; Hong, X. Blastic Plasmacytoid Dendritic Cell Neoplasm without Cutaneous Lesion at Presentation: Case Report and Literature Review. Acta Haematol. 2012, 127, 124–127. [Google Scholar] [CrossRef]
- Pagano, L.; Valentini, C.G.; Pulsoni, A.; Fisogni, S.; Carluccio, P.; Mannelli, F.; Lunghi, M.; Pica, G.; Onida, F.; Cattaneo, C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm with Leukemic Presentation: An Italian Multicenter Study. Haematologica 2013, 98, 239–246. [Google Scholar] [CrossRef]
- Endo, K.; Mihara, K.; Oiwa, H.; Yoshida, T.; Mino, T.; Sasaki, N.; Takihara, Y. Lung Involvement at Initial Presentation in Blastic Plasmacytoid Dendritic Cell Neoplasm Lacking Cutaneous Lesion. Ann. Hematol. 2013, 92, 269–270. [Google Scholar] [CrossRef]
- Ascani, S.; Massone, C.; Ferrara, G.; Rongioletti, F.; Papini, M.; Pileri, S.; Cerroni, L. CD4-Negative Variant of CD4+/CD56+ Hematodermic Neoplasm: Description of Three Cases. J. Cutan. Pathol. 2008, 35, 911–915. [Google Scholar] [CrossRef]
- Boiocchi, L.; Lonardi, S.; Vermi, W.; Fisogni, S.; Facchetti, F. BDCA-2 (CD303): A Highly Specific Marker for Normal and Neoplastic Plasmacytoid Dendritic Cells. Blood 2013, 122, 296–297. [Google Scholar] [CrossRef]
- Jaye, D.L.; Geigerman, C.M.; Herling, M.; Eastburn, K.; Waller, E.K.; Jones, D. Expression of the Plasmacytoid Dendritic Cell Marker BDCA-2 Supports a Spectrum of Maturation among CD4+ CD56+ Hematodermic Neoplasms. Mod. Pathol. 2006, 19, 1555–1562. [Google Scholar] [CrossRef]
- Pilichowska, M.E.; Fleming, M.D.; Pinkus, J.L.; Pinkus, G.S. CD4+/CD56+ Hematodermic Neoplasm (“Blastic Natural Killer Cell Lymphoma”): Neoplastic Cells Express the Immature Dendritic Cell Marker BDCA-2 and Produce Interferon. Am. J. Clin. Pathol. 2007, 128, 445–453. [Google Scholar] [CrossRef]
- Trimoreau, F.; Donnard, M.; Turlure, P.; Gachard, N.; Bordessoule, D.; Feuillard, J. The CD4+ CD56+ CD116- CD123+ CD45RA+ CD45RO- Profile Is Specific of DC2 Malignancies. Haematologica 2003, 88, ELT10. [Google Scholar]
- Deotare, U.; Yee, K.W.L.; Le, L.W.; Porwit, A.; Tierens, A.; Musani, R.; Barth, D.; Torlakovic, E.; Schimmer, A.; Schuh, A.C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm with Leukemic Presentation: 10-Color Flow Cytometry Diagnosis and HyperCVAD Therapy: BPDCN Diagnosis and Therapy. Am. J. Hematol. 2016, 91, 283–286. [Google Scholar] [CrossRef]
- Deotare, U.; Kim, D.; Dong, H.; Michelis, F.V.; Lipton, J.H. Allogeneic Hematopoietic Stem Cell Transplantions in Blastic Plasmacytoid Dendritic Cell Neoplasm in First Complete Remission: An Effective Therapy for a Rare Disease. Leuk. Lymphoma 2016, 57, 1942–1944. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef]
| Presentation | Diagnostic Approach | Princiapl Differential Diagnosis | Aggressivity | Possible Therapies | |
|---|---|---|---|---|---|
| Basal cell carcinoma | Single | Dermoscopic and Histological diagnosis | Squamous cell carcinoma and amelanotic melanoma | Locally invasive | Topical Therapy, surgical excision |
| Squamous cell carcinoma | Single | Dermoscopic and Histological diagnosis | Basal cell carcinoma and amelanotic melanoma | Aggressive | Topical Therapy, surgical excision, radiotherapy |
| Kaposi sarcoma | Multiple | Histological and immunohistochemistry | Lymphomatoid papulosis | Indolent or aggressive | Surgical excision, radiotherapy, systemic drugs |
| Pyogenic granuloma | Single | Histological | Amelanotic melanoma | Indolent | Topical beta-blockers, topical steroids, cryosurgery or electrodesiccation |
| Amelanotic melanoma | Single | Histological | Pyogenic granuloma | Aggressive | Surgical excision |
| Lymphomatoid papulosis | Multiple | Histological | Kaposi sarcoma and CTCL | Indolent | Include topical creams, laser therapy or surgical excision |
| Anaplastic large cell lymphoma | Single | Histological | Other CTCL | Indolent or aggressive | Chemotherapy, radiotherapy, rarely surgical excision |
| Primary cutaneous diffuse large b-cell lymphoma, leg type | Multiple | Histological and immunohistochemistry | Other CBCL | Aggressive | Chemoimmunotherapy |
| Primary cutaneous marginal zone lymphoma | Single | Histological and immunohistochemistry | Other CBCL | Indolent | Field radiotherapy, surgery, chemotherapy |
| Primary cutaneous follicle center lymphoma | Multiple | Histological and immunohistochemistry | Other CBCL | Indolent | Field radiotherapy, surgery, chemotherapy |
| Mycosis fungoides | Single to multiple | Histological, immunophenotype and molecular characterization | Other CTCL, eczematous dermatitis, psoriasis | From indolent to aggressive | Phototherapy, systemic regimens, chemotherapy, radiotherapy |
| Blastic plasmacytoid dendritic cell neoplasm | Single to multiple | Histological and immunohistochemistry | Amelanotic melanoma and other CTCL/CBCL | Aggressive | Chemotherapy, bone marrow transplant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pileri, A.; Orioni, G.; Zengarini, C.; Grandi, V.; Piraccini, B.M.; Gaspari, V. Violaceous Lesions on the Leg: What Else Apart from Kaposi Sarcoma? Differential Diagnosis with a Narrative Review of the Literature. Dermato 2023, 3, 56-68. https://doi.org/10.3390/dermato3010005
Pileri A, Orioni G, Zengarini C, Grandi V, Piraccini BM, Gaspari V. Violaceous Lesions on the Leg: What Else Apart from Kaposi Sarcoma? Differential Diagnosis with a Narrative Review of the Literature. Dermato. 2023; 3(1):56-68. https://doi.org/10.3390/dermato3010005
Chicago/Turabian StylePileri, Alessandro, Gionathan Orioni, Corrado Zengarini, Vieri Grandi, Bianca Maria Piraccini, and Valeria Gaspari. 2023. "Violaceous Lesions on the Leg: What Else Apart from Kaposi Sarcoma? Differential Diagnosis with a Narrative Review of the Literature" Dermato 3, no. 1: 56-68. https://doi.org/10.3390/dermato3010005
APA StylePileri, A., Orioni, G., Zengarini, C., Grandi, V., Piraccini, B. M., & Gaspari, V. (2023). Violaceous Lesions on the Leg: What Else Apart from Kaposi Sarcoma? Differential Diagnosis with a Narrative Review of the Literature. Dermato, 3(1), 56-68. https://doi.org/10.3390/dermato3010005








