A Review of Coronaviruses in Wild Birds and Opportunities for Future Research on Migratory Waterfowl
Simple Summary
Abstract
1. Introduction
1.1. Historical Context
1.2. Taxonomy
1.3. Pathogenesis and Clinical Signs
1.4. Transmission
1.5. Surveillance and Diagnostics
1.6. Control
1.7. Prevalence in Wild Birds
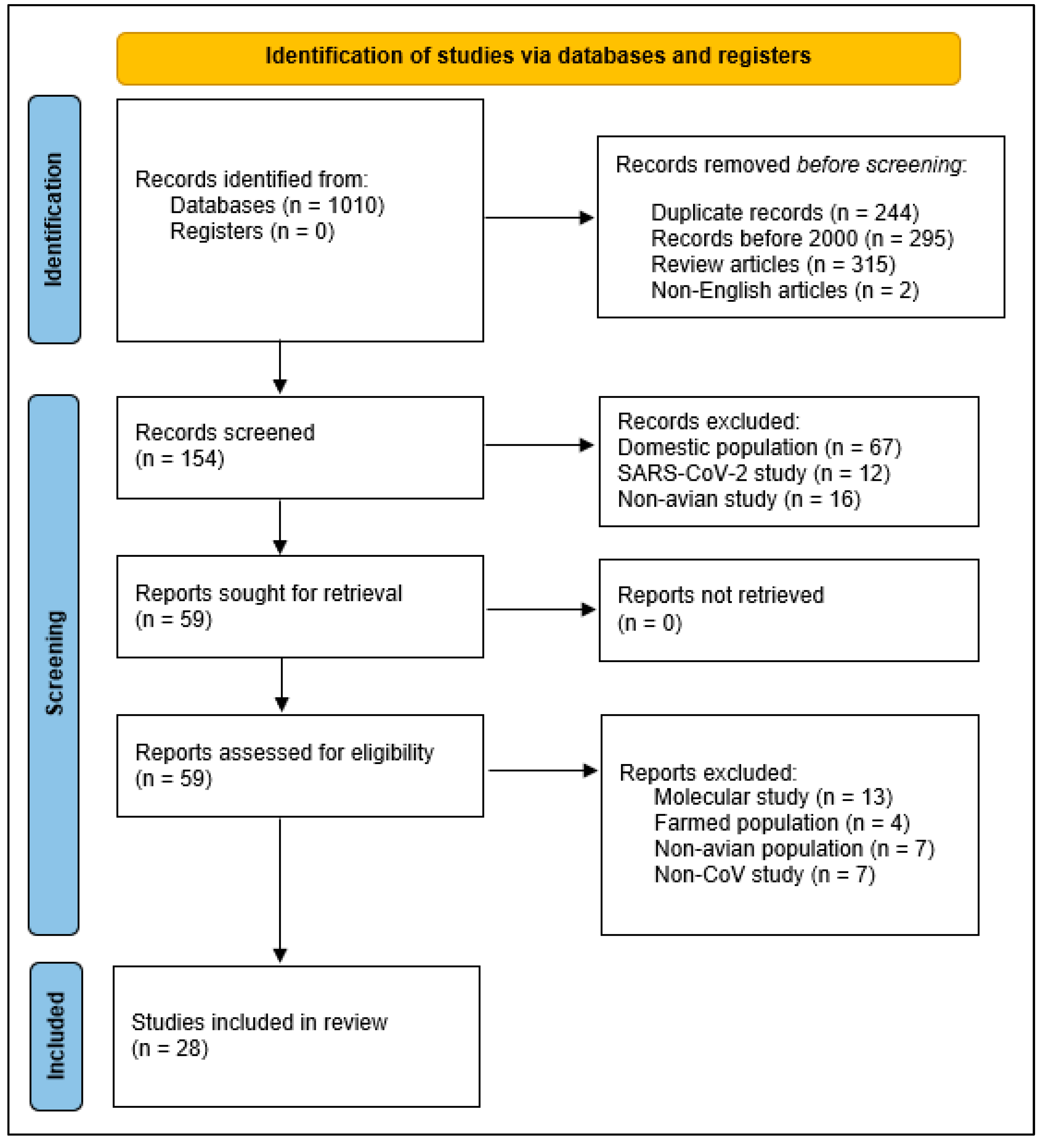
2. Materials and Methods
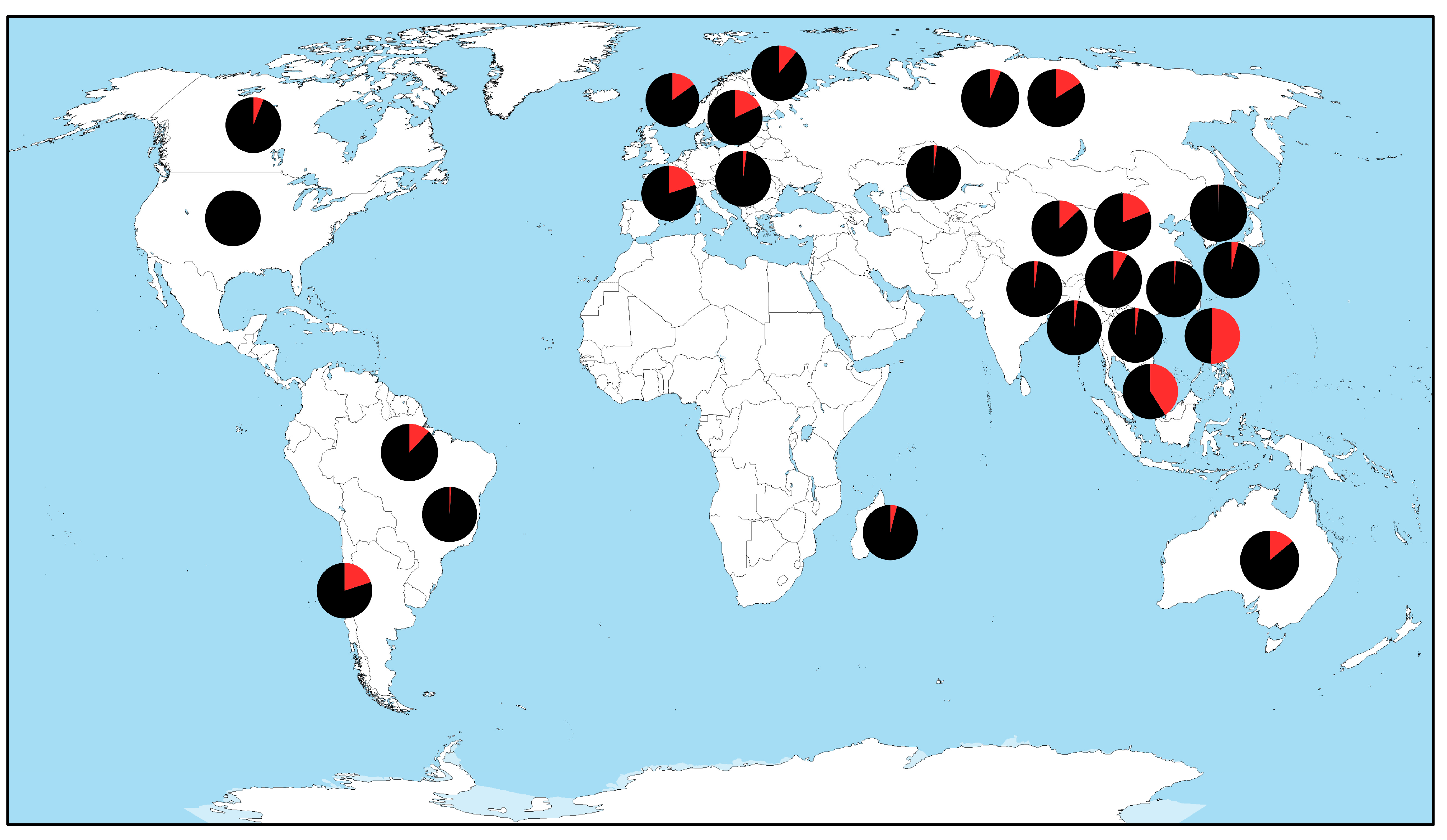
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wille, M.; Holmes, E.C. Wild birds as reservoirs for diverse and abundant gamma- and deltacoronaviruses. FEMS Microbiol. Rev. 2020, 44, 631–644. [Google Scholar] [CrossRef]
- Torres, C.A.; Listorti, V.; Lupini, C.; Franzo, G.; Drigo, M.; Catelli, E.; Brandao, P.E.; Cecchinato, M. Gamma and Deltacoronaviruses in quail and pheasants from Northern Italy. Poult. Sci. 2017, 96, 717–722. [Google Scholar] [CrossRef]
- Wille, M.; Lindqvist, K.; Muradrasoli, S.; Olsen, B.; Jarhult, J.D. Urbanization and the dynamics of RNA viruses in Mallards (Anas platyrhynchos). Infect. Genet. Evol. 2017, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.C.; Hu, G.M.; Chen, C.M. Phylogenetic network of infectious bronchitis virus: Exploring the impact of migratory birds on viral clustering, evolution, and recombination. Vet. Q. 2025, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schalk, A.F. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931, 78, 413–423. [Google Scholar]
- Cook, J.K.A.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, L.; Brandly, C. Laryngotracheitis in chicks. Poult. Sci. 1933, 12, 55–60. [Google Scholar] [CrossRef]
- May, H.G.; Tittsler, R.P. Tracheo-laryngitis in poultry. J. Am. Vet. Med. Assoc. 1925, 67, 229–231. [Google Scholar]
- Beach, J.; Schalm, O. A filterable virus, distinct from that of laryngotracheitis, the cause of a respiratory disease of chicks. Poult. Sci. 1936, 15, 199–206. [Google Scholar] [CrossRef]
- Chute, H.L.; O’Meara, D.C.; Witter, J.F. Controlling infectious bronchitis in Maine chickens. Maine Agric. Exp. Stn. Bull. 1959, 584, 1–26. [Google Scholar]
- Gharaibeh, S.; Mahmoud, K. Decay of maternal antibodies in broiler chickens. Poult. Sci. 2013, 92, 2333–2336. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.H.; Hymas, T.A. Antibiotics in the treatment of an unfamiliar turkey disease. Poult. Sci. 1951, 30, 466–468. [Google Scholar] [CrossRef]
- Pomeroy, B.; Sieburth, J. Bluecomb disease of turkeys. In Proceedings 90th Annual Meeting of the American Veterinary Medical Association; American Veterinary Medical Association: Schaumburg, IL, USA, 1954; pp. 3227–3231. [Google Scholar]
- Sieburth, J.M.; Johnson, E.P. Transmissible enteritis of turkeys (bluecomb disease): 1. Preliminary studies. Poult. Sci. 1957, 36, 256–261. [Google Scholar] [CrossRef]
- Tyrrell, D.A.; Bynoe, M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet 1966, 1, 76–77. [Google Scholar] [CrossRef]
- Almeida, J.D.; Tyrell, D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J. Gen. Virol. 1967, 1, 175–178. [Google Scholar] [CrossRef]
- Almeida, J.D.; Berry, D.; Cunningham, C.; Hamre, D.; Hofstad, M.; Mallucci, L.; McIntosh, K.; Tyrrell, D. Coronaviruses; Nature Publishing Group: London, UK, 1968. [Google Scholar]
- Panigrahy, B.; Naqi, S.; Hall, C. Isolation and characterization of viruses associated with transmissible enteritis (bluecomb) of turkeys. Avian Dis. 1973, 17, 430–438. [Google Scholar] [CrossRef]
- Ritchie, A.; Deshmukh, D.; Larsen, C.; Pomeroy, B. Electron microscopy of coronavirus-like particles characteristic of turkey bluecomb disease. Avian Dis. 1973, 17, 546–558. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, Y.; Ge, X. The taxonomy, host range and pathogenicity of coronaviruses and other viruses in the nidovirales order. Anim. Dis. 2021, 1, 5. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; Garcia, M.L.; Hendrickson, R.C.; et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; De Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; Hoek, L.V.; Wong, A.C.P.; Yeh, S.H. ICTV virus taxonomy profile 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar]
- Quinteros, J.A.; Noormohammadi, A.H.; Lee, S.W.; Browning, G.F.; Diaz-Mendez, A. Genomics and pathogenesis of the avian coronavirus infectious bronchitis virus. Aust. Vet. J. 2022, 100, 496–512. [Google Scholar] [CrossRef]
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.S.; Hamid, A.; Bhat, S.U. SARS-CoV-2: A critical review of its history, pathogenesis, transmission, diagnosis and treatment. Biosaf. Health 2020, 2, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.J.; Zhang, L.B.; Li, H.Y.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aravena, M.; Mckee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef]
- El-Sahly, H.M.; Atmar, R.L.; Glezen, W.P.; Greenberg, S.B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin. Infect. Dis. 2000, 31, 96–100. [Google Scholar] [CrossRef]
- Tao, Y.; Shi, M.; Chommanard, C.; Queen, K.; Zhang, J.; Markotter, W.; Kuzmin, I.V.; Holmes, E.C.; Tong, S.X. Surveillance of bat coronaviruses in Kenya identifies relatives of human coronaviruses NL63 and 229E and their recombination history. J. Virol. 2017, 91, e01953-16. [Google Scholar] [CrossRef]
- Naserghandi, A.; Allameh, S.F.; Saffarpour, R. All about COVID-19 in brief. New Microb. New Infect. 2020, 35, 100678. [Google Scholar] [CrossRef]
- Duraes-Carvalho, R.; Caserta, L.C.; Barnabe, A.C.S.; Martini, M.C.; Ferreira, H.L.; Felippe, P.N.; Santos, M.B.; Arns, C.W. Coronaviruses detected in Brazilian wild birds reveal close evolutionary relationships with beta- and deltacoronaviruses isolated from mammals. J. Mol. Evol. 2015, 81, 21–23. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Leung, C.Y.H.; Gilbert, M.; Joyner, P.H.; Ng, E.M.; Tse, T.M.; Guan, Y.; Peiris, J.S.M.; Poon, L.L.M. Avian coronavirus in wild aquatic birds. J. Virol. 2011, 85, 12815–12820. [Google Scholar] [CrossRef]
- Jackwood, M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Papineau, A.; Berhane, Y.; Wylie, T.N.; Wylie, K.M.; Sharpe, S.; Lung, O. Genome organization of Canada goose coronavirus, a novel species identified in a mass die-off of Canada geese. Sci. Rep. 2019, 9, 5954. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, K.A.; Wu, G.; Leger, J.S.; Nordhausen, R.W.; Wang, D. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 2008, 82, 5084–5088. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of seven novel mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Jackwood, M.W.; De Wit, S. Infectious bronchitis. In Diseases of Poultry, 13th ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 139–159. [Google Scholar]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Wickramasinghe, I.N.A.; De Vries, R.P.; Grone, A.; De Haan, C.M.; Verheije, M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011, 85, 8903–8912. [Google Scholar] [CrossRef]
- Milek, J.; Blicharz-Domanska, K. Coronaviruses in avian species—Review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef]
- Wickramasinghe, I.N.A.; De Vries, R.P.; Weerts, E.W.S.; Van Beurden, S.J.; Peng, W.; Mcbride, R.; Ducatez, M.; Guy, J.; Brown, P.; Eterradossi, N.; et al. Novel receptor specificity of avian gammacoronaviruses that cause enteritis. J. Virol. 2015, 89, 8783–8792. [Google Scholar] [CrossRef]
- Ignjatovic, J.; Sapats, S. Avian infectious bronchitis virus. Rev. Sci. Tech. 2000, 19, 493–508. [Google Scholar] [CrossRef]
- Hughes, L.A.; Savage, C.; Naylor, C.; Bennett, M.; Chantrey, J.; Jones, R. Genetically diverse coronaviruses in wild bird populations of Northern England. Emerg. Infect. Dis. 2009, 15, 1091–1094. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, K.; Chen, S.; Yan, K.; Du, X.; Zhang, C.; Guo, M.; Wu, Y. Evaluation of the reproductive system development and egg-laying performance of hens infected with tw i-type infectious bronchitis virus. Vet. Res. 2020, 51, 95. [Google Scholar] [CrossRef]
- Sevoian, M.; Levine, P. Effects of infectious bronchitis on the reproductive tracts, egg production, and egg quality of laying chickens. Avian Dis. 1957, 1, 136–164. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R. Ultrastructural study of infectious bronchitis virus infection in infundibulum and magnum of commercial laying hens. Vet. Microbiol. 2007, 122, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wei, J. Influences of infectious bronchitis in young chickens on their later production (Accession No. 002873357). Chin. J. Vet. Med. 1995, 21, 15. [Google Scholar]
- Crinion, R.A.P. Egg quality and production following infectious bronchitis virus exposure at one-day-old. Poult. Sci. 1972, 51, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef]
- Torres, C.A.; Villarreal, L.Y.B.; Ayres, G.R.R.; Richtzenhain, L.J.; Brandao, P.E. An aviancoronavirus in quail with respiratory and reproductive signs. Avian Dis. 2013, 57, 295–299. [Google Scholar] [CrossRef]
- Boltz, D.A.; Nakai, M.; Bahr, J.M. Avian infectious bronchitis virus: A possible cause of reduced fertility in the rooster. Avian Dis. 2004, 48, 909–915. [Google Scholar] [CrossRef]
- Villarreal, L.Y.B.; Brandao, P.E.; Chacon, J.L.; Assayag, M.S.; Maiorka, P.C.; Raffi, P.; Saidenberg, A.B.S.; Jones, R.C.; Ferreira, A.J.P. Orchitis in roosters with reduced fertility associated with avian infectious bronchitis virus and avian metapneumovirus infections. Avian Dis. 2007, 51, 900–904. [Google Scholar] [CrossRef]
- Vaz, F.F.; Raso, T.F.; Agius, J.E.; Hunt, T.; Leishman, A.; Eden, J.S.; Phalen, D.N. Opportunistic sampling of wild native and invasive birds reveals a rich diversity of adenoviruses in Australia. Virus Evol. 2020, 6, veaa024. [Google Scholar] [CrossRef]
- Sarker, S. Metagenomic detection and characterisation of multiple viruses in apparently healthy Australian neophema birds. Sci. Rep. 2021, 11, 20915. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Yang, S.; Wang, H.; Wang, H.; Zhang, J.; Gong, G.; Xiao, Y.; Yang, J.; Wang, X.; Lu, J. Virome in the cloaca of wild and breeding birds revealed a diversity of significant viruses. Microbiome 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, C.M.; Kofstad, T.; Larsen, I.L.; Lovland, A.; Handeland, K.; Follestad, A.A.; Lillehaug, A. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J. Gen. Virol. 2005, 86, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Gough, R.E. Isolation of avian infectious-bronchitis virus from experimentally infected chickens. Res. Vet. Sci. 1977, 23, 344–347. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Hewson, I. Coronaviruses in the sea. Front. Microbiol. 2020, 11, 1795. [Google Scholar] [CrossRef]
- Wartecki, A.; Rzymski, P. On the coronaviruses and their associations with the aquatic environment and wastewater. Water 2020, 12, 1598. [Google Scholar] [CrossRef]
- Wang, L.; Maddox, C.; Terio, K.; Lanka, S.; Fredrickson, R.; Novick, B.; Parry, C.; Mcclain, A.; Ross, K. Detection and characterization of new coronavirus in bottlenose dolphin, United States, 2019. Emerg. Infect. Dis. 2020, 26, 1610. [Google Scholar] [CrossRef]
- Calibeo-Hayes, D.; Denning, S.S.; Stringham, S.M.; Guy, J.S.; Smith, L.G.; Watson, D.W. Mechanical transmission of turkey coronavirus by domestic houseflies (Musca domestica linnaeaus). Avian Dis. 2003, 47, 149–153. [Google Scholar] [CrossRef]
- Guy, J.S. Isolation and propagation of coronaviruses in embryonated eggs. Methods Mol. Biol. 2008, 454, 109–117. [Google Scholar]
- Geerligs, H.J.; Boelm, G.J.; Meinders, C.M.; Stuurman, B.G.E.; Symons, J.; Tarres-Call, J.; Bru, T.; Vila, R.; Mombarg, M.; Karaca, K.; et al. Efficacy and safety of an attenuated live qx-like infectious bronchitis virus strain as a vaccine for chickens. Avian Pathol. 2011, 40, 93–102. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Casebolt, D.B.; Gangopadhyay, N.N. Phylogenetic analysis of a highly conserved region of the polymerase gene from 11 coronaviruses and development of a consensus polymerase chain reaction assay. Virus Res. 1999, 60, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Kappala, D. Avian infectious bronchitis virus. In Recent Advances in Animal Virology; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Springer: Singapore, 2019; pp. 301–319. [Google Scholar]
- Schilling, A.K.; Mazzamuto, M.V.; Romeo, C. A review of non-invasive sampling in wildlife disease and health research: What’s new? Animals 2022, 12, 1719. [Google Scholar] [CrossRef]
- Indriani, R.; Samaan, G.; Gultom, A.; Loth, L.; Indryani, S.; Adjid, R.; Dharmayanti, N.L.P.I.; Weaver, J.; Mumford, E.; Lokuge, K.; et al. Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg. Infect. Dis. 2010, 16, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Kishida, N.; Sakoda, Y.; Shiromoto, M.; Bai, G.R.; Isoda, N.; Takada, A.; Laver, G.; Kida, H. H2N5 influenza virus isolates from terns in Australia: Genetic reassortants between those of the Eurasian and American lineages. Virus Genes 2008, 37, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Diaz-Cao, J.M.; Fernandez-Antonio, R.; Panadero, R.; Diaz, P.; Lopez, C.; Morrondo, P.; Diez-Banos, P.; Fernandez, G. Application of real-time PCR to detect aleutian mink disease virus on environmental farm sources. Vet. Microbiol. 2014, 173, 355–359. [Google Scholar] [CrossRef]
- Costa, T.P.; Brown, J.D.; Howerth, E.W.; Stallknecht, D.E. Variation in viral shedding patterns between different wild bird species infected experimentally with low-pathogenicity avian influenza viruses that originated from wild birds. Avian Pathol. 2011, 40, 119–124. [Google Scholar] [CrossRef]
- Najimudeen, S.M.; Hassan, M.H.S.; Cork, S.C.; Abdul-Careem, M.F. Infectious bronchitis coronavirus infection in chickens: Multiple system disease with immune suppression. Pathogens 2020, 9, 779. [Google Scholar] [CrossRef]
- Pei, J.W.; Collisson, E.W. Specific antibody secreting cells from chickens can be detected by three days and memory b cells by three weeks post-infection with the avian respiratory coronavirus. Dev. Comp. Immunol. 2005, 29, 153–160. [Google Scholar] [CrossRef]
- Guzman, M.; Hidalgo, H. Live attenuated infectious bronchitis virus vaccines in poultry: Modifying local viral populations dynamics. Animals 2020, 10, 2058. [Google Scholar] [CrossRef]
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef]
- Silk, M.J.; Drewe, J.A.; Delahay, R.J.; Weber, N.; Steward, L.J.; Wilson-Aggarwal, J.; Boots, M.; Hodgson, D.J.; Croft, D.P.; McDonald, R.A. Quantifying direct and indirect contacts for the potential transmission of infection between species using a multilayer contact network. Behaviour 2018, 155, 731–757. [Google Scholar] [CrossRef]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef]
- Koch, G.; Elbers, A.R.W. Outdoor ranging of poultry: A major risk factor for the introduction and development of high-pathogenicity avian influenza. NJAS-Wagening. J. Life Sci. 2006, 54, 179–194. [Google Scholar] [CrossRef]
- Gonzales, J.L.; Pritz-Verschuren, S.; Bouwstra, R.; Wiegel, J.; Elbers, A.R.W.; Beerens, N. Seasonal risk of low pathogenic avian influenza virus introductions into free-range layer farms in the Netherlands. Transbound. Emerg. Dis. 2021, 68, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Buddle, E.A.; Stevens, K.; Bray, H.J.; Ankeny, R.A. The Chicken for the Egg: Australian Motivations for Raising Backyard Chickens. Anthrozoös 2024, 38, 299–310. [Google Scholar] [CrossRef]
- Oliver, C. Returning to ‘the good life’? Chickens and chicken-keeping during COVID-10 in Britain. Anim. Studi J. 2021, 10, 114–139. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Population biology of infectious-diseases: Part I. Nature 1979, 280, 361–367. [Google Scholar] [CrossRef]
- May, R.M.; Anderson, R.M. Population biology of infectious–diseases: Part II. Nature 1979, 280, 455–461. [Google Scholar] [CrossRef]
- De Castro, F.; Bolker, B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005, 8, 117–126. [Google Scholar] [CrossRef]
- Mathews, F. Zoonoses in wildlife: Integrating ecology into management. Adv. Parasitol. 2009, 68, 185–209. [Google Scholar] [PubMed]
- Dobson, A.P. Disease and conservation. Conserv. Biol. Sci. Scarcity Diversity 1986, 345–365. [Google Scholar]
- Bernstein, A.S.; Ando, A.W.; Loch-Temzelides, T.; Vale, M.M.; Li, B.V.; Li, H.Y.; Busch, J.; Chapman, C.A.; Kinnaird, M.; Nowak, K.; et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci. Adv. 2022, 8, eabl4183. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.B.; Mihaljevic, J.R.; Arellano, A.L.; Kueneman, G.; Preston, D.L.; Cross, P.C.; Johnson, P.T.J. Taming wildlife disease: Bridging the gap between science and management. J. Appl. Ecol. 2013, 50, 702–712. [Google Scholar] [CrossRef]
- Langwig, K.E.; Voyles, J.; Wilber, M.Q.; Frick, W.F.; Murray, K.A.; Bolker, B.M.; Collins, J.P.; Cheng, T.L.; Fisher, M.C.; Hoyt, J.R.; et al. Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 2015, 13, 195–202. [Google Scholar] [CrossRef]
- Deem, S.L.; Karesh, W.B.; Weisman, W. Putting theory into practice: Wildlife health in conservation. Conserv. Biol. 2001, 5, 1224–1233. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chen, J.; Kong, X.; Shao, Y.; Han, Z.; Feng, L.; Cai, X.; Gu, S.; Liu, M. Isolation of avian infectious bronchitis coronavirus from domestic peafowl (Pavo cristatus) and teal (anas). J. Gen. Virol. 2005, 86, 719–725. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronaviruses in poultry and other birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef]
- Domanska-Blicharz, K.; Jacukowicz, A.; Lisowska, A.; Wyrostek, K.; Minta, Z. Detection and molecular characterization of infectious bronchitis-like viruses in wild bird populations. Avian Pathol. 2014, 43, 406–413. [Google Scholar] [CrossRef]
- Wille, M.; Muradrasoli, S.; Nilsson, A.; Jarhult, J.D. High prevalence and putative lineage maintenance of avian coronaviruses in Scandinavian waterfowl. PLoS ONE 2016, 11, e0150198. [Google Scholar] [CrossRef]
- Muradrasoli, S.; Bálint, Á.; Wahlgren, J.; Waldenström, J.; Belák, S.; Blomberg, J.; Olsen, B. Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS ONE 2010, 5, e13640. [Google Scholar] [CrossRef]
- Marchenko, V.; Danilenko, A.; Kolosova, N.; Bragina, M.; Molchanova, M.; Bulanovich, Y.; Gorodov, V.; Leonov, S.; Gudymo, A.; Onkhonova, G.; et al. Diversity of gammacoronaviruses and deltacoronaviruses in wild birds and poultry in Russia. Sci. Rep. 2022, 12, 19412. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Oem, J.-K. Surveillance of avian coronaviruses in wild bird populations of Korea. J. Wildl. Dis. 2014, 50, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Núñez–Nogueira, G.; Valentino-Álvarez, J.A.; Granados-Berber, A.A.; Ramírez-Ayala, E.; Zepeda-González, F.A.; Tintos-Gómez, A. Aquatic biota is not exempt from coronavirus infections: An overview. Water 2021, 13, 2215. [Google Scholar] [CrossRef]
- Jordan, B.J.; Hilt, D.A.; Poulson, R.; Staliknecht, D.E.; Jackwood, M.W. Identification of avian coronavirus in wild aquatic birds of the central and eastern USA. J. Wildl. Dis. 2015, 51, 218–221. [Google Scholar] [CrossRef]
- Barbosa, C.M.; Durigon, E.L.; Thomazelli, L.M.; Ometto, T.; Marcatti, R.; Nardi, M.S.; De Aguiar, D.M.; Pinho, J.B.; Petry, M.V.; Neto, S.; et al. Divergent coronaviruses detected in wild birds in Brazil, including a central park in Sao Paulo. Braz. J. Microbiol. 2019, 50, 547–556. [Google Scholar] [CrossRef]
- Canuti, M.; Kroyer, A.N.K.; Ojkic, D.; Whitney, H.G.; Robertson, G.J.; Lang, A.S. Discovery and characterization of novel RNA viruses in aquatic North American wild birds. Viruses 2019, 11, 768. [Google Scholar] [CrossRef]
- Paim, F.C.; Bowman, A.S.; Miller, L.; Feehan, B.J.; Marthaler, D.; Saif, L.J.; Vlasova, A.N. Epidemiology of deltacoronaviruses (δ-cov) and gammacoronaviruses (γ-cov) in wild birds in the United States. Viruses 2019, 11, 897. [Google Scholar] [CrossRef]
- Khatun, M.N.; Tasnim, S.; Hossain, M.R.; Rahman, M.Z.; Hossain, M.T.; Chowdhury, E.H.; Parvin, R. Molecular epidemiology of avian influenza viruses and avian coronaviruses in environmental samples from migratory bird inhabitants in Bangladesh. Front. Vet. Sci. 2024, 11, 1446577. [Google Scholar] [CrossRef]
- Latorre-Margalef, N.; Gunnarsson, G.; Munster, V.J.; Fouchier, R.A.; Osterhaus, A.D.; Elmberg, J.; Olsen, B.; Wallesten, A.; Haemig, P.D.; Fransson, T.; et al. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. B Biol. Sci. 2009, 276, 1029–1036. [Google Scholar] [CrossRef]
- Groepper, S.R.; DeLiberto, T.J.; Vriska, M.P.; Pederson, K.; Swafford, S.R.; Hygnstrom, S.E. Avian influenza virus prevalence in migratory waterfowl in the United States, 2007–2009. Avian Dis. 2014, 58, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, S.; Lindh, E.; Vapalahti, O.; Huovilainen, A. Prevalence and genetic diversity of coronaviruses in wild birds, Finland. Infect. Ecol. Epidemiol. 2017, 7, 1408360. [Google Scholar] [CrossRef] [PubMed]
- de Sales Lima, F.E.; Gil, P.; Pedrono, M.; Minet, C.; Kwiatek, O.; Campos, F.S.; Spilki, F.R.; Roehe, P.M.; Franco, A.C.; Maminianina, O.F.; et al. Diverse gammacoronaviruses detected in wild birds from Madagascar. Eur. J. Wildl. Res. 2015, 61, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Blicharz, K.; Milek-Krupa, J.; Pikula, A. Diversity of coronaviruses in wild representatives of the Aves class in Poland. Viruses 2021, 13, 1497. [Google Scholar] [CrossRef]
- Verdugo, C.; Pinto, A.; Ariyama, N.; Moroni, M.; Hernandez, C. Molecular identification of avian viruses in neotropic cormorants (Phalacrocorax brasilianus) in Chile. J. Wildl. Dis. 2019, 55, 105–112. [Google Scholar] [CrossRef]
- Chamings, A.; Nelson, T.M.; Vibin, J.; Wille, M.; Klassen, M.; Alexandersen, S. Detection and characterisation of coronaviruses in migratory and non-migratory Australian wild birds. Sci. Rep. 2018, 8, 5980. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.J.; You, Z.; Wu, D.Y.; Liu, S.J.; Zhang, W.L.; Fan, K.R.; Luo, R.; Qiu, Y.; Ge, X.Y. Epidemiology and evolution of novel deltacoronaviruses in birds in central China. Transbound. Emerg. Dis. 2022, 69, 632–644. [Google Scholar] [CrossRef]
- Parvin, R.; Kabiraj, C.K.; Mumu, T.T.; Chowdhury, E.H.; Islam, M.R.; Beer, M.; Harder, T. Active virological surveillance in backyard ducks in Bangladesh: Detection of avian influenza and gammacoronaviruses. Avian Pathol. 2020, 49, 361–368. [Google Scholar] [CrossRef]
- Le Gall-Ladevèze, C.; Vollot, B.; Hirschinger, J.; Lèbre, L.; Aaziz, R.; Laroucau, K.; Guérin, J.L.; Paul, M.; Cappelle, J.; Le Loc’h, G. Limited transmission of avian influenza viruses, avulaviruses, coronaviruses and Chlamydia sp. at the interface between wild birds and a free-range duck farm. Vet. Res. 2025, 56, 36. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Xu, P.; Zhou, S.; Zhao, P.; Wang, Y.; Hu, J.; Ma, M.; Li, Z.; Bo, S.; et al. Avian Migration-Mediated Transmission and Recombination Driving the Diversity of Gammacoronaviruses and Deltacoronaviruses. Mol. Biol. Evol. 2025, 42, msaf045. [Google Scholar] [CrossRef]
- Marchenko, V.Y.; Kolosova, N.P.; Danilenko, A.V.; Bragina, M.K.; Nhai, T.T.; Ryzhikov, A. Diversity of coronaviruses in wild and domestic birds in Vietnam. Asian Pac. J. Trop. Med. 2022, 15, 442–450. [Google Scholar] [CrossRef]
- Ma, M.; Ji, L.; Ming, L.; Xu, Y.T.; Zhao, C.Y.; Wang, T.H.; He, G.M. Co-circulation of coronavirus and avian influenza virus in wild birds in Shanghai (2020–2021). Transbound. Emerg. Dis. 2022, 69, 3985–3991. [Google Scholar] [CrossRef]
- Zhigailov, A.V.; Maltseva, E.R.; Perfilyeva, Y.V.; Ostapchuk, Y.O.; Naizabayeva, D.A.; Berdygulova, Z.A.; Kuatbekova, S.A.; Nizkorodova, A.S.; Mashzhan, A.; Gavrilov, A.E.; et al. Prevalence and genetic diversity of coronaviruses, astroviruses and paramyxoviruses in wild birds in southeastern Kazakhstan. Heliyon 2022, 8, 11. [Google Scholar] [CrossRef]
- Sharshov, K.; Dubovitskiy, N.; Derko, A.; Loginova, A.; Kolotygin, I.; Zhirov, D.; Sobolev, I.; Kurskaya, O.; Alekseev, A.; Druzyaka, A.; et al. Does avian coronavirus co-circulate with avian paramyxovirus and avian influenza virus in wild ducks in Siberia? Viruses 2023, 15, 1121. [Google Scholar] [CrossRef] [PubMed]
- Gim, Y.; Jeong, S.H.; Lee, Y.J.; Jang, G.; Lee, C. Incidence and Genetic Investigation of Avian Coronaviruses in Migratory Ducks From South Korea. Transbound. Emerg. Dis. 2024, 1, 9502737. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Jenkins, K.M.; Bell, D.; Morris, P.J.; Kingsford, R.T. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 2008, 53, 380–392. [Google Scholar] [CrossRef]
- Gibbs, S.E. Avian biology, the human influence on global avian influenza transmission, and performing surveillance in wild birds. Anim. Health Res. Rev. 2010, 11, 35–41. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.; Hauser, R.; Matthes, D.; Stärk, K.D. Evaluation of effectiveness and efficiency of wild bird surveillance for avian influenza. Vet. Res. 2010, 41, 50. [Google Scholar] [CrossRef]
- Lefrançois, T.; Hendrikx, P.; Ehrhardt, N.; Millien, M.; Gomez, L.; Gouyet, L.; Gaidet, N.; Gerbier, G.; Vachiéry, N.; Petitclerc, F.; et al. Surveillance of avian influenza in the Caribbean through the Caribbean Animal Health Network: Surveillance tools and epidemiologic studies. Avian Dis. 2010, 54, 369–373. [Google Scholar] [CrossRef]
- Bande, F.; Arshad, S.S.; Omar, A.R.; Bejo, M.H.; Abubakar, M.S.; Abba, Y. Pathogenesis and diagnostic approaches of avian infectious bronchitis. Adv. Virol. 2016, 2016, 4621659. [Google Scholar] [CrossRef]
- Graham, R.L.; Baric, R.S. Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross-species transmission. J. Virol. 2010, 84, 3134–3146. [Google Scholar] [CrossRef]
- Eikenaar, C.; Hegemann, A. Migratory common blackbirds have lower innate immune function during autumn migration than resident conspecifics. Biol. Lett. 2016, 12, 20160078. [Google Scholar] [CrossRef]
- Posada-Cespedes, S.; Seifert, D.; Beerenwinkel, N. Recent advances in inferring viral diversity from high-throughput sequencing data. Virus Res. 2017, 239, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Smeele, Z.E.; Ainley, D.G.; Varsani, A. Viruses associated with antarctic wildlife: From serology based detection to identification of genomes using high throughput sequencing. Virus Res. 2018, 243, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, G.C.; Jerde, C.L.; Young, H.S. High-throughput sequencing for understanding the ecology of emerging infectious diseases at the wildlife-human interface. Front. Ecol. Evol. 2019, 7, 126. [Google Scholar] [CrossRef]
- Smith, K.F.; Sax, D.F.; Lafferty, K.D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 2006, 20, 1349–1357. [Google Scholar] [CrossRef]
- Wille, M.; Avril, A.; Tolf, C.; Schager, A.; Larsson, S.; Borg, O.; Olsen, B.; Waldenstrom, J. Temporal dynamics, diversity, and interplay in three components of the virodiversity of a mallard population: Influenza a virus, avian paramyxovirus and avian coronavirus. Infect. Genet. Evol. 2015, 29, 129–137. [Google Scholar] [CrossRef]
- Kong, L.C.; You, R.R.; Zhang, D.C.; Yuan, Q.L.; Xiang, B.; Liang, J.P.; Lin, Q.Y.; Ding, C.; Liao, M.; Chen, L.; et al. Infectious bronchitis virus infection increases pathogenicity of H9N2 avian influenza virus by inducing severe inflammatory response. Front. Vet. 2022, 8, 824179. [Google Scholar]
- Machalaba, C.C.; Elwood, S.E.; Forcella, S.; Smith, K.M.; Hamilton, K.; Jebara, K.B.; Swayne, D.E.; Webby, R.J.; Mumford, E.; Mazet, J.A.; et al. Global avian influenza surveillance in wild birds: A strategy to capture viral diversity. Emerg. Infect. Dis. 2015, 21, e1–e7. [Google Scholar] [CrossRef]
- Stallknecht, D.E. Impediments to wildlife disease surveillance, research, and diagnostics. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Richt, J.A., Mackenzie, J.S., Childs, J.E., Eds.; Springer: New York, NY, USA, 2007; pp. 445–461. [Google Scholar]
- Belant, J.L.; Deese, A.R. Importance of wildlife disease surveillance. HWI 2010, 4, 165–169. [Google Scholar]
- Hoerr, F.J. The pathology of infectious bronchitis. Avian Dis. 2021, 65, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; De Garine-Wichatitsky, M.; Gaidet, N.; Chiweshe, N.; Cumming, G.S. Estimating dynamic risk factors for pathogen transmission using community-level bird census data at the wildlife/domestic interface. Ecol. Soc. 2010, 15, 25. [Google Scholar] [CrossRef]
- Jourdain, E.; Gunnarsson, G.; Wahlgren, J.; Latorre-Margalef, N.; Bröjer, C.; Sahlin, S.; Svensson, L.; Waldenström, J.; Lundkvist, Å.; Olsen, B. Influenza virus in a natural host, the mallard: Experimental infection data. PLoS ONE 2010, 51, e8935. [Google Scholar] [CrossRef] [PubMed]
- Laudert, E.; Halvorson, D.; Sivanandan, V.; Shaw, D. Comparative Evaluation of Tissue Trophism Characteristics in Turkeys and Mallard Ducks after Intravenous Inoculation of Type A Influenza Viruses. Avian Dis. 1993, 37, 773–780. [Google Scholar] [CrossRef]
- Hill, N.J.; Takekawa, J.Y.; Ackerman, J.T.; Hobson, K.A.; Herring, G.; Cardona, C.J.; Runstadler, J.A.; Boyce, W.M. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos). Mol. Ecol. 2021, 21, 5986–5999. [Google Scholar] [CrossRef]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. B Biol. Sci. 2001, 268, 25–29. [Google Scholar] [CrossRef]
- Holmes, R.T.; Sherry, T.W. Thirty-year bird population trends in an unfragmented temperate deciduous forest: Importance of habitat change. Auk 2001, 118, 589–609. [Google Scholar] [CrossRef]
- Teitelbaum, C.S.; Ackerman, J.T.; Hill, M.A.; Satter, J.M.; Casazza, M.L.; De La Cruz, S.E.W.; Boyce, W.M.; Buck, E.J.; Eadie, J.M.; Herzog, M.P.; et al. Avian influenza antibody prevalence increases with mercury contamination in wild waterfowl. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221312. [Google Scholar] [CrossRef]
- Rushing, C.S.; Royle, J.A.; Ziolkowski, D.J.; Pardieck, K.L. Migratory behavior and winter geography drive differential range shifts of eastern birds in response to recent climate change. Proc. Natl. Acad. Sci. USA 2020, 117, 12897–12903. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird migration times, climate change, and changing population sizes. Glob. Change Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Marra, P.P.; Francis, C.M.; Mulvihill, R.S.; Moore, F.R. The influence of climate on the timing and rate of spring bird migration. Oecologia 2005, 142, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Yadav, S.; Haldar, C. Influence of environmental factors on avian immunity: An overview. J. Immunol. Res. 2017, 4, 1028–1033. [Google Scholar]
- Messina, S.; Edwards, D.P.; Eens, M.; Costantini, D. Physiological and immunological responses of birds and mammals to forest degradation: A meta-analysis. Biol. Conserv. 2018, 224, 223–229. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Destoumieux-Garzon, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Carey, C. Infectious disease and worldwide declines of amphibian populations, with comments on emerging diseases in coral reef organisms and in humans. Environ. Health Perspect. 2000, 108, 143–150. [Google Scholar]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Infectious disease and amphibian population declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef]
- Robinson, R.A.; Lawson, B.; Toms, M.P.; Peck, K.M.; Kirkwood, J.K.; Chantrey, J.; Clatworthy, I.R.; Evans, A.D.; Hughes, L.A.; Hutchinson, O.C.; et al. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 2010, 5, e12215. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, C.; Eden, P.; Reiss, A.; Ellis, T.; Knowles, G.; Wayne, A.F. Disease hazard identification and assessment associated with wildlife population declines. Ecol. Manag. Restor. 2015, 16, 142–152. [Google Scholar] [CrossRef]

| Genus | Subgenus | Pre-April 2024 [MSL #38] | Current [MSL #40] |
|---|---|---|---|
| Deltacoronavirus | Andecovirus | Wigeon coronavirus HKU20 | Deltacoronavirus marecae |
| Buldecovirus | Common moorhen coronavirus HKU21 | Deltacoronavirus gallinulae | |
| Munia coronavirus HKU13 | Deltacoronavirus lonchurae | ||
| Bulbul coronavirus HKU11 | Deltacoronavirus pycnonoti | ||
| Coronavirus HKU15 | Deltacoronavirus suis | ||
| White-eye coronavirus HKU16 | Deltacoronavirus zosteropis | ||
| Herdecovirus | Night heron coronavirus HKU19 | Deltacoronavirus nycticoracis | |
| Gammacoronavirus | Brangacovirus | Goose coronavirus CB17 | Gammacoronavirus brantae |
| Cegacovirus | Beluga whale coronavirus SW1 | Gammacoronavirus delphinapteri | |
| Igacovirus | Duck coronavirus 2714 | Gammacoronavirus anatis | |
| Avian coronavirus | Gammacoronavirus galli | ||
| Avian coronavirus 9203 | Gammacoronavirus pulli |
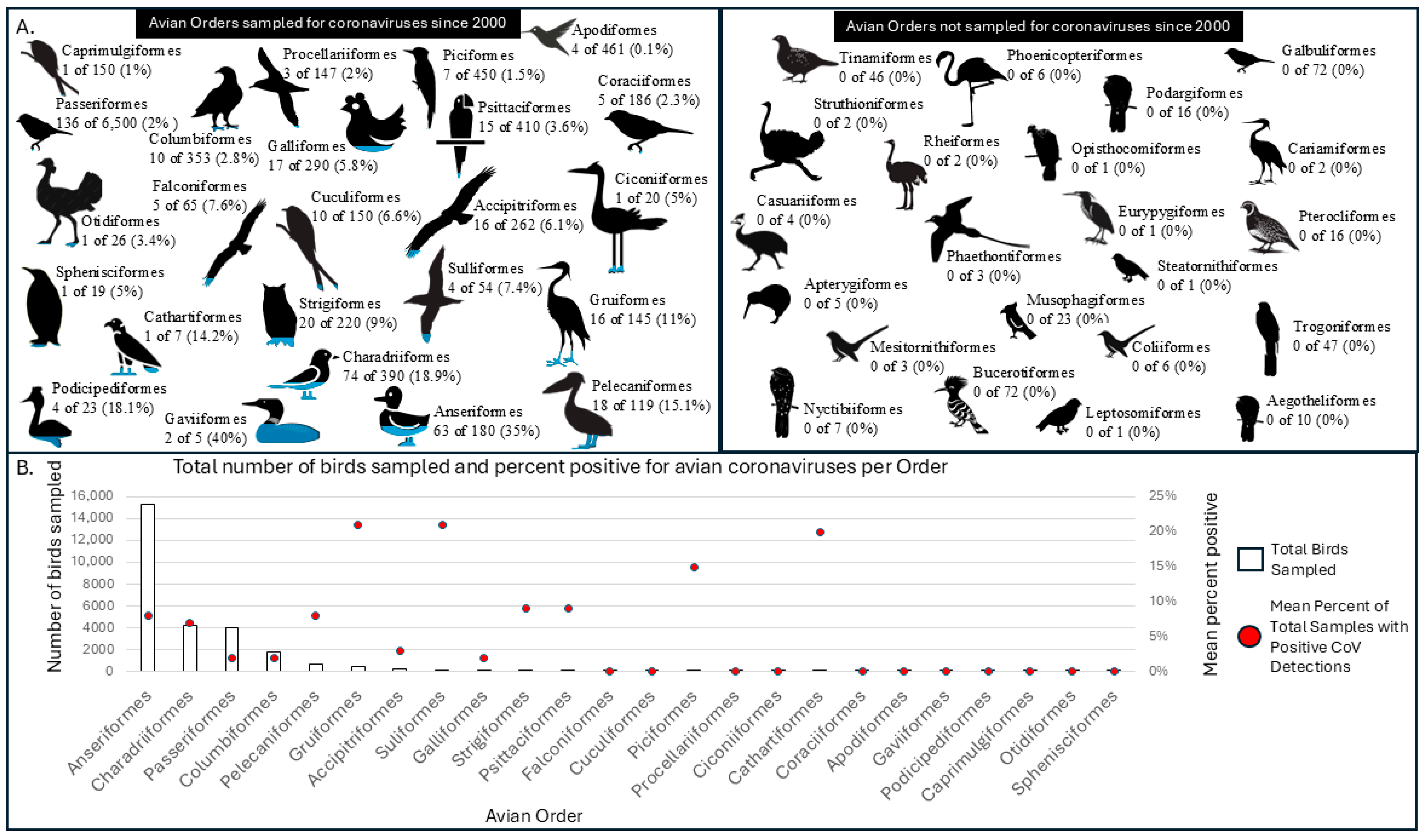
| Avian Order | Mean Percent of Total Samples with Positive CoV Detections | Number of Studies Since 2000 | Total Birds Sampled | Total Species Sampled | Species Found with Positive CoV Detections |
|---|---|---|---|---|---|
| Suliformes | 21% | 8 | 169 | 4 | 3 |
| Gruiformes | 21% | 8 | 427 | 16 | 5 |
| Cathartiformes | 20% | 1 | 24 | 1 | 1 |
| Piciformes | 15% | 4 | 36 | 7 | 1 |
| Strigiformes | 9% | 6 | 112 | 20 | 2 |
| Psittaciformes | 9% | 3 | 101 | 15 | 3 |
| Pelecaniformes | 8% | 11 | 678 | 18 | 8 |
| Anseriformes | 8% | 24 | 15,254 | 63 | 40 |
| Charadriiformes | 7% | 15 | 4228 | 74 | 31 |
| Accipitriformes | 3% | 8 | 251 | 16 | 1 |
| Passeriformes | 2% | 12 | 4034 | 136 | 16 |
| Columbiformes | 2% | 14 | 1756 | 10 | 2 |
| Galliformes | 2% | 6 | 144 | 17 | 1 |
| Apodiformes | 0 | 3 | 20 | 4 | |
| Caprimulgiformes | 0 | 2 | 7 | 1 | |
| Ciconiiformes | 0 | 1 | 28 | 1 | |
| Coraciiformes | 0 | 2 | 21 | 5 | |
| Cuculiformes | 0 | 3 | 58 | 10 | |
| Falconiformes | 0 | 5 | 66 | 5 | |
| Gaviiformes | 0 | 2 | 19 | 2 | |
| Otidiformes | 0 | 1 | 1 | 1 | |
| Podicipediformes | 0 | 4 | 13 | 4 | |
| Procellariiformes | 0 | 2 | 34 | 3 | |
| Sphenisciformes | 0 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vestal-Laborde, A.A.; Christofferson, R.C.; Ringelman, K.M.; Long, A.M. A Review of Coronaviruses in Wild Birds and Opportunities for Future Research on Migratory Waterfowl. Birds 2025, 6, 52. https://doi.org/10.3390/birds6040052
Vestal-Laborde AA, Christofferson RC, Ringelman KM, Long AM. A Review of Coronaviruses in Wild Birds and Opportunities for Future Research on Migratory Waterfowl. Birds. 2025; 6(4):52. https://doi.org/10.3390/birds6040052
Chicago/Turabian StyleVestal-Laborde, Allison A., Rebecca C. Christofferson, Kevin M. Ringelman, and Ashley M. Long. 2025. "A Review of Coronaviruses in Wild Birds and Opportunities for Future Research on Migratory Waterfowl" Birds 6, no. 4: 52. https://doi.org/10.3390/birds6040052
APA StyleVestal-Laborde, A. A., Christofferson, R. C., Ringelman, K. M., & Long, A. M. (2025). A Review of Coronaviruses in Wild Birds and Opportunities for Future Research on Migratory Waterfowl. Birds, 6(4), 52. https://doi.org/10.3390/birds6040052





