Bayesian Structure Learning Reveals Disconnected Correlation Patterns Between Morphometric Traits and Blood Biomarkers in White Stork Nestlings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
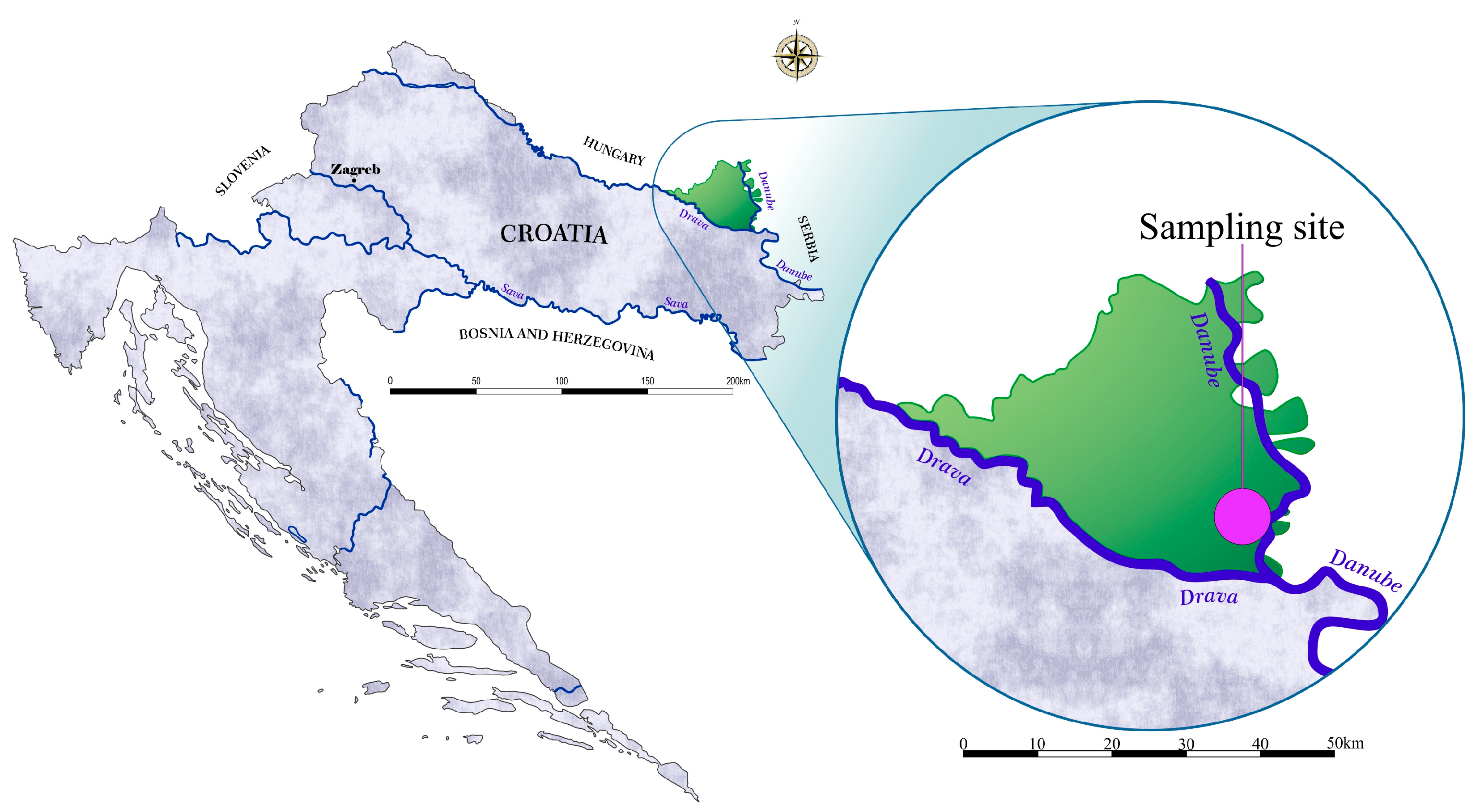
2.1. Sampling Procedure and Blood Processing
2.2. Chemicals
2.3. Biomarker Measurements
2.4. Body Condition Estimation and Descriptive Analysis
2.5. Multivariate Analysis
2.5.1. Imputation of Missing Records in BCI, TL, and S9 Fraction
2.5.2. Detection and Recoding of Multivariate Outliers
2.5.3. Evaluation of Multivariate Normality
2.6. Conditional and Structural Associations Between Blood Biomarkers and Morphometry of White Stork Nestlings
2.6.1. Conditional Associations
2.6.2. Bayesian Structure of Conditional Partial Associations
3. Results
3.1. Descriptive Aspects and Interannual Context
3.2. Conditional Associations
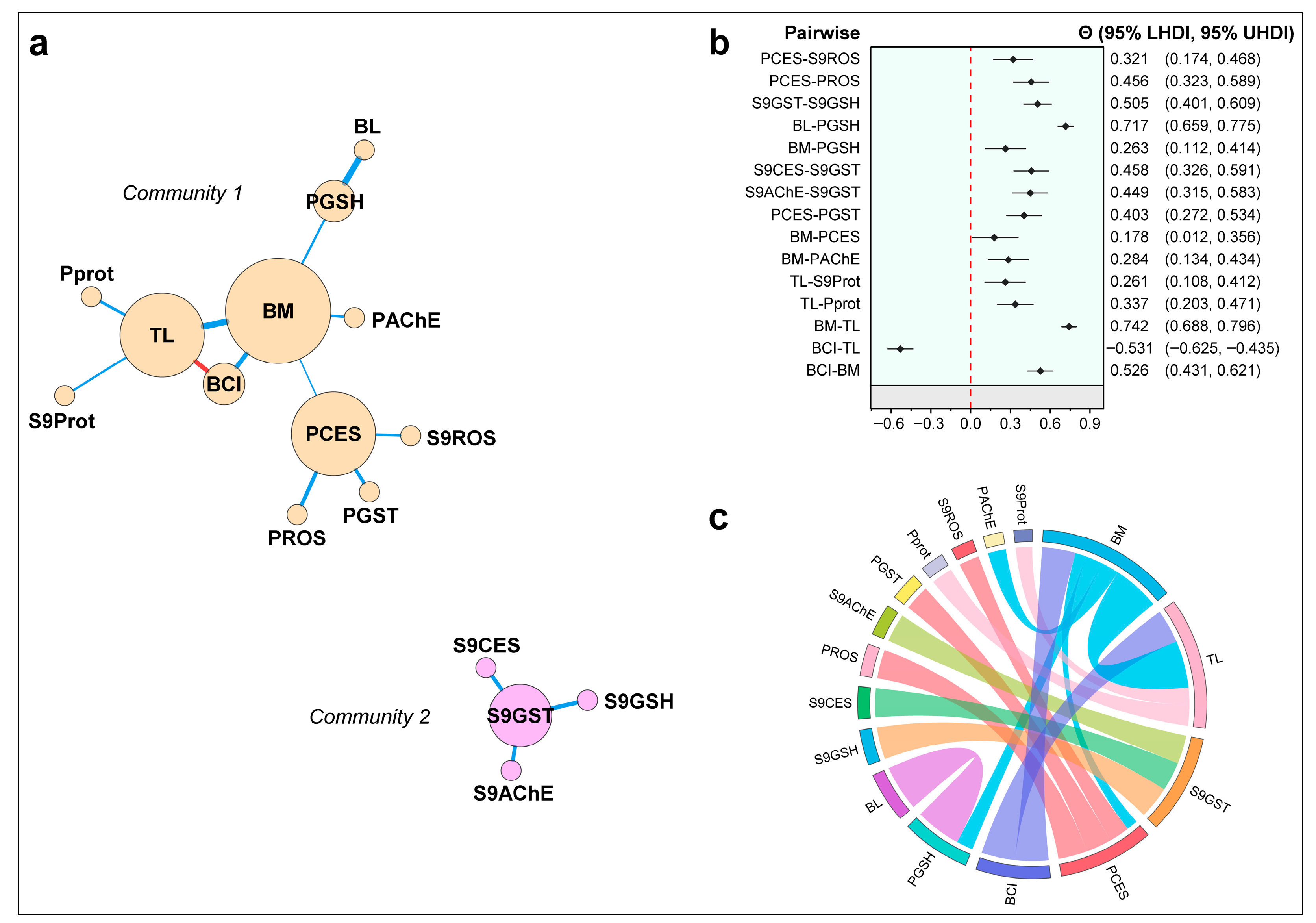
3.3. Bayesian Structure of Conditional Partial Associations
4. Discussion
4.1. Biomarker Response and Environmental Context
4.2. Hoeffding’s Dependence Analysis: Sparse but Informative Links
4.3. BUGM: Disconnected Corrleation Structure and Key Bridges
4.4. Broader Ecological and Biomonitoring Implications
4.5. Future Directions and Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barton, M.G.; Henderson, I.; Border, J.A.; Siriwardena, G. A Review of the Impacts of Air Pollution on Terrestrial Birds. Sci. Total Environ. 2023, 873, 162136. [Google Scholar] [CrossRef]
- Wang, L.; Nabi, G.; Yin, L.; Wang, Y.; Li, S.; Hao, Z.; Li, D. Birds and Plastic Pollution: Recent Advances. Avian Res. 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Baos, R.; Hiraldo, F.; Jovani, R.; Serrano, D.; Tella, J.L.; Gómez, G. Developmental Exposure to a Toxic Spill Compromises Long-Term Reproductive Performance in a Wild, Long-Lived Bird: The White Stork (Ciconia ciconia). PLoS ONE 2012, 7, 34716. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Ku, T.; Li, B.; Li, G.; Sang, N. Ambient Fine Particulate Matter Exposure Induces Cardiac Functional Injury and Metabolite Alterations in Middle-Aged Female Mice. Environ. Pollut. 2019, 248, 121–132. [Google Scholar] [CrossRef]
- Resano-Mayor, J.; Hernández-Matías, A.; Real, J.; Parés, F.; Moleón, M.; Mateo, R.; Ortiz-Santaliestra, M.E. The Influence of Diet on Nestling Body Condition of an Apex Predator: A Multi-Biomarker Approach. J. Comp. Physiol. B 2016, 186, 343–362. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Ciocan, C.M.; Hill, E.M.; Osada, M.; Kishida, M.; Itoh, N.; Kondo, R.; Minier, C.; Rotchell, J.M. Estrogens Disrupt Serotonin Receptor and Cyclooxygenase MRNA Expression in the Gonads of Mussels (Mytilus edulis). Aquat. Toxicol. 2010, 98, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Parsons, K.C.; Matz, A.C.; Hooper, M.J.; Pokras, M.A. Monitoring Wading Bird Exposure to Agricultural Chemicals Using Serum Cholinesterase Activity. Environ. Toxicol. Chem. 2000, 19, 1317–1323. [Google Scholar] [CrossRef]
- Burger, J. Metals in Avian Feathers: Bioindicators of Environmental Pollution. Rev. Environ. Toxicol. 1993, 5, 203–311. [Google Scholar]
- Furness, R.W. Birds as Monitors of Pollutants. In Birds as Monitors of Environmental Change; Springer: Dordrecht, The Netherlands, 1993; pp. 86–143. [Google Scholar]
- Janssens, E.; Dauwe, T.; Bervoets, L.; Eens, M. Inter- and Intraclutch Variability in Heavy Metals in Feathers of Great Tit Nestlings (Parus major) Along a Pollution Gradient. Arch. Environ. Contam. Toxicol. 2002, 43, 323–329. [Google Scholar] [CrossRef]
- Albayrak, T.; Pekgöz, A.K. Heavy Metal Effects on Bird Morphometry: A Case Study on the House Sparrow Passer domesticus. Chemosphere 2021, 276, 130056. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Yan, Q. Peroxymonosulfate Activation by Algal Carbocatalyst for Organic Dye Oxidation: Insights into Experimental and Theoretical. Sci. Total Environ. 2022, 816, 151611. [Google Scholar] [CrossRef]
- Janssens, E.; Dauwe, T.; Pinxten, R.; Bervoets, L.; Blust, R.; Eens, M. Effects of Heavy Metal Exposure on the Condition and Health of Nestlings of the Great Tit (Parus major), a Small Songbird Species. Environ. Pollut. 2003, 126, 267–274. [Google Scholar] [CrossRef]
- Gómez-Ramírez, P.; Martínez-López, E.; María-Mojica, P.; León-Ortega, M.; García-Fernández, A.J. Blood Lead Levels and δ-ALAD Inhibition in Nestlings of Eurasian Eagle Owl (Bubo bubo) to Assess Lead Exposure Associated to an Abandoned Mining Area. Ecotoxicology 2011, 20, 131–138. [Google Scholar] [CrossRef]
- Marsili, L.; Fossi, M.C.; Casini, S.; Focardi, S. PCB Levels in Bird Blood and Relationship to MFO Responses. Chemosphere 1996, 33, 699–710. [Google Scholar] [CrossRef]
- Bjedov, D.; Mikuška, A.; Lackmann, C.; Begović, L.; Mikuška, T.; Velki, M. Application of Non-Destructive Methods: Biomarker Assays in Blood of White Stork (Ciconia ciconia) Nestlings. Animals 2021, 11, 2341. [Google Scholar] [CrossRef]
- Bjedov, D.; Velki, M.; Lackmann, C.; Begović, L.; Mikuška, T.; Jurinović, L.; Mikuška, A. Blood Biomarkers in White Stork (Ciconia ciconia) Nestlings Show Different Responses in Several Areas of Croatia. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 547–558. [Google Scholar] [CrossRef]
- Bjedov, D.; Velki, M.; Toth, L.; Filipović Marijić, V.; Mikuška, T.; Jurinović, L.; Ečimović, S.; Turić, N.; Lončarić, Z.; Šariri, S.; et al. Heavy Metal(loid) Effect on Multi-Biomarker Responses in Apex Predator: Novel Assays in the Monitoring of White Stork Nestlings. Environ. Pollut. 2023, 324, 121398. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, D.; Velki, M.; Kovačić, L.S.; Begović, L.; Lešić, I.; Jurinović, L.; Mikuska, T.; Sudarić Bogojević, M.; Ečimović, S.; Mikuška, A. White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses. Agriculture 2023, 13, 1045. [Google Scholar] [CrossRef]
- Anam, K.K.; Maitra, S.K. Impact of Quinalphos on Blood Glucose and Acetylcholinesterase (AChE) Activity in Brain and Pancreas in a Roseringed Parakeet (Psittacula krameri Borealis: Newmann). Arch. Environ. Contam. Toxicol. 1995, 29, 20–23. [Google Scholar] [CrossRef]
- Hart, A.D.M. Relationships between Behavior and the Inhibition of Acetylcholinesterase in Birds Exposed to Organophosphorus Pesticides. Environ. Toxicol. Chem. 1993, 12, 321–336. [Google Scholar] [CrossRef]
- Schmoll, T.; Dietrich, V.; Winkel, W.; Lubjuhn, T. Blood Sampling Does Not Affect Fledging Success and Fledgling Local Recruitment in Coal Tits (Parus ater). J. Ornithol. 2004, 145, 79–80. [Google Scholar] [CrossRef]
- Lackmann, C.; Velki, M.; Bjedov, D.; Ečimović, S.; Seiler, T.-B.; Hollert, H. Commercial Preparations of Pesticides Exert Higher Toxicity and Cause Changes at Subcellular Level in Earthworm Eisenia andrei. Environ. Sci. Eur. 2021, 33, 12. [Google Scholar] [CrossRef]
- Lončarić, Ž.; Lackmann, C.; Bjedov, D.; Šimić, A.; Ečimović, S.; Seiler, T.-B.; Hollert, H.; Velki, M. Chronic Effects of Commercial Pesticide Preparations on Biomarkers and Reproductive Success in Earthworm Eisenia andrei. Environ. Sci. Eur. 2024, 36, 117. [Google Scholar] [CrossRef]
- Kosicki, J.Z.; Indykiewicz, P. Effects of Breeding Date and Weather on Nestling Development in White Storks Ciconia ciconia. Bird Study 2011, 58, 178–185. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Sparks, T.H.; Bochenski, M.; Dabert, M.; Kasprzak, M.; Kaminski, P.; Mroczkowski, S.; Wisniewska, E.; Jerzak, L. Do Males Hatch First and Dominate Sex Ratios in White Stork Ciconia ciconia Chicks? J. Ornithol. 2011, 152, 213–218. [Google Scholar] [CrossRef][Green Version]
- Jerzak, L.; Sparks, T.H.; Kasprzak, M.; Bochenski, M.; Kaminski, P.; Wiśniewska, E.; Mroczkowski, S.; Tryjanowski, P. Blood Chemistry in White Stork Ciconia ciconia Chicks Varies by Sex and Age. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, P.; Jerzak, L.; Sparks, T.H.; Johnston, A.; Bochenski, M.; Kasprzak, M.; Wiśniewska, E.; Mroczkowski, S.; Tryjanowski, P. Sex and Other Sources of Variation in the Haematological Parameters of White Stork Ciconia ciconia Chicks. J. Ornithol. 2014, 155, 307–314. [Google Scholar] [CrossRef]
- Tapia-Contreras, E.; Bernal-Alviz, J.; Bjedov, D.; Moyano-Salcedo, Á.; Córdoba-Tovar, L.; Buelvas-Soto, J.; Seña-Gaibao, A.; Marrugo-Negrete, J. Methylmercury, Cadmium and Plastron Length Topologically Modulate Multimetallic Bioaccumulation in Colombian Slider (Trachemys callirostris Gray, 1856): A Bayesian Structure Learning. J. Hazard. Mater. 2025, 496, 139487. [Google Scholar] [CrossRef]
- Boros, G.; Sály, P.; Vanni, M.J. Ontogenetic Variation in the Body Stoichiometry of Two Fish Species. Oecologia 2015, 179, 329–341. [Google Scholar] [CrossRef]
- Mangel, M.; Munch, S.B. A Life-History Perspective on Short- and Long-Term Consequences of Compensatory Growth. Am. Nat. 2005, 166, E155–E176. [Google Scholar] [CrossRef]
- Amiard, J.-C.; Caquet, T.; Lagadic, L. Use of Biomarkers for Environmental Quality Assessment; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781000445640. [Google Scholar]
- Cordi, B.; Fossi, C.; Depledge, M. Temporal Biomarker Responses in Wild Passerine Birds Exposed to Pesticide Spray Drift. Environ. Toxicol. Chem. 1997, 16, 2118–2124. [Google Scholar] [CrossRef]
- Bartkowiak, D.J.; Wilson, B.W. Avian Plasma Carboxylesterase Activity as a Potential Biomarker of Organophosphate Pesticide Exposure. Environ. Toxicol. Chem. 1995, 14, 2149–2153. [Google Scholar] [CrossRef]
- Fossi, M.C.; Leonzio, C.; Massi, A.; Lari, L.; Casini, S. Serum Esterase Inhibition in Birds: A Nondestructive Biomarker to Assess Organophosphorus and Carbamate Contamination. Arch. Environ. Contam. Toxicol. 1992, 23, 99–104. [Google Scholar] [CrossRef]
- Espín, S.; Martínez-López, E.; Jiménez, P.; María-Mojica, P.; García-Fernández, A.J. Effects of Heavy Metals on Biomarkers for Oxidative Stress in Griffon Vulture (Gyps fulvus). Environ. Res. 2014, 129, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species—Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Lee, H.; Kim, B.-W.; Lee, J.-W.; Hong, J.; Lee, J.-W.; Kim, H.-L.; Lee, J.-S.; Ko, Y.-G. Extracellular Reactive Oxygen Species Are Generated by a Plasma Membrane Oxidative Phosphorylation System. Free Radic. Biol. Med. 2017, 112, 504–514. [Google Scholar] [CrossRef]
- Yan, B. Carboxylesterases. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 695–698. [Google Scholar] [CrossRef]
- Satoh, T.; Hosokawa, M. Structure, Function and Regulation of Carboxylesterases. Chem. Biol. Interact. 2006, 162, 195–211. [Google Scholar] [CrossRef]
- Castro, F.L.d.S.; Kim, W.K. Secondary Functions of Arginine and Sulfur Amino Acids in Poultry Health: Review. Animals 2020, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Vilar da Silva, J.H.; González-Cerón, F.; Howerth, E.W.; Rekaya, R.; Aggrey, S.E. Alteration of Dietary Cysteine Affects Activities of Genes of the Transsulfuration and Glutathione Pathways, and Development of Skin Tissues and Feather Follicles in Chickens. Anim. Biotechnol. 2020, 31, 203–208. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for Differentiation of Glutathione S-Transferases. Methods Enzym. 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Kania, W. Investigations of White Stork (Ciconia ciconia). Ring 1988, 135, 13–19. [Google Scholar]
- Zurell, D.; von Wehrden, H.; Rotics, S.; Kaatz, M.; Groß, H.; Schlag, L.; Schäfer, M.; Sapir, N.; Turjeman, S.; Wikelski, M.; et al. Home Range Size and Resource Use of Breeding and Non-Breeding White Storks Along a Land Use Gradient. Front. Ecol. Evol. 2018, 6, 382083. [Google Scholar] [CrossRef]
- Standfuß, I.; Geiß, C.; Nathan, R.; Rotics, S.; Scacco, M.; Kerr, G.; Taubenböck, H. Time Series Enable the Characterization of Small-scale Vegetation Dynamics That Influence Fine-scale Animal Behavior—An Example from White Storks’ Foraging Behavior. Remote Sens. Ecol. Conserv. 2022, 8, 391–408. [Google Scholar] [CrossRef]
- Janiszewski, T.; Minias, P.; Wojciechowski, Z. Timing of Arrival at Breeding Grounds Determines Spatial Patterns of Productivity within the Population of White Stork (Ciconia ciconia). Popul. Ecol. 2014, 56, 217–225. [Google Scholar] [CrossRef]
- Carey, C. Avian Growth and Development Evolution within the Altrical-Precocial Spectrum. Auk 2000, 117, 839–840. [Google Scholar] [CrossRef]
- Bright, K.L.; Rausher, M.D. Natural Selection On A Leaf-Shape Polymorphism In The Ivyleaf Morning Glory (Ipomoea hederacea). Evolution 2008, 62, 1978–1990. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating Fitness: A Comparison of Body Condition Indices. Oikos 1996, 77, 61. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Hosokawa, M.; Satoh, T. Measurement of Carboxylesterase (CES) Activities. Curr. Protoc. Toxicol. 2001, 10, 4–7. [Google Scholar] [CrossRef]
- Schulte-Hostedde, A.I.; Millar, J.S.; Hickling, G.J. Evaluating Body Condition in Small Mammals. Can. J. Zool. 2001, 79, 1021–1029. [Google Scholar] [CrossRef]
- Van Der Laan, M.J.; Polley, E.C.; Hubbard, A.E. Super Learner. Stat. Appl. Genet. Mol. Biol. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Carpenito, T.; Manjourides, J. MISL: Multiple Imputation by Super Learning. Stat. Methods Med. Res. 2022, 31, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- El Badisy, I.; Graffeo, N.; Khalis, M.; Giorgi, R. Multi-Metric Comparison of Machine Learning Imputation Methods with Application to Breast Cancer Survival. BMC Med. Res. Methodol. 2024, 24, 191. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.W. Missing Data Analysis: Making It Work in the Real World. Annu. Rev. Psychol. 2009, 60, 549–576. [Google Scholar] [CrossRef]
- García, S.; Luengo, J.; Herrera, F. Dealing with Missing Values. Intell. Syst. Ref. Libr. 2015, 72, 59–105. [Google Scholar] [CrossRef]
- Enders, C.K. Applied Missing Data Analysis, 2nd ed.; Guilford Publications: New York, NY, USA, 2020; pp. 1–23. [Google Scholar]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Pereira, R.C.; Rodrigues, P.P.; Figueiredo, M.A.T.; Abreu, P.H. Automatic Delta-Adjustment Method Applied to Missing Not At Random Imputation. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Cham, Switzerland, 2023; Volume 14073 LNCS, pp. 481–493. [Google Scholar]
- Chandola, V.; Banerjee, A.; Kumar, V. Anomaly Detection. ACM Comput. Surv. 2009, 41, 1–58. [Google Scholar] [CrossRef]
- Horton, N.J.; Lipsitz, S.R. Multiple Imputation in Practice. Am. Stat. 2001, 55, 244–254. [Google Scholar] [CrossRef]
- Cai, M.; van Buuren, S.; Vink, G. Generalizing Univariate Predictive Mean Matching to Impute Multiple Variables Simultaneously. In Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2022; Volume 506 LNNS, pp. 75–91. [Google Scholar]
- Fujita, A.; Sato, J.R.; Demasi, M.A.A.; Sogayar, M.C.; Ferreira, C.E.; Miyano, S. Comparing Pearson, Spearman And Hoeffding’s D Measure For Gene Expression Association Analysis. J. Bioinform. Comput. Biol. 2009, 7, 663–684. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, A.D.; Chicken, E. Nonparametric Statistical Methods; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2015; ISBN 9780470387375. [Google Scholar]
- Hoeffding, W. A Non-Parametric Test of Independence; Springer: New York, NY, USA, 1994; pp. 214–226. [Google Scholar]
- Wilding, G.E.; Mudholkar, G.S. Empirical Approximations for Hoeffding’s Test of Bivariate Independence Using Two Weibull Extensions. Stat. Methodol. 2008, 5, 160–170. [Google Scholar] [CrossRef]
- Vogels, L.; Mohammadi, R.; Schoonhoven, M.; Birbil, Ş.İ. Bayesian Structure Learning in Undirected Gaussian Graphical Models: Literature Review with Empirical Comparison. J. Am. Stat. Assoc. 2024, 119, 3164–3182. [Google Scholar] [CrossRef]
- Mohammadi, R.; Wit, E.C. BDgraph: An R Package for Bayesian Structure Learning in Graphical Models. J. Stat. Softw. 2019, 89, 1–30. [Google Scholar] [CrossRef]
- Mohammadi, R.; Massam, H.; Letac, G. Accelerating Bayesian Structure Learning in Sparse Gaussian Graphical Models. J. Am. Stat. Assoc. 2023, 118, 1345–1358. [Google Scholar] [CrossRef]
- Cappé, O.; Robert, C.P.; Rydén, T. Reversible Jump, Birth-and-Death and More General Continuous Time Markov Chain Monte Carlo Samplers. J. R. Stat. Soc. Ser. B Stat. Methodol. 2003, 65, 679–700. [Google Scholar] [CrossRef]
- Zondervan-Zwijnenburg, M.; Peeters, M.; Depaoli, S.; Van de Schoot, R. Where Do Priors Come From? Applying Guidelines to Construct Informative Priors in Small Sample Research. Res. Hum. Dev. 2017, 14, 305–320. [Google Scholar] [CrossRef]
- Smid, S.C.; McNeish, D.; Miočević, M.; van de Schoot, R. Bayesian Versus Frequentist Estimation for Structural Equation Models in Small Sample Contexts: A Systematic Review. Struct. Equ. Model. 2020, 27, 131–161. [Google Scholar] [CrossRef]
- Kundu, S.; Cheng, Y.; Shin, M.; Manyam, G.; Mallick, B.K.; Baladandayuthapani, V. Bayesian Variable Selection with Graphical Structure Learning: Applications in Integrative Genomics. PLoS ONE 2018, 13, e0195070. [Google Scholar] [CrossRef]
- Lee, Y.; Song, J. Robustness of Model Averaging Methods for the Violation of Standard Linear Regression Assumptions. Commun. Stat. Appl. Methods 2021, 28, 189–204. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer Series in Statistics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Dyrba, M.; Mohammadi, R.; Grothe, M.J.; Kirste, T.; Teipel, S.J. Gaussian Graphical Models Reveal Inter-Modal and Inter-Regional Conditional Dependencies of Brain Alterations in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 507957. [Google Scholar] [CrossRef]
- Li, B.; Sun, Z.; He, Q.; Zhu, Y.; Qin, Z.S. Bayesian Inference with Historical Data-Based Informative Priors Improves Detection of Differentially Expressed Genes. Bioinformatics 2016, 32, 682–689. [Google Scholar] [CrossRef]
- Zitzmann, S.; Lüdtke, O.; Robitzsch, A.; Hecht, M. On the Performance of Bayesian Approaches in Small Samples: A Comment on Smid, McNeish, Miocevic, and van de Schoot (2020). Struct. Equ. Model. 2021, 28, 40–50. [Google Scholar] [CrossRef]
- Mukherjee, S.; Speed, T.P. Network Inference Using Informative Priors. Proc. Natl. Acad. Sci. USA 2008, 105, 14313–14318. [Google Scholar] [CrossRef]
- Leday, G.G.R.; Richardson, S. Fast Bayesian Inference in Large Gaussian Graphical Models. Biometrics 2019, 75, 1288–1298. [Google Scholar] [CrossRef]
- Huth, K.B.S.; de Ron, J.; Goudriaan, A.E.; Luigjes, J.; Mohammadi, R.; van Holst, R.J.; Wagenmakers, E.-J.; Marsman, M. Bayesian Analysis of Cross-Sectional Networks: A Tutorial in R and JASP. Adv. Methods Pract. Psychol. Sci. 2023, 6, 25152459231193334. [Google Scholar] [CrossRef]
- Mohammadi, A.; Abegaz, F.; Heuvel, E.; Wit, E.C. Bayesian Modelling of Dupuytren Disease by Using Gaussian Copula Graphical Models. J. R. Stat. Soc. Ser. C Appl. Stat. 2017, 66, 629–645. [Google Scholar] [CrossRef]
- Lynch, S.M. Bayesian Statistics. In Encyclopedia of Social Measurement; Elsevier: Amsterdam, The Netherlands, 2005; Volume 1, pp. 135–144. [Google Scholar]
- Sekulovski, N.; Keetelaar, S.; Huth, K.; Wagenmakers, E.-J.; van Bork, R.; van den Bergh, D.; Marsman, M. Testing Conditional Independence in Psychometric Networks: An Analysis of Three Bayesian Methods. Multivar. Behav. Res. 2024, 59, 913–933. [Google Scholar] [CrossRef]
- Huth, K.B.S.; Keetelaar, S.; Sekulovski, N.; van den Bergh, D.; Marsman, M. Simplifying Bayesian Analysis of Graphical Models for the Social Sciences with Easybgm: A User-Friendly R-Package. Adv. Psychol. 2024, 2, e66366. [Google Scholar] [CrossRef]
- Romić, D.; Hušnjak, S.; Mesić, M.; Salajpal, K.; Barić, K.; Poljak, M.; Romić, M.; Konjačić, M.; Vnučec, I.; Bakić, H. Utjecaj Poljoprivrede Na Onečišćenje Površinskih i Podzemnih Voda u Republici Hrvatskoj. Hrvat. vode. Elabor./Study 2015. [Google Scholar]
- Bašic, F.; Bogunović, M.; Božić, M.; Husnjak, S.; Jurić, I.; Kisić, I.; Mesić, M.; Mirošević, N.; Romić, D.; Žugec, I. Regionalisation of Croatian Agriculture. Agric. Conspec. Sci. 2007, 72, 27–38. [Google Scholar]
- Mikuska, T. Distribution and Population Status of White Storks in Croatia. In Proceedings of the 12th Meeting of the European Stork Villages, Čigoč, Croatia, 25–27 June 2015. [Google Scholar]
- Pandey, S.P.; Mohanty, B. The Neonicotinoid Pesticide Imidacloprid and the Dithiocarbamate Fungicide Mancozeb Disrupt the Pituitary–Thyroid Axis of a Wildlife Bird. Chemosphere 2015, 122, 227–234. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; He, W.; Liu, D. Regulations of Xenobiotics and Endobiotics on Carboxylesterases: A Comprehensive Review. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 321–330. [Google Scholar] [CrossRef]
- Di, L. The Impact of Carboxylesterases in Drug Metabolism and Pharmacokinetics. Curr. Drug Metab. 2019, 20, 91–102. [Google Scholar] [CrossRef]
- Dobreva, M.P.; Camacho, J.; Abzhanov, A. Time to Synchronize Our Clocks: Connecting Developmental Mechanisms and Evolutionary Consequences of Heterochrony. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Nichelmann, M.; Höchel, J.; Tzschentke, B. Biological Rhythms in Birds—Development, Insights and Perspectives. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 124, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Velando, A.; Noguera, J.C.; da Silva, A.; Kim, S.-Y. Redox-Regulation and Life-History Trade-Offs: Scavenging Mitochondrial ROS Improves Growth in a Wild Bird. Sci. Rep. 2019, 9, 2203. [Google Scholar] [CrossRef] [PubMed]
- Gabriela Jimenez, A. “The Same Thing That Makes You Live Can Kill You in the End”: Exploring the Effects of Growth Rates and Longevity on Cellular Metabolic Rates and Oxidative Stress in Mammals and Birds. Integr. Comp. Biol. 2018, 58, 544–558. [Google Scholar] [CrossRef]
- Rode, K.D.; Robbins, C.T.; Nelson, L.; Amstrup, S.C. Can Polar Bears Use Terrestrial Foods to Offset Lost Ice-based Hunting Opportunities? Front. Ecol. Environ. 2015, 13, 138–145. [Google Scholar] [CrossRef]
- Bright Ross, J.G.; Newman, C.; Buesching, C.D.; Connolly, E.; Nakagawa, S.; Macdonald, D.W. A Fat Chance of Survival: Body Condition Provides Life-History Dependent Buffering of Environmental Change in a Wild Mammal Population. Clim. Change Ecol. 2021, 2, 100022. [Google Scholar] [CrossRef]
- Resano-Mayor, J.; Hernández-Matías, A.; Real, J.; Parés, F.; Inger, R.; Bearhop, S. Comparing Pellet and Stable Isotope Analyses of Nestling Bonelli’s Eagle Aquila fasciata Diet. Ibis 2014, 156, 176–188. [Google Scholar] [CrossRef]
- Gallo, L.; Quintana, F.; Svagelj, W.S.; Uhart, M. Plasma Biochemistries and Morphometric Indices of Body Condition in Imperial Cormorant (Phalacrocorax atriceps) Chicks. Waterbirds 2017, 40, 118–128. [Google Scholar] [CrossRef]
| Method | Reaction Mixture | Measurement Settings | ||
|---|---|---|---|---|
| Enzymatic biomarkers | Acetylcholinesterase (AChE) Activity | Ellman et al. [51] | 5 µL plasma (5× dilution in 0.10 M phosphate buffer, pH 7.20) or 25 µL S9 (10× dilution in phosphate buffer) 180 µL phosphate buffer 10 µL DTNB (1.6 mM in buffer) 10 µL acetylthiocholine iodide (156 mM in distilled water) | Absorbance was recorded at 412 nm over 5 min. The specific activity was calculated using the molar extinction coefficient ε = 13.60 · 103 M−1·cm−1. |
| Carboxylesterase (CES) Activity | Hosokawa and Satoh [52] | 10 µL undiluted plasma sample or 20 µL S9 (10× dilution in phosphate buffer) 150 µL p-nitrophenyl acetate (1 mM in acetonitrile, diluted in distilled water) | Absorbance was recorded at 405 nm over 5 min. The specific activity was calculated using the molar extinction coefficient ε = 16.40 · 103 M−1·cm−1. | |
| Glutathione S-Transferase (GST) Activity | Habig and Jakoby [43] | 5 µL plasma or 20 µL S9 (10× dilution in phosphate buffer) 160 µL CDNB (1 mM in ethanol/phosphate buffer, 0.10 M, pH 7.2), 40 µL GSH (25 mM in distilled water) | Absorbance was recorded at 340 nm over 2 min (plasma) or 5 min (S9). The specific activity was calculated using the molar extinction coefficient ε = 9.60 · 103 M−1·cm−1. | |
| Non-enzymatic biomarkers | GSH Detection | Bjedov et al. [16] | 2 µL sample (for both plasma and S9) 90 µL phosphate buffer (0.10 M, pH 7.20) 5 µL CellTracker™ Green CMFDA (9.78 µM in DMSO) | Fluorescence was recorded at 5 min intervals for 15 min with excitation of 485 nm and emission of 530 nm (gain: 50). |
| ROS Detection | Bjedov et al. [16] | 10 µL sample (for both plasma and S9) 90 µL phosphate buffer 10 µL CM-H2DCFDA (7.87 µM in DMSO) for plasma or 5 µL CM-H2DCFDA (7.87 µM in DMSO) for S9 | Fluorescence was recorded at 5 min intervals for 15 min with excitation of 485 nm and emission of 530 nm (gain: 50). | |
| Proteins (mg∙mL−1) | AChE (nmol·min−1·mgPROT−1) | CES (nmol·min−1·mgPROT−1) | GST (nmol·min−1·mgPROT−1) | GSH (RFU) | ROS (RFU) | ||
|---|---|---|---|---|---|---|---|
| Plasma | n (individual) | 20 | 20 | 20 | 20 | 20 | 20 |
| Min | 10 | 11.30 | 2.57 | 2.65 | 5493 | 127 | |
| 25% Percentile (Q1) | 42.29 | 22.96 | 3.45 | 4.26 | 6851 | 135 | |
| Median | 54.01 | 29.61 | 4.65 | 6.15 | 7625 | 143 | |
| 75% Percentile (Q3) | 58.68 | 36.47 | 5.83 | 9.63 | 9513 | 150 | |
| Max | 74.09 | 120.30 | 26.25 | 34.64 | 13,382 | 205 | |
| Range | 64.09 | 109 | 23.69 | 31.99 | 7889 | 78 | |
| Mean | 50.43 | 33.21 | 5.68 | 8.39 | 8266 | 145 | |
| SD | 13.46 | 21.81 | 4.99 | 7.04 | 1848 | 17 | |
| SE | 3.00 | 4.88 | 1.12 | 1.58 | 413 | 4 | |
| S9 | n (individual) | 20 | 20 | 20 | 20 | 20 | 19 |
| Min | 91.74 | 0.62 | 1.04 | 0.82 | 10,575 | 59 | |
| 25% Percentile (Q1) | 173.40 | 0.75 | 1.13 | 1.09 | 16,684 | 82 | |
| Median | 193.00 | 0.88 | 1.23 | 1.48 | 20,155 | 94 | |
| 75% Percentile (Q3) | 235.90 | 0.96 | 1.53 | 1.68 | 22,435 | 118 | |
| Max | 322 | 2.28 | 2.74 | 3.28 | 30,113 | 290 | |
| Range | 230.20 | 1.66 | 1.71 | 2.46 | 19,538 | 231 | |
| Mean | 195.10 | 0.98 | 1.39 | 1.51 | 19,947 | 115 | |
| SD | 58.32 | 0.40 | 0.43 | 0.56 | 5004 | 61 | |
| SE | 13.04 | 0.09 | 0.09 | 0.12 | 1119 | 14 |
| Mass (kg) | Beak (mm) | Tarsus (mm) | Residual BCI | |
|---|---|---|---|---|
| n (individual) | 20 | 20 | 19 | 19 |
| Min | 2.35 | 97.00 | 136.00 | −0.72 |
| 25% Percentile (Q1) | 3.13 | 107.80 | 160.00 | −0.11 |
| Median | 3.40 | 115.50 | 174.00 | 0.01 |
| 75% Percentile (Q3) | 3.69 | 125.80 | 188.00 | 0.11 |
| Max | 4.20 | 140.00 | 237.00 | 0.58 |
| Range | 1.85 | 43.00 | 101.00 | 1.31 |
| Mean | 3.36 | 116.80 | 177.00 | 0 |
| SD | 0.43 | 12.30 | 23.73 | 0.32 |
| SE | 0.10 | 2.75 | 5.45 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikuška, A.; Alić, S.; Levak, I.; Bernal-Alviz, J.; Velki, M.; Nekić, R.; Ečimović, S.; Bjedov, D. Bayesian Structure Learning Reveals Disconnected Correlation Patterns Between Morphometric Traits and Blood Biomarkers in White Stork Nestlings. Birds 2025, 6, 51. https://doi.org/10.3390/birds6040051
Mikuška A, Alić S, Levak I, Bernal-Alviz J, Velki M, Nekić R, Ečimović S, Bjedov D. Bayesian Structure Learning Reveals Disconnected Correlation Patterns Between Morphometric Traits and Blood Biomarkers in White Stork Nestlings. Birds. 2025; 6(4):51. https://doi.org/10.3390/birds6040051
Chicago/Turabian StyleMikuška, Alma, Sabina Alić, Ivona Levak, Jorge Bernal-Alviz, Mirna Velki, Rocco Nekić, Sandra Ečimović, and Dora Bjedov. 2025. "Bayesian Structure Learning Reveals Disconnected Correlation Patterns Between Morphometric Traits and Blood Biomarkers in White Stork Nestlings" Birds 6, no. 4: 51. https://doi.org/10.3390/birds6040051
APA StyleMikuška, A., Alić, S., Levak, I., Bernal-Alviz, J., Velki, M., Nekić, R., Ečimović, S., & Bjedov, D. (2025). Bayesian Structure Learning Reveals Disconnected Correlation Patterns Between Morphometric Traits and Blood Biomarkers in White Stork Nestlings. Birds, 6(4), 51. https://doi.org/10.3390/birds6040051







