Assessing Parasite Prevalence and Health Status of the Eurasian Tree Sparrow (Passer montanus) in Green Urban Areas of a Southern European City
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
Description of the Habitat
2.2. Birds Sampling
2.3. Laboratory Work
2.3.1. Blood Parasite Detection and Leukocyte Profile
2.3.2. DNA Extraction and Nested PCR Analysis
2.3.3. Coprological Examinations
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentili, R.; Quaglini, L.A.; Galasso, G.; Montagnani, C.; Caronni, S.; Cardarelli, E.; Citterio, S. Urban refugia sheltering biodiversity across world cities. Urban Ecosyst. 2024, 27, 219–230. [Google Scholar] [CrossRef]
- Biella, P.; Bani, L.; Caprio, E.; Cochis, F.; Dondina, O.; Fiorilli, V.; Genre, A.; Gentili, R.; Orioli, V.; Ranalli, R.; et al. Biodiversity-friendly practices to support urban nature across ecosystem levels in green areas at different scales. Urban For. Urban Green. 2025, 105, 128682. [Google Scholar] [CrossRef]
- Łopucki, R.; Kitowski, I. How small cities affect the biodiversity of ground-dwelling mammals and the relevance of this knowledge in planning urban land expansion in terms of urban wildlife. Urban Ecosyst. 2017, 20, 933–943. [Google Scholar] [CrossRef]
- Collins, M.K. Global trends in urban wildlife ecology and conservation. Biol. Conserv. 2021, 261, 109–236. [Google Scholar] [CrossRef]
- Bertule, M.; Lloyd, G.J.; Korsgaard, L.; Dalton, J.; Welling, R. Green Infrastructure Guide for Water Management Ecosystem-Based Management Approaches for Water-Related Infrastructure Projects; UNEP-DHI: Copenhagen, Denmark, 2014; Available online: https://unepdhi.org/wp-content/uploads/sites/2/2020/05/WEB-UNEP-DhiGroup-Green-infrastructure-Guide-EN-20140814.pdf (accessed on 20 February 2025).
- Jones, L.; Anderson, S.; Læssøe, J.; Banzhaf, E.; Jensen, A.; Bird, D.N.; Miller, J.; Hutchins, M.G.; Yang, J.; Garrett, J.; et al. A typology for urban Green Infrastructure to guide multifunctional planning of nature-based solutions. Nat.-Based Solut. 2022, 2, 100041. [Google Scholar] [CrossRef]
- Frantzeskaki, N.; McPhearson, T.; Collier, M.J.; Kendal, D.; Bulkeley, H.; Dumitru, A.; Noble, K.; van-Wyk, E.; Ordóñez, C.; Oke, C.; et al. Nature-based solutions for urban climate change adaptation: Linking science, policy, and practice communities for evidence-based decision-making. BioScience 2019, 69, 455–466. [Google Scholar] [CrossRef]
- Wong, G.K.L. Urban-microclimate effect on vector mosquito abundance of tropical green roofs. Build. Environ. 2017, 112, 63–76. [Google Scholar] [CrossRef]
- Coutts, C.; Hahn, M. Green Infrastructure, Ecosystem Services, and Human Health. Int. J. Environ. Res. Public Health 2015, 12, 9768–9798. [Google Scholar] [CrossRef]
- Delgado-V, C.A.; French, K. Parasite–bird interactions in urban areas: Current evidence and emerging questions. Landsc. Urban Plan. 2012, 105, 5–14. [Google Scholar] [CrossRef]
- Lõhmus, M.; Balbus, J. Making green infrastructure healthier infrastructure. Infect. Ecol. Epidemiol. 2015, 5, 30082. [Google Scholar] [CrossRef]
- Kibret, S.; Alemu, Y.; Boelee, E.; Tekie, H.; Alemu, D.; Petros, B. The impact of a small-scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop. Med. Int. Health 2009, 15, 41–50. [Google Scholar] [CrossRef]
- Titcomb, G.; Mantas, J.N.; Hulke, J.; Rodriguez, I.; Branch, D.; Young, H. Water sources aggregate parasites with increasing effects in more arid conditions. Nat. Commun. 2021, 12, 7066. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.Y.; Sung, H.C. Nest site selection, nest characteristics, and breeding ecology of the Eurasian Tree Sparrow, Passer montanus, living in an urban area. Anim. Taxon. Ecol. 2024, 70, 46–60. [Google Scholar] [CrossRef]
- Mardiastuti, A.; Mulyani, Y.A.; Rinaldi, D.; Rumblat, W.; Dewi, L.K.; Kaban, A.; Sastranegara, H. Synurbic avian species in Greater Jakarta Area, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012001. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J.; Kaisanlahti-Jokimäki, M.-L. Differential Long-Term Population Responses of Two Closely Related Human-Associated Sparrow Species with Respect to Urbanization. Birds 2021, 2, 230–249. [Google Scholar] [CrossRef]
- BirdLife International. IUCN Red List of Threatened Species. 2018. Available online: http://www.birdlife.org (accessed on 17 July 2025).
- Mónus, F.; Barta, Z. Seasonality and sociality in tree sparrows “Passer montanus”. Intern. Stud. Sparrows 2010, 34, 18–22. [Google Scholar] [CrossRef]
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.; Melo, M.; Covas, R.; Doutrelant, C.; Pereira, H.; De Lima, R.; Loiseau, C. Influence of land use and host species on parasite richness, prevalence and co-infection patterns. Int. J. Parasitol. 2021, 51, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, A.; Clark, N.J.; Bell, J.A.; Skeen, H.R.; Lutz, H.L.; De La Torre, G.M.; Vaughan, J.A.; Tkach, V.V.; Schunck, F.; Ferreira, F.C.; et al. Global drivers of avian haemosporidian infections vary across zoogeographical regions. Glob. Ecol. Biogeogr. 2021, 30, 2393–2406. [Google Scholar] [CrossRef]
- Han, Y.; Hellgren, O.; Wu, Q.; Liu, J.; Jin, T.; Bensch, S.; Ding, P. Seasonal variations of intensity of avian malaria infection in the Thousand Island Lake System, China. Parasites Vectors 2023, 16, 218. [Google Scholar] [CrossRef]
- Kwasnoski, L.; Brown, J.; Taylor, J.; Watson, J.L.; Oleyar, D.; Ellis, V.A. Avian haemosporidian parasite prevalence and diversity in two populations of the American kestrel (Falco sparverius). Parasitol. Res. 2025, 124, 60. [Google Scholar] [CrossRef]
- Granthon, C.; Williams, D.A. Avian Malaria, Body Condition, and Blood Parameters in Four Species of Songbirds. Wilson J. Ornithol. 2017, 129, 492–508. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Mettler, R.; Segelbacher, G.; Schaefer, H.M. Haemosporidian parasitism in the blackcap Sylvia atricapilla in relation to spring arrival and body condition. J. Avian Biol. 2013, 44, 521–530. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Trade-off between immune response and body mass in wintering house sparrows (Passer domesticus). Ecol. Res. 2011, 26, 943–947. [Google Scholar] [CrossRef]
- Lashev, L.; Hubenov, H.; Nikolov, Y.; Lasheva, V.; Mihailov, R. Comparison of some haematological parameters between three bird species from the Columbidae family—Short communication. Veterinarski Arhiv. 2009, 79, 409–414. [Google Scholar]
- Wille, M.; Klaassen, M. Should I stay, should I go, or something in between? The potential for parasite-mediated and age-related differential migration strategies. Evol. Ecol. 2023, 37, 189–202. [Google Scholar] [CrossRef]
- Muñoz, F.; Soria, S.; Bautista, N.; Pino, C.; Ortiz, J.C. Plan de Fomento y Gestión de la Biodiversidad de la Ciudad de Madrid; Área del Gobierno de Medio Ambiente y Movilidad Dirección General de Gestión del Agua y Zonas Verdes; Ayuntamiento de Madrid: Madrid, Spain, 2020. [Google Scholar]
- Instituto Nacional de Estadística (INE). Padrón Municipal. Población por Municipios; INE: Madrid, Spain, 2024; Available online: https://www.ine.es (accessed on 17 July 2025).
- Agencia Estatal de Meteorología (AEMET). Anuario Climatológico 2023; AEMET: Madrid, Spain, 2023; Available online: https://www.aemet.es (accessed on 14 July 2025).
- Álvarez-Cobelas, M.; Cirujano-Bracamonte, S.; Molina_Benito, J.; Riolobos-Lopez, P.; Rubio-Olmo, A.; Soriano-Hernando, O.; Velasco-Diaz, J.L.; Vicente-Sanchez, J. Las lagunas de las Rozas de Madrid; Ayuntamiento de Las Rozas: Las Rozas, Spain, 2005; 179p. [Google Scholar]
- Fernández García, J.L.; Babamonte, A.; Barreiro, P.; Ruiz-del-Castillo, J. La Casa de Campo: Más de un millón de años de historia; Lunwerg, Ayuntamiento de Madrid: Madrid, Spain, 2003; 429p. [Google Scholar]
- Remón, J.F.; Añón-Feliú, C. Parques y jardines de Madrid. Vol. 8, Parque del Oeste, 2nd ed.; rev, and expanded; Fundación Caja de Madrid: Madrid, Spain, 2001. [Google Scholar]
- García-Gómez, L. Jardines Parque del Oeste Paso a Paso. Guía del Parque; Naperma-Autoediciones: Madrid, Spain, 2018; 229p. [Google Scholar]
- Esteban-Penelas, J.L.; Carrascosa-Campos, D.; Pérez-Hernández, M.I.; Pena-López, C.; Esteras-Martín, E. Parque Juan Carlos I: La puerta de Madrid; Ayuntamiento de Madrid, Junta Municipal del Distrito de Barajas, Unidad de Actividades Culturales, Formativas y Deportivas: Madrid, Spain, 2017. [Google Scholar]
- Hernández-Lamas, P.; Rubio Gavilán, A.; Bernabeu-Larena, J. Parks and roads build the cities: The M-30 and Madrid-Río project, building landscape. In Back to the Sense of the City: International Monograph Book (Ed.); Centre de Política de Sòl I Valoracions: Barcelona, Spain, 2016; pp. 415–428. [Google Scholar] [CrossRef]
- Liker, A.; Papp, Z.; Bókony, V.; Lendvai, Á.Z. Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 2008, 77, 789–795. [Google Scholar] [CrossRef]
- Arizaga, J.; Iraeta, A.; Crespo, A.; Azkona, A.; Laso, M.; Gutiérrez, Ó.; Banda, E. Programas de Monitorización de Aves a Largo Plazo de la Oficina de Anillamiento de la Sociedad de Ciencias Aranzadi; Sociedad de Ciencias Aranzadi: Donostia, Spain, 2023; Available online: https://www.aranzadi.eus/assets/files/manualprogramasv06.pdf (accessed on 10 February 2025).
- Robinson, R.A.; Julliard, R.; Saracco, J.F. Constant effort: Studying avian population processes using standardised ringing. Ringing Migr. 2009, 24, 199–204. [Google Scholar] [CrossRef]
- Pinilla, J. Manual Para el Anillamiento Científico de Aves; SEO/BirdLife and DGCN_MIMAM: Madrid, Spain, 2000; 160p. [Google Scholar]
- Demongin, L.; Moss, A. Identification Guide to Birds in the Hand: The 301 Species Most Frequently Caught in Western Europe: Identification, Measurements, Geographical Variation, Moult, Sex and Age; Demongin, L., Ed.; Laurent Demongin: Beauregard-Vendon, France, 2016. [Google Scholar]
- Blasco-Zumeta, J.; Heinze, G.M. Atlas de Identificación de las Aves Continentales de la Península Ibérica; Tundra Ediciones: Valencia, Spain, 2022; 564p. [Google Scholar]
- Bobby Fokidis, H.; Greiner, E.C.; Deviche, P. Interspecific variation in avian blood parasites and haematology associated with urbanization in a desert habitat. J. Avian Biol. 2008, 39, 300–310. [Google Scholar] [CrossRef]
- Lynton-Jenkins, J.G.; Chaine, A.S.; Russell, A.F.; Bonneaud, C. Parasite detection and quantification in avian blood is dependent on storage medium and duration. Ecol. Evol. 2023, 13, e9819. [Google Scholar] [CrossRef]
- Lozano, J.; Almeida, C.; Victório, A.C.; Melo, P.; Rodrigues, J.P.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; et al. Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Vet. Sci. 2021, 8, 160. [Google Scholar] [CrossRef]
- Carr, J.H. Atlas de Hematología Clínica, 6th ed.; Editorial Médica Panamericana: Madrid, Spain, 2023; 287p. [Google Scholar]
- Valkiūnas, G. Avian Malaria Parasites and other Haemosporidia, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Demina, I.; Tsvey, A.; Babushkina, O.; Bojarinova, J. Time-keeping programme can explain seasonal dynamics of leukocyte profile in a migrant bird. J. Avian Biol. 2019, 50, jav.02117. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Reina, R.D.; Lill, A. Interpreting indices of physiological stress in free-living vertebrates. J. Comp. Physiol. B 2012, 182, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Minias, P. The effects of urban life on animal immunity: Adaptations and constraints. Sci. Total Environ. 2023, 895, 165085. [Google Scholar] [CrossRef]
- Bensch, S.; Åkesson, S. Temporal and Spatial Variation of Hematozoans in Scandinavian Willow Warblers. J. Parasitol. 2003, 89, 388–391. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Schumm, Y.R.; Bakaloudis, D.; Barboutis, C.; Cecere, J.G.; Eraud, C.; Fischer, D.; Hering, J.; Hillerich, K.; Lormée, H.; Mader, V.; et al. Prevalence and genetic diversity of avian haemosporidian parasites in wild bird species of the order Columbiformes. Parasitol. Res. 2021, 120, 1405–1420. [Google Scholar] [CrossRef]
- Laverty, L.; Beer, L.C.; Martin, K.; Hernandez-Velasco, X.; Juarez-Estrada, M.A.; Arango-Cardona, M.; Forga, A.J.; Coles, M.E.; Vuong, C.N.; Latorre, J.D.; et al. In vitro and in vivo evaluation of chlorhexidine salts as potential alternatives to potassium dichromate for Eimeria maxima M6 oocyst preservation. Front. Vet. Sci. 2023, 10, 1226298. [Google Scholar] [CrossRef]
- Becker, A.C.; Kraemer, A.; Epe, C.; Strube, C. Sensitivity and efficiency of selected coproscopical methods—Sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol. Res. 2016, 115, 2581–2587. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 27 October 2024).
- Kruse, W. Ueber blutparasiten. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin 1890, 120, 541–560. [Google Scholar]
- Grassi, R.; Feletti, B. Nuova contribuzione allo studio della malaria. Bulletino Mensile della Accademia Gioenia di Scienze Naturali in Catania 1891, 16, 16–20. [Google Scholar]
- Grulet, O.; Landau, I.; Baccam, D. Les Isospora du Moineau domestique; multiplicité des espèces. Ann. De Parasitol. Hum. Et Comparée. 1982, 57, 209–235. [Google Scholar] [CrossRef]
- Fülöp, A.; Lukács, D.; Barta, Z. Space use of wintering Eurasian Tree Sparrows (Passer montanus) in a semi-urban area: A radiotelemetry-based case study. ORNIS Hung. 2022, 30, 124–133. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, J.H.; Jang, J.U.; Son, J.J.; Sung, H.C. The adaptation and fitness costs to urban noise in the calls of the tree sparrow (Passer montanus). Sci. Rep. 2025, 15, 5359. [Google Scholar] [CrossRef]
- Marinez-Miranzo, B.; Lopez-Garcia, A.; Payo-Payo, A.; Banda, E.; Aguirre, J.I. Fitness Consequences of Urban Green Space Management in a Passerine Bird: The Case of Eurasian Tree Sparrow (Passer montanus) in Madrid, Spain; Enara, E.A., Ed.; Madrid, Spain, 2025; p. 28231, manuscript in preparation. [Google Scholar]
- Kroth, N.; Cozzer, G.D.; de Carvalho, G.; Cassol, A.S.; Breaux, J.; Lutinski, J.A.; Busato, M.A.; Junior, W.A.R. Oviposition preferences of the mosquito Aedes aegypti Linnaeus, 1762 (Culicidae): An urban environment bioassay. Bull. Entomol. Res. 2019, 109, 726–770. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Charlwood, D.; Lindsay, S.W. The behaviour of adult Anopheles gambiae, sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: A review. Malar. J. 2024, 23, 161. [Google Scholar] [CrossRef]
- Erram, D.; Burkett-Cadena, N. Oviposition of Culicoides insignis (Diptera: Ceratopogonidae) under laboratory conditions with notes on the developmental life history traits of its immature stages. Parasites Vectors 2021, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Harvey-Samuel, T.; Ant, T.; Sutton, J.; Niebuhr, C.N.; Asigau, S.; Parker, P.; Sinkins, S.; Alphey, L. Culex quinquefasciatus: Status as a threat to island avifauna and options for genetic control. CABI Agric. Biosci. 2021, 2, 9. [Google Scholar] [CrossRef]
- López-Peña, D.; Kúdela, M.; Kúdelová, T.; Falcó-Garí, J.V. Simuliids from Madrid Autonomous Region (Spain): Update and new contributions. Cuad. Biodiversidad. 2024, 66, 15–27. [Google Scholar] [CrossRef]
- Baz, M.M.; Baeshen, R.S.; El-Shourbagy, N.M.; Hikal, W.M.; Darwish, A.B.; El-Sayed, Y.A.; Abououf, E.A. Ecological Factors Affecting Diversity and Abundance of Mosquito Larvae in Nile Delta, Egypt. Egypt. J. Vet. Sci. 2024, 55, 991–1006. [Google Scholar] [CrossRef]
- Kibret, S.; Wilson, G.G.; Tekie, H.; Petros, B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar. J. 2014, 13, 360. [Google Scholar] [CrossRef]
- González, M.; López, S.; Mullens, B.A.; Baldet, T.; Goldarazena, A. A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Vet. Parasitol. 2013, 191, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lara, C.; Carbó-Ramírez, P.; Santiago-Alarcon, D. Effects of land use change (rural-urban) on the diversity and epizootiological parameters of avian Haemosporida in a widespread neotropical bird. Acta Trop. 2020, 209, 105542. [Google Scholar] [CrossRef]
- Caizergues, A.E.; Robira, B.; Perrier, C.; Jeanneau, M.; Berthomieu, A.; Perret, S.; Gandon, S.; Charmantier, A. Cities as parasitic amplifiers? Malaria prevalence and diversity in great tits along an urbanization gradient. Peer Community J. 2024, 4, e38. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Rao, M.; Huang, X.; Bensch, S. Estimating prevalence of avian haemosporidians in natural populations: A comparative study on screening protocols. Parasites Vectors 2017, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.D.; Berto, B.P.; Neves, D.M.; Lopes, C.W.G. Periodicity and intensity of oocysts of the genus Isospora Schneider, 1881 shedding by passerines birds from wildlife. Rev. Bras. Med. Vet. 2016, 8, 75–79. [Google Scholar]
- Dolnik, O.V.; Dolnik, V.R.; Bairlein, F. The Effect of Host Foraging Ecology on the Prevalence and Intensity of Coccidian Infection in Wild Passerine Birds. Ardea 2010, 98, 97–103. [Google Scholar] [CrossRef]
- European Comission. EU Biodiversity Strategy for 2030: Bringing Nature Back into Our Lives (COM(2020) 380 Final); European Comission: Brussels, Belgium, 2020. [Google Scholar]
- Maes, J.; Zulian, G.; Günther, S.; Thijssen, M.; Raynal, J. Enhancing Resilience of Urban Ecosystems Through Green Infrastructure: Final Report (EUR 29630 EN); Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Heinrich, K.; Bach, M.; Breuer, L. Infectious disease research—What role is there for hydrologists? J. Water Resour. Prot. 2017, 9, 139. [Google Scholar] [CrossRef]
- Sorci, G. Immunity, resistance and tolerance in bird–parasite interactions. Parasite Immunol. 2013, 35, 350–361. [Google Scholar] [CrossRef]
- Navarro, C.; Marzal, A.; de Lope, F.; Møller, A.P. Dynamics of an immune response in house sparrows Passer domesticus in relation to time of day, body condition and blood parasite infection. Oikos 2003, 101, 291–298. [Google Scholar] [CrossRef]
- Cooper, N.W.; Sherry, T.W.; Marra, P.P. Experimental reduction of winter food decreases body condition and delays migration in a long-distance migratory bird. Ecology 2015, 96, 1933–1942. [Google Scholar] [CrossRef]
- Fast, P.L.F.; Grant Gilchrist, H.; Clark, R.G. Experimental evaluation of nest shelter effects on weight loss in incubating common eiders Somateria mollissima. J. Avian Biol. 2007, 38, 205–213. [Google Scholar] [CrossRef]
- García-Navas, V. Gorrión molinero—Passer montanus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Morales, M.B., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2016. [Google Scholar]
- Moe, B.; Langseth, I.; Fyhn, M.; Gabrielsen, G.W.; Bech, C. Changes in body condition in breeding kittiwakes Rissa tridactyla. J. Avian Biol. 2002, 33, 225–234. [Google Scholar] [CrossRef]
- Krams, R.; Krama, T.; Elferts, D.; Daukšte, J.; Raibarte, P.; Brūmelis, G.; Dauškane, I.; Strode, L.; Krams, I.A. High blood parasite infection rate and low fitness suggest that forest water bodies comprise ecological traps for pied flycatchers. Birds 2022, 3, 221–233. [Google Scholar] [CrossRef]
- Irizarry-Rovira, A.R. Avian Hematology. In Veterinary Clinical Pathology Secrets; Cowell, R.L., Ed.; Hanley & Belfus: Philadelphia, PA, USA, 2004; pp. 282–304. [Google Scholar]
- Samani, A.D.; Kheirabadi, K.P.; Mohebbi, A. Effect of Haemoproteus columbae infection on the hemogram of the Pigeons (Columba livia domestica). J. Parasit. Dis. 2016, 40, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.B.; Johns, J. Avian Hematology and Related Disorders. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 501–522. [Google Scholar] [CrossRef] [PubMed]

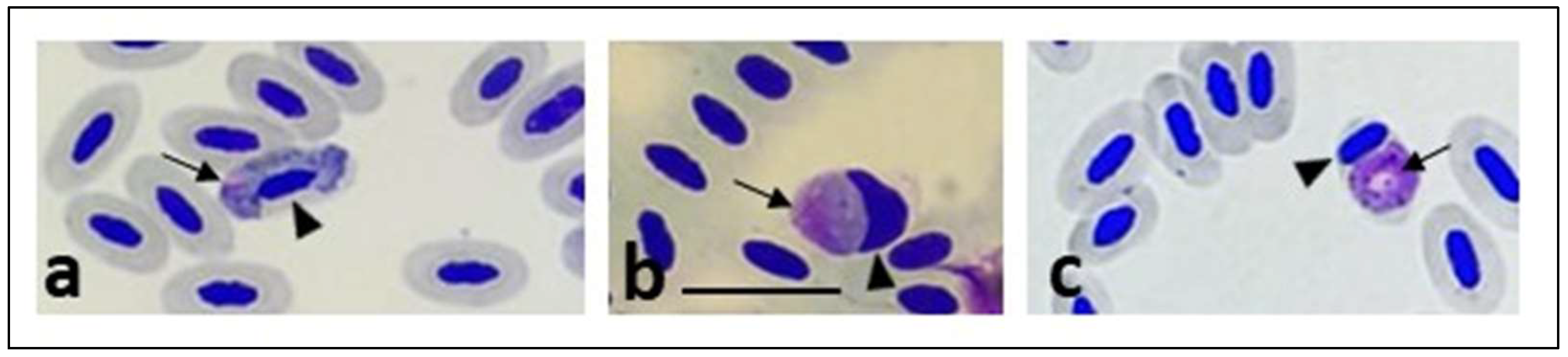
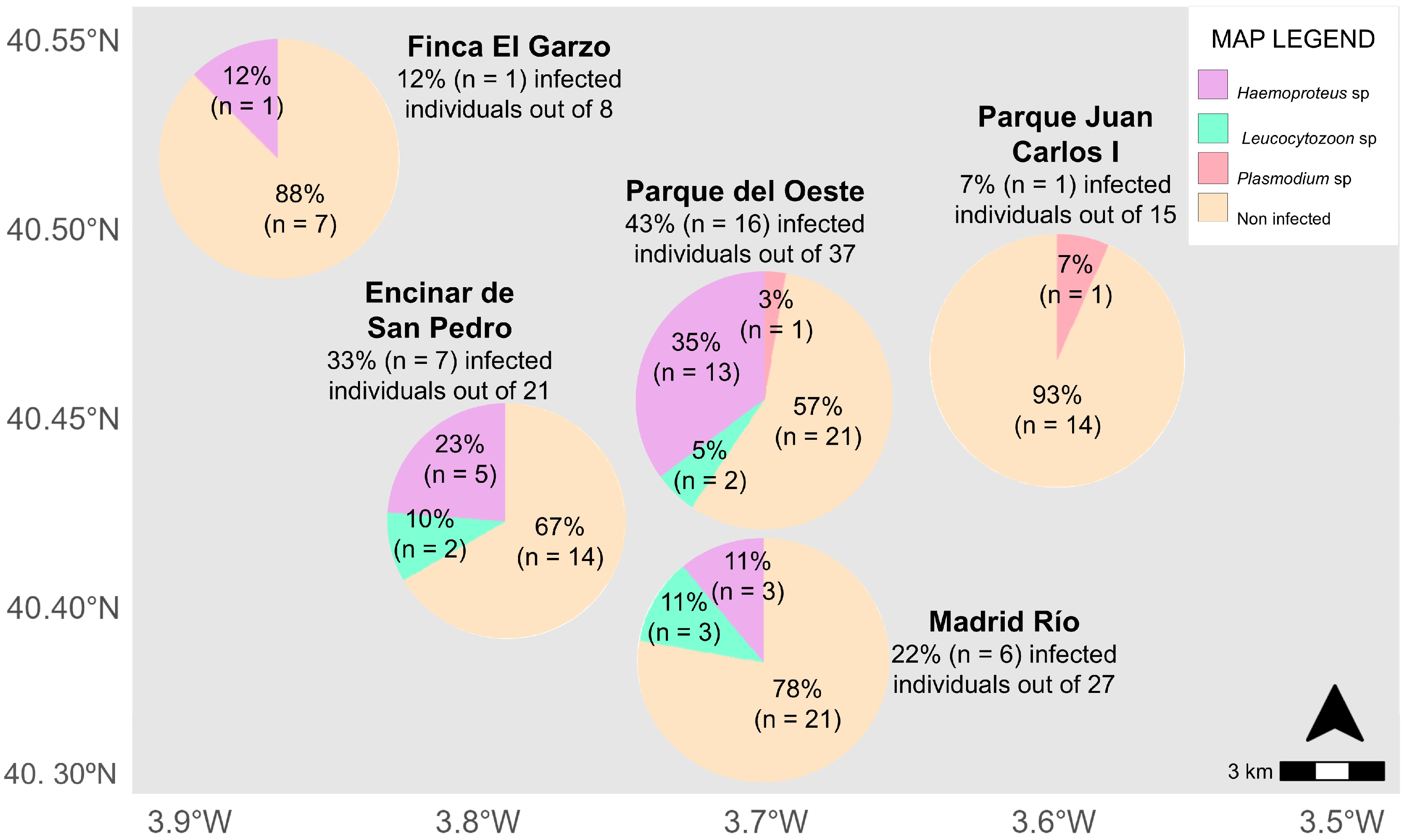




| Green Area | % Vegetation | % Buildings | % Pavement | % Water | Type of Water |
|---|---|---|---|---|---|
| Finca El Garzo | 94.5 | 2.4 | 2.3 | 0.8 | Untreated; Flowing; Unpaved shore |
| Encinar de San Pedro | 94.4 | 0.3 | 5.3 | 0.1 | Untreated; Stagnant; Unpaved shore |
| Parque del Oeste | 61.4 | 10.3 | 27.9 | 0.4 | Untreated; Stagnant; Paved shore |
| Parque Juan Carlos I | 55.4 | 15.7 | 23.4 | 5.5 | Treated; Stagnant; Paved shore |
| Madrid Río | 23.4 | 37.7 | 33.3 | 5.7 | Treated; Flowing; Paved shore |
| Technique | Genus | No. Positive | Prevalence (%; N = 108) |
|---|---|---|---|
| Microscopic technique | Haemoproteus | 22 | 20% |
| Leucocytozoon | 7 | 6% | |
| Plasmodium | 2 | 2% | |
| Nested PCR technique | Haemoproteus | 8 | 7% |
| Leucocytozoon | 0 | 0% | |
| Plasmodium | 2 | 2% | |
| Both techniques combined | Haemoproteus | 22 | 20% |
| Leucocytozoon | 7 | 6% | |
| Plasmodium | 2 | 2% |
| Independent Variable | Estimate | Std. Error | z-Value | p-Value |
|---|---|---|---|---|
| Finca El Garzo | 0.2924 | 0.4744 | 0.616 | 0.5377 |
| Encinar de San Pedro | 0.9502 | 0.4203 | 2.282 | 0.0225 |
| Parque del Oeste | −1.3422 | 0.3307 | −4.059 | <0.001 |
| Parque Juan Carlos I | 0.649 | 0.4878 | 1.331 | 0.1833 |
| Madrid Río | −1.2969 | 0.8673 | −1.495 | 0.1348 |
| Independent Variable | Estimate | Std. Error | z-Value | p-Value |
|---|---|---|---|---|
| Finca El Garzo | −30.93 | 30.3 | −1.021 | 0.3096 |
| Encinar de San Pedro | 32.9 | 18.01 | 1.827 | 0.0706 |
| Parque del Oeste | 80.63 | 32.98 | 2.445 | 0.0162 |
| Parque Juan Carlos I | 15.72 | 27.52 | 0.571 | 0.569 |
| Madrid Río | −32.9 | 37.32 | −0.881 | 0.3801 |
| Independent Variable | Estimate | Std. Error | z-Value | p-Value | |
|---|---|---|---|---|---|
| Lymphocyte | Finca El Garzo | −4.962 | 2.7123 | −1.829 | 0.0703 |
| Encinar de San Pedro | −0.2942 | 2.4848 | −0.118 | 0.906 | |
| Parque del Oeste | 76.0899 | 1.6109 | 47.234 | <0.001 | |
| Parque Juan Carlos I | 4.5043 | 2.9506 | 1.527 | 0.13 | |
| Madrid Río | −7.3413 | 3.467 | −2.117 | 0.0367 | |
| Body condition | −2.3877 | 1.0501 | −2.274 | 0.0251 | |
| Eosinophil | |||||
| Finca El Garzo | 2.7279 | 0.9346 | 2.191 | 0.004 | |
| Encinar de San Pedro | −2.0205 | 0.8789 | −2.299 | 0.0235 | |
| Parque del Oeste | 3.1895 | 0.6975 | 4.573 | <0.001 | |
| Parque Juan Carlos I | 0.1877 | 1.0188 | 0.184 | 0.8541 | |
| Madrid Río | 0.0453 | 1.1671 | 0.039 | 0.969 | |
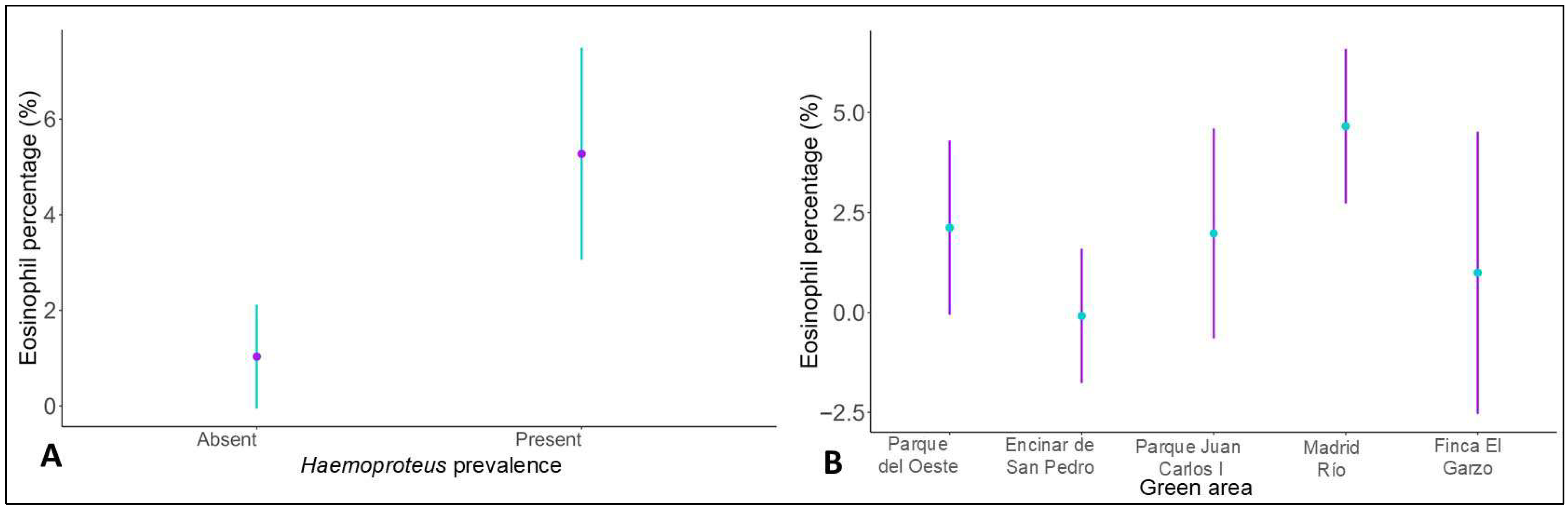
| Haemoproteus prevalence | −2.1215 | 0.6329 | −3.352 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, A.; Yabsley, M.J.; Hernández, S.M.; Garrett, K.B.; Aguirre, J.I.; Banda, E. Assessing Parasite Prevalence and Health Status of the Eurasian Tree Sparrow (Passer montanus) in Green Urban Areas of a Southern European City. Birds 2025, 6, 43. https://doi.org/10.3390/birds6030043
Vega A, Yabsley MJ, Hernández SM, Garrett KB, Aguirre JI, Banda E. Assessing Parasite Prevalence and Health Status of the Eurasian Tree Sparrow (Passer montanus) in Green Urban Areas of a Southern European City. Birds. 2025; 6(3):43. https://doi.org/10.3390/birds6030043
Chicago/Turabian StyleVega, Aida, Michael J. Yabsley, Sonia M. Hernández, Kayla B. Garrett, Jose I. Aguirre, and Eva Banda. 2025. "Assessing Parasite Prevalence and Health Status of the Eurasian Tree Sparrow (Passer montanus) in Green Urban Areas of a Southern European City" Birds 6, no. 3: 43. https://doi.org/10.3390/birds6030043
APA StyleVega, A., Yabsley, M. J., Hernández, S. M., Garrett, K. B., Aguirre, J. I., & Banda, E. (2025). Assessing Parasite Prevalence and Health Status of the Eurasian Tree Sparrow (Passer montanus) in Green Urban Areas of a Southern European City. Birds, 6(3), 43. https://doi.org/10.3390/birds6030043





