The Structure and Spatial Distribution of the Raptor Community in the Urban Landscapes of Kyzylorda, Kazakhstan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
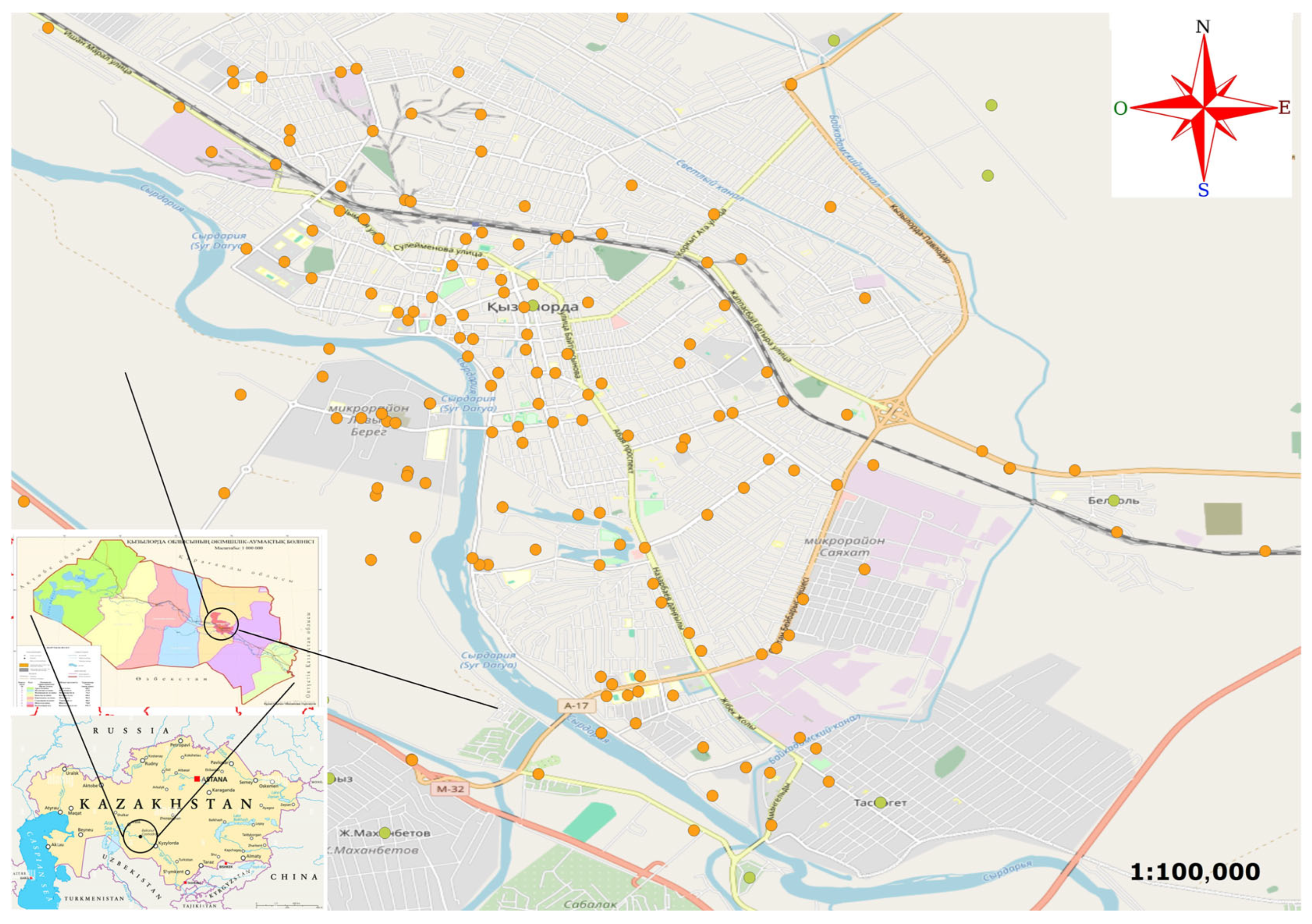
2.1. Study Area
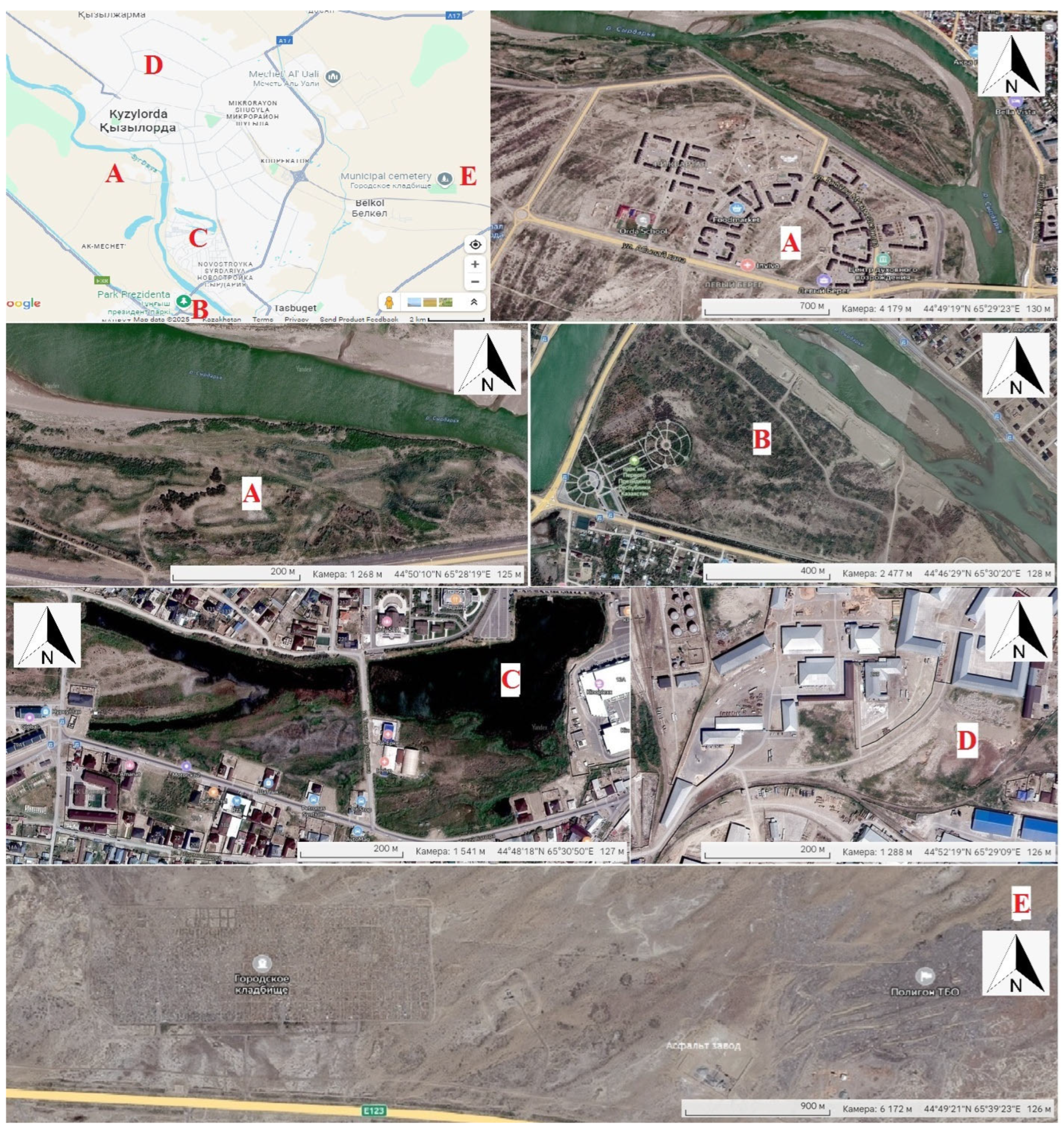
2.2. Delineating the Study Site
2.3. Raptor Surveys
2.4. Collection of Environmental Variables
2.5. Landscape Measurements
2.6. Statistical Methods
3. Results
3.1. Registration of Raptors
3.2. Recording Models
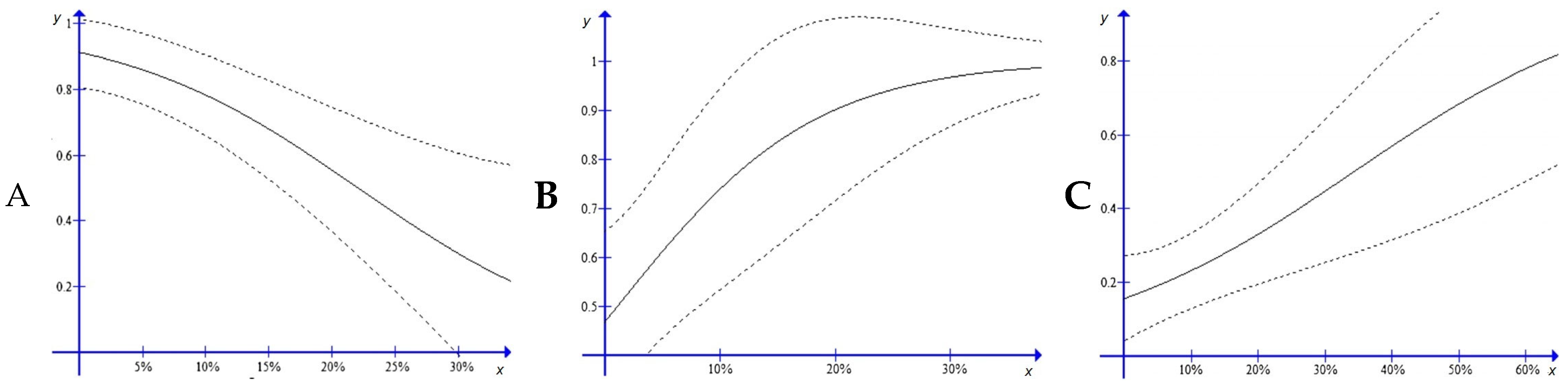
3.3. Landscape Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- Frantz, A.; Baneux, M.; Pichon, L.; Renier, S.; Vilanova, J. Flight initiation distance differs among eumelanin-based color morphs in feral pigeons. J. Zool. 2025, 325, 115–123. [Google Scholar] [CrossRef]
- Papouchis, C.M.; Singer, F.J.; Sloan, W.B. Responses of desert bighorn sheep to increased human recreation. J. Wildl. Manag. 2001, 65, 573–582. Available online: https://www.jstor.org/stable/3803110 (accessed on 1 March 2025). [CrossRef]
- Parris, K.M.; Schneider, A. Impacts of traffic noise and traffic volume on birds of roadside habitats. Ecol. Soc. 2009, 14, 29. Available online: https://www.ecologyandsociety.org/vol14/iss1/art29/ (accessed on 1 March 2025). [CrossRef]
- Rebolo-Ifrán, N.; Carrete, M.; Sanz-Aguilar, A.; Tella, J.L. Links between fear of humans, stress and survival support a non-random distribution of birds among urban and rural habitats. Sci. Rep. 2015, 5, 13723. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M.D.; Manley, P.N.; Holyoak, M. Distinguishing stressors acting on land bird communities in an urbanizing environment. Ecology 2008, 89, 2302–2314. [Google Scholar] [CrossRef]
- Smith, J.A.; McDaniels, M.E.; Peacor, S.D.; Bolas, E.C.; Cherry, M.J.; Dorn, N.J.; Feldman, O.K.; Kimbro, D.L.; Leonhardt, E.K.; Peckham, N.E.; et al. Population and community consequences of perceived risk from humans in wildlife. Ecol. Lett. 2024, 27, e14456. [Google Scholar] [CrossRef]
- Bildstein, K.L.; Therrien, J.F. Urban Raptors: A Lengthy History of Human-Raptor Cohabitation. In Urban Raptors; Boal, C.W., Dykstra, C.R., Eds.; Island Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Movalli, P.; Duke, G.; Helmick, K.; Katzner, T.; Krone, O.; Naidoo, V.; Pain, D.; Plaza, P.I.; Santangeli, A.; Taggart, M.; et al. Monitoring contaminants, emerging infectious diseases and environmental change with raptors, and links to human health. Bird. Study 2018, 65 (Suppl. S1), S96–S109. [Google Scholar] [CrossRef]
- Fischer, J.D.; Schneider, S.C.; Ahlers, A.A.; Miller, J.R. Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. BioScience 2012, 62, 809–818. [Google Scholar] [CrossRef]
- Newton, I. Population Ecology of Raptors; Academic Press: London, UK, 1998. [Google Scholar]
- Marzluff, J.M.; Ewing, K. Restoration of fragmented landscapes for the conservation of birds: A case study of urbanization and its effects on bird populations. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer: Boston, MA, USA, 2001; pp. 207–226. [Google Scholar]
- Evans, K.L.; Davidson, S.C. The role of urban areas in the ecology of birds of prey. Urban. Ecosyst. 2014, 17, 795–807. [Google Scholar]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef]
- Bell, C.P.; Baker, S.W.; Parkes, N.G.; Brooke, M.D.L.; Chamberlain, D.E. The role of the Eurasian sparrowhawk (Accipiter nisus) in the decline of the house sparrow (Passer domesticus) in Britain. Auk 2010, 127, 411–420. [Google Scholar] [CrossRef]
- McCabe, J.D.; Yin, H.; Cruz, J.; Radeloff, V.; Pidgeon, A.; Bonter, D.N.; Zuckerberg, B. Prey abundance and urbanization influence the establishment of avian predators in a metropolitan landscape. Proc. R. Soc. B 2018, 285, 20182120. [Google Scholar] [CrossRef] [PubMed]
- Solonen, T. Larger broods in the Northern Goshawk Accipiter gentilis near urban areas in southern Finland. Ornis Fenn. 2008, 85, 118–125. Available online: https://ornisfennica.journal.fi/article/view/133712 (accessed on 1 March 2025).
- Kettel, E.F.; Gentle, L.K.; Yarnell, R.W.; Quinn, J.L. The breeding performance of raptors in urban landscapes: A review and meta-analysis. J. Ornithol. 2018, 159, 1–18. [Google Scholar] [CrossRef]
- Rosenfield, R.N.; Bielefeldt, J.; Rosenfield, L.J.; Cava, J.A. Nesting density, nest area reoccupancy, and monitoring implications for Cooper’s Hawks in Wisconsin. J. Raptor Res. 1995, 29, 1. Available online: https://digitalcommons.usf.edu/jrr/vol29/iss1/1 (accessed on 1 March 2025).
- Charter, M.; Izhaki, I.; Bouskila, A.; Leshem, Y. Breeding success of the Eurasian Kestrel (Falco tinnunculus) nesting on buildings in Israel. J. Raptor Res. 2007, 41, 139–143. [Google Scholar] [CrossRef]
- Rutz, C. The establishment of an urban bird population. J. Anim. Ecol. 2008, 77, 1008–1019. [Google Scholar] [CrossRef]
- Sumasgutner, P.; Nemeth, E.; Tebb, G.; Krenn, H.W.; Gamauf, A. Hard times in the city—Attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Front. Zool. 2014, 11, 48. [Google Scholar] [CrossRef]
- Leveau, L.M.; Gorleri, F.C.; Roesler, I.; González-Táboas, F. What makes an urban raptor? Ibis 2022, 164, 1213–1226. [Google Scholar] [CrossRef]
- San Martín-Cruz, M.A.; Villegas-Patraca, R.; Martínez-Gómez, J.E.; Ruelas Inzunza, E. Raptors of a Neotropical city: Diversity and habitat relationships along an urbanization gradient. Urban. Ecosyst. 2024, 27, 927–940. [Google Scholar] [CrossRef]
- Jones, M.P.; Pierce, K.E., Jr.; Ward, D. Avian vision: A review of form and function with special consideration to raptors. J. Exot. Pet. Med. 2007, 16, 69–87. [Google Scholar] [CrossRef]
- Martin, G.R. Through birds’ eyes: Insights into avian sensory ecology. J. Ornithol. 2012, 153 (Suppl. 1), 23–48. [Google Scholar] [CrossRef]
- Martin, G.R. Avian vision. Curr. Biol. 2022, 32, R1079–R1085. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Major, J.C.; McKeown, C.; Knighton, R.W.; Puliafito, C.A.; Jiao, S. Retinal Structure of Raptor Revealed by Ultra-High Resolution Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5789–5795. [Google Scholar] [CrossRef]
- Tella, J.L.; Hiraldo, F.; Donázar-Sancho, J.A.; Negro, J.J. Costs and benefits of urban nesting in the Lesser Kestrel. In Raptors in Human Landscapes: Adaptations to Built and Cultivated Environments; Bird, D.M., Varland, D.E., Negro, J.J., Eds.; Academic Press: London, UK, 1996; pp. 53–60. [Google Scholar]
- Mazumdar, S.; Ghose, D.; Saha, G.K. Foraging strategies of Black Kites (Milvus migrans govinda) in urban garbage dumps. J. Ethol. 2016, 34, 243–247. [Google Scholar] [CrossRef]
- Krone, O.; Altenkamp, R.; Kenntner, N. Prevalence of Trichomonas gallinae in Northern Goshawks from the Berlin area of Northeastern Germany. J. Wildl. Dis. 2005, 41, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, A.; Russo, T.P.; Caputo, V.; Cozza, D.; Zapparoli, G.; Fioretti, A.; Pagnini, U. Occurrence of enteropathogenic bacteria in raptors in Italy. Lett. Appl. Microbiol. 2018, 66, 202–206. [Google Scholar] [CrossRef]
- Panter, C.T.; White, R.L.; Coutts, S.; Lindsell, J.A.; McDonald, R.A.; Bearhop, S. Causes, temporal trends, and the effects of urbanization on admissions of wild raptors to rehabilitation centers in England and Wales. Ecol. Evol. 2022, 12, e8856. [Google Scholar] [CrossRef]
- Sol, D.; Santos, D.M.; GarcÍa, J. Competition for food in urban pigeons: The cost of being juvenile. Condor 1998, 100, 298–304. [Google Scholar] [CrossRef]
- Padgett, D.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef]
- Bocelli, M.L.; Morelli, F.; Benedetti, Y.; Leveau, L. Estrategias de escape de aves en ambientes urbanos. El Hornero 2022, 37, 7. [Google Scholar] [CrossRef]
- Cade, T.J.; Martell, M.; Redig, P.; Septon, G.; Tordoff, H. Peregrine falcons in urban North America. In Raptors in Human Landscapes: Adaptations to Built and Cultivated Environments; Bird, D.M., Varland, D.E., Negro, J.J., Eds.; Academic Press: London, UK, 1996; pp. 3–13. [Google Scholar]
- Riegert, J.; Fainová, D.; Bystrická, D. Genetic variability, body characteristics and reproductive parameters of neighbouring rural and urban common kestrel (Falco tinnunculus) populations. Popul. Ecol. 2010, 52, 73–79. [Google Scholar] [CrossRef]
- Kolnegari, M.; Conway, G.J.; Basiri, A.A.; Panter, C.T.; Hazrati, M.; Rafiee, M.S.; Ferrer, M.; Dwyer, J.F. Electrical components involved in avian-caused outages in Iran. Bird. Conserv. Int. 2020, 31, 364–378. [Google Scholar] [CrossRef]
- Batyrova, K.I.; Valieva, G.M. To the issue of involuntary confinement of raptor in the conditions of Almaty zoo. Nauka I Mir. 2016, 1, 111–112. [Google Scholar]
- Pfander, P.V. Semi-species and unrecognized, hidden hybrids (on the example of raptor). Raptors Their Conserv. 2011, 23, 74–105. [Google Scholar]
- Berezovikov, N.N. Winter encounter of the Black-eared Kite Milvus migrans lineatus in the city of Almaty. Russ. J. Ornithol. 2014, 23, 3981. [Google Scholar]
- Berezovikov, N.N. On nesting of Kestrel Falco tinnunculus in dacha houses of Ust-Kamenogorsk. Russ. J. Ornithol. 2018, 27, 3394–3396. [Google Scholar]
- Tarasovskaya, N.E. Grey Crow and Common Kestrel Nesting in Magpie Nests on the South-Eastern Steppe Outskirts of Pavlodar. 2022. Available online: https://repo.kspi.kz/bitstream/handle/123456789/5963/konf-14-04-22_150-156.pdf?sequence=1&isAllowed=y (accessed on 1 March 2025).
- McDermid, S.S.; Winter, J. Anthropogenic forcings on the climate of the Aral Sea: A regional modeling perspective. Anthropocene 2017, 20, 48–60. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Li, Z.; Fang, G.; Wang, F.; Liu, H. The impact of climate change and human activities on the Aral Sea Basin over the past 50 years. Atmos. Res. 2020, 245, 105125. [Google Scholar] [CrossRef]
- Khaibullina, Z.; Amantaikyzy, A.; Ariphanova, D.; Temirbayeva, R.; Mitusov, A.; Zhurumbetova, Z. Socio-economic and public health impacts of climate change and water availability in Aral District, Kyzylorda Region, Kazakhstan. Asian J. Water Res. 2022, 8, 177–204. [Google Scholar] [CrossRef]
- Kassymova, S.; Yegemberdiyeva, S.; Mustafayev, K. Environmental and socio-economic aspects of sustainable development in Kyzylorda Region. Econ. Ser. Bull. L.N. Gumilyov ENU 2023, 23, 9–26. [Google Scholar] [CrossRef]
- Sihanova, N.S.; Sultankulov, B.; Toleubayev, S.; Zhumadilov, Z.; Sultangazina, M.; Kassenova, N.; Kassenov, M.; Zhakupov, T.; Zhumabayev, M.; Zhanabergenov, K.; et al. New data on avifauna of the city of Kyzylorda (Kazakhstan). Mod. Probl. Ornithol. Sib. Cent. Asia 2022, 208, 1–15. [Google Scholar]
- Kasimgaliev, S.; Kelinbaeva, R.; Sultanov, M.; Zhunisbekova, N. Geoinformation support, analysis, evaluation and forecasting of the use of land resources of Kyzylorda region. Bull. L.N. Gumilyov Eurasian Natl. Univ. Chem. Geogr. Ecol. Ser. 2023, 144, 105–118. [Google Scholar] [CrossRef]
- Mukhamedzhanov, M.; Arystanbaev, Y.; Iskakov, N.; Kazanbaeva, L.; Bekzhigitova, D. Prospects of use of underground waters torangesicle field of water supply for the growing needs of Kyzylorda. Int. Multidiscip. Sci. GeoConf. SGEM 2017, 17, 659–667. [Google Scholar] [CrossRef]
- Kassymgaliyev, S.; Turganaliyev, S.; Kaliyeva, M.; Dabylova, B.; Kozhakhmetov, B.; Khamit, N.; Zhumakan, A. Information support for the use of land resources in the Kyzylorda region, Kazakhstan: Analysis, assessment and forecasts. Casp. J. Environ. Sci. 2024, 22, 987–992. [Google Scholar] [CrossRef]
- Dukenov, Z.; Rakhimzhanov, A.; Akhmetov, R.; Dosmanbetov, D.; Abayeva, K.; Borissova, Y.; Trushin, M. Reforestation potential of tugai forests in the floodplains of Syr Darya and Ili Rivers in the territory of Kazakhstan. SABRAO J. Breed. Genet. 2023, 55, 1768–1777. [Google Scholar] [CrossRef]
- Schulz, C.; Kleinschmit, B. Monitoring the Condition of Wetlands in the Syr Darya Floodplain—How Healthy Are the Tugai Forests in Kazakhstan? Forests 2023, 14, 2305. [Google Scholar] [CrossRef]
- Bureau of National Statistics of the Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Population Size and Migration. 2025. Available online: https://stat.gov.kz/ru/region/kyzylorda/ (accessed on 1 March 2025).
- Nyussupova, G.N.; Isolde, B.; Kairova, S.G.; Kenespayeva, L.B. Social indicators of the quality of life of the population of the Republic of Kazakhstan: Analysis and evaluation. J. Geogr. Environ. Manag. 2019, 52, 48–56. [Google Scholar] [CrossRef]
- Salnikov, V.; Talanov, Y.; Polyakova, S.; Assylbekova, A.; Kauazov, A.; Bultekov, N.; Musralinova, G.; Kissebayev, D.; Beldeubayev, Y. An Assessment of the Present Trends in Temperature and Precipitation Extremes in Kazakhstan. Climate 2023, 11, 33. [Google Scholar] [CrossRef]
- Moyroud, N.; Portet, F. Introduction to QGIS. In QGIS and Generic Tools; Baghdadi, N., Mallet, C., Zribi, M., Eds.; Wiley-ISTE: London, UK; Hoboken, NJ, USA, 2018; pp. 1–17. [Google Scholar] [CrossRef]
- MAPS.ME. Offline Maps GPS Nav. 2025. Available online: https://play.google.com/store/apps/details?id=com.mapswithme.maps.pro (accessed on 1 March 2025).
- Conway, C.J.; Garcia, V.; Smith, M.D.; Hughes, K. Factors affecting detection of burrowing owl nests during standardized surveys. J. Wildl. Man. 2008, 72, 688–696. [Google Scholar] [CrossRef]
- Berthiaume, E.; Bélisle, M.; Savard, J. Incorporating detectability into analyses of population trends based on hawk counts, a double-observer approach. Condor 2009, 111, 43–58. [Google Scholar] [CrossRef]
- Dowling, J.L.; Luther, D.A.; Mara, P.P. Comparative effects of urban development and anthropogenic noise on bird songs. Behav. Ecol. 2011, 23, 201–209. [Google Scholar] [CrossRef]
- Hogg, J.R. Habitat Associations of Raptors in Urban Business Parks. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2013. [Google Scholar]
- Google Earth. 2025. Available online: https://earth.google.com/web/ (accessed on 1 March 2025).
- Klausnitzer, B. Ecology of Urban Fauna; Springer: München, Germany, 1990; p. 246. [Google Scholar] [CrossRef][Green Version]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Academic Press: San Diego, CA, USA, 2006; p. 648. [Google Scholar][Green Version]
- Hansen, C.P.; Millspaugh, J.L.; Rumble, M.A. Occupancy modeling of ruffed grouse in the Black Hills National Forest. J. Wildl. Man. 2011, 75, 71–77. [Google Scholar] [CrossRef]
- U.S. Department of the Interior. United States Geological Survey Patuxent Wildlife Research Center PRESENCE53. 2012. Available online: http://www.mbr-pwrc.usgs.gov/software/presence.html (accessed on 1 March 2025).[Green Version]
- Duren, K.R.; Buler, J.J.; Jones, W.; Williams, C.K. An improved multi-scale approach to modeling habitat occupancy of northern bobwhite. J. Wildl. Man. 2011, 75, 1700–1709. [Google Scholar] [CrossRef]
- Kroll, A.J.; Duke, S.D.; Runde, D.E.; Arnett, E.B.; Austin, K.A. Modeling habitat occupancy of orange-crowned warblers in managed forests of Oregon and Washington, USA. J. Wildl. Man. 2007, 71, 1089–1097. [Google Scholar] [CrossRef]
- Henneman, C.; Andersen, D.E. Occupancy patterns of nesting-season habitat associations of red-shouldered hawks in central Minnesota. J. Wildl. Man. 2009, 73, 1316–1324. [Google Scholar] [CrossRef]
- Bosakowski, T.; Smith, D.G. Distribution and species richness of a forest raptor community in relation to urbanization. J. Raptor Res. 1997, 31, 26–33. [Google Scholar]
- Miller, J.R.; Hobbs, R.J. Conservation where people live and work. Conserv. Biol. 2002, 16, 330–337. [Google Scholar] [CrossRef]
- Smallwood, J.A.; Wargo, P.J. Nest site habitat structure of American kestrels in northwestern New Jersey. Bull. NJ Acad. Sci. 1997, 42, 7–10. [Google Scholar]
- Snep, R.P.H. Biodiversity Conservation at Business Sites—Options and Opportunities. Ph.D. Thesis, Alterra, Wageningen University & Research, Wageningen UR, Wageningen, The Netherlands, 2009. [Google Scholar][Green Version]
- MacKenzie, D.I.; Bailey, L.L. Assessing the fit of site occupancy models. J. Agric. Biol. Environ. Stat. 2004, 9, 300–318. [Google Scholar] [CrossRef]
- Bennett, C. Evaluating the Influence of Habitat on Nest Distribution and Breeding Performance of the Marsh Harrier, Circus aeruginosus, in the UK. Ph.D. Thesis, Department of Life Sciences, Imperial College London, Silwood Park, UK, 2014. [Google Scholar][Green Version]
- Palomino, D.; Carrascal, L.M. Habitat associations of a raptor community in a mosaic landscape of Central Spain under urban development. Landsc. Urban. Plan. 2007, 83, 268–274. [Google Scholar] [CrossRef]
- Bibby, K.; Jones, M.; Marsden, S. Field Expeditionary Survey Methods: Bird Surveys and Records; Translation from English; Russian Bird Conservation Union: Moscow, Russia, 2000; p. 186. [Google Scholar][Green Version]
- Dickinson, E.C.; Remsen, J.V., Jr. The Howard Moore Complete Checklist of the Birds of the World, 4th ed.; JSTOR: Ann Arbor, MI, USA, 2013; Volume 1. [Google Scholar][Green Version]
- Del Hoyo, J.; Collar, N.J. HBW and BIRDLIFE International Illustrated Checklist of the Birds of the World; Aves Press: London, UK, 2003; Volume 1. [Google Scholar][Green Version]
- Village, K. Breeding performance of kestrels at Eskdalemuir, South Scotland. J. Zool. 1986, 208, 367–378. [Google Scholar] [CrossRef]
- Kostrzewa, A.A.; Kostrzewa, A.R. Der Bruterfolg des Turmfalken Falco tinnunculus in Deutschland: Ergebnisse 1985–1994 (Breeding success of the kestrel Falco tinnunculus in Germany: Results 1985–1994). J. Ornithol. 1997, 138, 73–82. [Google Scholar] [CrossRef]
- Sumasgutner, P.; Schulze, C.H.; Krenn, H.W.; Gamauf, A. Conservation related conflicts in nest-site selection of the Eurasian Kestrel (Falco tinnunculus) and the distribution of its avian prey. Landsc. Urban. Plan. 2014, 127, 94–103. [Google Scholar] [CrossRef]
- Rejt, Ł. Peregrine Falcon and Kestrel in urban environment—The case of Warsaw. In Naturschutz und Verhalten; Gottschalk, E., Barkow, A., Muehlenberg, M., Settele, J., Eds.; UFZ-Bericht: Leipzig, Germany, 2001; pp. 81–85. [Google Scholar][Green Version]
- Salvati, L. Spring weather and breeding success of the Eurasian Kestrel (Falco tinnunculus) in urban Rome, Italy. J. Raptor Res. 2002, 36, 15. Available online: https://digitalcommons.usf.edu/jrr/vol36/iss1/15 (accessed on 1 March 2025).
- Kübler, S.; Kupko, S.; Zeller, U. The kestrel (Falco tinnunculus L.) in Berlin: Investigation of breeding biology and feeding ecology. J. Ornithol. 2005, 146, 271–278. [Google Scholar] [CrossRef]
- Sánchez-Zapata, J.A.; Carrete, M.; Gravilov, A.; Sklyarenko, S.; Donázar, J.A. Land use changes and raptor conservation in steppe habitats of Eastern Kazakhstan. Biol. Conserv. 2003, 111, 71–77. [Google Scholar] [CrossRef]
- Ilyukh, M.P. Common Kestrel in the Predcaucasus. Cauc. Ornithol. Bull. 2009, 21, 64–134. [Google Scholar]
- Shcherbakov, B.V.; Berezovikov, N.N. To the ecology of the Common Kestrel Falco tinnunculus in the Western Altai. Russ. J. Ornithol. 2011, 20, 895–902. [Google Scholar]
- Lykov, E.L. Nesting of the Common Kestrel Falco tinnunculus in Palaearctic cities, a brief review. Russ. J. Ornithol. 2017, 26, 149–153. [Google Scholar]
- Panchenko, S.G. New data on avifauna of Semipalatinsk vicinities. Russ. J. Ornithol. 2011, 20, 2545–2549. [Google Scholar]
- Khromov, V.A.; Shupova, T.V. Biodiversity of avifauna of the right bank part of Semey city (Semipalatinsk). Ecol. Monit. Biodivers. 2018, 1, 148–152. [Google Scholar]
- Kuryashkin, A.N. Birds of the city of Kurchatov and its environs. Russ. J. Ornithol. 2021, 30, 3919–3940. [Google Scholar]
- Barashkova, A.; Smelansky, I.; Tomilenko, A.; Akentiev, A. Raptors of the Kazakh Upland—Indicators of steppe well-being. Ibis 2013, 155, 426–427. [Google Scholar] [CrossRef]
- Kolbintsev, V.G. Winter sightings of the Steppe Eagle Aquila nipalensis in Southern Kazakhstan. Russ. J. Ornithol. 2015, 24, 3923. [Google Scholar]
- Kovshar, V.A.; Karpov, F.F. On wintering of some birds from the Red Book of Kazakhstan on the eastern coast of the Caspian Sea in 2008–2019. Russ. J. Ornithol. 2020, 29, 2343–2349. [Google Scholar]
- Karyakin, I.V. What is happening to the steppe eagle. Steppe Bull. 2011, 33, 30–34. [Google Scholar]
- Khokhryakov, D.D. The reasons for the disappearance of raptor in Kazakhstan. Student science-a look into the future. 2023, 26, 445–447. [Google Scholar]
- Almasieh, K.; Cheraghi, M.; Khani, A.; Shahi, T. Evaluation of habitat suitability and migratory paths of an endangered raptor, Steppe Eagle (Aquila nipalensis) in Iran. Glob. Ecol. Conserv. 2024, 55, e03236. [Google Scholar] [CrossRef]
- Ram, M.; Gadhavi, D.; Sahu, A.; Srivastava, N.; Rather, T.A.; Modi, V.; Jhala, D. Aspects of Movement Ecology and Habitat Use of Migratory Raptors Using Satellite Telemetry from India to Central Asia. Birds 2024, 5, 487–508. [Google Scholar] [CrossRef]
- Zuban, I.A.; Sokolov, A.A.; Kuznetsova, N.P.; Petrova, M.V. Avifaunistic observations and findings in the North Kazakhstan region. Fauna Ural. Sib. 2010, 15, 43–74. [Google Scholar]
- Karpov, F.F.; Kovshar, V.A. Observations of wintering birds on the eastern coast of the Kazakhstan part of the Caspian Sea. Russ. J. Ornithol. 2017, 26, 3699–3704. [Google Scholar]
- Gubin, B.M. Wintering bird surveys in the South Kazakhstan region. Russ. J. Ornithol. 2018, 27, 847–868. [Google Scholar]
- Chalikova, E.S. Results of 20-year winter bird surveys at the Shardara reservoir (Kazakhstan). Russ. J. Ornithol. 2024, 33, 1916–1934. [Google Scholar]
- Ydenberg, R.C.; Butler, R.W.; Lank, D.B. Effects of predator landscapes on the evolutionary ecology of routing, timing and molt by long-distance migrants. J. Avian Biol. 2007, 38, 523–529. [Google Scholar] [CrossRef]
- Møller, A.P. Urban areas as refuges from predators and flight distance of prey. Behav. Ecol. 2012, 23, 1030–1035. [Google Scholar] [CrossRef]
- Martin, T.E.; Zhumabayev, R.; Bekmansurov, R.; Bekmansurov, T.; Zhumabayev, M.; Chudinov, A. Bird records from the arid and semi-arid areas in southern Kazakhstan, 2009–2017. Sandgrouse 2018, 40, 53–74. [Google Scholar]
- Kolarić, V. Habitat preferences of Common Kestrel Falco tinnunculus in Zagreb. Larus-Godišnjak Zavoda za ornitologiju. Hrvat. Akad. Znan. Umjet. 2018, 53, 7–18. [Google Scholar] [CrossRef]
- Kaf, A.; Saheb, M.; Bensaci, E. Preliminary data on breeding, habitat use and diet of Common Kestrel, Falco tinnunculus, in urban area in Algeria. Zool. Ecol. 2015, 25, 203–210. [Google Scholar] [CrossRef]
- Casagrande, S.; Nieder, L.; Di Minin, E.; La Fata, I.; Csermely, D. Habitat utilization and prey selection of the kestrel Falco tinnunculus in relation to small mammal abundance. Ital. J. Zool. 2008, 75, 401–409. [Google Scholar] [CrossRef]
- Sheridan, K.; Monaghan, J.; Tierney, T.D.; Doyle, S.; Tweney, C.; Redpath, S.M.; McMahon, B.J. The influence of habitat edge on a ground nesting bird species: Hen harrier Circus cyaneus. Wildl. Biol. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Arroyo, B.; Amar, A.; Leckie, F.; Buchanan, G.M.; Wilson, J.D.; Redpath, S. Hunting habitat selection by hen harriers on moorland: Implications for conservation management. Biol. Conserv. 2009, 142, 586–596. [Google Scholar] [CrossRef]
| Parameter | Variable | Mean | Mean Square Deviation |
|---|---|---|---|
| Proportion of the study area | Dense urban development with little or no landscaping | 0.109 | 0.006 |
| Commercial and industrial buildings | 0.018 | 0.034 | |
| Open green spaces | 0.079 | 0.004 | |
| Paved and concreted areas | 0.225 | 0.009 | |
| Vacant built-up areas | 0.223 | 0.017 | |
| Forested green spaces | 0.040 | 0.002 | |
| Dense urban development with limited green space | 0.195 | 0.014 | |
| Mean plot area (ha) | Open green spaces | 1.399 | 0.096 |
| Forested green spaces | 0.500 | 0.026 | |
| Edge ratio (perimeter/area) | Open green spaces | 0.063 | 0.003 |
| Forested green spaces | 0.055 | 0.005 | |
| Visibility | 1.358 | 0.083 |
| Model | Description |
|---|---|
| Ψ (dud) | Coverage of dense urban development with little or no landscaping |
| Ψ (comm) | Coverage of commercial premises |
| Ψ (vl) | Coverage of vacant land |
| Ψ (OS) | Open space model (% of area covered by wasteland and floodplain) |
| Ψ (NS) | Natural space model (% of area covered by grass and wasteland) |
| Ψ (CS) | Cleared space model (% of area covered by paved and concreted areas) |
| Ψ (TM) | Tree model (% of area covered by open green space and afforested green space) |
| Ψ (NP) | Mean area of natural plots (grass and wasteland) |
| Ψ (PT) | Mean area of plots with trees (open green space and afforested green space) |
| Ψ (AV) | All variables of mean area (open green space and afforested green space) |
| Ψ (NPRE) | Ratio of edges of natural plots (perimeter/area) |
| Ψ (.) | Null model |
| Ψ (GL.) | All variables |
| Species [80,81] | Scientific Name [80,81] | Total Number of Observations | Number of Observation Points (Where Species Is Detected) | Number of Points Detected Divided by Total Number of Survey Points |
|---|---|---|---|---|
| All target species | 210 | 93 | 0.6 | |
| Common Kestrel | Falco tinnunculus | 105 | 61 | 0.39 |
| Steppe Eagle | Aqulia nipalensis | 75 | 37 | 0.24 |
| Hen Harrier | Circus cyaneus | 25 | 20 | 0.13 |
| Eurasian Sparrowhawk | Accipiter nisus | 4 | 3 | 0.013 |
| Long-Legged Buzzard | Buteo rufinus | 3 | 2 | 0.013 |
| Other types | ||||
| Marsh Harrier | Circus aeroginosus | 2 | 2 | 0.013 |
| Eurasian Buzzard | Buteo buteo | 2 | 2 | 0.013 |
| Eurasian Hobby | Falco subbuteo | 1 | 1 | 0.007 |
| Unidentified | 7 | 7 | null | |
| Species | Model | QAIC | ΔAIC | AIC wt. | No. Par. | −2 × Log-Like |
|---|---|---|---|---|---|---|
| All five target species | Ψ (.), p (d + t + r) | 570.01 | 0 | 0.241 | 5 | 670.84 |
| Ψ (.), p (d + t) | 571.04 | 1.03 | 0.144 | 4 | 674.46 | |
| Ψ (.), p (t + r) | 571.14 | 1.13 | 0.137 | 4 | 674.59 | |
| Ψ (.), p (t) | 571.69 | 1.68 | 0.104 | 3 | 677.64 | |
| Ψ (.), p (ti) | 572.43 | 2.42 | 0.072 | 3 | 678.53 | |
| Ψ (.), p (ti + r) | 572.5 | 2.49 | 0.070 | 4 | 676.21 | |
| Ψ (.), p (.) | 572.7 | 2.69 | 0.063 | 2 | 681.24 | |
| Ψ (.), p (r) | 572.8 | 2.79 | 0.060 | 3 | 678.97 | |
| Ψ (.), p (c) | 574.29 | 4.28 | 0.028 | 3 | 680.76 | |
| Common Kestrel | Ψ (.), p (d + t + dt + r) | 479.45 | 0 | 0.431 | 6 | 467.45 |
| Ψ (.), p (d + t + dt + r + ti) | 479.69 | 0.24 | 0.382 | 7 | 465.69 | |
| Ψ (.), p (d + t + dt + ti) | 484.14 | 4.69 | 0.041 | 6 | 472.14 | |
| Ψ (.), p (d + t + dt) | 484.65 | 5.2 | 0.032 | 5 | 474.65 | |
| Ψ (.), p (ti + r) | 485.09 | 5.64 | 0.026 | 4 | 477.09 | |
| Steppe Eagle | Ψ (.), p (t) | 346.98 | 0 | 0.427 | 3 | 340.98 |
| Ψ (.), p (c + ti) | 347.18 | 0.2 | 0.386 | 4 | 339.18 | |
| Ψ (.), p (c) | 350.37 | 3.39 | 0.078 | 3 | 344.37 | |
| Ψ (.), p (d) | 351.6 | 4.62 | 0.042 | 3 | 345.6 | |
| Hen Harrier | Ψ (.), p (n + r) | 182.26 | 0 | 0.421 | 4 | 174.26 |
| Ψ (.), p (n) | 183.59 | 1.33 | 0.216 | 3 | 177.59 | |
| Ψ (.), p (d + r) | 185.31 | 3.05 | 0.092 | 4 | 177.31 | |
| Ψ (.), p (r) | 185.52 | 3.26 | 0.082 | 3 | 179.52 | |
| Ψ (.), p (d) | 186.67 | 4.41 | 0.046 | 3 | 180.67 | |
| Ψ (.), p (.) | 186.9 | 4.64 | 0.041 | 2 | 182.9 |
| Model | QAIC | ΔAIC | AIC wt. | No. Par. | −2 × Log-Like | |
|---|---|---|---|---|---|---|
| All five target species | Ψ (OS), p (d + t + r) | 570.64 | 0 | 0.239 | 7 | 660.34 |
| Ψ (FD), p (d + t + r) | 572.23 | 2.65 | 0.108 | 6 | 664.4 | |
| Ψ (CS), p (d + t + r) | 572.28 | 2.7 | 0.105 | 4 | 669.4 | |
| Ψ (CS), p (.) | 572.28 | 2.7 | 0.105 | 4 | 669.4 | |
| Ψ (Gl.), p (d + t + r) | 573.5 | 3.92 | 0.057 | 19 | 635.26 | |
| Common Kestrel | Ψ (TM), p (d + t + dt + r) | 380.02 | 0 | 0.228 | 8 | 459.03 |
| Ψ (NS), p (d + t + dt + r) | 381.36 | 1.34 | 0.117 | 8 | 460.72 | |
| Ψ (NP), p (d + t + dt + r) | 381.59 | 1.57 | 0.104 | 8 | 461.01 | |
| Ψ (PT), p (d + t + dt + r) | 381.59 | 1.57 | 0.104 | 8 | 431.01 | |
| Ψ (OS), p (d + t + dt + r) | 381.77 | 1.75 | 0.095 | 8 | 461.23 | |
| Ψ (CS), p (d + t + dt + r) | 382.1 | 2.08 | 0.081 | 8 | 461.65 | |
| Ψ (CIC), p (d + t + dt + r) | 382.52 | 2.5 | 0.065 | 7 | 464.71 | |
| Ψ (NM), p (d + t + dt + r) | 382.69 | 2.67 | 0.060 | 6 | 467.44 | |
| Ψ (FD), p (d + t + dt + r) | 382.23 | 3.21 | 0.046 | 7 | 465.6 | |
| Steppe Eagle | Ψ (NS), p (t) | 200.98 | 0 | 0.412 | 5 | 325.47 |
| Ψ (OS), p (t) | 202.62 | 1.64 | 0.181 | 5 | 328.26 | |
| Ψ (NP), p (t) | 203.41 | 2.43 | 0.122 | 5 | 329.61 | |
| Ψ (NS), p (.) | 204.48 | 3.5 | 0.072 | 4 | 334.84 | |
| Ψ (NS), p (t) | 204.78 | 3.8 | 0.062 | 6 | 328.53 | |
| Ψ (TM), p (t) | 205.2 | 4.22 | 0.050 | 5 | 332.66 | |
| Hen Harrier | Ψ (FD), p (n + r) | 179.98 | 0 | 0.436 | 5 | 169.98 |
| Ψ (FD), p (.) | 181.68 | 1.7 | 0.186 | 3 | 175.68 | |
| Ψ (CIC), p (n + r) | 182.46 | 2.48 | 0.126 | 5 | 172.46 | |
| Ψ (Gl.), p (n + r) | 183.11 | 3.13 | 0.091 | 18 | 147.11 | |
| Ψ (OS), p (n + r) | 183.14 | 3.16 | 0.090 | 6 | 171.14 |
| Variable | Coefficient | SE | Odds Ratio | 90% CI | |
|---|---|---|---|---|---|
| All five target species | Intercept | 1.372 | 0.644 | ||
| Floodplain * | −0.634 | 0.374 | 0.531 | 0.287–0.981 | |
| Pavement | –0.028 | 0.700 | 0.972 | 0.308–3.072 | |
| Grass | 1.104 | 1.809 | 3.015 | 0.154–59.15 | |
| Free Development | 2.712 | 2.786 | 15.06 | 0.154–1472 | |
| Common Kestrel | Intercept | 0.950 | 0.721 | ||
| Tree * | 1.231 | 0.704 | 3.425 | 1.075–10.91 | |
| Tree | 0.374 | 0.340 | 1.453 | 0.831–2.544 | |
| Grass_mean | 0.022 | 0.373 | 1.022 | 0.553–1.188 | |
| Wood_mean | 2.468 | 1.567 | 11.80 | 0.899–155.2 | |
| Tree_mean | 0.002 | 0.298 | 1.002 | 0.614–1.636 | |
| Grass | –0.047 | 0.313 | 0.954 | 0.571–1.596 | |
| Floodplain * | –0.675 | 0.305 | 0.509 | 0.308–0.842 | |
| Pavement | –0.002 | 0.282 | 0.998 | 0.628–1.587 | |
| Residential | 0.465 | 0.407 | 1.592 | 0.815–3.110 | |
| Commercial * | –0.434 | 0.263 | 0.648 | 0.420–0.999 | |
| Steppe Eagle | Intercept | –0.838 | 0.357 | ||
| Grass * | 0.772 | 0.351 | 2.164 | 1.215–3.853 | |
| Floodplain | –0.087 | 0.337 | 0.917 | 0.527–1.595 | |
| Tree | –0.434 | 0.348 | 0.648 | 0.366–1.148 | |
| Grass_mean | 0.841 | 0.413 | 2.319 | 1.176–4.573 | |
| Shrub_mean | –0.194 | 0.336 | 0.824 | 0.474–1.433 | |
| Tree_mean | –0.238 | 0.306 | 0.788 | 0.477–1.303 | |
| Tree | –0.501 | 0.406 | 0.606 | 0.311–1.182 | |
| Intercept | 1.263 | 3.986 | |||
| Hen Harrier | Residential * | 1.095 | 0.658 | 2.988 | 1.013–8.816 |
| Commercial | 15.01 | 39.39 | 3.6 × 10 6 | 2.6 × 10−22–5.0 × 1034 | |
| Grass * | –1.211 | 0.750 | 0.290 | 0.088–0.955 | |
| Floodplain | –0.498 | 0.478 | 0.608 | 0.277–1.335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sihanova, N.S.; Shynbergenov, Y.A.; Karabalayeva, A.B.; Togyzbayeva, N.A.; Abilova, S.B. The Structure and Spatial Distribution of the Raptor Community in the Urban Landscapes of Kyzylorda, Kazakhstan. Birds 2025, 6, 44. https://doi.org/10.3390/birds6030044
Sihanova NS, Shynbergenov YA, Karabalayeva AB, Togyzbayeva NA, Abilova SB. The Structure and Spatial Distribution of the Raptor Community in the Urban Landscapes of Kyzylorda, Kazakhstan. Birds. 2025; 6(3):44. https://doi.org/10.3390/birds6030044
Chicago/Turabian StyleSihanova, Nurgul S., Yerlan A. Shynbergenov, Aiman B. Karabalayeva, Nurila A. Togyzbayeva, and Sholpan B. Abilova. 2025. "The Structure and Spatial Distribution of the Raptor Community in the Urban Landscapes of Kyzylorda, Kazakhstan" Birds 6, no. 3: 44. https://doi.org/10.3390/birds6030044
APA StyleSihanova, N. S., Shynbergenov, Y. A., Karabalayeva, A. B., Togyzbayeva, N. A., & Abilova, S. B. (2025). The Structure and Spatial Distribution of the Raptor Community in the Urban Landscapes of Kyzylorda, Kazakhstan. Birds, 6(3), 44. https://doi.org/10.3390/birds6030044





