Simple Summary
Coronaviruses are widespread pathogens that affect both humans and animals. While their presence in wild animals is understudied, many species of wild birds have tested positive for coronaviruses in recent years. Free-ranging, wild waterfowl are of particular concern as potential reservoirs for coronaviruses due to their ecology and migratory behavior. This review describes our current knowledge of coronaviruses in birds, provides an updated list of global detections of coronaviruses in 124 species of wild birds since 2000, and highlights the need for future research on wild birds, especially waterfowl, as potential contributors to local and global virus transmission.
Abstract
Coronaviruses (CoVs) were first described in poultry in the early 1930s and formally recognized as pathogens of both animal and human populations in the late 1960s. They are now considered among the most abundant viral families in the world. Though their distribution and diversity remain understudied in wild animals, representatives from 13 orders of wild birds worldwide have tested positive for CoVs of the gamma and delta genera over the last 25 years. Many of these wild bird species are in the orders Charadriiformes (shorebirds and their relatives) and Anseriformes (waterfowl including ducks, geese, and swans). Waterfowl are particularly concerning as potential reservoirs for CoVs because they are globally distributed; often congregate in large, mixed-species flocks; and may exist in close proximity to humans and domesticated animals. This review describes the history and current knowledge of CoVs in birds, provides an updated list of global detections of CoVs in 124 species of wild birds as reported in the peer-reviewed literature since 2000, and highlights topics for future research that would help elucidate the role of waterfowl in CoV transmission. Our review reiterates the need for continuous surveillance to detect and monitor CoVs across all bird species and for standardization in data reporting and analysis of both negative and positive results. Such information is critical to understand the potential role of free-ranging birds in the maintenance, evolution, and transmission of the virus. Further, we believe that research on the potential impacts of coronavirus infections and coinfections on avian demographics, especially reproduction in waterfowl, is warranted given known consequences in domestic poultry.
1. Introduction
Coronaviruses (CoVs) were first described in poultry in 1931, formally recognized as animal and human pathogens in 1968, and continue to impact domestic poultry production worldwide. Beyond poultry, these viruses have been detected in 13 orders of wild birds since 2000,and are most commonly reported in representatives of Anseriformes (waterfowl including ducks, geese, and swans) and Charadriiformes (shorebirds, gulls, auks, and their relatives) [1]. Wild birds with CoV are typically described as asymptomatic, but some avian CoVs—including the highly contagious infectious bronchitis virus (IBV)—can cause distinct morbidities such as renal and respiratory failure, decreased egg production, and systemic comorbidities in domestic poultry [2].
In addition, wild birds could serve as important reservoirs for CoV transmission both among wild and to domestic birds [3], as illustrated by the clustering of IBV strains along migratory pathways of free-ranging wildlife [4]. Waterfowl are particularly concerning as potential reservoirs for CoVs because they occur worldwide, are migratory and congregate in large mixed-species flocks, play an important role in the connectivity of wetland habitats, and come in close contact with humans and other animals (including poultry) during their annual cycles. However, our understanding of CoVs and their potential impacts on wild birds, including waterfowl, is limited. Further, the methodology used to detect and report CoVs in wild birds is highly variable.
In this review, we summarize the historical context of CoVs, our current molecular and genetic understanding of CoVs established mainly by research on domestic poultry, and the reported prevalence of CoVs in wild birds worldwide. We concluded by identifying knowledge gaps as they pertain to CoVs in wild birds with a focus on waterfowl’s role as a reservoir for these viruses and potential avenues for future research on this topic that align with the One Health Initiative.
1.1. Historical Context
In 1931, Schalk and Hawn described “an apparently new respiratory disease” that emerged in domestic poultry during the late 1920s [5]. The chicks infected with this disease displayed clinical signs of gasping, listlessness, and depression, which resulted in 40–90% mortality; post-mortem findings were consistent with acute congestion in the lungs [6]. In 1933, Bushnell and Brandly [7] described a nearly identical respiratory disease that affected chicks and found the causative agent to be viral in origin. Bushnell and Brandly isolated this virus and cultivated it in chick embryos, which resulted in embryo death. The symptoms described by both Schalk and Hawn [5] and Bushnell and Brandley [7] were similar to a viral disease that had previously been described by May and Tittsler [8] in domestic poultry in 1925, infectious layngotracheitis. Due to the similarities in the observed signs, these respiratory diseases were initially attributed to the same viral agent. However, further work by Beach and Schalm (1936) [9] determined that there were two separate diseases caused by distinct viruses—infectious layngotracheitis virus and infectious bronchitis virus (IBV), the cause of the newly discovered “gaping disease.”
Given the speed of transmission, high mortality rates, and economic losses due to reduction in growth, poor health, and meat condemnations associated with IBV and secondary infections, there was an urgent need to understand the disease and develop control strategies for the poultry industry [10]. Early attempts at the prevention of IBV within domestic flocks included “controlled exposure,” whereby samples of the virus were passed through embryos to attenuate the virus [6]. The attenuated virus was then transmitted through the flock, providing a protective effect within the hens with an average half-life of four days [11].
Concurrent with work to control “gaping disease” in domestic poultry, Peterson and Hymas [12] described an outbreak of an unknown disease with a variety of signs in domestic turkeys of Washington state. The birds presented with sudden onset of near total morbidity with the entire flock going “off feed” resulting in loss of weight. The disease was called “mud fever” or “bluecomb,” so named for the resulting cyanosis of the head. Pomeroy and Sieburth [13] described a similar outbreak in Minnesota and demonstrated that this disease was highly transmissible. Subsequent filtration studies by Sieburth and Johnson [14] provided evidence that a viral-like agent was responsible for the disease; however, the specific virus would not be identified for nearly two decades.
In 1966, Tyrrell and Bynoe isolated viruses with a distinctive layer of projections on their surfaces from the respiratory tracts of adult humans who had symptoms attributed to the common cold [15]. In 1967, Almeida and Tyrrell noted that the human viruses described by Tyrrell and Bynoe [15] resembled those previously isolated from IBV in poultry [16]. Scientists would go on to identify similar viruses with crown-like protruding spike (S) proteins on their surfaces in other animal species, and in 1968, the Coronaviridae family, which includes IBV, was officially recognized as a distinct group of viruses [17]. The causative viral agent of “bluecomb” disease (initially named as a separate viral species, Turkey coronavirus), described above in domestic turkeys, was included in this family in 1973 after microscopy studies presented evidence of infectious particles morphologically similar to CoVs [18,19]. Historically, our knowledge of CoVs in birds was limited to these described reports in domestic poultry species of order Galliformes, but we now have evidence that CoVs occur throughout class Aves, including the orders Anseriformes, Charadriiformes, Columbiformes, and Passeriformes [20].
1.2. Taxonomy
Due to the high genetic diversity of CoVs and the large number of viral species found within birds and mammals, the taxonomic system to classify CoVs has been updated several times, with the most recent update approved in 2023 and ratified in April 2024 (Master Species List #39) [21]. Avian CoVs now fall within the Orthocoronavirinae subfamily of the Coronaviridae family. This family contains the subfamilies Letovirinae (the only species included being the fish virus, now called Alphaletovirus microhylae), Pitovirinae (the only species included being the fish virus, now called Alphapironavirus salmonis) and Orthocornavirinae. The 54 currently established species of the Coronaviridae family were renamed with the previously mentioned 2024 update to binomially formatted species names [21,22]. Based on the molecular clock analysis of CoVs, the most recent common ancestor of all current CoVs was estimated at 8100 BC [23].
The Orthocoronavirinae subfamily is divided into four genera: Alpha-, Beta-, Gamma- and Deltacoronavirus [24,25]. CoVs classified to the Alphacoronavirus and Betacoronavirus genera are typically isolated from mammals, where bats and rodents are the key reservoir species. Phylogenetic analysis demonstrates that bat hosts have a high level of connectivity, with 11 of the 19 viral species of Alphacoronavirus detected from 14 of the 21 bat families, allowing for significant cross-species transmission [26,27]. Alphacoronaviruses are the most numerous genus within the Orthocoronavirinae subfamily and are prevalent as respiratory infections in humans worldwide [28]. The human-associated Alphacoronaviruses share common ancestors with bat CoVs, suggesting a bat viral origin [29]. The CoVs associated with the human SARS-CoV, SARS-CoV-2 and MERS-CoV epidemics are Betacoronaviruses and these CoVs primarily affect mammals [30]. Interestingly, a survey of wild birds in Brazil observed Betacoronavirus-positive samples which clustered with murine coronavirus in the Betacoronavirus genera [31], demonstrating the high plasticity of CoVs and their cross-species transmission potential.
CoVs classified to the gamma and delta genera are typically isolated from birds [32,33,34]. Gamma- and Delta- coronaviruses are present in both wild and domesticated bird species, but our understanding of the disease ecology of CoVs in birds is derived primarily from research on CoVs in the gamma genus that infect domestic poultry, namely IBV. Initially all known as avian coronavirus, within the Gammacoronavirus genus, the Igacovirus subgenus now consists of Gammacoronavirus galli ([IBV], formerly known as avian coronavirus), Gammacoronavirus pulli (formerly known as avian coronavirus 9203), and Gammacoronavirus anatis (formerly known as duck coronavirus 2714) (Table 1) [21]. The Brangacovirus subgenus contains only one species which infects Canada goose (Branta canadensis), Gammacoronavirus brantae (formerly known as goose coronavirus CB17) [35]. CoVs isolated from whales and dolphins within the Cegacovirus subgenus also fall in the Gammacoronavirus genus [36], a notable exception to the remaining mammalian CoVs within the Alphacoronavirus and Betacoronavirus genera. Avian Gammacoronaviruses have been found throughout a wide range of orders, including Anseriformes, Ciconiiformes, Charadriiformes, Columbiformes, Galliformes, Passeriformes, Pelecaniformes, and Psittaciformes.

Table 1.
Binomial species names of coronavirus species within Gammacoronavirus and Deltacoronavirus updated in April 2024 in Master Species List (MSL) #39. All established coronavirus species were renamed to a unified binomial nomenclature. As species is the lowest rank recognized by the International Committee on Taxonomy of Viruses, formal subspecies categories were not created.
The Deltacoronavirus genus is subdivided into the Andecovirus, Buldecovirus, and Herdecovirus subgenera, which infect mammal and avian species. These viruses are found not only in larger waterfowl, but also smaller birds in the order Passeriformes, including thrushes, munias, and bulbuls [37]. In surveillance studies, Deltacoronaviruses are generally found less often in wild birds than Gammacoronaviruses, hindering efforts to assess the diversity and epidemiology of these viruses in wild avian species [1]. Notably, some screening methods for CoVs in birds are specifically designed to detect IBV, a Gammacoronavirus, and so, this bias may be due, in part, to the methodology used. The variety of methods previously used to detect CoVs from avian samples are described further below in Table S1. Concurrent infection and cocirculation of both Deltacoronaviruses and Gammacoronaviruses have been found in Anseriformes [32], providing opportunities for recombination and necessitating molecular protocols capable of detecting all possible CoVs present. While most species classified as Deltacoronaviruses are found in birds, one notable exception is Deltacoronavirus suis (previously coronavirus HKU15), which was initially detected in 2009 in Asia and has since been found in pigs and humans [1,17,20]. Deltacoronavirus suis shares a high sequence identity (i.e., a measure that describes similarity) with the proposed subspecies sparrow deltacoronavirus HKU17 [20]. Given the potentially close evolutionary relationship between the two viruses, some hypothesize that the porcine CoV evolved from an avian CoV [1]. Further study on the evolutionary relationships between these viruses could help elucidate the potential risks of spillovers from avian species and livestock to humans and other mammals.
1.3. Pathogenesis and Clinical Signs
In general, gammacoronaviruses can be classified into respiratory, reproductive, or nephropathogenic groups based on the main disease manifestations throughout the host tissues [38]. Our current knowledge of CoV pathogenesis and symptomology in nearly all avian species is lacking the depth of knowledge observed for IBV, a gammacoronavirus of domestic poultry, complicating surveillance efforts within avian populations. However, most CoVs infect respiratory or enteric originated cells after binding of host proteins by the viral S protein [39]. The genetic sequence diversity of the S gene allows for the observed variety of host tropism and pathogenesis [40]. The pathogenicity of CoVs in avian species varies widely by viral strain and physiological and environmental state of the infected bird [38]. The main attachment factor for the respiratory affiliated IBV strain is α2,3-linked sialic acid glycan, which is widely expressed, allowing for tropism throughout the body [41]. The receptor for S1 proteins of CoVs within the gastrointestinal tract has been reported as a set of nonsialylated type 2 poly-N-acetyl-lactosamines, distributed throughout the intestines of a variety of Galloanserae species, but not in Mallards (Anas platyrhynchos) [42].
The clinical signs of CoVs in avian species are best described from studies of IBV in domestic poultry as similar signs may not be present in other avian species and clinical signs are inherently difficult to study in wildlife. Gammacoronaviruses in domestic poultry can reduce egg laying by mature hens up to 50% [43] and cause renal and respiratory diseases that result in mortality rates up to 25–75% [44,45]. Reduction in egg production and egg quality in hens likely results from reduced or regressed oviduct and ovaries [46]. Lesions are produced in the ciliated and granular cells of the surface epithelia and the secretory epithelial cells of the tubular glands of the infundibulum and magnum [47]. These lesions result in both a smaller egg size and a reduction in the peak egg-laying time period in laying hens [48]. In chicks experimentally infected at one day of age, reduced egg production and quality persisted through sexual maturity six months later, thereby presenting long term effects from an earlier and seemingly resolved, disease state [49]. Both the viral strain and bird demographics (age and immune state) play a role in the scale of these reproductive effects [50]. The reproductive degradation found in domestic chickens also presented in farmed quail [51]. In addition to hen reproductive damage, IBV has also been implicated in testicular disease in roosters, influencing fertility [52,53].
In contrast to highly observed domestic flocks, morbidity and mortality descriptions of CoV infection in wild birds are few. As described above, this may be due to a difference in disease-presentation between these species, as newly developed metavirome studies in wildlife are providing evidence that disease with clinical signs is unique and not necessarily standard [54,55,56]. Some previous work has found CoV-positive birds associated with morbidity or mortality; however, a direct implication of a disease state as a result of CoV infection has yet to be determined. For example, hunter-harvested migrating Graylag Goose (Anser anser) with CoV infection presented with lower body weights than virus-negative birds [57]. In addition, CoVs were isolated from two Canada Geese (Branta canadensis) and a Snow Goose (Anser caerulescens), which were the only specimens tested following a large die-off in Nunavut, Canada during the fall of 2017 [35]. Further studies will be required to determine whether these morbidity and mortality reports in waterfowl species with CoVs are more common than currently documented.
1.4. Transmission
Many avian species that have been found to carry CoVs, including waterbirds of orders Anseriformes and Chadariiformes, are associated with aquatic environments in which they typically feed and defecate directly into the water, shedding CoV RNA. Experimentally inoculated chickens shed recoverable virus in their feces for more than 20 weeks after infection [58], suggesting that water could be an avenue for transmission among large flocks of waterfowl congregating in aquatic environments throughout the migration cycle. However, little work has been performed to determine the risk of water-based transmission of infectious viral particles, particularly in marine mammals cohabitating aquatic environments with these populations of birds [59,60]. This would be a valuable undertaking, as Gammacoronavirus delphinapteri (previously Cetacean coronavirus), found in both bottlenose dolphins (Delphinus truncatus) and beluga whale (Delphinapterus leucas), falls within the Gammacoronavirus genus along with Gammacoronavirus galli (previously avian coronavirus) [61]. Mechanical transmission of CoVs has also been reported, including transmission of Gammacoronavirus galli (previously Turkey coronavirus, included within avian coronavirus since 2009 along with Duck coronavirus, Goose coronavirus, Infectious bronchitis virus, Pheasant coronavirus, and Pigeon coronavirus) by domestic houseflies (Musca domestica) [62].
1.5. Surveillance and Diagnostics
Early diagnostic work for viruses required serial inoculations of embryonated hens’ eggs with infected tissue to amplify and identify the causative agent [63,64]. This method required several weeks to obtain results. Advances in diagnostic methodology allow for the identification of CoVs through virus-specific molecular tests, such as reverse transcriptase-polymerase chain reaction (RT-PCR) with several varying primer locations to amplify CoV specific sequences within the viral genetic code, despite small initial viral genetic code amounts or viral concentration in a sample. Initial studies amplified a 251 bp region of the replicase gene shared by all known CoVs at the time [65]. However, additional amplification targets now include the RNA-dependent RNA polymerase, 3′ UTR, 5′ UTR, or S protein gene fragments for monitoring studies [41]. Routine monitoring with techniques such as enzyme-linked immunosorbent assay (ELISA) does not differentiate between virus types.
Paired tracheal/oropharyngeal swabs and tissue samples are preferred for these tests as the trachea is the primary site of infection [66]. Passive environmental sampling of the aquatic environment and fecal samples also plays an important role in monitoring these viruses in free-ranging species [60]. Feces are one of the most widely used sampling materials for non-invasive health surveys of wildlife [67]. These environmental techniques have been used widely in animal health studies, including sampling live-bird markets [68], roosting free-ranging terns for avian influenza viruses (AIVs) [69], and mink farms for Aleutian mink virus [70]. Additionally, utilization of a variety of sampling techniques, including environmental and directly from specimens, is the best practice when studying a variety of species, including waterfowl. In birds experimentally inoculated with a low-pathology AIV, Mallards were found to excrete the virus predominately via feces [71]. This was in comparison to Wood Ducks (Aix sponsa), where more virus was detected via oropharyngeal swabs [71]. Therefore, cloacal swabs should be paired with oropharyngeal swabs in addition to environmental sampling, as the virus disseminates from the trachea to the gastrointestinal, reproductive, and urinary tracts [72]. Blood samples may also be collected to identify specific antibodies for disease with the previously mentioned ELISA kits. However, because there is a time lag between initial infection, disease onset, and the production of measurable antibodies (found to be 10 days post infection in domestic poultry [73]), an absence of antibodies does not rule out an ongoing infection in birds.
1.6. Control
Since 1941, vaccination has been the most effective method for control of IBV in domestic flocks [74]. This control effort is challenged by antigenically different strains of IBV, which do not induce cross-protection, creating the development of a wide assortment of type-specific IBV vaccines [75]. To control viral transmission between free-ranging Anseriformes and domestic flocks, mechanical and biological prevention strategies are always preferred, including high levels of biosecurity, single-age housing, and the cleaning and sanitization of equipment in contact with poultry. Good bioexclusion protocols depend on full compliance by everyone who enters poultry farms to prevent any possible contact between contaminated animals, outdoor areas, or abiotic vectors such as tools, as indirect transmission to poultry appears more probable in these settings [76]. Bioexclusion is particularly difficult, if not impossible, to achieve in free-range poultry farming. Notably, free-range or backyard poultry farming is the dominant form of poultry production in developing countries [77], presenting ample opportunity for disease transmission within, among, and outside the domestic flocks, as observed with AIV outbreaks [78,79]. The demand for backyard poultry has surged as well in developed countries, due to both the COVID-19 pandemic and increasing egg prices, with a 25% increase from 2019 to 2022 in Australia [80] and a 225% increase in bird rehoming numbers reported by chicken organizations in Great Britain from May to August 2020 [81].
Historically, pathogens within wildlife populations were viewed as a natural part of the biodiversity of the ecosystem and were theorized to play an important role in regulating populations [82,83]. Early modeling suggested that as population density decreased as a result of disease, transmission rates would fall to levels too low for the pathogen to continue to spread through a population. However, as further described below, complications due to anthropogenic changes have led to disease-induced population declines, necessitating further evaluation of this theory [84]. The rate of success in controlling pathogens in wildlife is poor, with measures traditionally consisting of crisis management due to an outbreak [85]. Management of disease in wildlife populations is best applied through understanding the factors that lead to outbreaks, including changes in the distribution or availability of habitat [86,87]. A thorough review of direct management applications as they relate to wildlife disease ecology concepts, such as corridor vaccination and host density reductions, can be found in Joseph et al. [88] and Langwig et al. [89], while suggestions on the implementation of an integration of wildlife health into conservation work can be found in Deem et al. [90].
1.7. Prevalence in Wild Birds
As the importance of the Coronaviradae family was established in mammals and domestic poultry in the decades following the initial discovery and viral description, focus shifted to avian species that could serve as reservoirs for CoVs, such as teal and peafowl [91]. Teal and peafowl tested positive for viruses in the Gamma- and Deltacoronavirus genera, but the birds tested were part of domestic flocks in close contact with poultry [92]. These CoV-positive domestic species raised the question of whether the strains of CoV found were the result of the known domestic poultry IBV jumping between avian species or novel CoVs of wild avian species not yet discovered [92]. Increased surveillance through subsequent surveys of wild bird populations detected additional CoVs in apparently healthy, free-ranging animals worldwide, including England [44], Europe [93,94], the Bering Strait [95], Egypt [38], Hong Kong [32], Russia [96], and Korea [97] (Further described in Table S1). Birds worldwide have the highest reported diversity of CoVs, with at least 88 genetically identified sequences, consisting of 75 Gammacoronavirus and 13 Deltacoronavirus varieties [98].
Surveillance for CoVs in free-ranging avian species in the Americas provided mixed results, with only three studies since 2000 finding these viruses within any wild population. Ruddy Turnstone (Arenaria interpres) samples collected as part of an AIV surveillance program in New Jersey in 2010–2011 resulted in one positive for CoV [99]. Individuals of species in the orders Anseriformes and Charadriiformes that were sampled from 2006 to 2013 in Brazil tested positive for CoV [100]. CoV-positive Great Black-backed Gulls (Larus marinus) and American Herring Gulls (Larus smithsonianus) were recorded in Canada in 2015 [101] and positive Anseriformes species were found along the Mississippi Flyway in 2015–2018 [102].
Although there has been increasing interest in the role of disease ecology in wild birds, knowledge of the role that CoVs may play in population dynamics is still very sparse, with most surveillance work occurring post-2000 on samples of Anseriformes initially obtained for AIV studies or samples of other orders in much smaller study sizes. Thus, our goal for this review was to identify avian species that tested positive for CoVs over the last 25 years and synthesize current knowledge about CoVs in these species to help guide future research.
2. Materials and Methods
We conducted a systematic literature search from January 2024 to June 2025 across Google Scholar and Web of Science databases to document all avian species since 2000 that have been tested for CoVs worldwide, and to determine which species have tested positive for a CoV. We limited article inclusion to those (1) published in English, (2) that measured CoVs in wild, free-ranging avian species, and (3) that were published since 1 January 2000. We searched for keywords including all variations and combinations of “avian coronavirus”, “avian CoV”, “avian”, “coronavirus”, “wild”,”waterfowl”, “bird.” We then consolidated the articles into a single repository and removed duplicates across databases. We then consolidated the articles into a single repository and removed duplicates across databases.
Next, we screened articles based on titles, abstracts, and methods to determine initial suitability. We excluded studies that solely measured CoV prevalence in farmed, domestic poultry. We also excluded studies that described molecular or genetic studies of CoVs without surveillance information for wild, free-ranging birds. We retrieved full texts of the remaining potentially relevant articles and assessed these against the three previously described inclusion criteria, which resulted in 28 relevant studies (Figure 1, with the 28 retained studies detailed in Table S1).
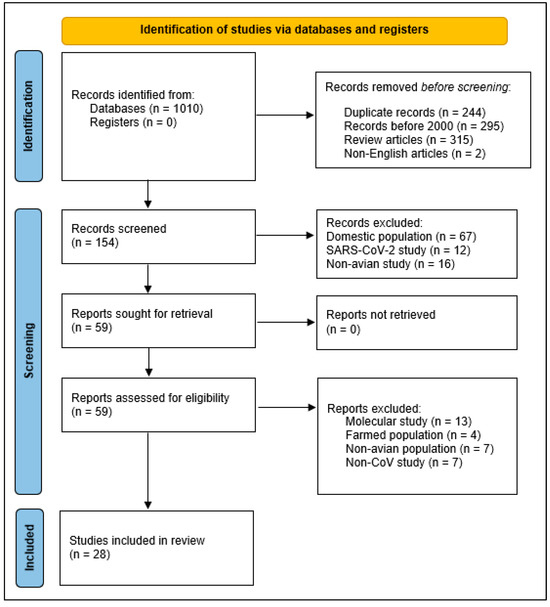
Figure 1.
PRISMA 2020 flow diagram depicting inclusion and exclusion criteria for the systematic review of avian coronaviruses in wild birds since 2000 that we conducted from January 2024 to June 2025.
For each retained study, we collected information regarding sampling location, study dates, avian species and orders sampled, number of samples per order, and swab and molecular techniques utilized. We did not use automation tools during this data collection process and we did not contact study authors for any further information regarding their work. We included both order and species because some studies pooled their samples prior to analysis and did not include species-specific information. We documented all CoV detections reported for wild birds in peer-reviewed literature since 2000 in Table S1, with measured prevalence of the reported avian orders tested in each study, as taxonomic species identity may have changed since publication. All sampled avian species since 2000 listed by avian order may be found in Table S2, with current AviList species nomenclature and taxonomy as of September 2025.
We also summarized reported positive CoV detections by order in Table 2, including number of studies which sampled for each order, number of species sampled within each order, and the number of these species which were found with a positive sample for CoVs. We also recorded how many individual avian samples were collected and tested within each order, as opportunistic testing for wildlife health studies can result in small sample numbers. We calculated the percentage of samples in each study that tested positive for CoVs per avian order, as presented in Table S1. We then calculated the weighted mean of samples for each order that tested positive for CoVs across all included studies to account for differences in sample size. We weighed the means for each order by the sample size of each tested order in the study. We excluded three studies from our calculations due to reporting methods. Hughes et al. [44] pooled samples among related species into pools of five and reported RT-PCR-positive fecal pools. Paim et al. [102] reported avian species which tested positive for CoVs, but did not include the complete 16,672 sample information for this collection previously obtained for AIV surveillance. Khatun et al. [103] utilized fresh environmental samples from free-ranging waterfowl but did not report species. We used Spearman’s Rho correlation analysis to examine the relationship between the number of species tested for CoVs and the mean percent positivity found for CoVs across orders, as well as the relationship between the number of individual birds sampled and the mean percent positivity found in CoVs across orders.

Table 2.
Mean percent of samples per order that tested positive for avian coronaviruses since 2000 as reported in the peer-reviewed literature. Means weighted by total number of samples tested per study and rounded to the nearest whole number.
3. Results
Since 2000, 24 of 46 avian orders worldwide have had at least one representative bird sampled and tested for CoV. CoVs have been detected in representatives of 13 out of the 24 tested avian orders worldwide, namely Accipitriformes (2 of 17 species sampled), Anseriformes (45 of 72 species sampled), Charafriiformes (39 of 90 species sampled), Columbiformes (2 of 10 species sampled), Galliformes (1 of 17 species sampled), Gruiformes (4 of 16 species sampled), Passeriformes (15 of 145 species sampled), Pelecaniformes (8 of 19 species sampled), Piciformes (1 of 7 species sampled), Psittaciformes (3 of 16 species sampled), Strigiformes (2 of 20 species sampled), and Sulliformes (3 of 4 species sampled) (Table 2 and Figure 2). These orders include the 124 species in which at least one individual has tested positive for a CoV out of the 435 avian species tested and reported since 2000.
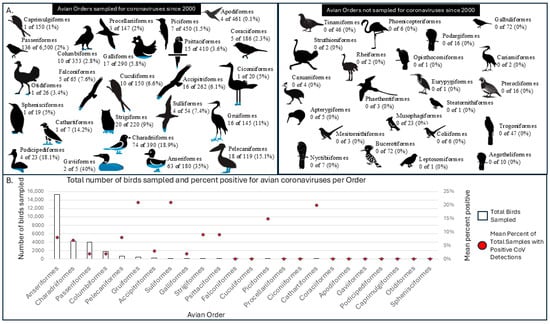
Figure 2.
(A) Avian orders sampled for coronaviruses with the percentage of species that tested positive per order (left) and number of avian orders not sampled for avian coronaviruses (right) as reported in the peer-reviewed literature since 2000. (B) Total number of individual birds sampled per avian order and the percent found positive for avian coronavirus as reported in the peer-reviewed literature since 2000.
Among avian orders sampled since 2000 with no positive detections, both the sampled number of species and the total number of individual birds sampled were notably lower compared to orders with positive detections (Figure 2). The orders without a positive sampled species include Apodiformes (5 species sampled), Caprimulgiformes (1 species sampled), Ciconiiformes (1 species sampled), Coraciiformes (5 species sampled), Cuculiformes (10 species sampled), Falconiformes (5 species sampled), Gaviiformes (2 species sampled), Otidiformes (1 species sampled), Podicipediformes (4 species sampled), Procellariiformes (3 species sampled), and Sphenisciformes (1 species sampled). These include two orders in which a single bird of an order was sampled and reported only once since 2000, namely Otidiformes and Sphenisciformes. Previous compilation studies of CoVs within avian populations have included positive samples from species of order Sphenisciformes utilizing indirect, secondary environmental sampling [1].
The overall weighted mean for positive CoV detections across all sampled avian orders was 7.05%, though most CoV-positive wildlife were described as asymptomatic. We acknowledge that this percentage may be high due to publication bias or variation in methods (e.g., the variety of genetic amplification targets utilized across studies) but does provide insight into the percentage of positive CoV detections per order based on the current literature. The weighted mean for positive CoV detections in the order Anseriformes was 8.42%, slightly higher than overall positivity, but similar to the positivity for AIV in seemingly asymptomatic free-ranging waterfowl (e.g., 10.2–23.4% across 6 years in Sweden [104], 7.3–8.9% across 3 years within the Atlantic Flyway of the United States [105]). Anseriformes, followed by Charadriiformes, have been sampled for CoVs the most often of any avian order since 2000, partially due to concurrent AIV sampling or convenience testing of samples obtained from prior AIV surveillance efforts. Individuals from the order Anseriformes tested positive for CoVs in 24 of 25 studies in which they were sampled (Table S1) [31,32,37,44,57,94,96,97,99,100,101,102,103,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. In the 2015 study without a positive case in an Anseriformes species, samples were pooled in groups of 10 and amplified in embryonated domestic chicken eggs prior to RT-PCR [99]. While it is certainly possible that none of the 665 ducks were positive for a CoV at the time of sampling, the sampling methods they used may not be sufficient to detect CoVs in wild bird samples [99].
We found a moderate positive correlation (ρ = 0.52, p < 0.01) between the number of species tested for CoVs and the mean percent positivity found for CoVs across orders. Additionally, we found a moderate positive correlation (ρ = 0.64, p < 0.01) between the number of individual birds sampled and the mean percent positivity found in CoVs across orders.
Figure 3 illustrates the study locations and overall positivity for avian CoVs across 28 studies included in our study, with each pie chart representing the country where free-ranging bird species were sampled. Six studies sampled birds from the Americas Flyways System. Eight studies sampled birds within the African–Eurasian Flyway, although notably, no studies since 2000 included birds from the mainland Africa. Fifteen studies included birds from the Central Asian and East Asian-Australasian Flyways. The proportion of avian CoV positivity within each study is shown in red on each pie chart. Figure 4 presents a timeline of the retained studies alongside their study location by continent. This visualization highlights disparities in study coverage, with the majority of recent research (since 2018) focusing on free-ranging wild birds from Asia. In contrast, although birds from North and South America were included in six peer-reviewed studies, no new reporting from these regions has occurred since 2018. Together, these published datasets consistently report the presence of CoVs in Anseriformes across global regions. In contrast, other avian orders either lack reported detections or have been sampled less intensively, leading to greater uncertainty and highlighting the need for broader surveillance of wild birds worldwide.
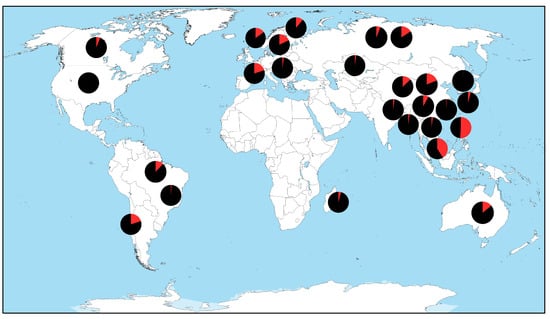
Figure 3.
Map of countries with studies that tested for avian coronaviruses in free-ranging birds as reported in the peer-reviewed literature since 2000. Location of each pie chart does not represent a specific study site. Black and red coloration of each pie chart represent the percentage of negative and positive test results across studies.
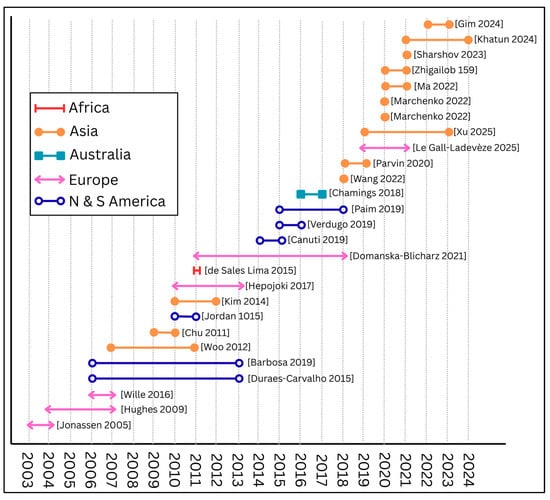
Figure 4.
Timeline and location of studies of avian coronaviruses in wild birds as reported in peer-reviewed literature since 2000. Bars represent beginning and end of wild bird sampling efforts as reported by the literature [31,32,37,44,57,94,96,97,99,100,101,102,103,106,107,108,109].
4. Discussion
Since 2000, CoVs have been documented in 124 avian species across various geographic regions and taxonomic orders. However, our understanding of the disease ecology of these viruses in wild bird populations remains limited. Notably, avian orders without reported CoV detections tend to have substantially fewer individuals and species sampled compared to those with positive detections, as shown in Table 2. Furthermore, only 24 of the 46 recognized avian orders have been sampled since 2000. These uneven sampling efforts are compounded by the nature of wildlife health surveillance initiatives, which often rely on convenience sampling rather than systematic, long-term monitoring, particularly in groups such as waterfowl (order Anseriformes). Reporting biases may also contribute to these disparities, as surveillance efforts that do not detect CoCs are less likely to be published or reported than those that do. Our review was limited to English-language publications, which may have excluded relevant studies published in other languages. This decision was made to ensure consistency in language interpretation and accessibility, but future reviews would benefit from incorporating multilingual sources to provide a more comprehensive perspective.
Of the 25 studies that reported testing results for waterfowl in the order Anseriformes since 2000, 24 reported positive CoV detections. However, testing for CoVs typically occurred as a secondary research objective as part of a testing program for AIVs or well after samples were initially collected, limiting our ability to identify the species, prevalence, and spatial distributions of CoV infections in waterfowl [99,102]. Establishing a robust, continuous surveillance system to detect and monitor CoVs in wild waterfowl is important to ensure the health of their populations, detect potential outbreaks, and implement measures to mitigate the spread of disease if necessary [120,121,122,123]. Further, a more robust surveillance system for CoVs would provide food animal health and veterinary medicine professionals with information to prepare for and prevent viral incursions into the food animal system. Our correlation analyses demonstrating moderate positive correlation between positivity for CoVs and number of birds studied further demonstrate the need for additional testing across a wider variety of avian species and in greater number to fully appreciate CoV prevalence in wild birds.
With adequate resources and support, more thorough testing to monitor CoVs could be conducted within existing networks as part of ongoing surveillance of waterfowl for AIV (e.g., Global Influenza Programme established by the World Health Organization, National Wildlife Disease Program in the United States). Laboratory testing and genetic analyses of the pathogens themselves is critical. Diagnosis of CoVs relies on detection through virus-specific molecular tests, such as RT-PCR, with a number of varying primer locations in order to amply CoV-specific sequences within the viral genetic code [41,124]. Initial tests amplified a 251 bp region of the replicase gene shared by all known CoVs at the time [65]. However, additional amplification targets now include the RNA-dependent RNA polymerase, 3′ UTR, 5′ UTR, or S protein gene fragments for monitoring studies [41,125]. The disparate amplification strategies described in Table S1 demonstrate the need for unified and consistent sampling of free-ranging waterfowl and other wild birds for CoVs. Increased use of more advanced technologies, such as high-throughput sequencing, could also provide genomic-wide analysis of viral relationships and movement through populations [126,127,128,129]. Due to risks associated with recombination of CoVs in domesticated animal and human populations or coinfection with other disease in avian populations [39], these sampling and analysis measures are warranted. Indeed, coinfections or subsequent infections appear to be a norm rather than an exception, and theinteractions of pathogens with CoVs may have consequences yet to be determined. Various studies worldwide have found significant coinfections of CoV and AIVs [94,130,131], presenting opportunities for an increase in pathogenicity, morbidity and mortality, as observed in domestic poultry [132]. These relationships within wildlife disease ecology also warrant further investigation, perhaps by leveraging the ongoing long-term avian influenza monitoring efforts previously described.
Beyond basic surveillance, mortality and morbidities associated with CoV infections are well-documented in domestic poultry and a small number of studies suggest that CoVs could have similar impacts on wild birds (e.g., [57]). Nevertheless, this topic remains virtually unexplored in free-ranging species, and understandably so, given the many pressing issues with respect to wildlife disease that have come to fruition in recent years, and the challenges posed by assessing disease outcomes in wild populations [132,133,134,135,136]. However, it is possible that wild birds with CoV infections, particularly free-ranging waterfowl, could experience morbidities that impact their reproductive success, as found in domestic poultry with IBV (e.g., reduction in egg quantity and quality due to lesions in the reproductive tract) [45,137], and as found in wild waterfowl with low pathogenic AIVs displaying no or few clinical signs of disease (e.g., lower body weight [104]; increased body temperature [138]; lower egg production [139]; decreased movements [104,140]). Future studies could aim to better understand whether these morbidities are present in additional free-ranging species, particularly those with currently low sampling efforts. One practical approach would be to leverage individuals already captured and handled during ongoing banding, movement, or habitat use studies. For many avian orders in which less than 100 individuals have been tested and reported for CoVs (Table 2 and Figure 2), utilizing these bird-in-hand opportunities would help expand our understanding of CoV prevalence across a broader taxonomic spectrum.
Given the ecological, social, and economic implications of a major disease outbreak in migratory waterfowl and waterbird species, monitoring their disease dynamics in relation to environmental and land use change is critical for achieving the goals of a “One Health” approach that ensures the well-being of people, animals, plants, and our environment. Global changes to climate and land use during the Anthropocene have altered migratory pathways, distances, and stopover durations for waterfowl and may compound an individual’s risk of exposure to reservoir hosts and pathogens as birds congregate in increasingly smaller areas for access to food resources and protective cover [141,142,143,144,145,146]. Modeling for AIVs along the East Asia-Australasian Flyway demonstrated that habitat loss and subsequent relocation of Greater White-fronted Goose (Anser albifrons) promoted the spread and transmission of AIV [145]. In addition, poor nutrition and reduced body condition due to habitat loss can compromise the immune response and increase host susceptibility to disease [147]. Spillover and spillback events along an expanding wildlife–urban interface could threaten biodiversity and ecosystem stability, require substantial financial investments for prevention and control, and result in economic losses due to trade and travel restrictions, disruptions to food systems, changes in consumer confidence, among other potential consequences [148,149,150,151,152,153,154]. Given the flocking, roosting, and nesting behaviors associated with these CoV-positive orders (e.g., Suliformes and Charadriiformes) and the relatively low numbers of individuals sampled from these groups, further testing and surveillance for these viruses would support the goals of the “One Health” framework.
5. Conclusions
Understanding the potential role of free-ranging birds in the maintenance, evolution, and transmission of diseases is imperative given their mobility and the wide diversity of pathogens that can infect this taxon. Wild birds can play a significant role in the circulation of pathogens, in part, due to their physiological, ecological, and behavioral characteristics. Migrating birds can become long-range vectors, creating the potential for disease spread along migration routes. The ecology and evolution of diseases in wild birds are complex, and disease dynamics in these species are further complicated by climate change, environmental degradation (e.g., pollution), and land conversion [153,154]. These factors have led to range shifts [144], both increases and decreases in populations [145], novel interactions among species [145], and increased stress on avian immune systems [146,147]. Further, they have simultaneously contributed to the prevalence and severity of many zoonotic pathogens [149]. Determining the distributions, causes, and potential impacts of diseases on wild birds, especially waterfowl, is necessary to achieve the goals of a “One Health” approach toward the health and well-being of people, domestic and free-ranging animals, plants, and our environment [150]. Additionally, a more thorough understanding is critical to slow and reverse population declines experienced by many species of wild birds and associated wildlife over the last century [151], as disease is increasingly recognized as a risk factor for declining wildlife populations worldwide [151,152,153,154].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/birds6040052/s1, Table S1: Mean percent of samples per order that tested positive for avian coronaviruses since 2000 as reported in the peer-reviewed literature. Table S2. Summary of avian species individually tested for coronavirus in free ranging birds since 2000 as reported in the peer-reviewed literature by avian order.
Author Contributions
Conceptualization, A.A.V.-L. and A.M.L.; writing—original draft preparation, A.A.V.-L.; writing—review and editing, A.A.V.-L., R.C.C., K.M.R. and A.M.L.; supervision, R.C.C., K.M.R. and A.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the reviewers and Associate Editor for their comments on previous versions of our manuscript. We did not conduct field research for the purposes of this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wille, M.; Holmes, E.C. Wild birds as reservoirs for diverse and abundant gamma- and deltacoronaviruses. FEMS Microbiol. Rev. 2020, 44, 631–644. [Google Scholar] [CrossRef]
- Torres, C.A.; Listorti, V.; Lupini, C.; Franzo, G.; Drigo, M.; Catelli, E.; Brandao, P.E.; Cecchinato, M. Gamma and Deltacoronaviruses in quail and pheasants from Northern Italy. Poult. Sci. 2017, 96, 717–722. [Google Scholar] [CrossRef]
- Wille, M.; Lindqvist, K.; Muradrasoli, S.; Olsen, B.; Jarhult, J.D. Urbanization and the dynamics of RNA viruses in Mallards (Anas platyrhynchos). Infect. Genet. Evol. 2017, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.C.; Hu, G.M.; Chen, C.M. Phylogenetic network of infectious bronchitis virus: Exploring the impact of migratory birds on viral clustering, evolution, and recombination. Vet. Q. 2025, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schalk, A.F. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931, 78, 413–423. [Google Scholar]
- Cook, J.K.A.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, L.; Brandly, C. Laryngotracheitis in chicks. Poult. Sci. 1933, 12, 55–60. [Google Scholar] [CrossRef]
- May, H.G.; Tittsler, R.P. Tracheo-laryngitis in poultry. J. Am. Vet. Med. Assoc. 1925, 67, 229–231. [Google Scholar]
- Beach, J.; Schalm, O. A filterable virus, distinct from that of laryngotracheitis, the cause of a respiratory disease of chicks. Poult. Sci. 1936, 15, 199–206. [Google Scholar] [CrossRef]
- Chute, H.L.; O’Meara, D.C.; Witter, J.F. Controlling infectious bronchitis in Maine chickens. Maine Agric. Exp. Stn. Bull. 1959, 584, 1–26. [Google Scholar]
- Gharaibeh, S.; Mahmoud, K. Decay of maternal antibodies in broiler chickens. Poult. Sci. 2013, 92, 2333–2336. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.H.; Hymas, T.A. Antibiotics in the treatment of an unfamiliar turkey disease. Poult. Sci. 1951, 30, 466–468. [Google Scholar] [CrossRef]
- Pomeroy, B.; Sieburth, J. Bluecomb disease of turkeys. In Proceedings 90th Annual Meeting of the American Veterinary Medical Association; American Veterinary Medical Association: Schaumburg, IL, USA, 1954; pp. 3227–3231. [Google Scholar]
- Sieburth, J.M.; Johnson, E.P. Transmissible enteritis of turkeys (bluecomb disease): 1. Preliminary studies. Poult. Sci. 1957, 36, 256–261. [Google Scholar] [CrossRef]
- Tyrrell, D.A.; Bynoe, M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet 1966, 1, 76–77. [Google Scholar] [CrossRef]
- Almeida, J.D.; Tyrell, D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J. Gen. Virol. 1967, 1, 175–178. [Google Scholar] [CrossRef]
- Almeida, J.D.; Berry, D.; Cunningham, C.; Hamre, D.; Hofstad, M.; Mallucci, L.; McIntosh, K.; Tyrrell, D. Coronaviruses; Nature Publishing Group: London, UK, 1968. [Google Scholar]
- Panigrahy, B.; Naqi, S.; Hall, C. Isolation and characterization of viruses associated with transmissible enteritis (bluecomb) of turkeys. Avian Dis. 1973, 17, 430–438. [Google Scholar] [CrossRef]
- Ritchie, A.; Deshmukh, D.; Larsen, C.; Pomeroy, B. Electron microscopy of coronavirus-like particles characteristic of turkey bluecomb disease. Avian Dis. 1973, 17, 546–558. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, Y.; Ge, X. The taxonomy, host range and pathogenicity of coronaviruses and other viruses in the nidovirales order. Anim. Dis. 2021, 1, 5. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; Garcia, M.L.; Hendrickson, R.C.; et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; De Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; Hoek, L.V.; Wong, A.C.P.; Yeh, S.H. ICTV virus taxonomy profile 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar]
- Quinteros, J.A.; Noormohammadi, A.H.; Lee, S.W.; Browning, G.F.; Diaz-Mendez, A. Genomics and pathogenesis of the avian coronavirus infectious bronchitis virus. Aust. Vet. J. 2022, 100, 496–512. [Google Scholar] [CrossRef]
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.S.; Hamid, A.; Bhat, S.U. SARS-CoV-2: A critical review of its history, pathogenesis, transmission, diagnosis and treatment. Biosaf. Health 2020, 2, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.J.; Zhang, L.B.; Li, H.Y.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aravena, M.; Mckee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef]
- El-Sahly, H.M.; Atmar, R.L.; Glezen, W.P.; Greenberg, S.B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin. Infect. Dis. 2000, 31, 96–100. [Google Scholar] [CrossRef]
- Tao, Y.; Shi, M.; Chommanard, C.; Queen, K.; Zhang, J.; Markotter, W.; Kuzmin, I.V.; Holmes, E.C.; Tong, S.X. Surveillance of bat coronaviruses in Kenya identifies relatives of human coronaviruses NL63 and 229E and their recombination history. J. Virol. 2017, 91, e01953-16. [Google Scholar] [CrossRef]
- Naserghandi, A.; Allameh, S.F.; Saffarpour, R. All about COVID-19 in brief. New Microb. New Infect. 2020, 35, 100678. [Google Scholar] [CrossRef]
- Duraes-Carvalho, R.; Caserta, L.C.; Barnabe, A.C.S.; Martini, M.C.; Ferreira, H.L.; Felippe, P.N.; Santos, M.B.; Arns, C.W. Coronaviruses detected in Brazilian wild birds reveal close evolutionary relationships with beta- and deltacoronaviruses isolated from mammals. J. Mol. Evol. 2015, 81, 21–23. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Leung, C.Y.H.; Gilbert, M.; Joyner, P.H.; Ng, E.M.; Tse, T.M.; Guan, Y.; Peiris, J.S.M.; Poon, L.L.M. Avian coronavirus in wild aquatic birds. J. Virol. 2011, 85, 12815–12820. [Google Scholar] [CrossRef]
- Jackwood, M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Papineau, A.; Berhane, Y.; Wylie, T.N.; Wylie, K.M.; Sharpe, S.; Lung, O. Genome organization of Canada goose coronavirus, a novel species identified in a mass die-off of Canada geese. Sci. Rep. 2019, 9, 5954. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, K.A.; Wu, G.; Leger, J.S.; Nordhausen, R.W.; Wang, D. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 2008, 82, 5084–5088. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of seven novel mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Jackwood, M.W.; De Wit, S. Infectious bronchitis. In Diseases of Poultry, 13th ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 139–159. [Google Scholar]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Wickramasinghe, I.N.A.; De Vries, R.P.; Grone, A.; De Haan, C.M.; Verheije, M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011, 85, 8903–8912. [Google Scholar] [CrossRef]
- Milek, J.; Blicharz-Domanska, K. Coronaviruses in avian species—Review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef]
- Wickramasinghe, I.N.A.; De Vries, R.P.; Weerts, E.W.S.; Van Beurden, S.J.; Peng, W.; Mcbride, R.; Ducatez, M.; Guy, J.; Brown, P.; Eterradossi, N.; et al. Novel receptor specificity of avian gammacoronaviruses that cause enteritis. J. Virol. 2015, 89, 8783–8792. [Google Scholar] [CrossRef]
- Ignjatovic, J.; Sapats, S. Avian infectious bronchitis virus. Rev. Sci. Tech. 2000, 19, 493–508. [Google Scholar] [CrossRef]
- Hughes, L.A.; Savage, C.; Naylor, C.; Bennett, M.; Chantrey, J.; Jones, R. Genetically diverse coronaviruses in wild bird populations of Northern England. Emerg. Infect. Dis. 2009, 15, 1091–1094. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, K.; Chen, S.; Yan, K.; Du, X.; Zhang, C.; Guo, M.; Wu, Y. Evaluation of the reproductive system development and egg-laying performance of hens infected with tw i-type infectious bronchitis virus. Vet. Res. 2020, 51, 95. [Google Scholar] [CrossRef]
- Sevoian, M.; Levine, P. Effects of infectious bronchitis on the reproductive tracts, egg production, and egg quality of laying chickens. Avian Dis. 1957, 1, 136–164. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R. Ultrastructural study of infectious bronchitis virus infection in infundibulum and magnum of commercial laying hens. Vet. Microbiol. 2007, 122, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wei, J. Influences of infectious bronchitis in young chickens on their later production (Accession No. 002873357). Chin. J. Vet. Med. 1995, 21, 15. [Google Scholar]
- Crinion, R.A.P. Egg quality and production following infectious bronchitis virus exposure at one-day-old. Poult. Sci. 1972, 51, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef]
- Torres, C.A.; Villarreal, L.Y.B.; Ayres, G.R.R.; Richtzenhain, L.J.; Brandao, P.E. An aviancoronavirus in quail with respiratory and reproductive signs. Avian Dis. 2013, 57, 295–299. [Google Scholar] [CrossRef]
- Boltz, D.A.; Nakai, M.; Bahr, J.M. Avian infectious bronchitis virus: A possible cause of reduced fertility in the rooster. Avian Dis. 2004, 48, 909–915. [Google Scholar] [CrossRef]
- Villarreal, L.Y.B.; Brandao, P.E.; Chacon, J.L.; Assayag, M.S.; Maiorka, P.C.; Raffi, P.; Saidenberg, A.B.S.; Jones, R.C.; Ferreira, A.J.P. Orchitis in roosters with reduced fertility associated with avian infectious bronchitis virus and avian metapneumovirus infections. Avian Dis. 2007, 51, 900–904. [Google Scholar] [CrossRef][Green Version]
- Vaz, F.F.; Raso, T.F.; Agius, J.E.; Hunt, T.; Leishman, A.; Eden, J.S.; Phalen, D.N. Opportunistic sampling of wild native and invasive birds reveals a rich diversity of adenoviruses in Australia. Virus Evol. 2020, 6, veaa024. [Google Scholar] [CrossRef]
- Sarker, S. Metagenomic detection and characterisation of multiple viruses in apparently healthy Australian neophema birds. Sci. Rep. 2021, 11, 20915. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Yang, S.; Wang, H.; Wang, H.; Zhang, J.; Gong, G.; Xiao, Y.; Yang, J.; Wang, X.; Lu, J. Virome in the cloaca of wild and breeding birds revealed a diversity of significant viruses. Microbiome 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, C.M.; Kofstad, T.; Larsen, I.L.; Lovland, A.; Handeland, K.; Follestad, A.A.; Lillehaug, A. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J. Gen. Virol. 2005, 86, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Gough, R.E. Isolation of avian infectious-bronchitis virus from experimentally infected chickens. Res. Vet. Sci. 1977, 23, 344–347. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Hewson, I. Coronaviruses in the sea. Front. Microbiol. 2020, 11, 1795. [Google Scholar] [CrossRef]
- Wartecki, A.; Rzymski, P. On the coronaviruses and their associations with the aquatic environment and wastewater. Water 2020, 12, 1598. [Google Scholar] [CrossRef]
- Wang, L.; Maddox, C.; Terio, K.; Lanka, S.; Fredrickson, R.; Novick, B.; Parry, C.; Mcclain, A.; Ross, K. Detection and characterization of new coronavirus in bottlenose dolphin, United States, 2019. Emerg. Infect. Dis. 2020, 26, 1610. [Google Scholar] [CrossRef]
- Calibeo-Hayes, D.; Denning, S.S.; Stringham, S.M.; Guy, J.S.; Smith, L.G.; Watson, D.W. Mechanical transmission of turkey coronavirus by domestic houseflies (Musca domestica linnaeaus). Avian Dis. 2003, 47, 149–153. [Google Scholar] [CrossRef]
- Guy, J.S. Isolation and propagation of coronaviruses in embryonated eggs. Methods Mol. Biol. 2008, 454, 109–117. [Google Scholar]
- Geerligs, H.J.; Boelm, G.J.; Meinders, C.M.; Stuurman, B.G.E.; Symons, J.; Tarres-Call, J.; Bru, T.; Vila, R.; Mombarg, M.; Karaca, K.; et al. Efficacy and safety of an attenuated live qx-like infectious bronchitis virus strain as a vaccine for chickens. Avian Pathol. 2011, 40, 93–102. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Casebolt, D.B.; Gangopadhyay, N.N. Phylogenetic analysis of a highly conserved region of the polymerase gene from 11 coronaviruses and development of a consensus polymerase chain reaction assay. Virus Res. 1999, 60, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Kappala, D. Avian infectious bronchitis virus. In Recent Advances in Animal Virology; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Springer: Singapore, 2019; pp. 301–319. [Google Scholar]
- Schilling, A.K.; Mazzamuto, M.V.; Romeo, C. A review of non-invasive sampling in wildlife disease and health research: What’s new? Animals 2022, 12, 1719. [Google Scholar] [CrossRef]
- Indriani, R.; Samaan, G.; Gultom, A.; Loth, L.; Indryani, S.; Adjid, R.; Dharmayanti, N.L.P.I.; Weaver, J.; Mumford, E.; Lokuge, K.; et al. Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg. Infect. Dis. 2010, 16, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Kishida, N.; Sakoda, Y.; Shiromoto, M.; Bai, G.R.; Isoda, N.; Takada, A.; Laver, G.; Kida, H. H2N5 influenza virus isolates from terns in Australia: Genetic reassortants between those of the Eurasian and American lineages. Virus Genes 2008, 37, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Diaz-Cao, J.M.; Fernandez-Antonio, R.; Panadero, R.; Diaz, P.; Lopez, C.; Morrondo, P.; Diez-Banos, P.; Fernandez, G. Application of real-time PCR to detect aleutian mink disease virus on environmental farm sources. Vet. Microbiol. 2014, 173, 355–359. [Google Scholar] [CrossRef]
- Costa, T.P.; Brown, J.D.; Howerth, E.W.; Stallknecht, D.E. Variation in viral shedding patterns between different wild bird species infected experimentally with low-pathogenicity avian influenza viruses that originated from wild birds. Avian Pathol. 2011, 40, 119–124. [Google Scholar] [CrossRef]
- Najimudeen, S.M.; Hassan, M.H.S.; Cork, S.C.; Abdul-Careem, M.F. Infectious bronchitis coronavirus infection in chickens: Multiple system disease with immune suppression. Pathogens 2020, 9, 779. [Google Scholar] [CrossRef]
- Pei, J.W.; Collisson, E.W. Specific antibody secreting cells from chickens can be detected by three days and memory b cells by three weeks post-infection with the avian respiratory coronavirus. Dev. Comp. Immunol. 2005, 29, 153–160. [Google Scholar] [CrossRef]
- Guzman, M.; Hidalgo, H. Live attenuated infectious bronchitis virus vaccines in poultry: Modifying local viral populations dynamics. Animals 2020, 10, 2058. [Google Scholar] [CrossRef]
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef]
- Silk, M.J.; Drewe, J.A.; Delahay, R.J.; Weber, N.; Steward, L.J.; Wilson-Aggarwal, J.; Boots, M.; Hodgson, D.J.; Croft, D.P.; McDonald, R.A. Quantifying direct and indirect contacts for the potential transmission of infection between species using a multilayer contact network. Behaviour 2018, 155, 731–757. [Google Scholar] [CrossRef]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef]
- Koch, G.; Elbers, A.R.W. Outdoor ranging of poultry: A major risk factor for the introduction and development of high-pathogenicity avian influenza. NJAS-Wagening. J. Life Sci. 2006, 54, 179–194. [Google Scholar] [CrossRef]
- Gonzales, J.L.; Pritz-Verschuren, S.; Bouwstra, R.; Wiegel, J.; Elbers, A.R.W.; Beerens, N. Seasonal risk of low pathogenic avian influenza virus introductions into free-range layer farms in the Netherlands. Transbound. Emerg. Dis. 2021, 68, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Buddle, E.A.; Stevens, K.; Bray, H.J.; Ankeny, R.A. The Chicken for the Egg: Australian Motivations for Raising Backyard Chickens. Anthrozoös 2024, 38, 299–310. [Google Scholar] [CrossRef]
- Oliver, C. Returning to ‘the good life’? Chickens and chicken-keeping during COVID-10 in Britain. Anim. Studi J. 2021, 10, 114–139. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Population biology of infectious-diseases: Part I. Nature 1979, 280, 361–367. [Google Scholar] [CrossRef]
- May, R.M.; Anderson, R.M. Population biology of infectious–diseases: Part II. Nature 1979, 280, 455–461. [Google Scholar] [CrossRef]
- De Castro, F.; Bolker, B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005, 8, 117–126. [Google Scholar] [CrossRef]
- Mathews, F. Zoonoses in wildlife: Integrating ecology into management. Adv. Parasitol. 2009, 68, 185–209. [Google Scholar] [PubMed]
- Dobson, A.P. Disease and conservation. Conserv. Biol. Sci. Scarcity Diversity 1986, 345–365. [Google Scholar]
- Bernstein, A.S.; Ando, A.W.; Loch-Temzelides, T.; Vale, M.M.; Li, B.V.; Li, H.Y.; Busch, J.; Chapman, C.A.; Kinnaird, M.; Nowak, K.; et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci. Adv. 2022, 8, eabl4183. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.B.; Mihaljevic, J.R.; Arellano, A.L.; Kueneman, G.; Preston, D.L.; Cross, P.C.; Johnson, P.T.J. Taming wildlife disease: Bridging the gap between science and management. J. Appl. Ecol. 2013, 50, 702–712. [Google Scholar] [CrossRef]
- Langwig, K.E.; Voyles, J.; Wilber, M.Q.; Frick, W.F.; Murray, K.A.; Bolker, B.M.; Collins, J.P.; Cheng, T.L.; Fisher, M.C.; Hoyt, J.R.; et al. Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 2015, 13, 195–202. [Google Scholar] [CrossRef]
- Deem, S.L.; Karesh, W.B.; Weisman, W. Putting theory into practice: Wildlife health in conservation. Conserv. Biol. 2001, 5, 1224–1233. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chen, J.; Kong, X.; Shao, Y.; Han, Z.; Feng, L.; Cai, X.; Gu, S.; Liu, M. Isolation of avian infectious bronchitis coronavirus from domestic peafowl (Pavo cristatus) and teal (anas). J. Gen. Virol. 2005, 86, 719–725. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronaviruses in poultry and other birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef]
- Domanska-Blicharz, K.; Jacukowicz, A.; Lisowska, A.; Wyrostek, K.; Minta, Z. Detection and molecular characterization of infectious bronchitis-like viruses in wild bird populations. Avian Pathol. 2014, 43, 406–413. [Google Scholar] [CrossRef]
- Wille, M.; Muradrasoli, S.; Nilsson, A.; Jarhult, J.D. High prevalence and putative lineage maintenance of avian coronaviruses in Scandinavian waterfowl. PLoS ONE 2016, 11, e0150198. [Google Scholar] [CrossRef]
- Muradrasoli, S.; Bálint, Á.; Wahlgren, J.; Waldenström, J.; Belák, S.; Blomberg, J.; Olsen, B. Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS ONE 2010, 5, e13640. [Google Scholar] [CrossRef]
- Marchenko, V.; Danilenko, A.; Kolosova, N.; Bragina, M.; Molchanova, M.; Bulanovich, Y.; Gorodov, V.; Leonov, S.; Gudymo, A.; Onkhonova, G.; et al. Diversity of gammacoronaviruses and deltacoronaviruses in wild birds and poultry in Russia. Sci. Rep. 2022, 12, 19412. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Oem, J.-K. Surveillance of avian coronaviruses in wild bird populations of Korea. J. Wildl. Dis. 2014, 50, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Núñez–Nogueira, G.; Valentino-Álvarez, J.A.; Granados-Berber, A.A.; Ramírez-Ayala, E.; Zepeda-González, F.A.; Tintos-Gómez, A. Aquatic biota is not exempt from coronavirus infections: An overview. Water 2021, 13, 2215. [Google Scholar] [CrossRef]
- Jordan, B.J.; Hilt, D.A.; Poulson, R.; Staliknecht, D.E.; Jackwood, M.W. Identification of avian coronavirus in wild aquatic birds of the central and eastern USA. J. Wildl. Dis. 2015, 51, 218–221. [Google Scholar] [CrossRef]
- Barbosa, C.M.; Durigon, E.L.; Thomazelli, L.M.; Ometto, T.; Marcatti, R.; Nardi, M.S.; De Aguiar, D.M.; Pinho, J.B.; Petry, M.V.; Neto, S.; et al. Divergent coronaviruses detected in wild birds in Brazil, including a central park in Sao Paulo. Braz. J. Microbiol. 2019, 50, 547–556. [Google Scholar] [CrossRef]
- Canuti, M.; Kroyer, A.N.K.; Ojkic, D.; Whitney, H.G.; Robertson, G.J.; Lang, A.S. Discovery and characterization of novel RNA viruses in aquatic North American wild birds. Viruses 2019, 11, 768. [Google Scholar] [CrossRef]
- Paim, F.C.; Bowman, A.S.; Miller, L.; Feehan, B.J.; Marthaler, D.; Saif, L.J.; Vlasova, A.N. Epidemiology of deltacoronaviruses (δ-cov) and gammacoronaviruses (γ-cov) in wild birds in the United States. Viruses 2019, 11, 897. [Google Scholar] [CrossRef]
- Khatun, M.N.; Tasnim, S.; Hossain, M.R.; Rahman, M.Z.; Hossain, M.T.; Chowdhury, E.H.; Parvin, R. Molecular epidemiology of avian influenza viruses and avian coronaviruses in environmental samples from migratory bird inhabitants in Bangladesh. Front. Vet. Sci. 2024, 11, 1446577. [Google Scholar] [CrossRef]
- Latorre-Margalef, N.; Gunnarsson, G.; Munster, V.J.; Fouchier, R.A.; Osterhaus, A.D.; Elmberg, J.; Olsen, B.; Wallesten, A.; Haemig, P.D.; Fransson, T.; et al. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. B Biol. Sci. 2009, 276, 1029–1036. [Google Scholar] [CrossRef]
- Groepper, S.R.; DeLiberto, T.J.; Vriska, M.P.; Pederson, K.; Swafford, S.R.; Hygnstrom, S.E. Avian influenza virus prevalence in migratory waterfowl in the United States, 2007–2009. Avian Dis. 2014, 58, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, S.; Lindh, E.; Vapalahti, O.; Huovilainen, A. Prevalence and genetic diversity of coronaviruses in wild birds, Finland. Infect. Ecol. Epidemiol. 2017, 7, 1408360. [Google Scholar] [CrossRef] [PubMed]
- de Sales Lima, F.E.; Gil, P.; Pedrono, M.; Minet, C.; Kwiatek, O.; Campos, F.S.; Spilki, F.R.; Roehe, P.M.; Franco, A.C.; Maminianina, O.F.; et al. Diverse gammacoronaviruses detected in wild birds from Madagascar. Eur. J. Wildl. Res. 2015, 61, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Blicharz, K.; Milek-Krupa, J.; Pikula, A. Diversity of coronaviruses in wild representatives of the Aves class in Poland. Viruses 2021, 13, 1497. [Google Scholar] [CrossRef]
- Verdugo, C.; Pinto, A.; Ariyama, N.; Moroni, M.; Hernandez, C. Molecular identification of avian viruses in neotropic cormorants (Phalacrocorax brasilianus) in Chile. J. Wildl. Dis. 2019, 55, 105–112. [Google Scholar] [CrossRef]
- Chamings, A.; Nelson, T.M.; Vibin, J.; Wille, M.; Klassen, M.; Alexandersen, S. Detection and characterisation of coronaviruses in migratory and non-migratory Australian wild birds. Sci. Rep. 2018, 8, 5980. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.J.; You, Z.; Wu, D.Y.; Liu, S.J.; Zhang, W.L.; Fan, K.R.; Luo, R.; Qiu, Y.; Ge, X.Y. Epidemiology and evolution of novel deltacoronaviruses in birds in central China. Transbound. Emerg. Dis. 2022, 69, 632–644. [Google Scholar] [CrossRef]
- Parvin, R.; Kabiraj, C.K.; Mumu, T.T.; Chowdhury, E.H.; Islam, M.R.; Beer, M.; Harder, T. Active virological surveillance in backyard ducks in Bangladesh: Detection of avian influenza and gammacoronaviruses. Avian Pathol. 2020, 49, 361–368. [Google Scholar] [CrossRef]
- Le Gall-Ladevèze, C.; Vollot, B.; Hirschinger, J.; Lèbre, L.; Aaziz, R.; Laroucau, K.; Guérin, J.L.; Paul, M.; Cappelle, J.; Le Loc’h, G. Limited transmission of avian influenza viruses, avulaviruses, coronaviruses and Chlamydia sp. at the interface between wild birds and a free-range duck farm. Vet. Res. 2025, 56, 36. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Xu, P.; Zhou, S.; Zhao, P.; Wang, Y.; Hu, J.; Ma, M.; Li, Z.; Bo, S.; et al. Avian Migration-Mediated Transmission and Recombination Driving the Diversity of Gammacoronaviruses and Deltacoronaviruses. Mol. Biol. Evol. 2025, 42, msaf045. [Google Scholar] [CrossRef]
- Marchenko, V.Y.; Kolosova, N.P.; Danilenko, A.V.; Bragina, M.K.; Nhai, T.T.; Ryzhikov, A. Diversity of coronaviruses in wild and domestic birds in Vietnam. Asian Pac. J. Trop. Med. 2022, 15, 442–450. [Google Scholar] [CrossRef]
- Ma, M.; Ji, L.; Ming, L.; Xu, Y.T.; Zhao, C.Y.; Wang, T.H.; He, G.M. Co-circulation of coronavirus and avian influenza virus in wild birds in Shanghai (2020–2021). Transbound. Emerg. Dis. 2022, 69, 3985–3991. [Google Scholar] [CrossRef]
- Zhigailov, A.V.; Maltseva, E.R.; Perfilyeva, Y.V.; Ostapchuk, Y.O.; Naizabayeva, D.A.; Berdygulova, Z.A.; Kuatbekova, S.A.; Nizkorodova, A.S.; Mashzhan, A.; Gavrilov, A.E.; et al. Prevalence and genetic diversity of coronaviruses, astroviruses and paramyxoviruses in wild birds in southeastern Kazakhstan. Heliyon 2022, 8, 11. [Google Scholar] [CrossRef]
- Sharshov, K.; Dubovitskiy, N.; Derko, A.; Loginova, A.; Kolotygin, I.; Zhirov, D.; Sobolev, I.; Kurskaya, O.; Alekseev, A.; Druzyaka, A.; et al. Does avian coronavirus co-circulate with avian paramyxovirus and avian influenza virus in wild ducks in Siberia? Viruses 2023, 15, 1121. [Google Scholar] [CrossRef] [PubMed]
- Gim, Y.; Jeong, S.H.; Lee, Y.J.; Jang, G.; Lee, C. Incidence and Genetic Investigation of Avian Coronaviruses in Migratory Ducks From South Korea. Transbound. Emerg. Dis. 2024, 1, 9502737. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Jenkins, K.M.; Bell, D.; Morris, P.J.; Kingsford, R.T. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 2008, 53, 380–392. [Google Scholar] [CrossRef]
- Gibbs, S.E. Avian biology, the human influence on global avian influenza transmission, and performing surveillance in wild birds. Anim. Health Res. Rev. 2010, 11, 35–41. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.; Hauser, R.; Matthes, D.; Stärk, K.D. Evaluation of effectiveness and efficiency of wild bird surveillance for avian influenza. Vet. Res. 2010, 41, 50. [Google Scholar] [CrossRef]
- Lefrançois, T.; Hendrikx, P.; Ehrhardt, N.; Millien, M.; Gomez, L.; Gouyet, L.; Gaidet, N.; Gerbier, G.; Vachiéry, N.; Petitclerc, F.; et al. Surveillance of avian influenza in the Caribbean through the Caribbean Animal Health Network: Surveillance tools and epidemiologic studies. Avian Dis. 2010, 54, 369–373. [Google Scholar] [CrossRef]
- Bande, F.; Arshad, S.S.; Omar, A.R.; Bejo, M.H.; Abubakar, M.S.; Abba, Y. Pathogenesis and diagnostic approaches of avian infectious bronchitis. Adv. Virol. 2016, 2016, 4621659. [Google Scholar] [CrossRef]
- Graham, R.L.; Baric, R.S. Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross-species transmission. J. Virol. 2010, 84, 3134–3146. [Google Scholar] [CrossRef]
- Eikenaar, C.; Hegemann, A. Migratory common blackbirds have lower innate immune function during autumn migration than resident conspecifics. Biol. Lett. 2016, 12, 20160078. [Google Scholar] [CrossRef]
- Posada-Cespedes, S.; Seifert, D.; Beerenwinkel, N. Recent advances in inferring viral diversity from high-throughput sequencing data. Virus Res. 2017, 239, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Smeele, Z.E.; Ainley, D.G.; Varsani, A. Viruses associated with antarctic wildlife: From serology based detection to identification of genomes using high throughput sequencing. Virus Res. 2018, 243, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, G.C.; Jerde, C.L.; Young, H.S. High-throughput sequencing for understanding the ecology of emerging infectious diseases at the wildlife-human interface. Front. Ecol. Evol. 2019, 7, 126. [Google Scholar] [CrossRef]
- Smith, K.F.; Sax, D.F.; Lafferty, K.D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 2006, 20, 1349–1357. [Google Scholar] [CrossRef]
- Wille, M.; Avril, A.; Tolf, C.; Schager, A.; Larsson, S.; Borg, O.; Olsen, B.; Waldenstrom, J. Temporal dynamics, diversity, and interplay in three components of the virodiversity of a mallard population: Influenza a virus, avian paramyxovirus and avian coronavirus. Infect. Genet. Evol. 2015, 29, 129–137. [Google Scholar] [CrossRef]
- Kong, L.C.; You, R.R.; Zhang, D.C.; Yuan, Q.L.; Xiang, B.; Liang, J.P.; Lin, Q.Y.; Ding, C.; Liao, M.; Chen, L.; et al. Infectious bronchitis virus infection increases pathogenicity of H9N2 avian influenza virus by inducing severe inflammatory response. Front. Vet. 2022, 8, 824179. [Google Scholar]
- Machalaba, C.C.; Elwood, S.E.; Forcella, S.; Smith, K.M.; Hamilton, K.; Jebara, K.B.; Swayne, D.E.; Webby, R.J.; Mumford, E.; Mazet, J.A.; et al. Global avian influenza surveillance in wild birds: A strategy to capture viral diversity. Emerg. Infect. Dis. 2015, 21, e1–e7. [Google Scholar] [CrossRef]
- Stallknecht, D.E. Impediments to wildlife disease surveillance, research, and diagnostics. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Richt, J.A., Mackenzie, J.S., Childs, J.E., Eds.; Springer: New York, NY, USA, 2007; pp. 445–461. [Google Scholar]
- Belant, J.L.; Deese, A.R. Importance of wildlife disease surveillance. HWI 2010, 4, 165–169. [Google Scholar]
- Hoerr, F.J. The pathology of infectious bronchitis. Avian Dis. 2021, 65, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; De Garine-Wichatitsky, M.; Gaidet, N.; Chiweshe, N.; Cumming, G.S. Estimating dynamic risk factors for pathogen transmission using community-level bird census data at the wildlife/domestic interface. Ecol. Soc. 2010, 15, 25. [Google Scholar] [CrossRef]
- Jourdain, E.; Gunnarsson, G.; Wahlgren, J.; Latorre-Margalef, N.; Bröjer, C.; Sahlin, S.; Svensson, L.; Waldenström, J.; Lundkvist, Å.; Olsen, B. Influenza virus in a natural host, the mallard: Experimental infection data. PLoS ONE 2010, 51, e8935. [Google Scholar] [CrossRef] [PubMed]
- Laudert, E.; Halvorson, D.; Sivanandan, V.; Shaw, D. Comparative Evaluation of Tissue Trophism Characteristics in Turkeys and Mallard Ducks after Intravenous Inoculation of Type A Influenza Viruses. Avian Dis. 1993, 37, 773–780. [Google Scholar] [CrossRef]
- Hill, N.J.; Takekawa, J.Y.; Ackerman, J.T.; Hobson, K.A.; Herring, G.; Cardona, C.J.; Runstadler, J.A.; Boyce, W.M. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos). Mol. Ecol. 2021, 21, 5986–5999. [Google Scholar] [CrossRef]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. B Biol. Sci. 2001, 268, 25–29. [Google Scholar] [CrossRef]
- Holmes, R.T.; Sherry, T.W. Thirty-year bird population trends in an unfragmented temperate deciduous forest: Importance of habitat change. Auk 2001, 118, 589–609. [Google Scholar] [CrossRef]
- Teitelbaum, C.S.; Ackerman, J.T.; Hill, M.A.; Satter, J.M.; Casazza, M.L.; De La Cruz, S.E.W.; Boyce, W.M.; Buck, E.J.; Eadie, J.M.; Herzog, M.P.; et al. Avian influenza antibody prevalence increases with mercury contamination in wild waterfowl. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221312. [Google Scholar] [CrossRef]
- Rushing, C.S.; Royle, J.A.; Ziolkowski, D.J.; Pardieck, K.L. Migratory behavior and winter geography drive differential range shifts of eastern birds in response to recent climate change. Proc. Natl. Acad. Sci. USA 2020, 117, 12897–12903. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird migration times, climate change, and changing population sizes. Glob. Change Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Marra, P.P.; Francis, C.M.; Mulvihill, R.S.; Moore, F.R. The influence of climate on the timing and rate of spring bird migration. Oecologia 2005, 142, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Yadav, S.; Haldar, C. Influence of environmental factors on avian immunity: An overview. J. Immunol. Res. 2017, 4, 1028–1033. [Google Scholar]
- Messina, S.; Edwards, D.P.; Eens, M.; Costantini, D. Physiological and immunological responses of birds and mammals to forest degradation: A meta-analysis. Biol. Conserv. 2018, 224, 223–229. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Destoumieux-Garzon, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Carey, C. Infectious disease and worldwide declines of amphibian populations, with comments on emerging diseases in coral reef organisms and in humans. Environ. Health Perspect. 2000, 108, 143–150. [Google Scholar]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Infectious disease and amphibian population declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef]
- Robinson, R.A.; Lawson, B.; Toms, M.P.; Peck, K.M.; Kirkwood, J.K.; Chantrey, J.; Clatworthy, I.R.; Evans, A.D.; Hughes, L.A.; Hutchinson, O.C.; et al. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 2010, 5, e12215. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, C.; Eden, P.; Reiss, A.; Ellis, T.; Knowles, G.; Wayne, A.F. Disease hazard identification and assessment associated with wildlife population declines. Ecol. Manag. Restor. 2015, 16, 142–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).