Simple Summary
The study focused on comparing the diversity of ascomycetous, potentially pathogenic yeast species (Candida spp.) in the fresh and dry feces of five synanthropic birds (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow). Significant differences in the microbiome of the pathogens were found in the fresh feces of the different hosts. The most diverse pathogen–yeast complexes were found in the fresh feces of pigeons. In contrast to the fresh feces, the dry samples did not differ significantly in terms of pathogen diversity between the different birds. And, they were generally characterized by a lower number of culturable, potentially pathogenic yeasts. Fresh and dry feces from synanthropic birds in the city (especially pigeons) are thus a source of a large number of potentially pathogenic yeasts. Contact with them (including indirect contact, e.g., through shoe soles carrying contaminants indoors from the environment) should be avoided to prevent the transmission of infection from synanthropic birds to humans. While such infection is not a risk for healthy people, it is a potential health hazard for immunocompromised people or children whose immune systems are not yet sufficiently developed.
Abstract
Public health in a densely populated city is inextricably linked to the state of the urban environment. The microclimate, the condition of water sources and sanitary well-being are just some of the many environmental factors that have a strong influence on people’s health. The presence of urban green spaces and various birds in cities is extremely important, also to create a more favorable psychological atmosphere for the people who live and/or work there. At the same time, it should not be forgotten that the feces of synanthropic birds are a favorable environment for various potentially pathogenic species of microorganisms, including yeasts of the genus Candida. Here, we investigated the culturable, potentially pathogenic ascomycetous yeast microbiome in the fresh and dry feces of five synanthropic birds (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow). The samples were collected in spring (May 2024). In total, 48 Rock Pigeon, 47 European Starling, 38 White Wagtail, 32 Great Tit and 30 House Sparrow droppings were collected and analyzed. The selective medium Brilliance Candida Agar was used for cultivation. A total of 638 strains were isolated belonging to 9 yeast species (Arxiozyma bovina, Candida albicans, Nakaseomyces glabratus, Clavispora lusitaniae, C. tropicalis, C. parapsilosis, Pichia kudriavzevii, Debaryomyces hansenii and D. fabryi). All detected yeast species were molecularly identified using the ITS rDNA region. The microbiome of potential pathogens in fresh feces proved to be significantly host-dependent. Most pathogenic yeasts (7 species)—A. bovina, C. albicans, N. glabratus, Cl. lusitaniae, C. tropicalis, C. parapsilosis and P. kudriavzevii—were only detected in fresh feces from pigeons. This list contains five out of six ascomycetous species from the list of critical, high and medium-important yeast pathogens published in the World Health Organization fungal list. Of the potentially pathogenic yeasts, two species were observed in the dry droppings of various birds: C. parapsilosis and P. kudriavzevii. No significant differences in the diversity of culturable pathogens in dry droppings were observed between the different hosts. Fresh droppings from synanthropic birds, especially pigeons (and to a lesser extent dry droppings), therefore pose a health risk. In this study, we did not find any feces from synanthropic birds in which potentially pathogenic ascomycetous yeasts were not detected. To maintain the sanitary safety and well-being of citizens, it is very important to regulate the number of synanthropic birds (primarily pigeons), especially in sensitive areas such as playgrounds, hospital territories, etc.
1. Introduction
Urban areas consist mainly of true synanthropes and semi-synanthropic species whose food sources and nesting sites are largely dependent on human activities [1,2]. These species (Common Swift (Apus apus), European Starlings (Sturnus vulgaris), Fieldfare (Turdus pilaris), House Sparrow (Passer domesticus) and Rock Pigeon (Columbia livia) nest directly in buildings (attics, ventilation holes and cracks in walls), using holes in lampposts and metal pipes to build their nests. They are characterized by their adaptive behavior and the ability to find new food sources quickly enough in various niches in the cities [3,4,5,6].
It is extremely important and necessary to maintain the presence of urban green spaces and various birds in cities, also to create a more favorable psychological atmosphere for people living and/or working in cities [7,8,9]. At the same time, it should not be forgotten that bird droppings (microorganic) are a favorable environment for the development of various potentially pathogenic species of microorganisms, including yeasts of the genera Candida, Cryptococcus, Rhodotorula, Trichosporon and some others that can be hazardous to human health [10]. While the vast majority of potentially pathogenic yeasts, once they have invaded the body of healthy people, do not find suitable conditions or are unable to withstand the protective reactions of the human body, therefore preventing the development of the infection, the likelihood of a yeast infection is significantly higher in people whose immune systems are suppressed [11,12]. Therefore, the spread of microorganisms via droppings becomes a public health concern, especially in sensitive hospital areas where immunocompromised people are more likely to come into contact with pathogens from bird droppings, or in playground areas where children whose immune systems are not yet sufficiently developed are present [10]. However, bird droppings can also be spread everywhere in various urban areas with shoes or clothing, and they can enter private houses and apartments, workplaces, schools or daycares, where the pathogens can be transmitted to humans. Bird droppings can become particles in indoor dust and the pathogens can be transmitted by inhalation, contaminate the mucous membranes of the eyes via the hands and penetrate through microcracks and wounds on the skin [13,14,15,16,17]. They can also pose a contamination risk after rainfall in sewage systems [18].
To date, most data on the presence of pathogenic yeasts in fresh feces of synanthropic birds worldwide have been collected from Rock Pigeons. A large proportion of these studies have been conducted in countries in South America, Africa and Asia [13,19,20,21,22,23]. Populations of these particular birds have been shown to increase with human density, probably due to the fact that pigeons are omnivores [24]. In a recent study of fungal pathogens in fresh pigeon droppings, based on the World Health Organization’s priority pathogen list published in 2022 [25], metagenomic analysis showed for the first time that the samples collected in Uppsala contained all critical, high and medium-important yeast pathogens, such as C. albicans, C. auris, Cryptococcus neoformans, N. glabratus, C. tropicalis, C. parapsilosis, P. kudriavzeveii and Cr. gattii [10]. To our knowledge, this was the first detection of C. auris (Candidozyma auris), an emerging drug-resistant human pathogenic yeast in bird feces. C. auris is a serious threat to public health, possibly caused by climate change. It persists on human skin and inanimate objects and often causes difficult-to-control outbreaks in healthcare facilities [26].
Much of the research on the feces of synanthropic birds as reservoirs for the development of potential pathogens is also devoted to various gull species (Yellow-legged Gull (Larus michahellis), Herring Gull (Larus argentatus), Kelp Gull (L. dominicanus), Lesser Black-backed Gull (L. fuscus) and Mew Gull (L. canus) [27,28,29,30,31]. The feces of wild, migratory, domestic and ornamental birds are also under investigation [32,33,34,35,36,37]. To our knowledge, potentially pathogenic ascomycetous yeasts have not yet been investigated in the feces of various synanthropic birds such as European Starling, White Wagtail (Motacilla alba), Great Tit (Parus major) and House Sparrow, which are common throughout Europe.
Despite the fact that all synanthropic birds use “junk” food in their diet (as probably one of the most important sources of potentially pathogenic yeasts), the reliance on it nevertheless varies from species to species. Diet is reported to be the most influential factor affecting the avian gut microbiome, followed by preferred habitats, nesting environments, social interactions and seasonal variation [38,39,40]. However, the current scarcity and wide variation in reported patterns limit a clear understanding of how avian ecology influences the gut microbiome [41]. Yeast complexes are commonly examined in fresh excretions. However, there is little comparative data on yeast complexes of potential pathogens in feces of different “ages”. In one study comparing yeast complexes in fresh and dry feces of gulls, no significant differences were found [29].
In this context, we tried to compare the complexes of potentially pathogenic ascomycetous yeasts in the fresh and dry (45 and 90 days “old”) feces of five synanthropic bird species, Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow, with the highest population density in the city of Moscow. We hypothesized that the fresh feces of various synanthropic host birds in one area have a different “pathogenic yeast load” and therefore differ in the probability and intensity of being a potential health hazard.
2. Materials and Methods
2.1. Study Sites
Fecal samples were collected in Moscow (55°44′24″ N 37°36′36″ E). Moscow is one of the most populous cities in Russia with a permanent population of around 14 million people. It is located in the center of the Eastern European Plain in a temperate continental climate zone with clearly defined seasons. The vast majority of plants in the city belong to the Magnoliophyta section. Spore and coniferous plants make up about 2% of the species. In general, herbaceous plants make up about 90% of the city’s flora. Trees, shrubs, bushes and semi-shrubs make up only 10% of the flora. Among the woody plants, trees predominate [42]. The samples were collected in the northern, north-eastern, eastern and south-eastern districts of the city. These are the districts where most working city residents and migrant workers live and where most of the typical small city parks like kindergartens, hospitals, industrial plants and landfills are located. Currently, the order of the number of birds we studied in Moscow is approximately as follows: Rock Pigeon (300–500 individuals/km2); House Sparrow (100–500 individuals/km2, with an upward trend); Great Tit (2–10 individuals/km2); White Wagtail (10–15 pairs/km2, with an upward trend); and European Starling (1–10 pairs/km2, with an upward trend) [42,43].
The samples were taken in May 2024 on nine days of clear weather without precipitation (May 19, 20, 22, 24, 25, 27, 28, 29 and 30) [44] in city parks, yards near benches and feeding areas, playgrounds, hospital areas, near metro stations and large supermarkets and on sidewalks with green areas along major highways. The required samples were taken from the fresh droppings of each bird found on soil, in the grass, on leaves of shrubs and young trees (at a height of no more than 2 m), on asphalt paths and curbs, on wooden and iron parts of benches, on swings and on the rubberized surface of playgrounds, taking care not to harm the birds. Sampling was carried out during the day from 11 am to 4 pm. The average daily air temperature during the sampling period was +24 °C and the average humidity was 35% [44].
2.2. Sampling and Processing
In total, 48 Rock Pigeon, 47 European Starling, 38 White Wagtail, 32 Great Tit and 30 House Sparrow droppings were collected over nine days. Three subsamples were taken from each fecal sample of each bird and analyzed for three tested “age” categories (fresh—tested no later than 6 h after defecation; 45 days old—tested after 45 days of storage in the laboratory; and 90 days old—tested after 90 days of storage in the laboratory). Thus, 195 fresh subsamples (48 + 47 + 38 + 32 + 30), 195 dry 45-days “old” subsamples and 195 dry 90-days “old” subsamples were analyzed. Each subsample (swab) was plated in three replicates. Subsamples of different “ages” from one bird species and between bird species were compared.
We waited for cases of defecation. The temperature of fresh feces measured with a DT-8865 pyrometer (Sem Test Instrument, Shenzhen, China) within 5 min after defecation of the bird and before sampling was in the range of +32–35 °C. To minimize errors when sampling different bird species in the same area, each team member was “assigned” to a specific bird species prior to sampling to observe defecation and collect samples on that day. Never did a team member “work” with different bird species on the same day. Any doubt about which bird species the fecal sample belonged to was interpreted by the sampler as “if in doubt, do not take”. In addition, team members had to take random photos during sampling and send them to the supervisor to confirm the correctness of the task performed. The bird species selected for the study can be distinguished quite well by non-professional ornithologists based on phenotype. There could be confusion between House Sparrow (whose droppings we examined) and Tree Sparrow (Passer montanus). However, the number of the latter is currently lower in Moscow [45]. Samples were collected using sterile gloves and sterile viscose swabs (1.8 ± 0.5 mm). After observation, three subsamples (three swabs) were collected from each fresh fecal sample. The first swab was dipped in 3 mL of sterile saline (0.9% NaCl) supplemented with 0.5 g/L chloramphenicol transport medium, and the second and third swabs were without transport medium. In this way, three swabs per one fecal sample were collected and delivered to the laboratory for analysis. Each sample and subsample were assigned a unique identifier according to origin and date. All microbiological manipulations in the laboratory, including plating, were performed in a sterile laminar box (Esco Airstream Gen 3 LVG-AG-F, Esco Lifesciences Group, Singapore) with sterile gloves. In addition, the hands of the operators were pre-tested for the absence of opportunistic yeasts on the skin to prevent contamination of the material to be tested. Potentially pathogenic ascomycetous yeasts are easily removed from the skin if a disinfectant is used. At the same time, contamination of the material and obtaining false positive results is not acceptable when testing a potentially pathogenic yeast community from different substrates. The first of three swabs (with transport medium) was processed within 6 h of collection. The swab was shaken in the transport medium on a Multi Reax Vortexer (Heidolph Instruments, Schwabach, Germany) for 15 min at 2000 rpm and then allowed to sediment for 15 min. After the swab was removed, the supernatant was transferred to a sterile conical tube. Subsequently, 100 μL of the supernatant was inoculated onto chromogenic medium Brilliance Candida Agar (Thermo Scientific™, Waltham, MA, USA) for rapid isolation and presumptive identification of clinically important Candida species. The chromogenic color reactions on an opaque background allow a quick and easy differentiation of Candida spp. especially when diverse complexes are present. Chloramphenicol inhibits bacterial growth, even after prolonged incubation. Grown colonies that did not show characteristic coloration were also counted; representatives were isolated in pure culture and then identified without rapid identification using only molecular methods (see below). It should be noted that organisms with an atypical enzyme pattern may give anomalous reactions on Brilliance Candida Agar. Therefore, we randomly selected colonies with typical coloration and performed molecular identification to minimize the number of errors. The plates were incubated aerobically at 30 °C. Colony growth was examined after 24, 48, 72, 96 and 120 h. Candida albicans ATCC 10231, Candida parapsilosis ATCC 22019 and Candida tropicalis ATCC 750 were used as positive media quality controls and Escherichia coli ATCC 25922 as negative media quality control. The second and third swabs (without transport medium) were stored in the laboratory at 22 °C and 40% humidity for 45 and 90 days, respectively. The control of the microclimate in the laboratory was carried out with an autonomous thermohygrometer IVA-6 (Microfor, Russia). On day 45 and on day 90, 3 mL of sterile saline (0.9% NaCl) supplemented with 0.5 g/L chloramphenicol was added to the second and third swab, respectively, and then, the same manipulations were performed as for the first swab.
2.3. Molecular Identification of Pure Cultures
All grown yeast colonies were counted and representatives were isolated in a pure culture. Representatives of each colony type were molecularly identified, including colonies of potentially pathogenic Candida with a typical colony appearance (expected phenotypic colony characteristics on Brilliance Candida Agar), to verify and validate rapid presumptive identification and colonies without a characteristic phenotype on the chromogenic medium. The yeast species (pure cultures) were molecularly identified using the ITS rDNA region as a universal DNA-barcoding for fungi [46]. The nuclear ribosomal ITS1-5.8S-ITS2 region was amplified and sequenced using the ITS5 primer. The criteria described in Vu [47] were used to separate the yeast species. DNA isolation and PCR were performed according to the previously described procedure [48,49]. DNA sequencing was performed using the Big Dye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) with subsequent analysis of reaction products on an Applied Biosystems 3130xl Genetic Analyzer at the facilities of Evrogen (Moscow, Russia). For sequencing, the ITS5 primer (5′-GGA AGT AAA AGT CGT AAC AAG G) was used [48]. For species identification, the nucleotide sequences were compared with those in public databases using the BLAST NCBI (www.ncbi.nlm.nih.gov (accessed on 20 January 2025)) and the MycoID (www.mycobank.org (accessed 20 January 2025)) tools. The ITS regions of the strains studied were 99.8–100%, similarly to the type strains. Sequences obtained in the present study for yeast species were deposited in the GenBank database (PP905608; PV089567–PV089574). The isolated and sequenced yeast strains were cryopreserved in 10% (v/v) glycerol in water solution at −80 °C in the yeast collection of the Soil Biology Department at Lomonosov Moscow State University.
2.4. Statistical Data Analyses
All statistical analyses were performed in PAST.5.02. (Oslo, Norway). The species diversity of the yeasts was estimated using the Shannon index. Species evenness was assessed using the Pielou index. To visualize the relative importance of the effect of a bird host on the beta diversity of ascomycetous, potentially pathogenic yeasts, we ran non-metric multidimensional scaling (NMDS) ordination using the Bray–Curtis dissimilarity index combined with permutation testing (one-way ANOSIM). Due to the non-normal distribution of the data (Shapiro–Wilk test: p < 0.05), the non-parametric Kruskal–Wallis test was applied, followed by a post hoc Dunn’s test for multiple pairwise comparisons at a significance level of p ≤ 0.01 with Bonferroni adjustment, to determine the significant differences in the relative abundance of eight potentially pathogenic yeast species in the fresh feces of five synanthropic birds (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow). Row normalized length was used to transform table of data. Linear discriminant analysis (LDA) combined with permutation testing (one-way ANOSIM and one-way PERMANOVA) was used to account for the maximum significant separability among feces of three “ages” (fresh, 6 h “old”; and dry, 45 and 90 days “old”) based on relative abundance of culturable yeast species.
3. Results
In this study, a total of nine species of culturable ascomycetous yeasts, seven potentially pathogenic species (A. bovina, C. albicans, N. glabratus, Cl. lusitaniae, C. tropicalis, C. parapsilosis and P. kudriavzevii) and two eurybionts (Debaryomyces hansenii and D. fabryi) were found in fresh feces (Figure 1, Table 1).
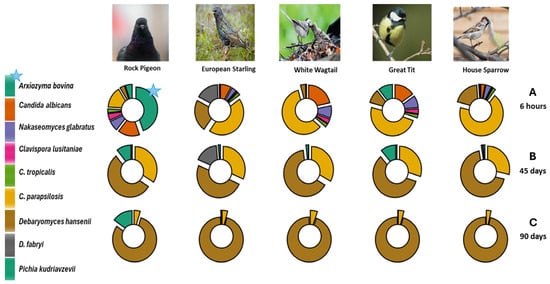
Figure 1.
Species of ascomycetous yeast taxa found in fresh (6 h) (A) and dry (45 days “old” (B) and 90 days “old” (C)) feces of five synanthropic birds (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow) collected in Moscow (May 2024). Photo: Rock Pigeon by Safron Golikov; European Starling from [50]; White Wagtail by Vadim Boyarkin and Yulia Nahimova; Great Tit from [51]; and House Sparrow by Ilya Ukolov.

Table 1.
Species list and relative abundance of yeast taxa isolated from fresh and dry feces. The indices of species diversity and community evenness can be found below.
All nine detected yeast species were cultured in different ratios from fresh feces only; a maximum of five yeast species were cultured from dry feces (45 days “old”); and a maximum of three yeast species were cultured from dry feces (90 days “old”). All seven potential pathogens were only detected in the fresh droppings of Rock Pigeon; six species were found in the fresh droppings of the other four synanthropic birds (European Starling, White Wagtail, Great Tit and House Sparrow). The potentially pathogenic yeast A. bovina was detected in high relative abundance only in the fresh droppings of Rock Pigeon. Four potential pathogens were only found in fresh droppings (6 h) and were not cultured from dry samples (45 and 90 days “old”). These species were A. bovina, C. albicans, N. glabratus and Cl. lusitaniae. Only two potential pathogens, C. parapsilosis and P. kudriavzevii, and eurybionts of the genus Debaryomyces were cultured from maximally dry feces (90 days “old”) (Figure 1).
Differences in the relative abundance of cultured ascomycetous yeasts were found in fresh feces from different synanthropic host birds. Multiple group comparison (eight yeast species from fresh feces of five bird species; A. bovina was excluded from the comparison as it was only found in fresh feces of Rock Pigeon) showed significant differences in some pairs (Figure 2, Table 2).

Figure 2.
Multiple group comparison of the relative abundance (row normalized length) of eight ascomycetous yeast species ((A)—C. albicans; (B)—N. glabratus; (C)—Cl. lusitaniae; (D)—C. parapsilosis; (E)—C. tropicalis; (F)—P. kudriavzevii; (G)—D. hansenii; and (H)—D. fabryi) from the fresh feces of five synanthropic birds (1—Rock Pigeon; 2—European Starling; 3—White Wagtail; 4—Great Tit; and 5—House Sparrow) collected in Moscow (May 2024). Kruskal–Wallis test followed by post hoc Dunn’s test with the Bonferroni adjustment. Cross line represents the median. Vertical spread of the box depicts interquartile range encompassing the middle 50% of data. Dots denote outliers.

Table 2.
Multiple group comparison of the relative abundance of eight ascomycetous yeast species from the fresh feces of five synanthropic birds (Kruskal–Wallis test followed by post hoc Dunn’s test with the Bonferroni adjustment). p ≤ 0.01 was used as the statistical significance cut-off value.
The community structure of cultured ascomycetous, potentially pathogenic yeasts differed significantly in fresh feces (6 h) of different species of synanthropic birds. As drying progressed, these differences decreased (45 days), and in dry feces (90 days), no significant differences were detected between cultured ascomycetous yeast complexes in feces of different bird species. Non-metric multidimensional scaling (NMDS) ordinations of yeast communities in feces of different “ages” (6 h, 45 days, and 90 days) from five synanthropic bird species (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow) based on Bray–Curtis dissimilarities showed that the origin of the sample (host bird) was the main factor determining the composition of ascomycetous, potentially pathogenic yeast communities in fresh feces (Figure 3).
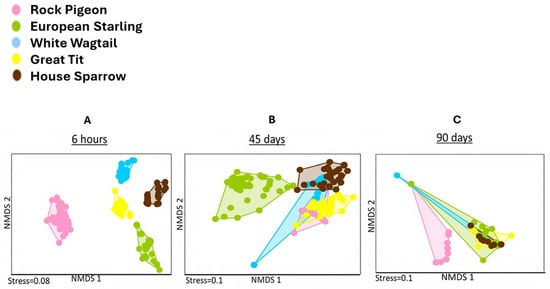
Figure 3.
Non-metric multidimensional scaling (NMDS) ordinations of potentially pathogenic ascomycetous yeast complexes based on Bray–Curtis dissimilarities between 6 h “old” (A), 45-days “old” (B) and 90-days “old” (C) feces of five synanthropic birds (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow) collected in Moscow (May 2024) show that the bird species is the main driver of the yeast community composition in the fresh (6 h) feces (one-way ANOSIM pairwise test with Bonferroni-corrected p-values, p = 0.001, R = 0.999) and in the dry 45-days “old” feces (one-way ANOSIM pairwise test with Bonferroni-corrected p-values, p = 0.001, R = 0.781) but not in the dry 90-days “old” feces (one-way ANOSIM pairwise test with Bonferroni-corrected p-values, p > 0.05, R = 0.446). Each point represents one sample of feces. The colors of points indicate the bird species.
To maximize the separability between the yeast complexes in fresh and dry feces, we used linear discriminant analysis (Figure 4).

Figure 4.
Linear discriminant analysis (LDA) plot displaying significant (one-way PERMANOVA pairwise test with Bonferroni-corrected p-values, p = 0.0003 and one-way ANOSIM pairwise test with Bonferroni-corrected p-values, p = 0.0003, R = 0.834) separability between three groups (“ages”) of feces (fresh 6 h “old” and dry 45 days “old” and 90 days “old” since defecation) in ascomycetous yeast complexes. The designated yeasts on the plot are the species cultured and contributed to the three “age” groups of feces’ separability. Each point represents one sample of feces. The colors represent feces age.
It was found that the potentially pathogenic ascomycetous yeast complexes were strongly shaped by the “age” of the feces. The potentially pathogenic yeasts A. bovina, C. albicans, N. glabratus and Cl. lusitaniae were only cultured from fresh feces. In 45-days “old” feces, the potentially pathogenic ascomycetes C. tropicalis and P. kudriavzevii were cultured in low relative abundance and about 30% of the potential pathogens was C. parapsilosis. In 90-days “old” feces, more than 90% of the cultured ascomycetous yeast complex was already the eurybiont D. hansenii and 3–5% was a potential pathogen C. parapsilosis. Only 90-days “old” Rock Pigeon droppings were a significant exception, in which the proportion of the cultured potential pathogen P. kudriavzevii was more than 15%, but the main proportion (more than 80%) was, as in all other 90-days “old” droppings, the eurybiont D. hansenii.
4. Discussion
This study showed significant differences in the relative abundance of clinically important ascomycetous yeast pathogens in the fresh droppings of different hosts. The most diverse pathogenic yeast complexes were found in the fresh droppings of Rock Pigeons. As far as we know, this is the first time—with the exception of Rock Pigeon—that clinically important pathogenic ascomycetous yeasts have been studied in the feces of different synanthropic birds simultaneously in the same urban area in Europe. In contrast to the fresh droppings, the dry samples did not differ significantly between the different birds in terms of pathogen diversity and were characterized by a lower number of culturable pathogenic yeasts.
Five of the six potentially pathogenic yeasts from the list of critical, high and medium-important yeast pathogens published in the World Health Organization fungal list, C. albicans, C. parapsilosis, C. tropicalis, N. glabratus (Candida glabrata) and P. kudriavzevii (Candida krusei) were detected in the fresh feces of all five synanthropic birds investigated (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow), representing the shared core of the pathogenic ascomycetous yeast microbiome of these synanthropic wild birds. The presence of these pathogens in the feces of the best-studied birds to date—pigeons [19,20,21,22,23,24,52], gulls [27,28,29,30,31], parrots [36,53] and certain species of wild and domestic birds [32,33,34,35,37,54]—has been demonstrated in many studies worldwide. Here, we find the marked difference in the relative abundance of two species of potential pathogens, C. albicans and C. parapsilosis, interesting. The proportion of C. albicans in the fresh droppings of Rock Pigeon, White Wagtail and Great Tit was between 15 and 20%, compared to 8% in the fresh droppings of European Starling and only 3% in the fresh droppings of House Sparrow. On the other hand, the proportion of C. parapsilosis in the fresh droppings of House Sparrow was almost 70%; in the fresh droppings of European Starling, Great Tit and White Wagtail, the values were also high, namely 44%, 49% and 59%, respectively; and in the droppings of Rock Pigeon, it was only slightly more than 15%. We do not yet have a clear understanding of this difference in relative shares. Based on field observations, we hypothesize that the proportion of these yeasts in the fresh droppings of the host birds studied could be influenced by dietary peculiarities: Rock Pigeon, White Wagtail and Great Tit consumed “junk” food more frequently than European Starling and House Sparrow. This observation is consistent with the results of a study in which the gut yeast communities in five breeding colonies of Yellow-legged Gulls along the Mediterranean littoral in southern France were analyzed: The frequency of anthropogenic yeast species in the gut of these gulls increased with the synanthropy of the gull colonies [29]. The regional factor could also be considered as studies of various natural substrates under anthropogenic pressure (topsoil, leaves of plants, and fruits) in the Moscow region over the last 10 years have shown that the proportion of the potential pathogen C. parapsilosis was always significantly higher than the proportion of C. albicans [48,55]. The species of synanthropic birds that were generally more oriented towards foraging in natural substrates in anthropophized environments (European Starling and House Sparrow) showed a higher relative abundance of C. parapsilosis in fresh feces. The high proportion of C. parapsilosis in the feces of some species of synanthropic birds gives rise to the observation that resistance of C. parapsilosis to fluconazole (a widely used, inexpensive antifungal drug worldwide) has become a major global health problem, with resistance rates increasing dramatically in recent years [56].
The potentially pathogenic yeast Cl. lusitaniae was found in low relative abundance in the feces of all birds examined. The literature refers to the observation of this species in pigeon feces [22]. In general, this species is considered an emerging pathogen. Cl. lusitaniae has been detected in various substrates, e.g., soil, water, fruit, vegetables, plants and the gastrointestinal tract of animals and humans. However, its importance lies in the fact that it has been isolated in invasive infections, especially in pediatric patients [57].
Another ascomycetous pathogen, the yeast A. bovina, which is also not yet included in the World Health Organization fungal list, was found by us in high relative abundance only in fresh droppings of Rock Pigeon. Two type strains of this species were also isolated from pigeons [58]. The phylogenetically closely related species, A. telluris, was also found in the feces of pigeons in three districts of Chon Buri province, Thailand [22]. Another phylogenetically closely related species, A. slooffiae, was the dominant yeast in pig intestines in Germany [59]. Tolerance to heat stress was investigated in a type strain of A. bovina (CBS 2760). It was shown that the acquisition of heat shock tolerance and the synthesis of the heat shock proteins hsp 104, hsp 90, hsp 70 and hsp 60 were induced by mild heat shock at temperatures of 35 to 40 °C for 30 min [60]. It can be cautiously assumed that the yeast A. bovina is a thermophilic species that could be part of the gut microbiome of pigeons (all strains isolated from pigeon droppings grew at +43 °C). However, this assumption requires separate detailed studies. In general, the yeast A. bovina is not widespread but can cause severe invasive fungal infections, especially in immunocompromised individuals. In a recent clinical trial in France, two patients with invasive fungal infections caused by A. bovina were found to have had previous contact with pigeons. A strain of A. bovina was also isolated from the feces of one of the pigeons with which one of the two patients had contact. To our knowledge, this is the first described case of possible zoonotic transmission reported for this yeast species [61].
Two eurybiontic yeast species, D. hansenii and D. fabryi, were also observed in fresh feces (when cultured on a selective medium to identify ascomycetous potential pathogens). Both species were found with a rather high proportion (23 and 17%, respectively) only in the feces of European Starling (the proportion of D. hansenii in the fresh feces of House Sparrow was also about 20%, but there was almost no D. fabryi, which amounted to less than 1%). On the one hand, these are eurybiontic yeasts that occur in both natural and anthropogenic niches [62]. On the other hand, associations of these yeast species with some soil invertebrates (ants, diplopods) [63,64] and wild Drosophila spp. [65] are known in nature. Of the five synanthropic host birds studied, we observed that European Starling fed mainly on earthworms, insect larvae and millipedes and did not consume abundant “junk” food as often. We hypothesize that the high relative abundance of Debaryomyces yeasts in the fresh feces of European Starling could primarily be related to a diet containing a greater number of invertebrates, but this needs future research.
In agreement with the hypothesis, the main factor for the formation of a potentially pathogenic ascomycetous microbiome in the fresh feces of five synanthropic birds (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow) in our study appeared to be an avian species. At the same time, the host species is undoubtedly a complex multicomponent system whose different parts influence the microbiome of potentially pathogenic yeasts to varying and still poorly understood extents. Dietary niches could be considered the most influential factor shaping the structure of potentially pathogenic yeast complexes in fresh feces. However, habitats (including individual preferences for certain habitats), environmental conditions (topsoil, water), nest environment, seasonal variation and health status of the birds should also be considered. The extension of sampling to different phylogenetic host lineages is also very important [41,66,67]. This study could be a starting point to continue research and gain a clear understanding of how a potentially pathogenic yeast microbiome is formed in different wild birds.
As expected, the number of culturable, potentially pathogenic ascomycetous yeasts in the feces decreased as drying progressed. No significant differences were found between the yeast complexes in the 90-days “old” feces of the different bird species. In contrast, in the study in which the gut yeast communities in five breeding colonies of Yellow-legged Gulls along the Mediterranean littoral in southern France were analyzed, whether dropping samples were dry or fresh had no significant effect on the growth of the colonies [29]. Perhaps because the study focused on geographical differences, not as much attention was paid to the condition of the samples, whether fresh or dry (in the article, the samples were described as fresh and relatively dry). The large difference in the number of fresh (90) and relatively dry (23) samples tested may also have had an influence. However, the difference in the results only shows that further investigation is needed. Only three ascomycetous, potentially pathogenic yeasts were found in dry 45-days “old” feces, two of them in low relative abundance, C. tropicalis and P. kudriavzevii. The proportion of the third, C. parapsilosis, increased to 30% and the majority of the complex, 50–70%, consisted of the eurybiontic yeasts, D. hansenii. Only one potential pathogen, C. parapsilosis, was already cultured from dry 90-days “old” feces (a proportion of approx. 5%, except for the Rock Pigeon, from whose feces another species, P. kudriavzevii, was also cultured). And approx. 80–90% was the eurybiontic yeast, D. hansenii, which is also considered a polyextremophilic yeast species that can develop in a variety of stressful habitat conditions [68,69].
Fresh feces from various synanthropic bird species therefore pose the greatest potential risk to the health of immunocompromised humans and children. Of the five synanthropic species we studied (Rock Pigeon, European Starling, White Wagtail, Great Tit and House Sparrow), pigeons were found to be the most dangerous for them. This should be taken into account and efforts should be made to regulate the number of synanthropic birds. However, raising awareness and educating citizens on this matter can also play a positive role in maintaining public health. In general, dry feces pose less of a health risk because the proportion of active pathogen cells in them is lower. However, the health risk posed by dry feces cannot be completely eliminated and should be taken into account when considering the sanitary safety of the city as a whole and each individual home or facility where people spend extended periods of time. One of the likely routes by which particulate matter from dry feces enter indoor spaces (private homes, workplaces, schools, daycare centers, libraries, etc.) is from topsoil particles on the soles of shoes. The tradition established in some countries (Finland, Sweden) of leaving shoes outside the door to keep indoor spaces safe deserves a particularly positive mention. Perhaps the no-shoe policy in homes should be encouraged as a standard practice in households where this is possible. Careful hand hygiene is also critical when handling or touching footwear, especially in households with immunocompromised individuals.
Limitations
Our study has some limitations. Firstly, we examined the feces of only five of the most common synanthropic birds in the study region (city of Moscow). Secondly, our study focused only on a specific group of potentially pathogenic ascomycetous yeasts from the critical, high and medium-important yeast pathogens published in the World Health Organization fungal list. Thirdly, a selective Brilliance Candida Agar medium was used for rapid isolation and presumptive identification of clinically important Candida species, which has certain limitations and drawbacks. Fourthly, the fecal yeast microbiome we examined likely consisted of species from both the colon and cecum, suggesting a mixture of fecal microbiota from the different segments of the gastrointestinal tract. Fecal yeasts can therefore only be considered as a proxy for the intestinal yeast microbiome.
5. Conclusions
The diversity of ascomycetous, potentially pathogenic yeasts in fresh feces of synanthropic birds depended significantly on the host species (Rock Pigeon, European Starling, White Wagtail, Great Tit or House Sparrow). The greatest diversity of potential pathogens was detected in fresh feces of pigeons: five out of six pathogens from the list of critical, high and medium-important yeast pathogens published in the World Health Organization fungal list (C. albicans, C. parapsilosis, C. tropicalis, N. glabratus and P. kudriavzevii), as well as two pathogens not yet included in the list, A. bovina and Cl. lusitaniae. No significant differences were found between the yeast complexes of different bird species in dry droppings, and the diversity of pathogens in them decreased. This study could be a starting point to continue research and gain a clear understanding of how a potentially pathogenic yeast microbiome forms in different wild birds. Furthermore, considering the One Health approach and the influence of climate change and global warming on fungal ecology and host–pathogen interactions, it seems important to evaluate the virulence and heat adaptation mechanisms of isolated strains both in vitro and in vivo (e.g., using insect host models).
Author Contributions
Conceptualization; data curation; formal analysis; methodology; visualization; writing—original draft, A.K. and A.G.; Funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was conducted under the state assignment of Lomonosov Moscow State University (no. 121040800174-6).
Institutional Review Board Statement
The study was approved by the Ethics Committee of Lomonosov Moscow State University (№ 144-5/3/2008-2020), https://bioethics.msu.ru, accessed on 1 January 2020. Additional ethical review and approval were waived for this study because the study did not involve the capturing or manipulation of birds but only their observation.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The study was carried out with the scientific equipment of the Collective Usage Center “I.I. Mechnikov NIIVS”, Moscow, Russia, with the financial support of the project by the Russian Federation represented by the Ministry of Science of Russia, Agreement No. 075-15-2021-676 dated 28.07.2021. We would like to thank Olga Rodionova and Evgenia Rodionova for their help with the sampling.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
References
- Marzluff, J.M.; Bowman, R.; Donnelly, R. Avian Ecology and Conservation in an Urbanizing World, 1st ed.; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Veech, J.A.; Small, M.F.; Baccus, J.T. The effect of habitat on the range expansion of a native and an introduced bird species. J. Biogeogr. 2011, 38, 69–77. [Google Scholar] [CrossRef]
- James Reynolds, S.; Ibáñez-Álamo, J.D.; Sumasgutner, P.; Mainwaring, M.C. Urbanisation and nest building in birds: A review of threats and opportunities. J. Ornithol. 2019, 160, 841–860. [Google Scholar] [CrossRef]
- Liordos, V.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L.; Valsamidis, E.; Kontsiotis, V.J. Niche analysis and conservation of bird species using urban core areas. Sustainability 2021, 13, 6327. [Google Scholar] [CrossRef]
- Kurucz, K.; Purger, J.J.; Batary, P. Urbanization shapes bird communities and nest survival, but not their food quantity. Glob. Ecol. Conserv. 2021, 26, e01475. [Google Scholar] [CrossRef]
- Jokimäki, J.; Ramos-Chernenko, A. Innovative foraging behavior of urban birds: Use of insect food provided by cars. Birds 2024, 5, 469–486. [Google Scholar] [CrossRef]
- Ratcliffe, E.; Gatersleben, B.; Sowden, P.T. Bird sounds and their contributions to perceived attention restoration and stress recovery. J. Environ. Psychol. 2013, 36, 221–228. [Google Scholar] [CrossRef]
- Taylor, L.; Hochuli, D.F. Creating better cities: How biodiversity and ecosystem functioning enhance urban residents’ wellbeing. Urban Ecosyst. 2015, 18, 747–762. [Google Scholar] [CrossRef]
- Kruize, H.; van der Vliet, N.; Staatsen, B.; Bell, R.; Chiabai, A.; Muiños, G.; Higgins, S.; Quiroga, S.; Martinez-Juarez, P.; Aberg Yngwe, M.; et al. Urban green space: Creating a triple win for environmental sustainability, health, and health equity through behavior change. Int. J. Environ. Res. Public Health 2019, 16, 4403. [Google Scholar] [CrossRef]
- Hermann, E.; Van Damme, R.; Bongcam-Rudloff, E.; Nasirzadeh, L. Urban pigeons as reservoirs of critical pathogens: Improved protocol for sequencing pigeon faeces in disease monitoring. EMBnet. J. 2024, 30, 1059. [Google Scholar] [CrossRef]
- Howard, D.H. Pathogenic Fungi in Humans and Animals, 2nd ed.; Howard, D.H., Ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Haag-Wackernagel, D.; Moch, H. Health hazards posed by feral pigeons. J. Infect. 2004, 48, 307–313. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.; Brandão, J.; Gordon, B.; Ferguson, A. Recreational environment: Pathogenic fungi in public places, information gaps in assessing public health risk. In Environmental Mycology in Public Health, 1st ed.; Viegas, C., Pinheiro, C., Verissimo, C., Sabino, R., Brandão, J., Viegas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–192. [Google Scholar] [CrossRef]
- Pereira, R.S.; Dos Santos, H.D.H.; Moraes, O.S.; Júnior, D.P.L.; Hahn, R.C. Children’s public health: Danger of exposure to pathogenic fungi in recreational places in the middle-west region of Brazil. J. Infect. Public Health 2020, 13, 51–57. [Google Scholar] [CrossRef]
- Moschetti, G.; Alfonzo, A.; Francesca, N. Yeasts in birds. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 435–454. [Google Scholar] [CrossRef]
- Caetano, C.F.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A.; Rolo, J. The role of yeasts in human health: A review. Life 2023, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, T.K.; Sunderland, D.; Rule, A.M.; da Silva, A.J.; Moura, I.N.S.; Tamang, L.; Girouard, A.S.; Schwab, K.J.; Breysse, P.N. Urban Feral Pigeons (Columba livia) as a source for air- and waterborne contamination with Enterocytozoon bieneusi spores. Appl. Environ. Microbiol. 2007, 73, 4357–4358. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.K.; Sidrim, J.J.; Cordeiro, R.A.; Brilhante, R.S.; Monteiro, A.J.; Rocha, M.F. Urban pigeons (Columba livia) as a potential source of pathogenic yeasts: A focus on antifungal susceptibility of Cryptococcus strains in Northeast Brazil. Mycopathologia 2010, 169, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Du, P.C.; Li, W.G.; Lu, J.-X. Identification and molecular analysis of pathogenic yeasts in droppings of Domestic Pigeons in Beijing, China. Mycopathologia 2012, 174, 203–214. [Google Scholar] [CrossRef]
- Abulreesh, H.H.; Organji, S.R.; Elbanna, K.; Osman, G.E.; Almalki, M.H.; Abdel-Malek, A.Y.; Ghyathuddin, A.A.K.; Ahmad, I. Diversity, virulence factors, and antifungal susceptibility patterns of pathogenic and opportunistic yeast species in Rock Pigeon fecal droppings in Western Saudi Arabia. Pol. J. Microbiol. 2019, 68, 493–504. [Google Scholar] [CrossRef]
- Nualmalang, R.; Thanomsridetchai, N.; Teethaisong, Y.; Sukphopetch, P.; Tangwattanachuleeporn, M. Identification of pathogenic and opportunistic yeasts in pigeon excreta by MALDI-TOF mass spectrometry and their prevalence in Chon Buri province, Thailand. Int. J. Environ. Res. Public Health 2023, 20, 3191. [Google Scholar] [CrossRef]
- Hermann, E. Health-Hazardous Fungi in Feces from Feral Pigeons. Bachelor’s Thesis, Disciplinary Domain of Science and Technology, Biology Education Centre Sweden, Uppsala University, Uppsala, Sweden, 2023. [Google Scholar]
- Jokimäki, J.; Suhonen, J. Distribution and habitat selection of wintering birds in urban environments. Landsc. Urban Plan. 1998, 39, 253–263. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 13 December 2024).
- Lionakis, M.S.; Chowdhary, A. Candida auris infections. N. Engl. J. Med. 2024, 391, 1924–1935. Available online: https://www.nejm.org/doi/abs/10.1056/NEJMra2402635 (accessed on 8 March 2025). [CrossRef]
- Banks, T.L. Identification of Potentially Pathogenic Yeast Species in Seagull Guano by Molecular Techniques. Honors Theses, Coastal Carolina University, Conway, SC, USA, 2009. Available online: https://digitalcommons.coastal.edu/honors-theses/144 (accessed on 8 March 2025).
- Al-Yasiri, M.H.; Normand, A.C.; L’ollivier, C.; Lachaud, L.; Bourgeois, N.; Rebaudet, S.; Piarroux, R.; Mauffrey, J.-F.; Ranque, S. Opportunistic fungal pathogen Candida glabrata circulates between humans and yellow-legged gulls. Sci. Rep. 2016, 6, 36157. [Google Scholar] [CrossRef]
- Al-Yasiri, M.H.; Normand, A.C.; Piarroux, R.; Ranque, S.; Mauffrey, J.F. Gut yeast communities in Larus michahellis from various breeding colonies. Med. Mycol. 2017, 55, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Reusch, K. Foraging Ecology of Kelp Gulls in Natural and Anthropogenically Modified Environments. Ph.D. Dissertation, School of Environmental Sciences, Faculty of Sciences, Nelson Mandela University, Port Elizabeth, South Africa, 2021. Available online: http://hdl.handle.net/10948/54106 (accessed on 8 March 2025).
- Glushakova, A.; Kachalkin, A. Diversity of culturable yeasts in the feces of Mew Gulls breeding in natural and urban habitats, with insights into the antifungal susceptibility of the observed pathogens. Birds 2024, 5, 543–557. [Google Scholar] [CrossRef]
- Cafarchia, C.; Camarda, A.; Romito, D.; Campolo, M.; Quaglia, N.C.; Tullio, D.; Otranto, D. Occurrence of yeasts in cloacae of migratory birds. Mycopathologia 2006, 161, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Romito, D.; Iatta, R.; Camarda, A.; Montagna, M.T.; Otranto, D. Role of birds of prey as carriers and spreaders of Cryptococcus neoformans and other zoonotic yeasts. Med. Mycol. 2006, 44, 485–492. [Google Scholar] [CrossRef]
- Latham, B.; Leishman, A.; Martin, J.; Phalen, D. Establishing normal fecal flora in wild Australian passerine birds by use of the fecal gram stain. J. Zoo Wildl. Med. 2017, 48, 786–793. [Google Scholar] [CrossRef]
- Cafarchia, C.; Iatta, R.; Danesi, P.; Camarda, A.; Capelli, G.; Otranto, D. Yeasts isolated from cloacal swabs, feces, and eggs of laying hens. Med. Mycol. 2018, 57, 340–345. [Google Scholar] [CrossRef]
- Simi, W.B.; Leite, D.P., Jr.; Paula, C.R.; Hoffmann-Santos, H.D.; Takahara, D.T.; Hahn, R.C. Yeasts and filamentous fungi in psittacidae and birds of prey droppings in midwest region of Brazil: A potential hazard to human health. Braz. J. Biol. 2018, 79, 414–422. [Google Scholar] [CrossRef]
- Mirhosseini, Z.; Khosravi, A. Fungal pathogens: Emerging threats to birds and human health, assessment the relative frequency of pathogenic fungi in ornamental bird feces. J. Poult. Sci. Avian Dis. 2023, 1, 20–24. [Google Scholar] [CrossRef]
- Teyssier, A. Influence of Urbanisation on the Gut Microbiota of Avian Hosts and Implications for Host Fitness. Ph.D. Dissertation in Sciences: Biology, University of Antwerp, Antwerp, Belgium, 2020. Available online: https://repository.uantwerpen.be/docstore/d:irua:2652 (accessed on 8 March 2025).
- Teyssier, A.; Matthysen, E.; Hudin, N.S.; De Neve, L.; White, J.; Lens, L. Diet contributes to urban-induced alterations in gut microbiota: Experimental evidence from a wild passerine. Proc. R. Soc. B 2020, 287, 20192182. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, Y.; Huang, J.; Liu, Y.; Li, S. Comparative study of gut microbiome in urban and rural Eurasian tree sparrows. Animals 2024, 14, 3497. [Google Scholar] [CrossRef]
- Matheen, M.I.A.; Gillings, M.R.; Dudaniec, R.Y. Dominant factors shaping the gut microbiota of wild birds. Emu Austral Ornithol. 2022, 122, 255–268. [Google Scholar] [CrossRef]
- Kalyakin, M.V.; Voltzit, O.V. Atlas. Birds of Moscow City and the Moscow Region, 1st ed.; Pensoft: Sofia, Bulgaria, 2006; 372p. [Google Scholar]
- Morkovin, A.A.; Kalyakin, M.V.; Voltzit, O.V. First steps of a common birds monitoring scheme in the Moscow region, Russia. Die Vogelwelt 2017, 137, 89–98. [Google Scholar]
- World-Weather. Available online: https://world-weather.ru/pogoda/russia/moscow/may-2024/ (accessed on 13 June 2025).
- Kalyakin, M.V.; Voltsit, O.V.; Stroganova, A.A. Results of monitoring the number of sparrows at several survey sites in Moscow. Baikal Zool. J. 2023, 1, 30–37. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. Available online: https://www.pnas.org/doi/10.1073/pnas.1117018109 (accessed on 8 March 2025). [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; de Vries, M.; Verkleij, G.J.M.; Crous, P.W.; Boekhout, T.; et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. Available online: https://pubmed.ncbi.nlm.nih.gov/28050055/ (accessed on 8 March 2025). [CrossRef]
- Glushakova, A.M.; Kachalkin, A.V. Endophytic yeasts in Malus domestica and Pyrus communis fruits under anthropogenic impact. Microbiology 2017, 86, 128–135. [Google Scholar] [CrossRef]
- Kachalkin, A.V.; Glushakova, A.M.; Venzhik, A.S. Presence of clinically significant endophytic yeasts in agricultural crops: Monitoring and ecological safety assessment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 723, p. 042005. [Google Scholar] [CrossRef]
- Flickr. Available online: https://www.flickr.com/ (accessed on 4 January 2025).
- Hlasek: Wildlife Photo Gallery Birds Mammals Plants. Available online: https://hlasek.com/ (accessed on 4 January 2025).
- Medina, I.R.; Fuentes, L.R.; Arteaga, M.B.; Valcárcel, F.R.; Arbelo, F.A.; Del Castillo, D.P.; Suárez, S.D.; Quintana, O.F.; Gutiérrez, B.V.; Sergent, F.S.; et al. Pigeons and their droppings as reservoirs of Candida and other zoonotic yeasts. Rev. Iberoam. Micol. 2017, 34, 211–214. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Castelo-Branco, D.D.S.C.M.; Soares, G.D.P.; Astete-Medrano, D.J.; Monteiro, A.J.; Cordeiro, R.D.A.; Sidrim, J.J.C.; Rocha, M.F.G. Characterization of the gastrointestinal yeast microbiota of cockatiels (Nymphicus hollandicus): A potential hazard to human health. J. Med. Microbiol. 2010, 59, 718–723. [Google Scholar] [CrossRef]
- Rhimi, W.; Aneke, C.I.; Annoscia, G.; Camarda, A.; Mosca, A.; Cantacessi, C.; Otranto, D.; Cafarchia, C. Virulence and in vitro antifungal susceptibility of Candida albicans and Candida catenulata from laying hens. Int. Microbiol. 2021, 24, 57–63. [Google Scholar] [CrossRef]
- Glushakova, A.; Tepeeva, A.; Prokof’eva, T.; Kachalkin, A. Culturable yeast diversity in urban topsoil influenced by various anthropogenic impacts. Int. Microbiol. 2024, 27, 1383–1403. [Google Scholar] [CrossRef]
- Yamin, D.; Akanmu, M.H.; Al Mutair, A.; Alhumaid, S.; Rabaan, A.A.; Hajissa, K. Global prevalence of antifungal-resistant Candida parapsilosis: A systematic review and meta-analysis. Trop. Med. Infect. Dis. 2022, 7, 188. [Google Scholar] [CrossRef]
- Rojas, O.C.; Montoya, A.M.; Treviño-Rangel, R.D.J. Clavispora lusitaniae: From a saprophytic yeast to an emergent pathogen. Fungal Biol. 2024, 128, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J.; Ward, J.M.; Brayton, C.; Gorelick, P.; Walsh, T.J. Multigene phylogenetic analysis of pathogenic Candida species in the Kazachstania (Arxiozyma) telluris complex and description of their ascosporic states as Kazachstania bovina sp. nov., K. heterogenica sp. nov., K. pintolopesii sp. nov., and K. slooffiae sp. nov. J. Clin. Microbiol. 2005, 43, 101–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urubschurov, V.; Büsing, K.; Janczyk, P.; Souffrant, W.-B.; Zeyner, A. Development and evaluation of qPCR assay for quantitation of Kazachstania slooffiae and total yeasts occurring in the porcine gut. Curr. Microbiol. 2015, 71, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Deegenaars, M.L.; Watson, K. Heat shock response in the thermophilic enteric yeast Arxiozyma telluris. Appl. Environ. Microbiol. 1998, 64, 3063–3065. [Google Scholar] [CrossRef]
- Kaeuffer, C.; Baldacini, M.; Ruge, T.; Ruch, Y.; Zhu, Y.J.; De Cian, M.; Philouze, G.; Bachellier, P.; Denis, J.; Lefebvre, N.; et al. Fungal infections caused by Kazachstania spp., Strasbourg, France, 2007–2020. Emerg. Infect. Dis. 2022, 28, 29–34. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Byzov, B.A.; Thanh, V.N.; Babjeva, I.P. Yeasts associated with soil invertebrates. Biol. Fertil. Soils 1993, 16, 183–187. [Google Scholar] [CrossRef]
- Maksimova, I.A.; Glushakova, A.M.; Kachalkin, A.V.; Chernov, I.Y.; Panteleeva, S.N.; Reznikova, Z.I. Yeast communities of Formica aquilonia colonies. Microbiology 2016, 85, 124–129. [Google Scholar] [CrossRef]
- Hoang, D.; Kopp, A.; Chandler, J.A. Interactions between Drosophila and its natural yeast symbionts—Is Saccharomyces cerevisiae a good model for studying the fly–yeast relationship? Peer J. 2015, 3, e1116. Available online: https://peerj.com/articles/1116 (accessed on 8 March 2025). [CrossRef]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The avian gut microbiota: Community, physiology and function in wild birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar] [CrossRef]
- Ječmenica, B.; Kralj, J.; Taylor, L.T.; Jurinović, L. Habitat use of urban nesting yellow-legged gulls in Croatia during the breeding season. Nat. Croat. Period. Musei Hist. Nat. Croat. 2023, 32, 399–412. [Google Scholar] [CrossRef]
- Breuer, U.; Harms, H. Debaryomyces hansenii—An extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- Aggarwal, M.; Mondal, A.K. Debaryomyces hansenii: An osmotolerant and halotolerant yeast. In Yeast Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer: Dordrecht, The Netherland, 2009; pp. 65–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).