Climate Changes Can Restore Allopatry Between Two Congeneric Birds in the Atlantic Forest
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
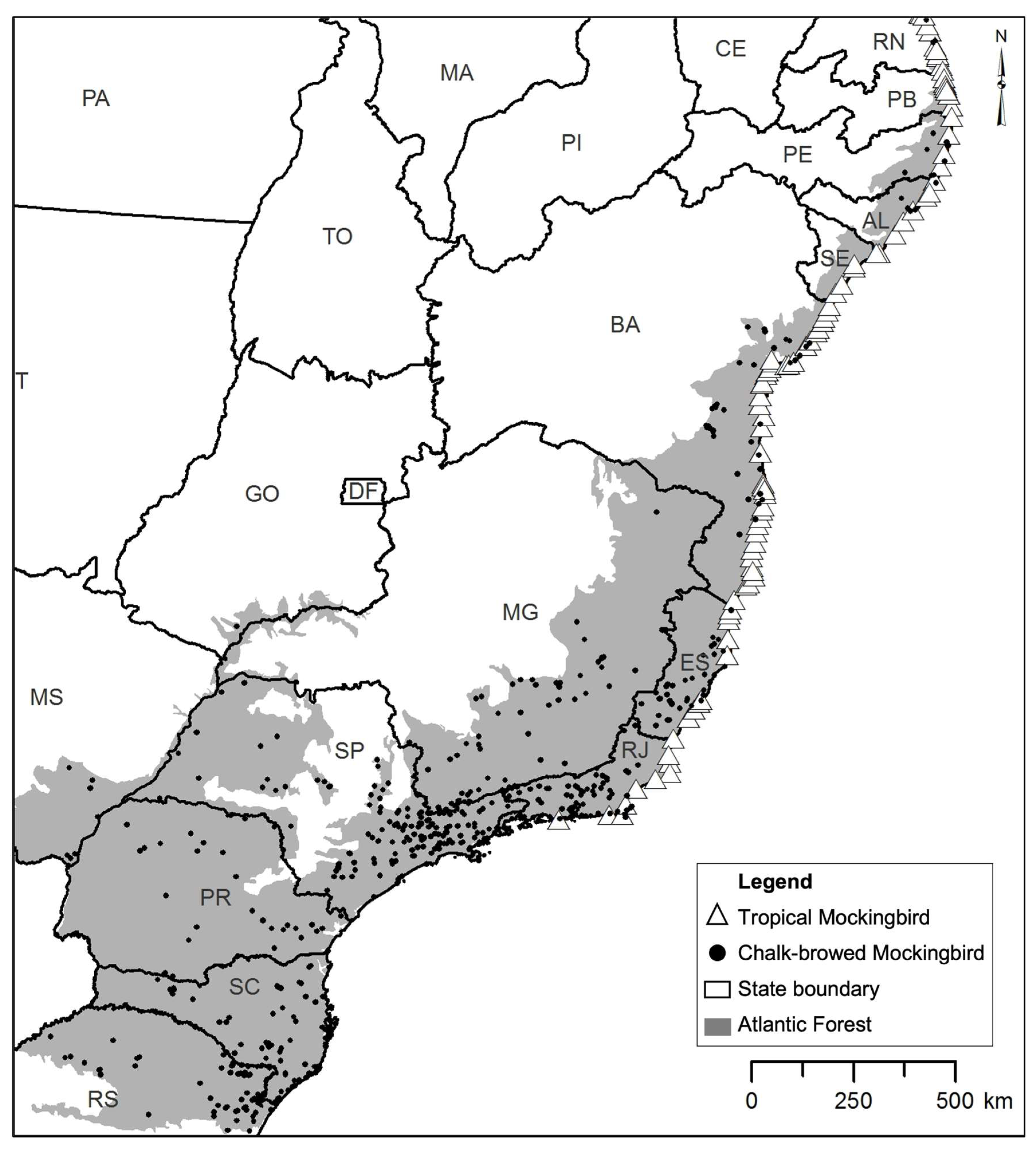
2.3. Bird Species Data
2.4. Climate Data
2.5. Statistical Analysis and Modeling Work
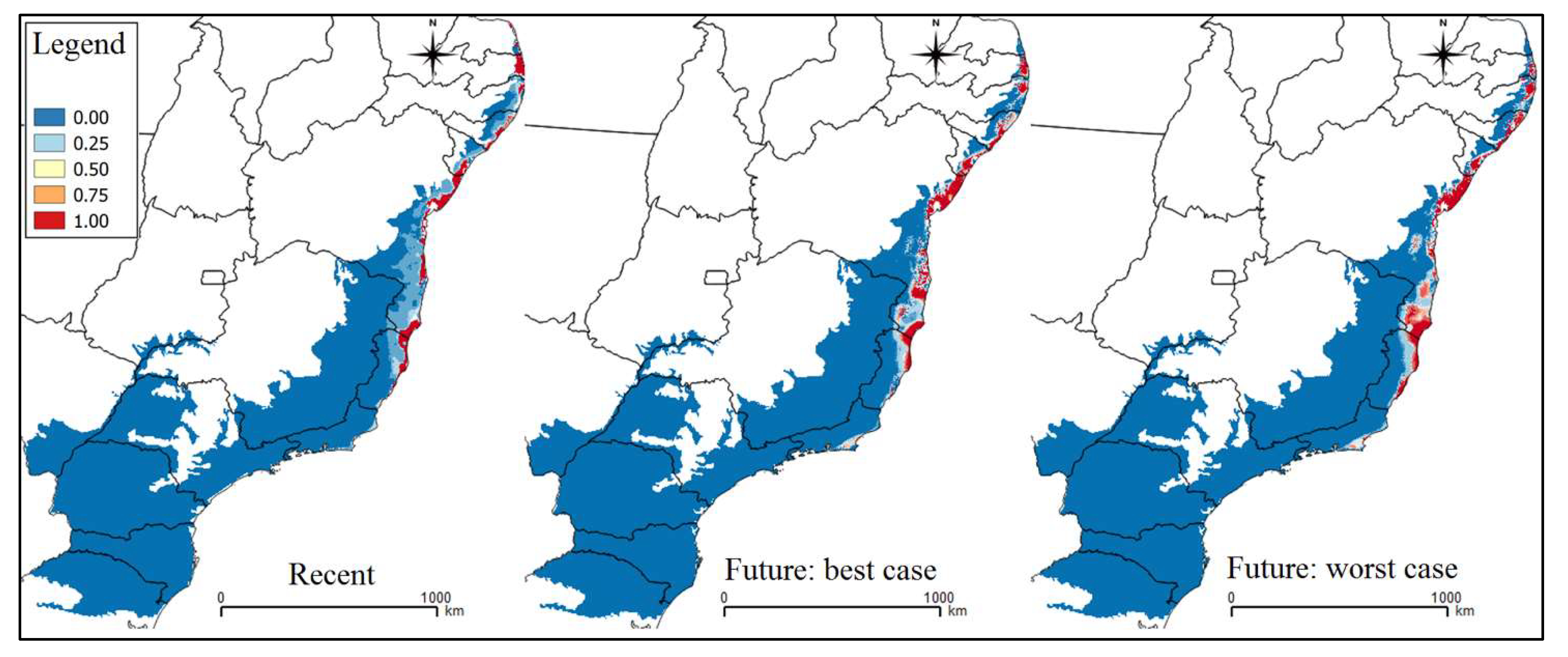
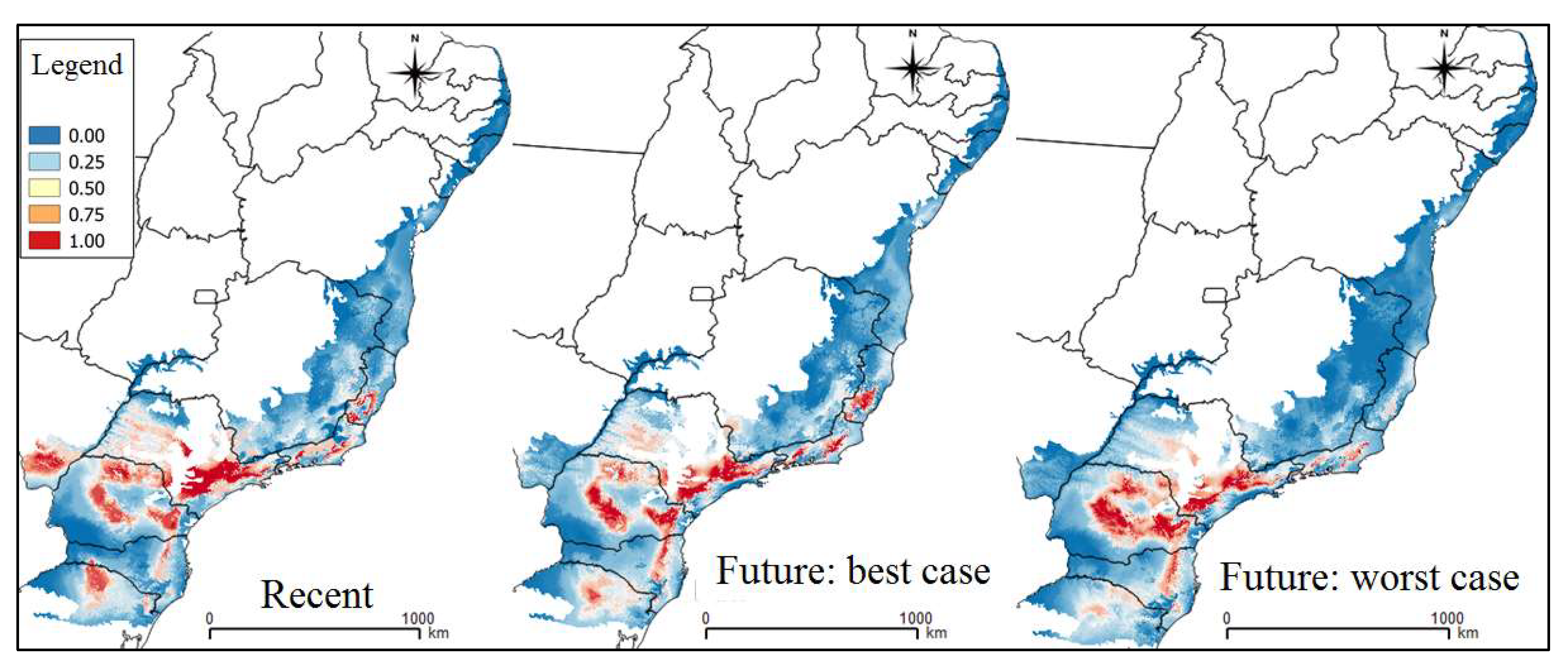
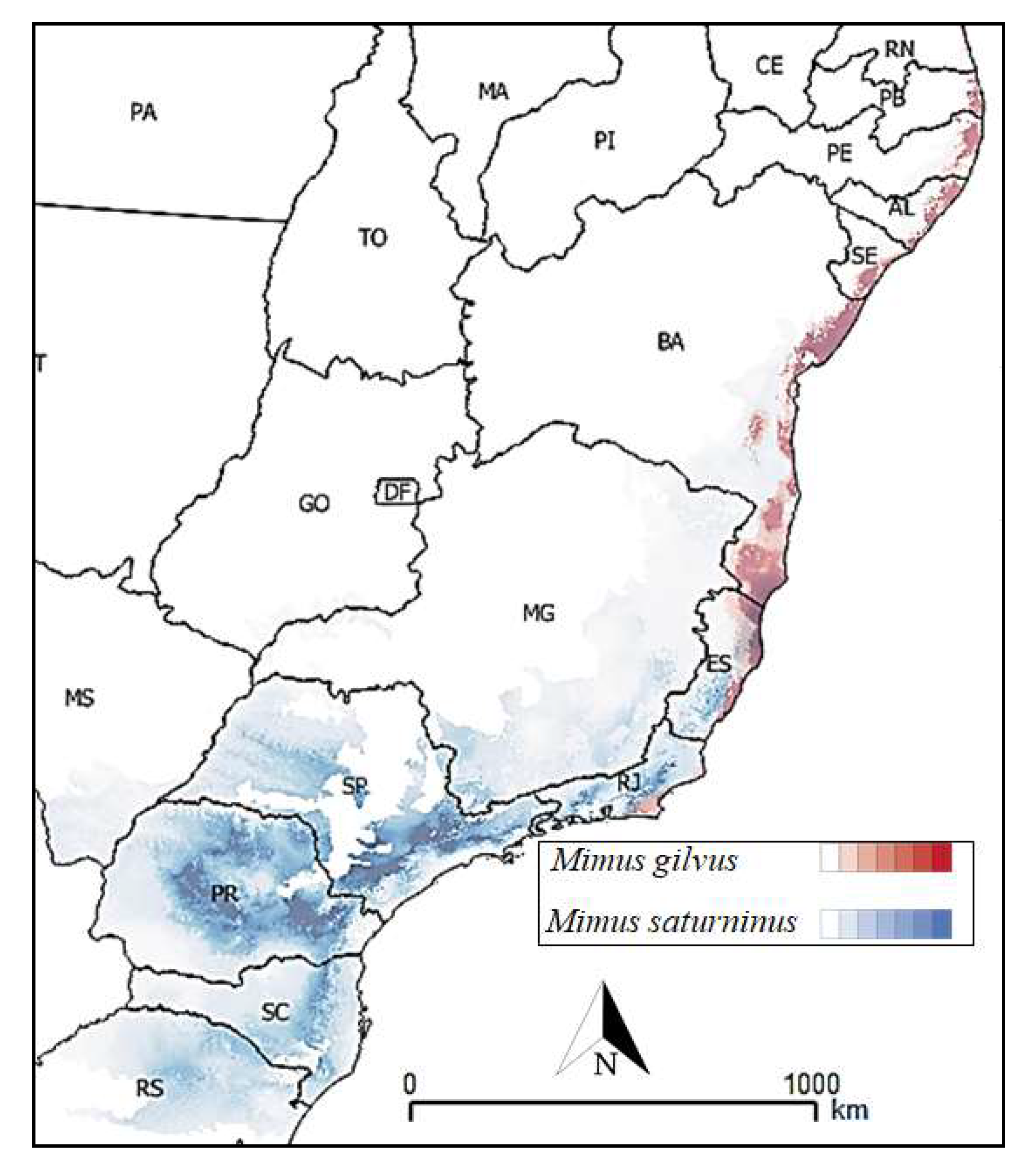
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate change 2013: The physical science basis. In Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2013; pp. 159–254. [Google Scholar]
- Thomas, C.D.; Lennon, J.J. Birds extend their ranges northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- McCarty, J.P. Ecological consequences of recent climate change. Conserv. Biol. 2001, 15, 320–331. [Google Scholar] [CrossRef]
- Diniz, M.H. Defaunação: A atual crise da biodiversidade. Rev. Bras. Dir. Animal 2017, 12, 15–52. [Google Scholar]
- Glasser, C.M.; Gomes, A.D.C. Clima e sobreposição da distribuição de Aedes aegypti e Aedes albopictus na infestação do Estado de São Paulo. Rev. Saúde Púb. 2002, 36, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.K. Response of a mammalian faunal element to climatic changes. J. Mammal. 1992, 73, 43–50. [Google Scholar] [CrossRef]
- Hamilton, C.D.; Kovacs, K.M.; Ims, R.A.; Aars, J.; Lydersen, C. An Arctic predator-prey system in flux: Climate change impacts on coastal space use by polar bears and ringed seals. J. Anim. Ecol. 2017, 86, 1054–1064. [Google Scholar] [CrossRef]
- López-Ramírez, S.; Chamorro, D.; Real, R.; Muñoz, A.R. Southern Europe is becoming climatically favourable for African birds: Anticipating the establishment of a new species. Front. Zool. 2023, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Weeks, T.L.; Betts, M.G.; Pfeifer, M.; Wolf, C.; Banks-Leite, C.; Barbaro, L.; Barlow, J.; Cerezo, A.; Kennedy, C.M.; Kormann, U.G.; et al. Climate-driven variation in dispersal ability predicts responses to forest fragmentation in birds. Nat. Ecol. Evol. 2023, 7, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.Â.; Barbet-Massin, M.; Lopes, L.E.; Jiguet, F. Major current and future gaps of Brazilian reserves to protect Neotropical savanna birds. Biol. Conserv. 2009, 142, 3039–3050. [Google Scholar] [CrossRef]
- Marini, M.Â.; Barbet-Massin, M.; Lopes, L.E.; Jiguet, F. Predicted climate-driven bird distribution changes and forecasted conservation conflicts in a Neotropical savanna. Conserv. Biol. 2009, 23, 1558–1567. [Google Scholar] [CrossRef]
- Loyola, R.D.; Lemes, P.; Faleiro, F.V.; Trindade-Filho, J.; Machado, R.B. Severe loss of suitable climatic conditions for marsupial species in Brazil: Challenges and opportunities for conservation. PLoS ONE 2012, 7, e46257. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Valdés, O.; Dávila-Aranda, P. Protected areas and climate change: A case study of the Cacti in the Tehuacán-Cuicatlán Biosphere Reserve, México. Conserv. Biol. 2003, 17, 846–853. [Google Scholar] [CrossRef]
- Berg, M.P.; Kiers, E.T.; Driessen, G.; Van Der Heijden, M.; Kooi, B.W.; Kuenen, F.; Liefting, M.; Verhoef, H.A.; Ellers, J. Adapt or disperse: Understanding species persistence in a changing world. Glob. Change Biol. 2010, 16, 587–598. [Google Scholar] [CrossRef]
- Merilä, J.; Hendry, A.P. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. Evol. Appl. 2014, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.R.; Cassemiro, F.A. Potenciais efeitos das mudanças climáticas futuras sobre a distribuição de um anuro da Caatinga Rhinella granulosa (Anura, Bufonidae). Iheringia Sér. Zool. 2013, 103, 272–279. [Google Scholar] [CrossRef]
- Peh, K.S. Potential effects of climate change on elevational distributions of tropical birds in Southeast Asia. Condor 2007, 109, 437–441. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Jetz, W. Avian distributions under climate change: Towards improved projections. J. Exp. Biol. 2010, 213, 862–869. [Google Scholar] [CrossRef] [PubMed]
- De Kort, S.R.; Den Hartog, P.M.; Ten-Cate, C. Diverge or merge? The effect of sympatric occurrence on the territorial vocalizations of the vinaceous dove Streptopelia vinacea and the ring-necked dove S. capicola. J. Avian Biol. 2002, 33, 150–158. [Google Scholar] [CrossRef]
- Araujo-Lima, V.; Ferreira, R.B.; Oliveira, R.S.; Ferreira-Santos, K.; Garbin, M.L.; Duca, C. Spatial segregation between the native Tropical mockingbird and the invader Chalk-browed mockingbird along a Neotropical natural-urban gradiente. Zoologia 2023, 40, e22061. [Google Scholar] [CrossRef]
- Cody, M. Family Mimidae (Mockinbird and Thrashers). In Handbook of the Birds of the World: Cuckoos-Shrikes to Thrashers; del Hoyo, J.D., Elliot, A., Christie, A.D., Eds.; Lynx Editions: Barcelona, Spain, 2005; Volume 10, pp. 448–495. [Google Scholar]
- Argel-de-Oliveira, M.M. A Família Mimidae. Boletim CEO 1994, 10, 3–15. [Google Scholar]
- Sick, H. Ornitologia Brasileira; Editora Nova Fronteira: Rio de Janeiro, Brazil, 1997; p. 912. [Google Scholar]
- Ridgely, R.S.; Tudor, G. Birds of South America–Passerines; Christopher Helm Publishers Ltd.: London, UK, 2009; p. 750. [Google Scholar]
- Alves, M.A.S.; Pacheco, J.F.; Gonzaga, L.A.P.; Cavalcanti, R.B.; Raposo, M.; Yamashita, C.; Maciel, N.C.; Castanheira, M. Aves. In A Fauna Ameaçada de Extinção do Estado do Rio de Janeiro; Bergallo, H.G., Rocha., C.F.D., Alves, M.A.S., Van-Sluys, M., Eds.; Editora UERJ: Rio de Janeiro, Brazil, 2000; pp. 113–124. [Google Scholar]
- Chaves, F.G.; Duca, C.; Pinto, G.O.; Rosa, G.A.B.; Magnago, G.R.; Daros-Filho, H.J.; Passamani, J.A.; Silva, J.N.; Silva, J.P.; Bissoli, L.B.; et al. Aves ameaçadas de extinção no estado do Espírito Santo. In Fauna e Flora Ameaçada de Extinção no Estado do Espírito Santo; Fraga, C.N., Formigoni, H., Chaves, F.G., Eds.; Instituto Nacional da Mata Atlântica: Santa Teresa, Brazil, 2019; pp. 295–313. [Google Scholar]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridization and introgression. Ann. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Jetz, W.; Wilcove, D.S.; Dobson, A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007, 5, e157. [Google Scholar] [CrossRef]
- Gonzaga, L.P.; Castiglioni, G.D.A.; Reis, H.B.R. Avifauna das restingas do Sudeste: Estado do conhecimento e potencial para futuros estudos. In Ecologia de Restingas e Lagoas Costeiras; Esteves, F.A., Lacerda, L.D., Eds.; Computer & Publish Editoração Gráfica, Núcleo de Pesquisas Ecológicas de Macaé (NUPEM/UFRJ): Rio de Janeiro, Brazil, 2000; Volume 1, pp. 151–163. [Google Scholar]
- Reis, H.B.R.; Gonzaga, L.P. Análise da distribuição geográfica das aves das restingas do Estado do Rio de Janeiro. In Ecologia de Restingas e Lagoas Costeiras; Esteves, F.A., Lacerda, L.D., Eds.; Computer & Publish Editoração Gráfica; Núcleo de Pesquisas Ecológicas de Macaé (NUPEM/UFRJ): Rio de Janeiro, Brazil, 2000; Volume 1, pp. 165–178. [Google Scholar]
- Leveau, L.M.; Leveau, C.M. Comunidades de ave en un gradient urbano de la Ciudad de Mar del Plata, Argentina. Hornero 2004, 19, 13–21. [Google Scholar] [CrossRef]
- Argel-de-Oliveira, M.M.; Pacheco, J.F. Um resumo da situação: Mimus saturninus e M. gilvus no litoral sudeste brasileiro. Bol. FBCN 1998, 25, 53–69. [Google Scholar]
- IBGE-Instituto Brasileiro de Geografia e Estatística. Mapa de Vegetação do Brasil; IBGE: Brasília, Brazil, 1988. [Google Scholar]
- Pereira, O.J. Restinga: Origem, estrutura e diversidade. In Desafios da Botânica Brasileira no Novo Milênio: Inventário, Sistematização e Conservação da Diversidade Vegetal; Jardim, M.A.G., Bastos, N.N.C., Santos, J.U.M., Eds.; Embrapa, Museu Paraense Emílio Goeldi, UFRA: Belém, Brazil, 2003; pp. 177–179. [Google Scholar]
- Oliveira-Filho, A.T.; Fontes, M.A.L. Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate 1. Biotropica 2000, 32, 793–810. [Google Scholar] [CrossRef]
- Mantovani, W. Delimitação do bioma Mata Atlântica: Implicações legais e conservacionistas. In Ecossistemas Brasileiros: Manejo e Conservação, 1st ed.; Expressão Gráfica e Editora: Fortaleza, Brazil, 2003; pp. 287–295. [Google Scholar]
- Gomes, V.S.M.; Loiselle, B.A.; Alves, M.A.S. Birds foraging for fruits and insects in shrubby resting vegetation, southeasthern Brazil. Biota Neotrop. 2008, 7, 21–31. [Google Scholar] [CrossRef]
- Morton, E.S.; Stutchbury, B.J.M.; Piper, W.H. Cooperative breeding in the Tropical Mockingbird (Mimus gilvus) in the Panama canal zone. Ornitol. Neotrop. 2004, 15, 417–421. [Google Scholar]
- Morais, R.; Araújo, L.C.; Silva, G.R.; Duca, C. Multiple nesting attempts and long breending seasons of Mimus gilvus (Aves: Mimidae) in southeastern Brazil. Zoologia 2019, 36, e25717. [Google Scholar] [CrossRef]
- Oliveira, D.V.; Pichorim, M. Breeding biology of the tropical mockingbird Mimus gilvus (Aves: Mimidae) in northeastern Brazil. Ornithol. Res. 2024, 32, 179–189. [Google Scholar] [CrossRef]
- Araujo, L.C. Conservação do Sabiá-da-Praia Mimus gilvus (Aves: Mimidae) um uma Reserva de Restinga na Região Sudeste do Brasil. Ph.D. Thesis, Universidade Vila Velha, Vila Velha, ES, Brazil, 2016. [Google Scholar]
- Zanon, M.S.; Valeand, M.M.; Alves, M.A.S. Missing for the last twenty years: The case of the southernmost populations of the Tropical mokingbird Mimus gilvus (Passeriformes: Mimidae). Zoologia 2015, 32, 1–8. [Google Scholar] [CrossRef]
- Argel-de-Oliveira, M.M. Eco-Etologia do Sabiá-do-Campo Mimus saturninus (Lichtenstein, 1823) (Passeriformes, Mimidae) no Estado de São Paulo. Master’s Thesis, Universidade Estadual de Campinas, Campinas, SP, Brazil, 1989. [Google Scholar]
- Rodrigues, S.S.; Lopes, L.E.; Marini, M.Â. Breeding biology of Chalk-browed Mockingbird Mimus saturninus in a natural savanna of central Brazil. Rev. Brasil. Ornit. 2017, 25, 237–244. [Google Scholar] [CrossRef]
- de la Colina, M.A.; Pompilio, L.; Hauber, M.E.; Reboreda, J.C.; Mahler, B. Parasitic egg rejection decisions of chalk-browed mockingbirds Mimus saturninus are independent of clutch composition. Anim. Cogn. 2018, 21, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Fiorini, V.D.; Crudele, I.; Reboreda, J.C.; Pladas, S.A.; Watson, A.P.; Bush, S.E.; Clayton, D.H. Co-parasitism in the face of predation: Effects of natural enemies on a neotropical mockingbird. J. Anim. Ecol. 2023, 92, 1992–2004. [Google Scholar] [CrossRef] [PubMed]
- CRIA (Centro de Referência e Informação Ambiental). Available online: http://splink.cria.org.br (accessed on 31 July 2018).
- GBIF Occurrence Download. Available online: http://doi.org/10.15468/dl.ackhc2 (accessed on 12 February 2018).
- Chapman, F.M. A List of Birds Observed at Santarem, Brazil. Auk 1890, 7, 131–137. [Google Scholar] [CrossRef]
- Hellmayr, C.E. An account of the birds collected by Mons. G.A. Baer in the state of Goyaz, Brazil. Novit. Zool. 1908, 15, 13–102. [Google Scholar]
- Rogers, S. CM Birds Collection, Version 9.1; Carnegie Museums: Pittsburgh, PA, USA, 1926. [Google Scholar]
- Smyth, C.H. Descripción de una colección de huevos de aves argentinas. Hornero 1928, 4, 125–152. [Google Scholar] [CrossRef]
- Cicero, C. MVZ Bird Collection (Arctos), Version 43.9; Museum of Vertebrate Zoology: Berkeley, CA, USA, 1930. [Google Scholar]
- Ridgely, R.S.; Tudor, G. The Birds of South America: The Oscine Passerines; University of Texas Press: Austin, TX, USA, 1989; Volume 1, p. 516. [Google Scholar]
- Mageski, M.M.; Varela, S.; Roper, J.J. Consequences of dispersal limitation and habitat fragmentation for the Brazilian heart-tongued frogs (Phyllodytes spp.). Austral Ecol. 2018, 43, 547–557. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.; Parra, J.; Jones, P.G.; Jarvis, A.; Richardson, K. WorldClim, Version 1.3; University of California: Berkeley, CA, USA, 2005. [Google Scholar]
- Martínez-Gutiérrez, P.G.; Martínez-Meyer, E.; Palomares, F.; Fernández, N. Niche centrality and human influence predict rangewide variation in population abundance of a widespread mammal: The collared peccary (Pecari tajacu). Divers. Distrib. 2018, 24, 103–115. [Google Scholar] [CrossRef]
- Wright, A.N.; Hijmans, R.J.; Schwartz, M.W.; Shaffer, H.B. Multiple sources of uncertainty affect metrics for ranking conservation risk under climate change. Divers. Distrib. 2015, 21, 111–122. [Google Scholar] [CrossRef]
- O’Donnel, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. US Geol. Surv. Data Ser. 2012, 691, 10. [Google Scholar]
- Ornelas, J.F.; Licona-Vera, Y.; Ortiz-Rodriguez, A.E. Contrasting responses of generalized/specialized mistletoe-host interactions under climate change. Écoscience 2018, 25, 223–234. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.J.; Leathwick, J.R.; Elith, J. Dismo: Species Distribution Modeling, R package version 1.0-15; 2016. Available online: https://CRAN.R-project.org/package=dismo (accessed on 12 February 2018).
- Booth, T.H. Why understanding the pioneering and continuing contributions of BIOCLIM to species distribution modelling is important. Austral Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef]
- Williams, S.E.; Bolitho, E.E.; Fox, S. Climate change in Australian tropical rainforests: An impending environmental catastrophe. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, L.J.; Hughes, L.; Poulsen, M. Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol. Model. 2005, 186, 251–270. [Google Scholar] [CrossRef]
- Palhano, S.; Raposo, M.A.; Cordeiro, P.H.; Weksler, M. Genealogical and niche modeling analyses reveal recent expansion and limited genetic divergence in the Formicivora serrana complex (Passeriformes: Thamnophilidae). J. Ornithol. 2018, 159, 79–92. [Google Scholar] [CrossRef]
- Vale, M.M.; Souza, T.V.; Alves, M.A.S.; Crouzeilles, R. Planning protected areas network that are relevant today and under future climate change is possible: The case of Atlantic Forest endemic birds. PeerJ 2018, 6, e4689. [Google Scholar] [CrossRef] [PubMed]
- Kindt, R. Ensemble species distribution modelling with transformed suitability values. Environ. Modell. Softw. 2018, 100, 136–145. [Google Scholar] [CrossRef]
- Giliba, R.A.; Yengoh, G.T. Predicting suitable habitats of the African cherry (Prunus africana) under climate change in Tanzania. Atmosphere 2020, 11, 988. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Tjoelker, M.G.; Lenoir, J.; Baumgartner, J.B.; Beaumont, L.J.; Nipperess, D.A.; Power, S.A.; Richard, B.; Rymer, P.F.; Gallagher, R.V. Climate change increases global risk to urban forests. Nat. Clim. Change 2022, 12, 950–955. [Google Scholar] [CrossRef]
- Booth, T.H. Species distribution modelling tools and databases to assist managing forests under climate change. Forest Ecol. Manag. 2018, 430, 196–203. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- QGIS 3.2.3 Development Team. QGIS Geographic Information System, Version 3.2.3; Open-Source Geospatial Foundation: Beaverton, OR, USA, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Ding, C.; Newbold, T.; Ameca, E.I. Assessing the global vulnerability of dryland birds to heatwaves. Glob. Change Biol. 2024, 30, e17136. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Heikkinen, R.K.; Kuussaari, M.; Lehikoinen, A.; Luoto, M.; Pöyry, J.; Virkkala, R.; Saastamoinen, M.; Kujala, H. Recent range shifts of moths, butterflies, and birds are driven by the breadth of their climatic niche. Evol. Lett. 2024, 8, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M. Understanding the effect of climate change on migratory birds: A review. Int. J. Zool. Animal Biol. 2024, 7, e000560. [Google Scholar] [CrossRef]
- Naves-Alegre, L.; García-Mayoral, H.; Morant, J.; Pérez-García, J.M.; Dias, A.; Cano-Montes, E.; Sánchez, A.; García-Matarranz, V. Differential flight responses of sympatric raptor species to weather conditions and extreme temperature events. Ecol. Evol. 2025, 15, e70658. [Google Scholar] [CrossRef] [PubMed]
- Duca, C.; Yokomizo, H.; Marini, M.Â.; Possingham, H.P. Cost-efficient conservation for the White-banded Tanager (Neothraupis fasciata) in the Cerrado, central Brazil. Biol. Conserv. 2009, 142, 563–574. [Google Scholar] [CrossRef]
- Duca, C.; Mairni, M.Â. Variation in breeding of the Shrike-like Tanager in central Brazil. Wilson J. Ornithol. 2011, 123, 259–265. [Google Scholar] [CrossRef]
- Chamberlain, D.; Lehikoinen, A.; Scridel, D.; Martin, K. Mountain birds and their habitats. In Ecology and Conservation of Mountain Birds; Chamberlain, D., Lehikoinen, A., Martin, K., Eds.; Cambridge University Press: Cambridge, UK, 2023; pp. 1–34. [Google Scholar]
- Bencke, G.A.; Maurício, G.N.; Develey, P.F.; Goerck, J.M. Áreas Importantes para a Conservação das Aves no Brasil, Parte I–Estados do Domínio da Mata Atlântica; SAVE Brasil: São Paulo, SP, Brazil, 2006. [Google Scholar]
- Fokidis, H.B.; Greiner, E.C.; Deviche, P. Interspecific variation in avian blood parasites and haematology associated with urbanization in a desert habitat. J. Avian Biol. 2008, 39, 300–310. [Google Scholar] [CrossRef]
- Hannah, L. Protected areas and climate change. Ann. N. Y. Acad. Sci. 2008, 1134, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Pressey, R.L.; Cabeza, M.; Watts, M.E.; Cowling, R.M.; Wilson, K.A. Conservation planning in a changing world. Trends Ecol. Evol. 2007, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-Lima, V.; Oliveira, R.d.S.; Mageski, M.; Ferreira, R.B.; Duca, C. Climate Changes Can Restore Allopatry Between Two Congeneric Birds in the Atlantic Forest. Birds 2025, 6, 42. https://doi.org/10.3390/birds6030042
Araujo-Lima V, Oliveira RdS, Mageski M, Ferreira RB, Duca C. Climate Changes Can Restore Allopatry Between Two Congeneric Birds in the Atlantic Forest. Birds. 2025; 6(3):42. https://doi.org/10.3390/birds6030042
Chicago/Turabian StyleAraujo-Lima, Vitor, Rayane dos Santos Oliveira, Marcio Mageski, Rodrigo Barbosa Ferreira, and Charles Duca. 2025. "Climate Changes Can Restore Allopatry Between Two Congeneric Birds in the Atlantic Forest" Birds 6, no. 3: 42. https://doi.org/10.3390/birds6030042
APA StyleAraujo-Lima, V., Oliveira, R. d. S., Mageski, M., Ferreira, R. B., & Duca, C. (2025). Climate Changes Can Restore Allopatry Between Two Congeneric Birds in the Atlantic Forest. Birds, 6(3), 42. https://doi.org/10.3390/birds6030042




