Arrival and Peak Abundance of Barn Swallows Hirundo rustica in Three Regions of South Africa in Relation to Climate Indices, Deduced from Bird Atlas Data
Simple Summary
Abstract
1. Introduction
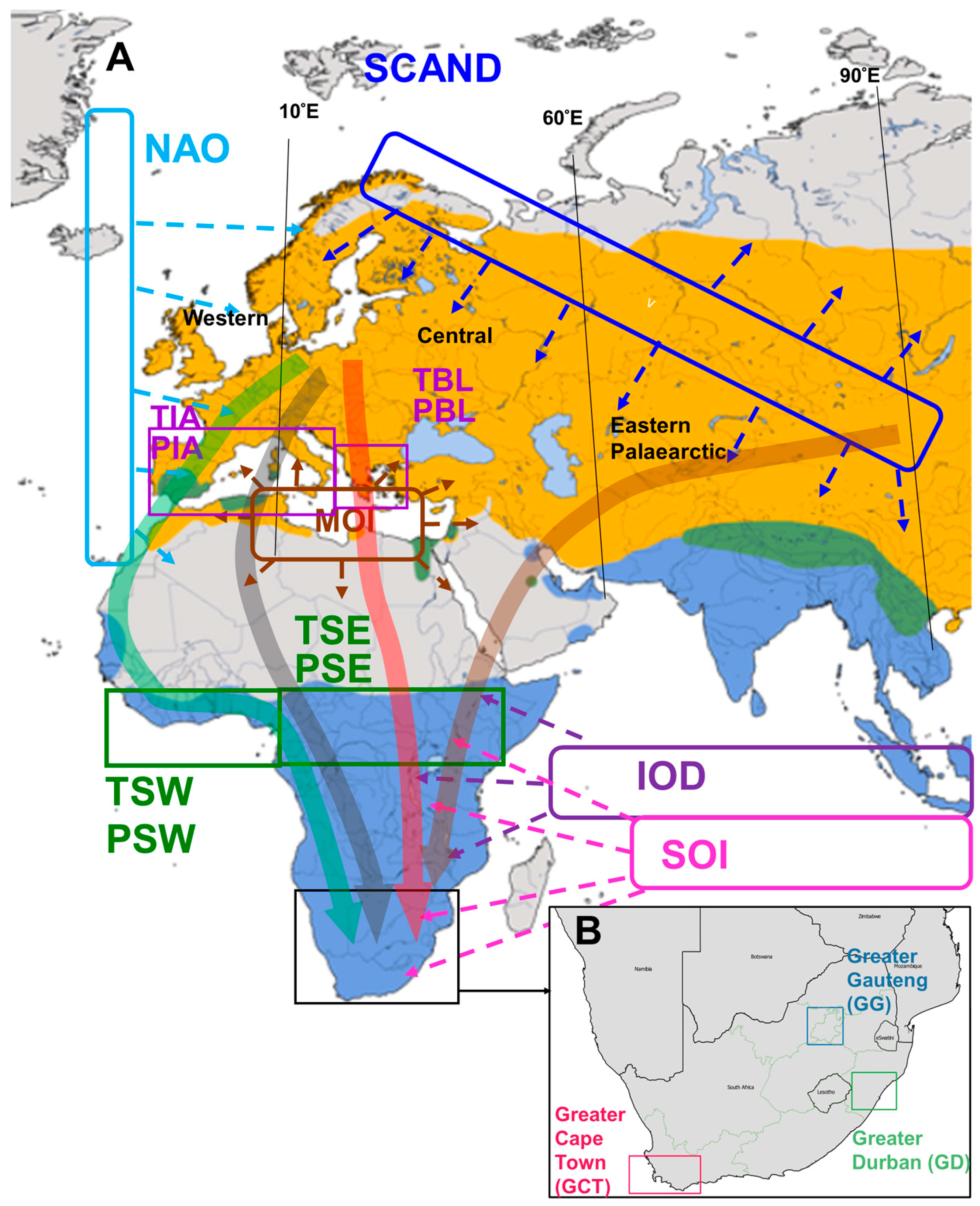
2. Materials and Methods
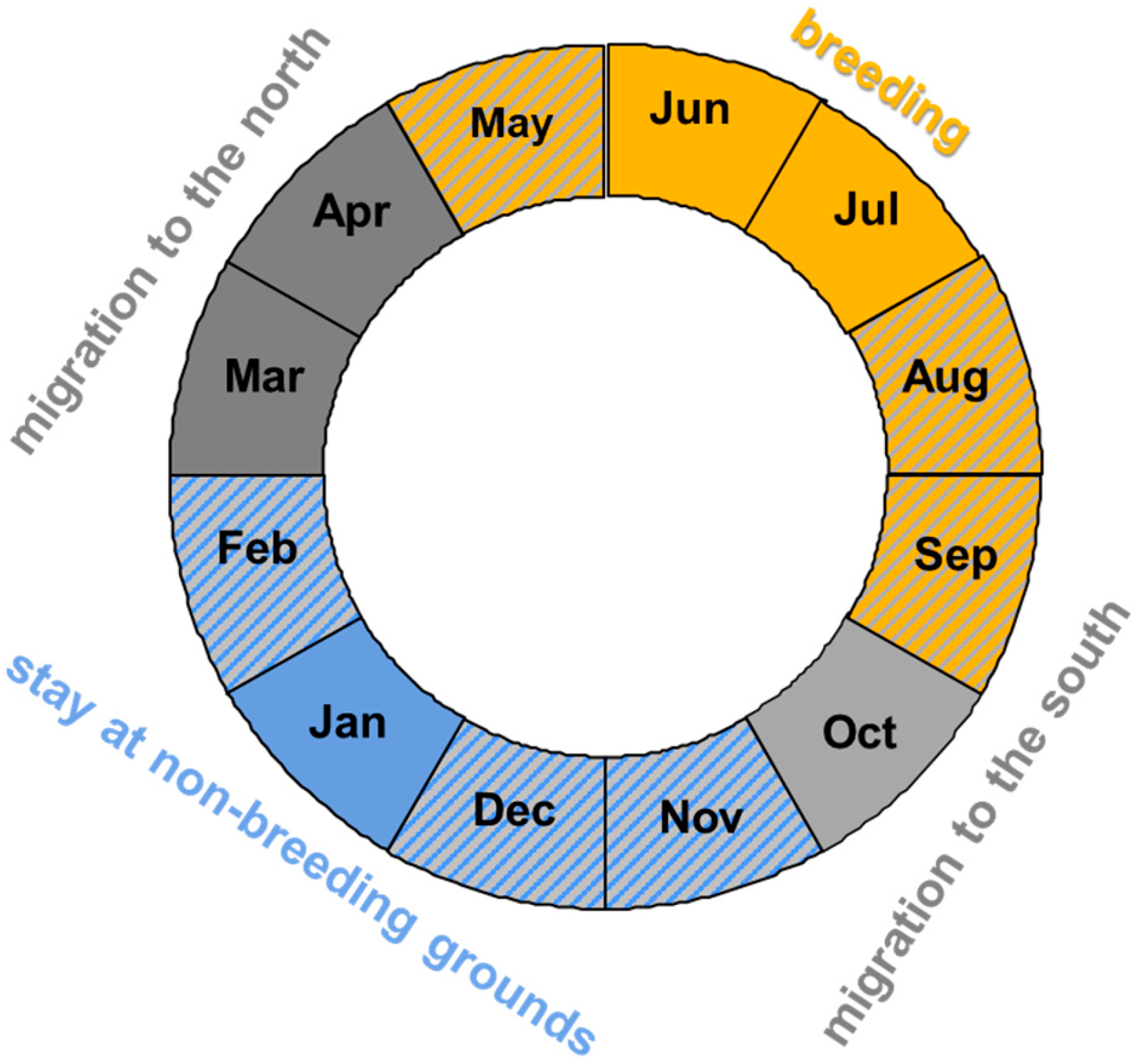
2.1. Bird Data
2.2. Climate Variables
2.2.1. Selection of Climate Variables for Analysis
| Symbol | Climate Index | Index Description [Main References] | Influenced Region | Winter (Nov–Feb) | Spring (Mar–Apr) | Summer (May–July) | Autumn (Aug–Oct) |
|---|---|---|---|---|---|---|---|
| SCAND | Scandinavian Pattern Index [40] | Gradient of pressure systems over western Europe and eastern Russia [41,42,43] | N Europe | warm, dry ↓ | warm, dry ↓ | warm, dry ↓ | |
| W Europe | cool, wet | cool, wet | cool, wet | ||||
| C Europe | cool, wet | cool, wet | cool, wet | ||||
| E Europe | cool, wet | cool, wet | cool, wet | ||||
| NAO | North Atlantic Oscillation Index [44] | Difference in the air pressure between Ponta Delgada (Azores) and Reykjavik (SW Iceland) [45,46,47,48,49] | N Europe | warm, wet − | warm, wet − | warm, dry ↓ | warm, dry − |
| W Europe | warm, wet | warm, wet | hot, dry | hot, dry | |||
| C Europe | warm, dry | warm, dry | hot, dry | hot, dry | |||
| W Mediterranean | warm, dry | warm, dry | cool, wet | cool, wet | |||
| NW Africa | warm, dry | warm, dry | |||||
| MOI | Mediterranean Oscillation Index [50] | Difference in the air pressure between Algiers (Algeria) and Cairo (Egypt) [51,52,53,54,55] | E Europe | warm, dry | warm, dry | ||
| E Mediterranean | warm, dry | warm, dry | |||||
| IOD | Indian Ocean Dipole [56] | Difference in the anomalies in sea surface temperature between the western and the eastern Indian Ocean near the equator [57,58,59,60,61] | Middle East | cool, wet ↑ | cool, wet ↑ | cool, wet ↑ | cool, wet ↑ |
| E Africa | cool, wet ↑ | cool, wet ↑ | cool, wet ↑ | cool, wet ↑ | |||
| SOI | Southern Oscillation Index [62] | Difference in the air pressure between Tahiti and Darwin (Australia). La Niña = positive SOI; El Niño = negative SOI [63,64] | E Africa (Tanzania) | cool, wet ↑ | cool, wet ↑ | cool, wet ↑ | cool, dry − |
| E Africa (Lake Victoria) | cool, dry | cool, dry | cool, dry | cool, dry | |||
| SE Africa | cool, wet | cool, dry | cool, dry | cool, dry | |||
| TIA | Monthly average temperature in the Iberian and Apennine Peninsulas [65] | Averaged daily mean temperatures for the area within 35°32′ N–46°29′ N; 10°32′ W–18°07′ E | W Mediteranean | hot ↑ | hot ↑ | hot ↑ | hot ↑ |
| PIA | Monthly average precipitation in the Iberian and Apennine Peninsulas [66] | Averaged daily total precipitation for the area within 35° 32′ N–46° 29′ N; 10° 32′ W–18°07′ E | W Mediterranean | wet | wet | wet | wet |
| TBL | Monthly mean temperature in the Balkan Peninsula [65] | Averaged daily mean temperatures for the area within 34°43′ N–44°58′ N; 18°51′–26°31′ E | E Mediterranean | hot ↑ | hot ↑ | hot ↑ | hot ↑ |
| PBL | Monthly average precipitation in the Balkan Peninsula [66] | Averaged daily total precipitation for the area within 34°43′ N–44°58′ N; 18°51′–26°31′ E | E Mediterranean | wet | wet | wet | wet |
| TSW | Monthly average temperature for the Western Sahel [65] | Averaged daily mean temperatures for the area within 15–20° N, 20° W–10° E | WAfrica | hot ↑ | hot ↑ | hot ↑ | hot ↑ |
| PSW | Monthly average precipitation for the Western Sahel [66] | Averaged daily total precipitation for the area within 5–20° N, 20° W–10° E | WAfrica | wet − | wet | wet ↑ | wet |
| TSE | Monthly average temperature for Eastern Sahel [65] | Averaged daily total precipitation for the area within 15–20° N, 10° E–35° E | E Africa | hot ↑ | hot↑ | hot ↑ | hot |
| PSE | Monthly average precipitation Anomaly for Eastern Sahel [66] | Averaged daily total precipitation for the area within 15–20° N, 10° E–35° E | E Africa | wet − | wet | wet ↑ | wet |
2.2.2. Large-Scale Climate Indices
2.2.3. Precipitation and Temperature
2.3. Statistical Analyses
3. Results
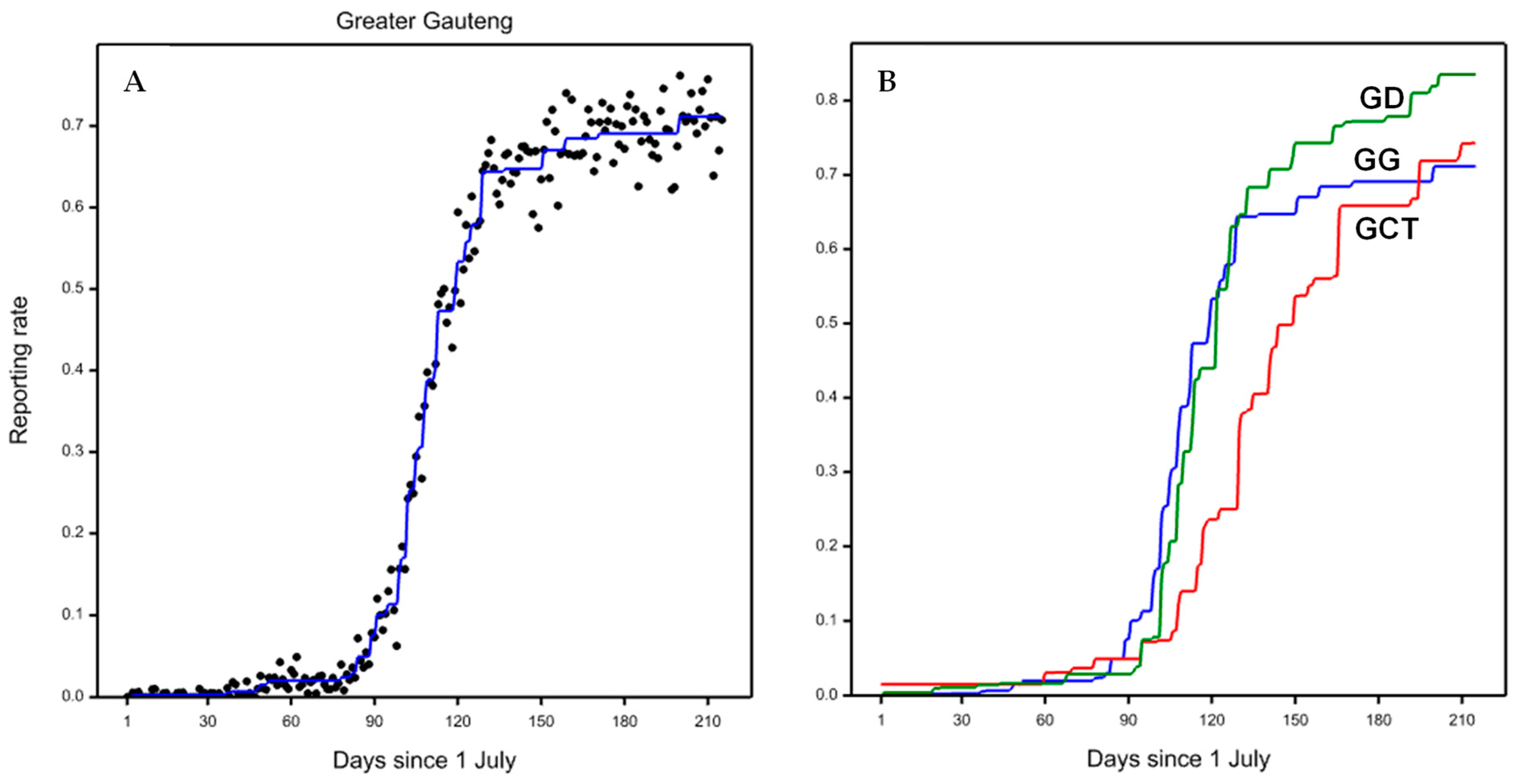
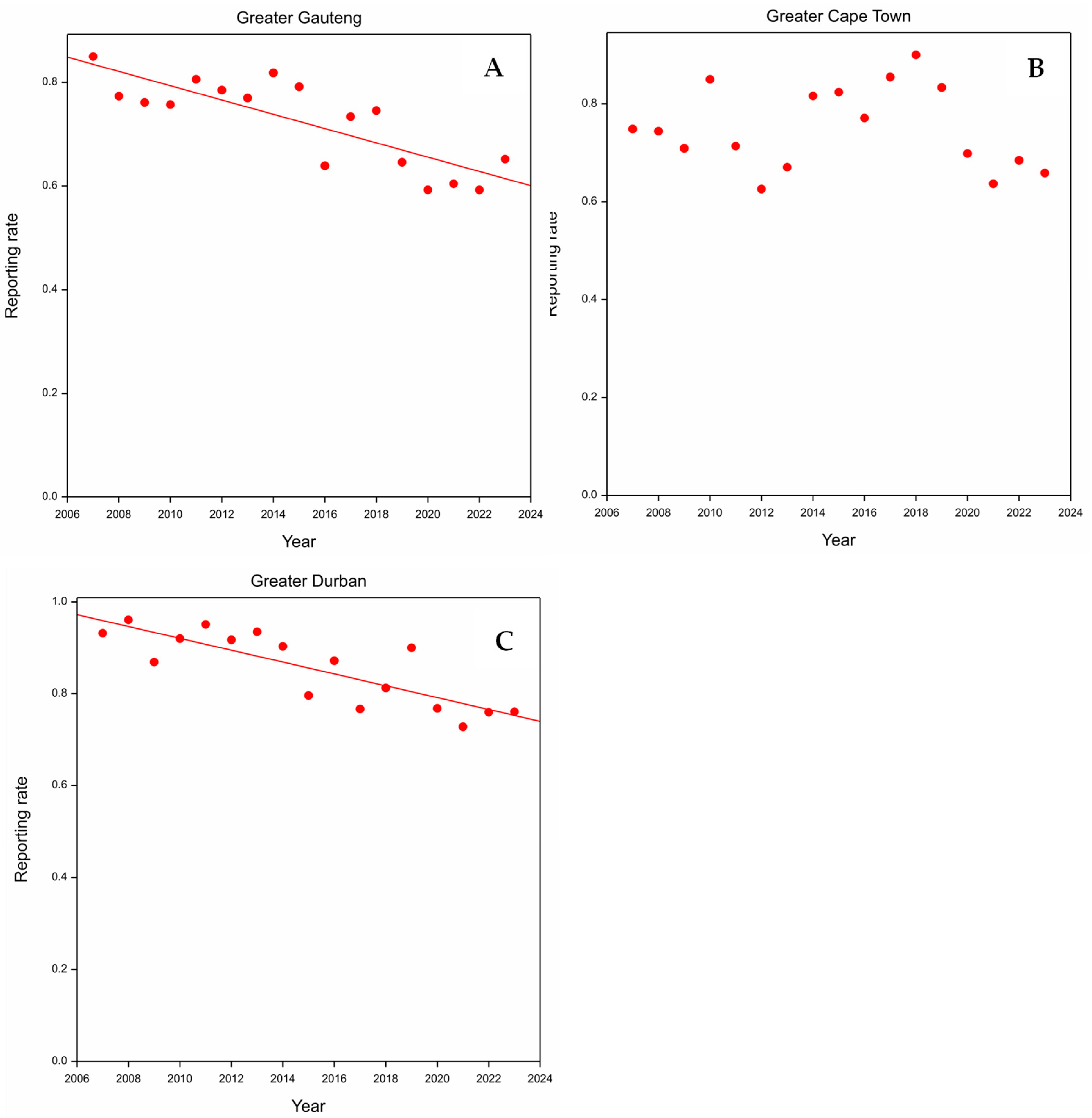
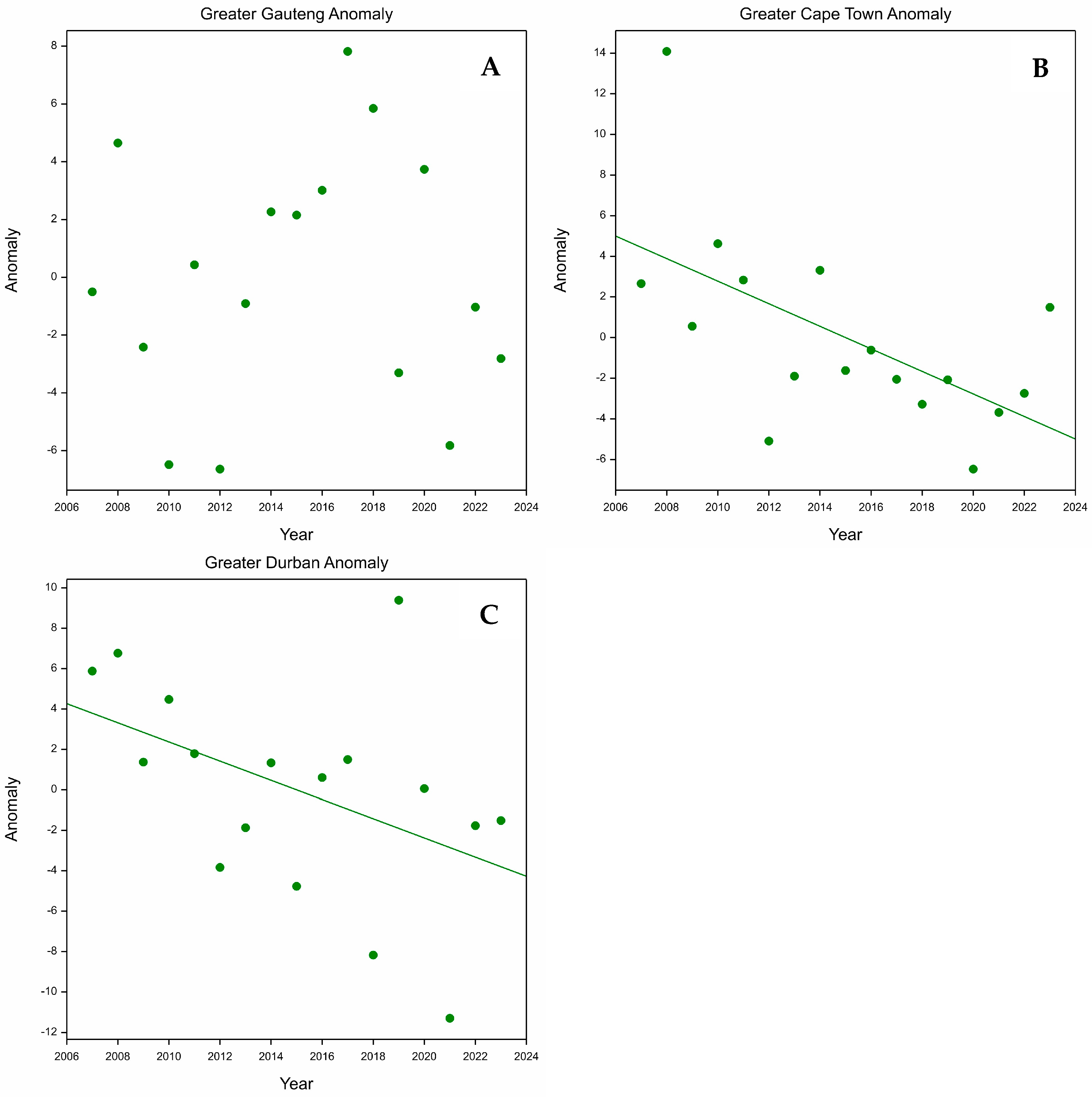
3.1. Greater Gauteng
3.2. Greater Cape Town
3.3. Greater Durban
4. Discussion
4.1. Effects of Climate Factors on the Timing of Barn Swallow Arrivals in South Africa
4.1.1. Effects of Precipitation
4.1.2. Effects of Temperature
4.1.3. The Effect of the Scandinavian Pattern Mediterranean Oscillation Index on Barn Swallow Arrivals in Greater Gauteng
4.1.4. The Effect of the Northern Oscillation Index on Barn Swallows’ Arrivals in South Africa
4.1.5. The Effect of the Mediterranean Oscillation Index on Barn Swallows’ Arrivals in South Africa
4.1.6. The Effect of the Indian Ocean Dipole in June–July on Barn Swallows’ Arrivals in Greater Gauteng and Greater Durban Areas
4.1.7. The Effect of the Southern Oscillation Index in November-February on Barn Swallows in Greater Cape Town Area
4.2. Effect of the Year
4.3. Different Patterns of Influence of Climate Indices Between the Three Regions of South Africa
4.4. Effects of Climate Factors on the Reporting Rates of Barn Swallow in South Africa
4.5. The Expected Effect of Climate Change on the Migration Patterns of Barn Swallows Between Eurasia and South Africa
4.6. Second Southern African Bird Atlas Project (SABAP2)
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Symbol of Variable | Climate Index | Source |
|---|---|---|---|
| 1 | SCAND_MAY_JUL | Scandinavian Pattern Index | [40] |
| 2 | SCAND_MAY_AUG | ||
| 3 | SCAND_JUN_JUL | ||
| 4 | SCAND_JUN_AUG | ||
| 5 | SCAND_AUG_OCT | ||
| 6 | SCAND_SEP_OCT | ||
| 7 | NAO_MAY_JUL | North Atlantic Oscillation Index | [44] |
| 8 | NAO_MAY_AUG | ||
| 9 | NAO_JUN_AUG | ||
| 10 | NAO_JUN_AUG | ||
| 11 | NAO_AUG_OCT | ||
| 12 | NAO_SEP_OCT | ||
| 13 | MOI_MAY_JUL | Mediterranean Oscillation Index Algiers/Cairo | [50] |
| 14 | MOI_MAY_AUG | ||
| 15 | MOI_JUN_JUL | ||
| 16 | MOI_JUN_AUG | ||
| 17 | MOI_AUG_OCT | ||
| 18 | MOI_SEP_OCT | ||
| 19 | IOD_MAY_JUL | Indian Ocean Dipole | [56] |
| 20 | IOD_MAY_AUG | ||
| 21 | IOD_JUN_AUG | ||
| 22 | IOD_JUN_JUL | ||
| 23 | IOD_SEP_OCT | ||
| 24 | IOD_AUG_OCT | ||
| 25 | IOD_NOV_FEB | ||
| 26 | SOI_MAY_JUL | Southern Oscillation Index | [62] |
| 27 | SOI_MAY_AUG | ||
| 28 | SOI_JUN_AUG | ||
| 29 | SOI_JUN_JUL | ||
| 30 | SOI_SEP_OCT | ||
| 31 | SOI_AUG_OCT | ||
| 32 | SOI_NOV_FEB | ||
| 33 | TIA_MAY_AUG | Monthly average temperature in the Iberian and Apennine Peninsulas (35°32′ N–46°29′ N; 10°32′ W–18°07′ E) | [65] |
| 34 | TIA_MAY_JUL | ||
| 35 | TIA_JUN_JUL | ||
| 36 | TIA_JUN_AUG | ||
| 37 | TIA_AUG_OCT | ||
| 38 | TIA_SEP_OCT | ||
| 39 | PIA_MAY_JUL | Monthly average precipitation in the Iberian and Apennine Peninsulas (35°32′ N–46°29′ N; 10°32′ W–18°07′ E) | [66] |
| 40 | PIA_MAY_AUG | ||
| 41 | PIA_JUN_JUL | ||
| 42 | PIA_JUN_AUG | ||
| 43 | PIA_AUG_OCT | ||
| 44 | PIA_SEP_OCT | ||
| 45 | TBLK_MAY_JUL | Monthly average temperature in the Balkan Peninsula (34°43′ N–44°58′ N; 18°51’–26°31’ E) | [65] |
| 46 | TBLK_MAY_AUG | ||
| 47 | TBLK_JUN_JUL | ||
| 48 | TBLK_JUN_AUG | ||
| 49 | TBLK_AUG_OCT | ||
| 50 | TBLK_SEP_OCT | ||
| 51 | PBLK_MAY_JUL | Monthly average precipitation in the Balkan Peninsula (34°43’ N–44°58’ N; 18°51’–26°31’ E) | [66] |
| 52 | PBLK_MAY_AUG | ||
| 53 | PBLK_JUN_JUL | ||
| 54 | PBLK_JUN_AUG | ||
| 55 | PBLK_AUG_OCT | ||
| 56 | PBLK_SEP_OCT | ||
| 57 | TSAHW_MAY_JUL | Monthly average temperature for the Western Sahel (15–20° N, 20° W–10° E) | [65] |
| 58 | TSAHW_MAY_AUG | ||
| 59 | TSAHW_JUN_AUG | ||
| 60 | TSAHW_JUN_JUL | ||
| 61 | TSAHW_SEP_OCT | ||
| 62 | TSAHW_AUG_OCT | ||
| 63 | TSAHW_NOV_FEB | ||
| 64 | PSAHW_MAY_JUL | Monthly average precipitation for the Western Sahel (15–20° N, 20° W–10° E) | [66] |
| 65 | PSAHW_MAY_AUG | ||
| 66 | PSAHW_JUN_JUL | ||
| 67 | PSAHW_JUN_AUG | ||
| 68 | PSAHW_AUG_OCT | ||
| 69 | PSAHW_SEP_OCT | ||
| 70 | PSAHW_NOV_FEB | ||
| 71 | TSAHE_MAY_JUL | Monthly average temperature for Eastern Sahel (15–20° N, 10° E–35° E) | [65] |
| 72 | TSAHE_MAY_AUG | ||
| 73 | TSAHE_JUN_JUL | ||
| 74 | TSAHE_JUN_AUG | ||
| 75 | TSAHE_AUG_OCT | ||
| 76 | TSAHE_SEP_OCT | ||
| 77 | TSAHE_NOV_FEB | ||
| 78 | PSAHE_MAY_JUL | Monthly average precipitation for Eastern Sahel (15–20° N, 10° E–35° E) | [66] |
| 79 | PSAHE_MAY_AUG | ||
| 80 | PSAHE_JUN_JUL | ||
| 81 | PSAHE_JUN_AUG | ||
| 82 | PSAHE_AUG_OCT | ||
| 83 | PSAHE_SEP_OCT | ||
| 84 | PSAHE_NOV_FEB |
References
- Rubolini, D.; Pastor, A.G.; Pilastro, A.; Spina, F. Ecological barriers shaping fuel stores in barn swallows Hirundo rustica following the central and western Mediterranean flyways. J. Avian Biol. 2002, 33, 15–22. [Google Scholar] [CrossRef]
- Saino, N.; Szép, T.; Romano, M.; Rubolini, D.; Spina, F.; Møller, A.P. Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol. Lett. 2004, 7, 21–25. [Google Scholar] [CrossRef]
- Gordo, O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 2007, 35, 37–58. [Google Scholar] [CrossRef]
- Robinson, R.A.; Balmer, D.E.; Marchant, J.H. Survival rates of Hirundines in relation to British and African rainfall. Ringing Migr. 2008, 24, 1–6. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Underhill, L.G. Climatic variation in Africa and Europe has combined effects on timing of spring migration in a long-distance migrant Willow Warbler Phylloscopus trochilus. PeerJ 2020, 8, e8770. [Google Scholar] [CrossRef] [PubMed]
- Remisiewicz, M.; Underhill, L.G. Large-scale climatic patterns have stronger carry-over effects than local temperatures on spring phenology of long-distance passerine migrants between Europe and Africa. Animals 2022, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Remisiewicz, M.; Underhill, L.G. Climate in Africa sequentially shapes within-season spring passage of Willow Warbler Phylloscopus trochilus through the Baltic coast. PeerJ 2022, 10, e12964. [Google Scholar] [CrossRef]
- Gołębiewski, I.; Remisiewicz, M. Carry-over effects of climate variability at breeding and non-breeding grounds on spring migration in the European Wren Troglodytes troglodytes at the Baltic Coast. Animals 2023, 13, 2015. [Google Scholar] [CrossRef]
- Pinszke, A.; Remisiewicz, M. Long-term changes in autumn migration timing of Garden Warblers Sylvia borin at the southern Baltic coast in response to spring, summer and autumn temperatures. Eur. Zool. J. 2023, 90, 283–295. [Google Scholar] [CrossRef]
- Maciag, T.; Remisiewicz, M. Climate change impact on the populations of Goldcrest Regulus regulus and Firecrest Regulus ignicapilla migrating through the southern Baltic coast. Sustainability 2025, 17, 1243. [Google Scholar] [CrossRef]
- BirdLife International. Hirundo rustica. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/22712252/137668645 (accessed on 28 July 2025).
- PECBMS. Pan-European Common Bird Monitoring Scheme. Available online: https://pecbms.info/trends-and-indicators/species-trends/species/hirundo-rustica/confidential/yes/?search=Hirundo%20rustica (accessed on 27 July 2025).
- Cramp, S. (Ed.) Handbook of the Birds of Europe, the Middle East and North Africa: The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1998; Volume 5. [Google Scholar]
- Zwarts, L.; Bijlsma, R.G.; van der Kamp, J.; Wymenga, E. Living on the Edge: Wetlands and Birds in a Changing Sahel; KNNV Publishing: Zeist, The Netherlands, 2009. [Google Scholar]
- Briedis, M.; Kurlavičius, P.; Mackevičienė, R.; Vaišvilienė, R.; Hahn, S. Loop migration, induced by seasonally different flyway use, in northern European barn swallows. J. Ornithol. 2018, 159, 885–891. [Google Scholar] [CrossRef]
- Pancerasa, M.; Ambrosini, R.; Romano, A.; Rubolini, D.; Winkler, D.W.; Casagrandi, R. Across the deserts and sea: Inter-individual variation in migration routes of south-central European barn swallows (Hirundo rustica). Mov. Ecol. 2022, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Baillie, S.R.; Bairlein, F.; Fiedler, W.; Thorup, K. The Eurasian African Bird Migration Atlas. 2022. Available online: https://migrationatlas.org (accessed on 29 May 2025).
- Turbek, S.P.; Schield, D.R.; Scordato, E.S.; Contina, A.; Da, X.W.; Liu, Y.; Liu, Y.; Pagani-Núñez, E.; Ren, Q.-M.; Smith, C.C.R.; et al. A migratory divide spanning two continents is associated with genomic and ecological divergence. Evolution 2022, 76, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Burman, M.; Underhill, L.G.; Altwegg, R.; Erni, B.; Remisiewicz, M.; MacLeod, J. Migratory connectivity of Barn Swallows in South Africa to their Palearctic breeding grounds. Divers. Distrib. 2018, 24, 1699–1708. [Google Scholar] [CrossRef]
- Szép, T.; Møller, A.P.; Piper, S.; Nuttall, R.; Szabó, Z.D.; Pap, P.L. Searching for potential wintering and migration areas of a Danish Barn Swallow population in South Africa by correlating NDVI with survival estimates. J. Ornithol. 2006, 147, 245–253. [Google Scholar] [CrossRef]
- Trepte, A. Rauchschwalbe—Steckbrief, Verbreitung, Bilder—Vogellexikon. 2023. Available online: https://www.avi-fauna.info/sperlingsvoegel/schwalben/rauchschwalbe/ (accessed on 29 May 2025).
- Dunn, E.H. Bird observatories: An underutilized resource for migration study. Wilson Bull. 2016, 128, 691–703. [Google Scholar] [CrossRef]
- EUFLYNET. EUFLYNET, COST Action CA22117. 2023. Available online: www.euflynet.eu (accessed on 29 May 2025).
- Underhill, L.G. Opening of the Alte Kalköfen Bird Observatory in southern Namibia, February 2025. Biodivers. Obs. 2025, 15, 23–33. [Google Scholar]
- Underhill, L.G. The fundamentals of the SABAP2 protocol. Biodivers. Obs. 2016, 7.42, 1–12. [Google Scholar]
- Underhill, L.G.; Brooks, M.; Loftie-Eaton, M. The Second Southern African Bird Atlas Project: Protocol, process, product. Vogelwelt 2017, 137, 64–70. [Google Scholar]
- Brooks, M.; Rose, S.; Altwegg, R.; Lee, A.T.K.; Nel, H.; Ottosson, U.; Retief, E.; Reynold, C.; Ryan, P.G.; Shema, S.; et al. The African Bird Atlas Project: A description of the project and BirdMap data collection protocol. Ostrich 2022, 93, 223–232. [Google Scholar] [CrossRef]
- Lee, A.T.K.; Brooks, M.; Underhill, L.G. The SABAP2 legacy: A review of the history and use of data generated by a long running citizen science project. S. Afr. J. Sci. 2022, 118, 12030. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.A.; Underhill, L.G.; Barnard, P. The seminal legacy of the Southern African Bird Atlas Project. S. Afr. J. Sci. 2008, 102, 82–84. [Google Scholar]
- Underhill, L.G.; Prŷs-Jones, R.P.; Harrison, J.A.; Martinez, P. Seasonal patterns of occurrence of Palaearctic migrants in southern Africa using atlas data. Ibis 1992, 134 (Suppl. S1), 99–108. [Google Scholar] [CrossRef]
- Harrison, J.A.; Underhill, L.G. Introduction and methods. In The Atlas of Southern African Birds. Volume 1: Non Passerines; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife South Africa: Johannesburg, South Africa, 1997; pp. xliii–lxiv. [Google Scholar]
- Earlé, R.A. European Swallow Hirundo rustica. In The Atlas of Southern African Birds: Volume 2: Passerines; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife South Africa: Johannesburg, South Africa, 1997; pp. 48–49. [Google Scholar]
- Martin, A.P. Cattle Egret Bubulcus ibis. In The Atlas of Southern African Birds. Volume 1: Non-Passerines; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife South Africa: Johannesburg, South Africa, 1997; pp. 51–53. [Google Scholar]
- Tarboton, W.R.; Kemp, M.K.; Kemp, A.C. Birds of the Transvaal; Transvaal Museum: Pretoria, South Africa, 1987. [Google Scholar]
- Linsdale, J.M. A method of showing relative frequency of occurrence of birds. Condor 1928, 30, 180–184. [Google Scholar] [CrossRef]
- Temple, S.A.; Temple, A.J. Geographic distributions and patterns of relative abundance of Wisconsin birds: A WSO research project. Passeng. Pigeon 1986, 48, 58–68. [Google Scholar]
- Miles, R.E. The complete amalgamation into blocks, by weighted means, of a finite set of real numbers. Biometrika 1959, 46, 317–327. [Google Scholar] [CrossRef]
- Kruskal, J. Multidimensional scaling by optimizing goodness-of-fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Kruskal, J. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- NOAA. National Oceanic and Atmospheric Administration US Department of Commerce, National Weather Service. Climate Prediction Centre. Northern Hemisphere Teleconnection Patterns. Scandinavia (SCAND). Available online: https://ftp.cpc.ncep.noaa.gov/wd52dg/data/indices/scand_index.tim (accessed on 30 April 2025).
- Barstone, A.G.; Livezey, R.E. Classification, Seasonality and Persistence of Low–Frequency Atmospheric Circulation Patterns. Mon. Weather Rev. 1987, 115, 1083–1126. [Google Scholar] [CrossRef]
- Bueh, C.; Nakamura, H. Scandinavian Pattern and its climatic impact. Q. J. R. Meteorol. Soc. 2007, 133, 2117–2131. [Google Scholar] [CrossRef]
- NOAA. National Oceanic and Atmospheric Administration US Department of Commerce, National Weather Service. Climate Prediction Center. Climate & Weather Linkage. Available online: https://www.noaa.gov/ (accessed on 29 May 2025).
- NOAA. National Oceanic and Atmospheric Administration US Department of Commerce, National Weather Service. Climate Prediction Centre. Climate & Weather Linkage. Teleconnections. North Atlantic Oscillation (NAO). Available online: https://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/norm.nao.monthly.b5001.current.ascii.table (accessed on 30 April 2025).
- Hurrell, J.W. Decadal trends in the North Atlantic Oscillation: Regional temperatures and precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef]
- Osborn, T.J. A historical and climatological note on snowfalls associated with cold pools in southern Britain. Weather 2011, 66, 19–21. [Google Scholar] [CrossRef]
- Wang, L.; Ting, M. Stratosphere-troposphere coupling leading to extended seasonal predictability of Summer North Atlantic Oscillation and boreal climate. Geophys. Res. Lett. 2022, 49, e2021GL096362. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Ottersen, G.; Hurrell, J.W.; Mysterud, A.; Lima, M.; Chan, K.S.; Yoccoz, N.G.; Ådlandsvik, B. Studying climate effects on ecology through the use of climate indices: The North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc. R. Soc. B Biol. Sci. 2003, 270, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.; Hanna, E.; Baker, L.; Sun, Y.; Wei, H.L. North Atlantic atmospheric circulation indices: Links with summer and winter temperature and precipitation in north-west Europe, including persistence and variability. Int. J. Clim. 2024, 44, 902–922. [Google Scholar] [CrossRef]
- Climatic Research Unit, University of East Anglia. Climate Data. Available online: https://crudata.uea.ac.uk/cru/data/moi/ (accessed on 30 April 2025).
- Conte, M.; Giuffrida, A.; Tedesco, S. The Mediterranean Oscillation. In Impact on Precipitation and Hydrology in Italy Climate Water; Publications of the Academy of Finland: Helsinki, Finland, 1989. [Google Scholar]
- Palutikof, J.P.; Conte, M.; Casimiro Mendes, J.; Goodess, C.M.; Espirito Santo, F. Climate and climate change. In Mediterranean Desertification and Land Use; Brandt, C.J., Thornes, J.B., Eds.; John Wiley and Sons: London, UK, 1996. [Google Scholar]
- Palutikof, J.P. Analysis of Mediterranean climate data: Measured and modelled. In Mediterranean Climate: Variability and Trends; Bolle, H.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Criado-Aldeanueva, F.; Soto-Navarro, J. Climatic indices over the Mediterranean Sea: A review. Appl. Sci. 2020, 10, 5790. [Google Scholar] [CrossRef]
- Zittis, G.; Hadjinicolaou, P.; Klangidou, M.; Proestos, Y.; Lelieveld, J.A. Multi-model, multi-scenario, and multi-domain analysis of regional climate projections for the Mediterranean. Reg. Environ. Change 2019, 19, 2621–2635. [Google Scholar] [CrossRef]
- Royal Netherlands Meteorological Institute (KNMI) and World Meteorological Organization (WMO). Climate Explorer. Dipole Mode Index (DMI)/Indian Ocean Dipole (IOD) dataset. Available online: http://climexp.knmi.nl/getindices.cgi?WMO=UKMOData/hadisst1_dmi&STATION=DMI_HadISST1&TYPE=i&id=someone@somewhere (accessed on 30 April 2025).
- Marchant, R.; Mumbi, C.; Behera, S.; Yamagata, T. The Indian Ocean Dipole—The unsung driver of climatic variability in East Africa. Afr. J. Ecol. 2007, 45, 4–16. [Google Scholar] [CrossRef]
- Saji, N.H.; Vinayachandran, P.N. A dipole mode in the tropical Indian Ocean. Nature 1999, 401, 360–364. [Google Scholar] [CrossRef]
- Stige, L.C.; Stave, J.; Chan, K.S.; Ciannelli, L.; Pettorelli, N.; Glantz, M.; Herren, H.R.; Stenseth, N.C. The effect of climate variation on agro-pastoral production in Africa. Proc. Natl. Acad. Sci. USA 2006, 103, 3049–3053. [Google Scholar] [CrossRef]
- Hirons, L.; Turner, A. The impact of Indian Ocean mean-state biases in climate models on the representation of the East African short rains. J. Clim. 2018, 31, 6611–6631. [Google Scholar] [CrossRef]
- Ashok, K.; Guan, Z.; Yamagata, T. A look at the relationship between the ENSO and the Indian Ocean Dipole. J. Meteorol. Soc. Jpn. 2003, 81, 41–56. [Google Scholar] [CrossRef]
- Royal Netherlands Meteorological Institute (KNMI) and World Meteorological Organization (WMO). Climate Explorer. Southern Oscillation Index (SOI) Dataset. Available online: http://climexp.knmi.nl/getindices.cgi?WMO=CRUData/soi&STATION=SOI&TYPE=i&id=someone@somewhere (accessed on 30 April 2025).
- McPhaden, M.J.; Santoso, A.; Cai, W. (Eds.) El Niño Southern Oscillation in a Changing Climate; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 253. [Google Scholar]
- Benkenstein, A. Climate change adaptation readiness: Lessons from the 2015/16 El Niño for climate readiness in southern Africa. SAIIA Occas. Pap. 2017, 250, 1–18. [Google Scholar]
- Royal Netherlands Meteorological Institute (KNMI) and World Meteorological Organization (WMO). Climate Explorer. Temperature dataset. Available online: http://climexp.knmi.nl/select.cgi?era5_t2m_daily (accessed on 30 April 2025).
- Royal Netherlands Meteorological Institute (KNMI) and World Meteorological Organization (WMO). Climate Explorer. Precipitation dataset. Available online: http://climexp.knmi.nl/select.cgi?field=era5_prcp_daily (accessed on 30 April 2025).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carr, G.; Garc, J.R.; Gruber, B.; Lafourcade, B.; Leit, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Tobolka, M.; Dylewski, L.; Wozna, J.T.; Zolnierowicz, K.M. How weather conditions in non-breeding and breeding grounds affect the phenology and breeding abilities of White Storks. Sci. Total Environ. 2018, 636, 512–518. [Google Scholar] [CrossRef]
- Gordo, O.; Barriocanal, C.; Robson, D. Ecological impacts of the North Atlantic Oscillation (NAO) in Mediterranean ecosystems. In Hydrological, Socioeconomic and Ecological Impacts of the North Atlantic Oscillation in the Mediterranean Region; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2011; pp. 153–170. [Google Scholar]
- Finch, T.; Pearce-Higgins, J.W.; Leech, D.I.; Evans, K.L. Carry-over effects from passage regions are more important than breeding climate in determining the breeding phenology and performance of three avian migrants of conservation concern. Biodivers. Conserv. 2014, 23, 2427–2444. [Google Scholar] [CrossRef]
- Guichard, F.; Kergoat, L.; Léauthaud, C.; Barbier, J.; Mougin, E.; Diarra, B. Climate warming observed in the Sahel since 1950. In Rural Societies in the Face of Climatic and Environmental Changes in West Africa, IRD éd.; Sultan, B., Lalou, R., Sanni, M.A., Oumarou, A., Soumaré, M.A., Eds.; OpenEdition Books: Marseille, France, 2017; pp. 23–41. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gillies, R.R. Observed change in Sahel rainfall, circulations, African easterly waves, and Atlantic hurricanes since 1979. Int. J. Geophys. 2011, 259529. [Google Scholar] [CrossRef]
- Biasutti, M. Rainfall trends in the African Sahel: Characteristics, processes, and causes. Wiley Interdiscip. Rev. Clim. Change 2019, 10, e591. [Google Scholar] [CrossRef]
- Frost, J. Regression Analysis: An intuitive Guide for Using and Interpreting Linear Models; First Edition; Statistics By Jim Publishing: Glendale, CA, USA, 2019. [Google Scholar]
- Breiman, L.; Freedman, D. How many variables should be entered in a regression equation? J. Am. Statist. Assoc. 1983, 78, 131–136. [Google Scholar] [CrossRef]
- Hawkins, D.M. The problem of overfitting. J. Chem. Inf. Comp. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows, 22nd ed.; VSN International: Hemel Hempstead, UK, 2022. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Meth. Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Deng, Y.; Haest, B.; Belotti, M.C.; Zhao, W.; Perez, G.; Tielens, E.K.; Sheldon, D.R.; Maji, S.; Kelly, J.F.; Horton, K.G. Continental connections: Changing temperature, wind and precipitation advance the postbreeding roosting phenology of avian aerial insectivores. Glob. Ecol. Biogeogr. 2025, 34, e70052. [Google Scholar] [CrossRef]
- Allan, D.G.; Harrison, J.A.; Herremans, M.; Navarro, R.; Underhill, L.G. Southern African geography: Its relevance to birds. In The Atlas of Southern African Birds. Volume 1: Non-Passerines; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife South Africa: Johannesburg, South Africa, 1997; pp. lxv–ci. [Google Scholar]
- Lingbeek, B.J.; Higgins, C.L.; Muir, J.P.; Kattes, D.H.; Schwertner, T.W. Arthropod diversity and assemblage structure response to deforestation and desertification in the Sahel of western Senegal. Glob. Ecol. Conserv. 2017, 11, 165–176. [Google Scholar] [CrossRef]
- Thorup, K.; Tøttrup, A.P.; Willemoes, M.; Klaassen, R.H.; Strandberg, R.; Vega, M.L.; Rahbek, C. Resource tracking within and across continents in long-distance bird migrants. Sci. Adv. 2017, 3, e1601360. [Google Scholar] [CrossRef] [PubMed]
- Peach, W.; Baillie, S.; Underhill, L.G. Survival of British Sedge Warblers Acrocephalus schoenobaenus in relation to West African rainfall. Ibis 1991, 133, 300–305. [Google Scholar] [CrossRef]
- Studds, C.E.; Marra, P.P. Rainfall-induced changes in food availability modify the spring departure programme of a migratory bird. Proc. R. Soc. B Biol Sci. 2011, 278, 3437–3443. [Google Scholar] [CrossRef]
- Altwegg, R.; Broms, K.; Erni, B.; Barnard, P.; Midgley, G.F.; Underhill, L.G. Novel methods reveal shifts in migration phenology of Barn Swallows in South Africa. Proc. R. Soc. Lond. B 2012, 279, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Salewski, V.; Altwegg, R.; Erni, B.; Falk, K.H.; Bairlein, F.; Leisler, B. Moult of three Palaearctic migrants in their West African winter quarters. J. Ornithol. 2004, 145, 109–116. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Bernitz, Z.; Bernitz, H.; Burman, M.S.; Raijmakers, J.; Raijmakers, J.; Underhill, L.G.; Rostkowska, A.; Barshep, Y.; Soloviev, S.A.; et al. Contrasting strategies for wing-moult and pre-migratory fuelling in western and eastern populations of Common Whitethroat Sylvia communis. Ibis 2019, 161, 824–838. [Google Scholar] [CrossRef]
- Gessesse, A.A.; Melesse, A.M. Temporal relationships between time series CHIRPS-rainfall estimation and eMODIS-NDVI satellite images in Amhara Region, Ethiopia. In Extreme Hydrology and Climate Variability; Gessesse, A.A., Melesse, A.M., Abtew, W., Senay, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 81–92. [Google Scholar] [CrossRef]
- Wen, L.; Yang, X.; Saintilan, N. Local climate determines the NDVI-based primary productivity and flooding creates heterogeneity in semi-arid floodplain ecosystem. Ecol. Model. 2012, 242, 116–126. [Google Scholar] [CrossRef]
- Van den Brink, B.; Bijlsma, R.G.; Van der Have, T.M.; De Roder, F.E. European Swallows Hirundo rustica in Botswana; WIWO-report (no. 56); WIWO: Zeist, The Netherlands, 1997. [Google Scholar]
- Van den Brink, B.; Bijlsma, R.G.; van der Have, T.M. European Swallows Hirundo rustica in Botswana during three non-breeding seasons: The effects of rainfall on moult. Ostrich 2000, 71, 198–204. [Google Scholar] [CrossRef]
- Ambrosini, R.; Rubolini, D.; Trovo, P.; Liberini, G.; Bandini, M.; Romano, A.; Sicurella, B.; Scandolara, C.; Romano, M.; Saino, N. Maintenance of livestock farming may buffer population decline of the Barn Swallow Hirundo rustica. Bird Conserv. Int. 2012, 22, 411–428. [Google Scholar] [CrossRef]
- Cucco, M.; Malacarne, G. Reproduction of the pallid swift (Apus pallidus) in relation to weather and aerial insect abundance. Ital. J. Zool. 1996, 63, 247–253. [Google Scholar] [CrossRef]
- Turner, A.K. The Use of Time and Energy by Aerial-Feeding Birds. Ph.D. Thesis, University of Stirling, Stirling, UK, 1980. [Google Scholar]
- Turner, A.K. Optimal foraging by the swallow (Hirundo rustica, L.): Prey size selection. Anim. Behav. 1982, 30, 862–872. [Google Scholar] [CrossRef]
- Imlay, T.L.; Leonard, M.L. A review of the threats to adult survival for swallows (Family: Hirundinidae). Bird Study 2019, 66, 251–263. [Google Scholar] [CrossRef]
- Nicholson, S. Climate of the Sahel and West Africa. Oxford Research Encyclopedia of Climate Science 2018. Available online: https://oxfordre.com/climatescience/view/10.1093/acrefore/9780190228620.001.0001/acrefore-9780190228620-e-510 (accessed on 29 May 2025).
- Swapna, P.; Sandeep, N.; Alsumaina, K.N.; Krishnan, R.; Ajinkya, A.; Shamal, M.; Sreejith, O.P. Contrasting tropical precipitation and ecosystem response to Indian Ocean Dipole in a warming climate. Sci. Total Environ. 2025, 971, 179081. [Google Scholar] [CrossRef]
- Burman, M. Citizen Science Reveals Complex Changes in Barn Swallow Phenology in South Africa over Three Decades. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2016. [Google Scholar]
- Grüebler, M.U.; Korner-Nievergelt, F.; Von Hirschheydt, J. The reproductive benefits of livestock farming in barn swallows Hirundo rustica: Quality of nest site or foraging habitat? J. Appl. Ecol. 2010, 47, 1340–1347. [Google Scholar] [CrossRef]
- Orłowski, G.; Karg, J. Partitioning the effects of livestock farming on the diet of an aerial insectivorous passerine, the Barn Swallow Hirundo rustica. Bird Study 2012, 60, 111–123. [Google Scholar] [CrossRef]
- Spiller, K.J.; Dettmers, R. Evidence for multiple drivers of aerial insectivore declines in North America. Condor. Ornithol. Appl. 2019, 121, duz010. [Google Scholar] [CrossRef]
- McClenaghan, B.; Nol, E.; Kerr, K.C. DNA metabarcoding reveals the broad and flexible diet of a declining aerial insectivore. Auk 2019, 136, uky003. [Google Scholar] [CrossRef]
- Sillett, T.S.; Holmes, R.T.; Sherry, T.W. Impacts of a global climate cycle on population dynamics of a migratory songbird. Science 2000, 288, 2040–2042. [Google Scholar] [CrossRef]
- Stokke, B.G.; Møller, A.P.; Sæther, B.E.; Rheinwald, G.; Gutscher, H. Weather in the breeding area and during migration affects the demography of a small long-distance passerine migrant. Auk 2005, 122, 637647. [Google Scholar] [CrossRef]
- Nebel, S.; Mills, A.; McCracken, J.D.; Taylor, P.D. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv. Ecol. 2010, 5, 1. [Google Scholar] [CrossRef]
- Toronto Wildlife Centre. Heat Wave Causes Baby Barn Swallows to Jump from Nest. 2023. Available online: https://youtu.be/ISqm3L1idmA?si=YNZqXFZ21dm9S1ht (accessed on 26 July 2025).
- World Meteorological Organisation. El Niño is Forecast to Swing to La Niña Later This Year. 2024. Available online: https://wmo.int/news/media-centre/el-nino-forecast-swing-la-nina-later-year (accessed on 27 July 2025).
- Ambrosini, R.; Rubolini, D.; Møller, A.P.; Bani, L.; Clark, J.; Karcza, Z.; Vangeluve, D.; du Feu, C.; Saino, N. Climate change and the long-term northward shift in the African wintering range of the Barn Swallow Hirundo rustica. Clim. Res. 2011, 49, 131–141. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Underhill, L.G. Climate in Europe and Africa sequentially shapes the spring passage of long-distance migrants at the Baltic coast in Europe. Diversity 2025, 17, 528. [Google Scholar] [CrossRef]
- Hockey, P.A.R.; Underhill, L.G.; Neatherway, M.; Ryan, P.G. Atlas of the Birds of the Southwestern Cape; Cape Bird Club: Cape Town, South Africa, 1989. [Google Scholar]
- Daniel, K.; Underhill, L.G. Temporal dimensions of data quality in bird atlases: The case of the Second Southern African Bird Atlas Project. Citiz. Sci. Theory Pract. 2023, 8, 31. [Google Scholar] [CrossRef]
- Daniel, K.; Underhill, L.G.; van Rooyen, J. Bird atlas in action: Generating alerts for population trends using citizen science data in Hessequa, South Africa. Front. Bird Sci. 2024, 3, 1214800. [Google Scholar] [CrossRef]
- Brooks, M.; Ryan, P. Southern African Bird Atlas Project 2, Version 1.81, Occurrence Dataset; FitzPatrick Institute of African Ornithology: Cape Town, South Africa, 2025; Available online: https://www.gbif.org/ (accessed on 27 August 2025).
| Variable | Year | Mid Greater Gauteng | Mid Greater Cape Town | Mid Greater Durban | Anomaly Greater Gauteng | Anomaly Greater Cape Town |
|---|---|---|---|---|---|---|
| Mid Greater Gauteng | −0.822 *** | |||||
| Mid Greater Cape Town | −0.103 | 0.261 | ||||
| Mid Greater Durban | −0.823 *** | 0.677 ** | 0.082 | |||
| Anomaly Greater Gauteng | 0.038 | 0.096 | 0.498 * | −0.132 | ||
| Anomaly Greater Cape Town | −0.588 * | 0.451 | 0.161 | 0.568 * | 0.120 | |
| Anomaly Greater Durban | −0.457 | 0.232 | 0.268 | 0.602 * | 0.065 | 0.552 * |
| Model | % | AIC | Explanatory Variables |
|---|---|---|---|
| 1 | 0.7 | 92.51 | Year (nbm) |
| 2 | 19.0 | 89.24 | Precipitation Sahel West (May–Aug) (+) |
| 3 | 11.1 | 90.73 | Temperature Sahel East (Sep–Oct) (–) |
| 4 | 34.4 | 86.77 | Precipitation Sahal West (May–Aug) (+), Temperature Sahel East (Sep–Oct) (–) |
| 5 | 28.9 | 88.06 | Precipitation Sahel West (May–Aug) (+), Indian Ocean Dipole (Jun–Jul) (–) |
| 6 | 27.4 | 88.40 | Precipitation Sahel West (May–Aug) (+), Scandinavian Pattern (Sep–Oct) (+) |
| Model | % | AIC | Explanatory Variables |
|---|---|---|---|
| 1 | 0.7 | 92.51 | Year (nbm) |
| 2 | 19.0 | 89.24 | Precipitation Sahel West (May–Aug) (+) |
| 3 | 11.1 | 90.73 | Temperature Sahel East (Sep–Oct) (–) |
| 4 | 34.4 | 86.77 | Precipitation Sahal West (May–Aug) (+), Temperature Sahel East (Sep-Oct) (–) |
| 5 | 28.9 | 88.06 | Precipitation Sahel West (May–Aug) (+), Indian Ocean Dipole (Jun–Jul) (–) |
| 6 | 27.4 | 88.40 | Precipitation Sahel West (May–Aug) (+), Scandinavian Pattern (Sep–Oct) (+) |
| Model | % | AIC | Explanatory Variables |
|---|---|---|---|
| 1 | 21.9 | 95.30 | Year (–) |
| 2 | 56.8 | 86.71 | Scandinavian Pattern (May–Aug) (+), Precipitation Iberia-Apennines (Aug–Oct) (+) |
| 3 | 39.6 | 91.19 | Scandinavian (May–Aug) (+) |
| 4 | 11.6 | 97.28 | Temperature Iberian+Apennine Peninsulas (Aug–Oct) (+) |
| 5 | 52.7 | 88.17 | Scandinavian (May–Aug) (+), Temperature Sahel East (Sep–Oct) (+) |
| 6 | 49.6 | 89.17 | Scandinavian (May–Aug) (+), Mediterranean Oscillation Index (Aug–Oct) (–) |
| 7 | 50.1 | 89.02 | Precipitation Sahel East (Sep–Oct) (+), Indian Ocean Dipole (Jun–Jul) (–) |
| 8 | 55.6 | 87.17 | Precipitation Sahel East (Sep–Oct) (+), Mediterranean Oscillation Index (May–Aug) (+) |
| Model | % | AIC | Explanatory Variables |
|---|---|---|---|
| 1 | 65.3 | 29.53 | Year (–) |
| 2 | 70.9 | 27.54 | Year (–), Precipitation Sahel East (Sep–Oct) (+) |
| Model | % | AIC | Explanatory Variables |
|---|---|---|---|
| 1 | 0.2 | 49.09 | Year (nbm) |
| 2 | 26.6 | 43.88 | Southern Oscillation Index (Nov–Feb) (–) |
| 3 | 12.7 | 46.83 | North Atlantic Oscillation Index (May–Jul) (+) |
| 4 | 35.4 | 42.61 | Southern Oscillation Index (Nov–Feb) (–), North Atlantic Oscillation Index (May–Jul) (+) |
| Model | % | AIC | Explanatory Variables |
|---|---|---|---|
| 1 | 64.4 | 28.22 | Year (–) |
| 2 | 75.2 | 23.32 | Year (–), Mediterranean Oscillation Index (May-Jul) (+) |
| 3 | 74.0 | 24.12 | Year (–), Temperature Balkans (May–Aug) (+) |
| 4 | 63.0 | 29.72 | Mediterranean Osc, Index (May–Jul) (+), Scandinavian Pattern (May–Aug) (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Underhill, L.G.; Remisiewicz, M. Arrival and Peak Abundance of Barn Swallows Hirundo rustica in Three Regions of South Africa in Relation to Climate Indices, Deduced from Bird Atlas Data. Birds 2025, 6, 48. https://doi.org/10.3390/birds6030048
Underhill LG, Remisiewicz M. Arrival and Peak Abundance of Barn Swallows Hirundo rustica in Three Regions of South Africa in Relation to Climate Indices, Deduced from Bird Atlas Data. Birds. 2025; 6(3):48. https://doi.org/10.3390/birds6030048
Chicago/Turabian StyleUnderhill, Les G., and Magdalena Remisiewicz. 2025. "Arrival and Peak Abundance of Barn Swallows Hirundo rustica in Three Regions of South Africa in Relation to Climate Indices, Deduced from Bird Atlas Data" Birds 6, no. 3: 48. https://doi.org/10.3390/birds6030048
APA StyleUnderhill, L. G., & Remisiewicz, M. (2025). Arrival and Peak Abundance of Barn Swallows Hirundo rustica in Three Regions of South Africa in Relation to Climate Indices, Deduced from Bird Atlas Data. Birds, 6(3), 48. https://doi.org/10.3390/birds6030048





