Migration Phenology and Spatial Distribution of Soaring Birds in Greece: From Citizen Science Data to Implications for Monitoring and Conservation Strategies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analyses
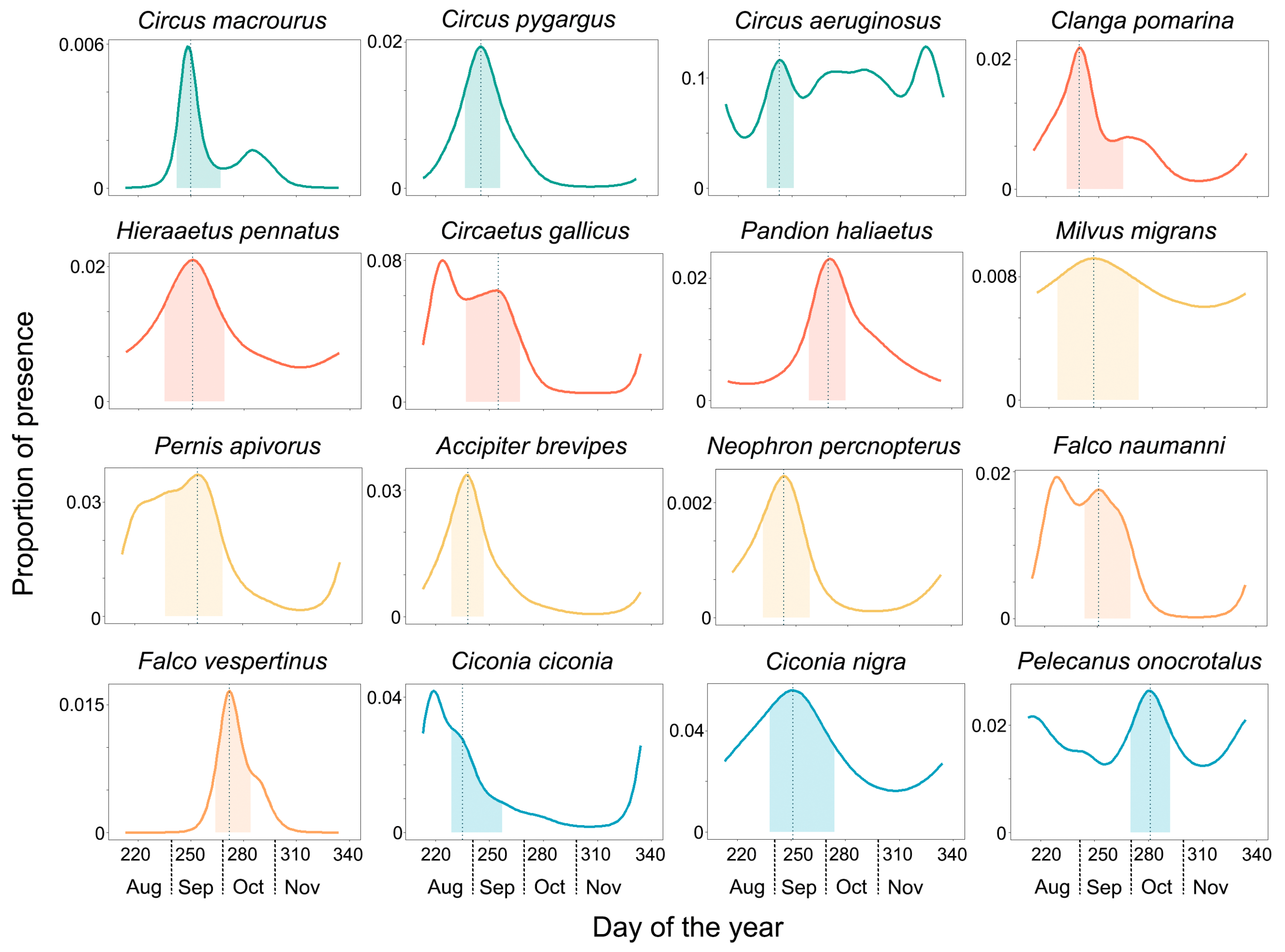
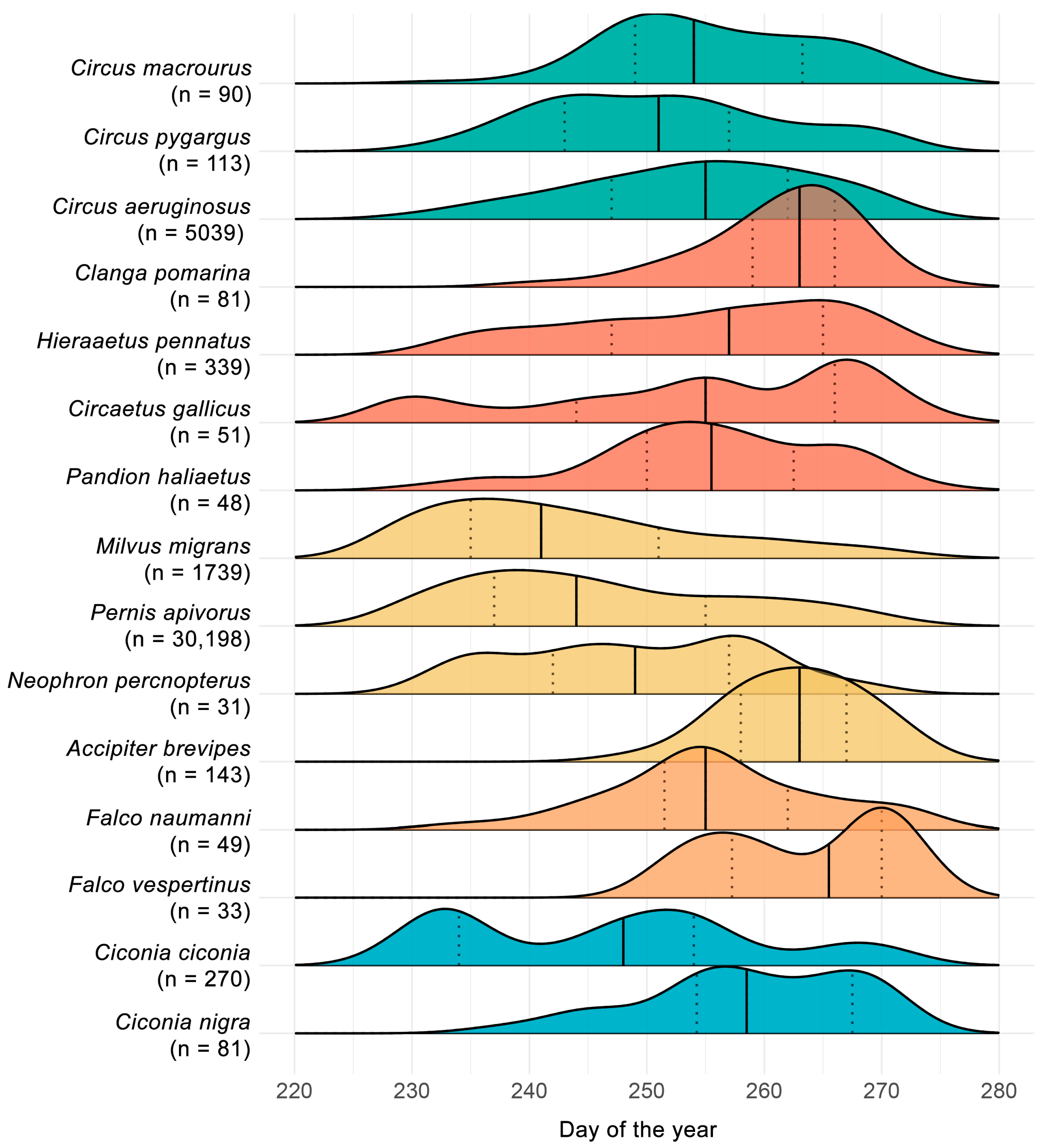
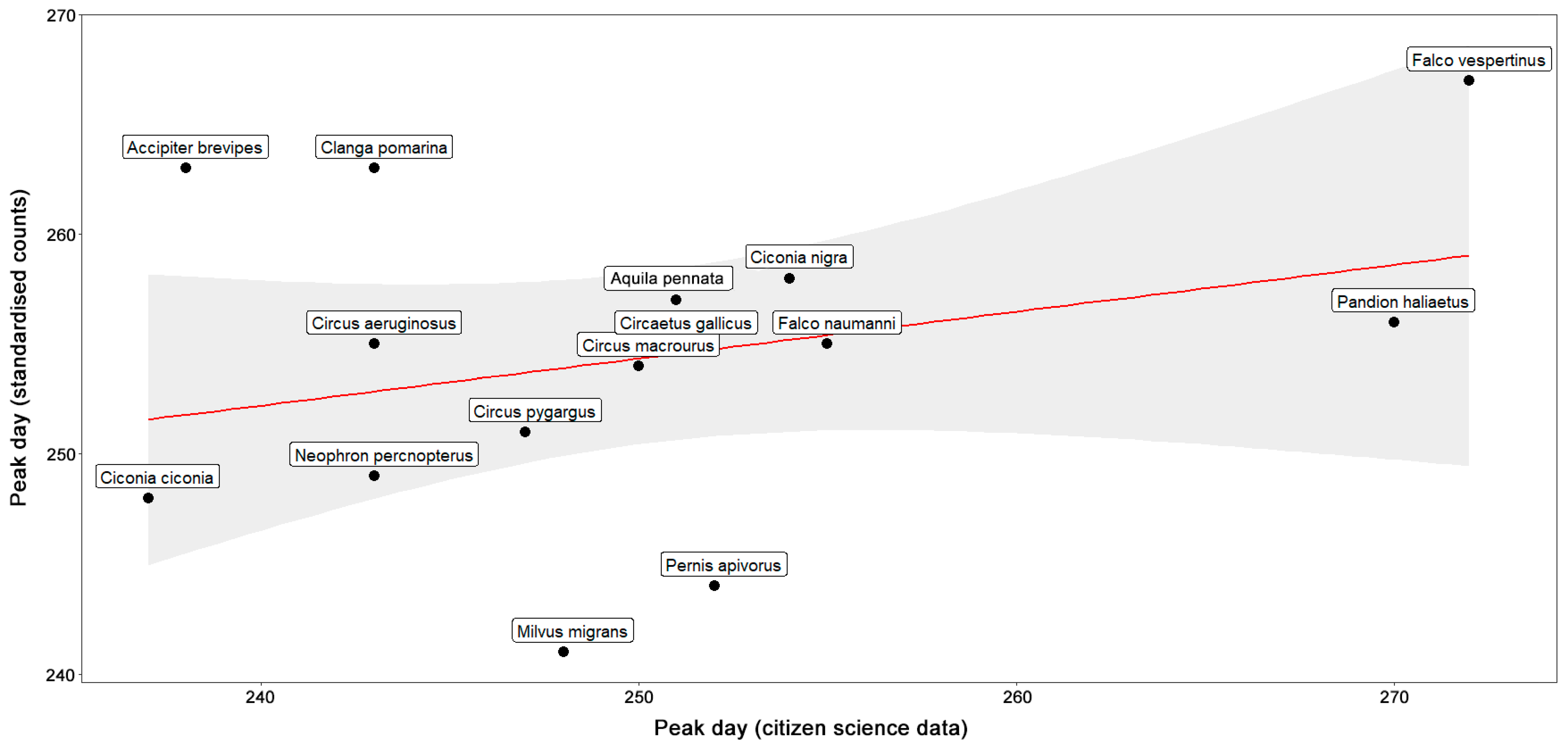
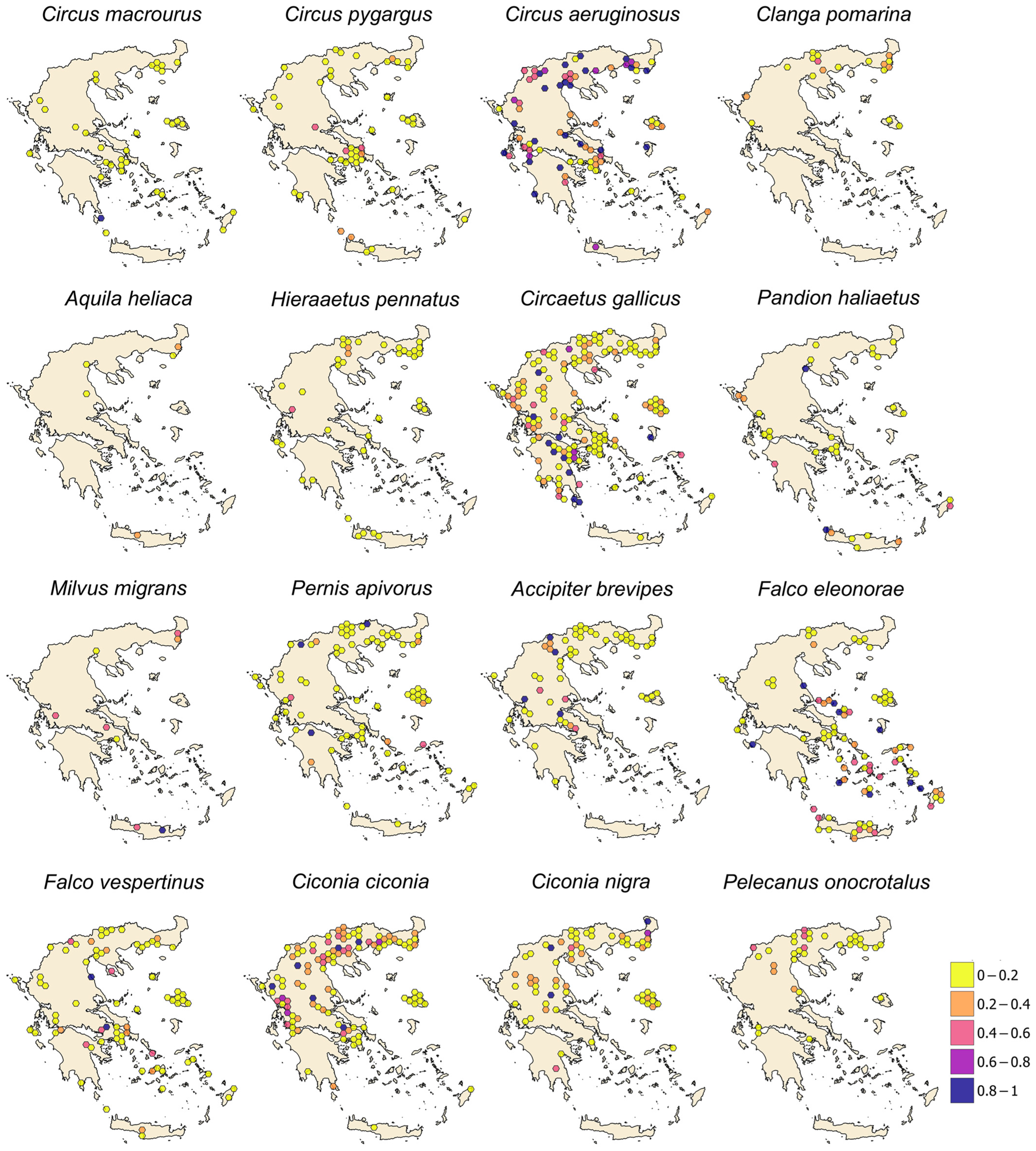
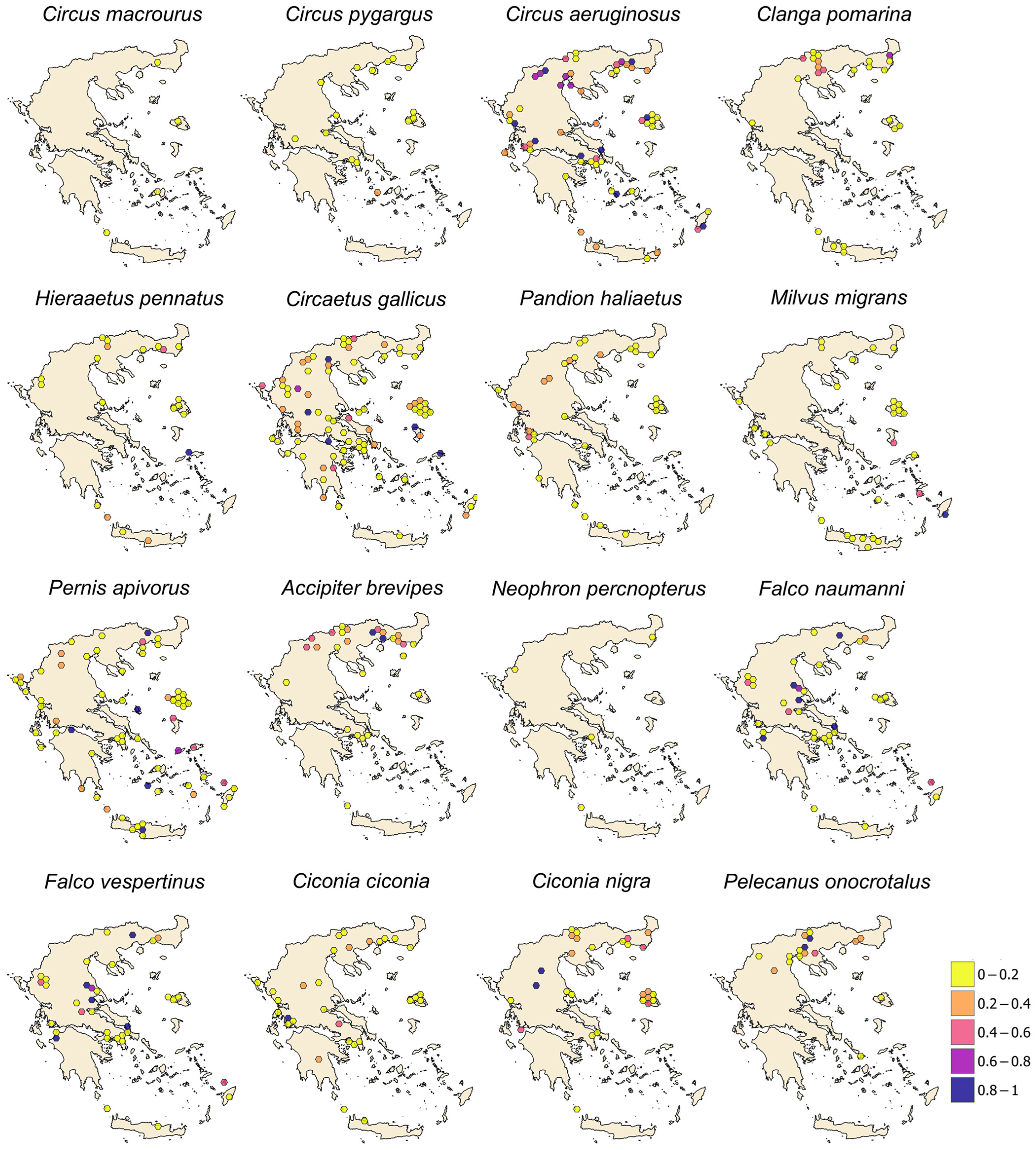
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berthold, P. Bird Migration: A General Survey; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Salewski, V.; Bruderer, B. The evolution of bird migration—A synthesis. Naturwissenschaften 2007, 94, 268–279. [Google Scholar] [CrossRef]
- Porter, R.; Beaman, M. A resume of raptor migration in Europe and the Middle East. Conserv. Stud. Raptors ICBP Tech. Publ. 1985, 5, 237–242. [Google Scholar]
- Agostini, N.; Panuccio, M.; Pasquaretta, C. Morphology, flight performance, and water crossing tendencies of Afro-Palearctic raptors during migration. Curr. Zool. 2015, 61, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.; Poirazidis, K.; Ruiz, C.; Scandolara, C.; Cárcamo, B.; Eastham, C.; Catsadorakis, G. At the crossroads from Asia to Europe: Spring migration of raptors and black storks in Dadia National Park (Greece). J. Nat. Hist. 2015, 49, 285–300. [Google Scholar] [CrossRef]
- Bildstein, K.L. Migrating Raptors of the World; Cornell University Press: Ithaca, NY, USA, 2006. [Google Scholar]
- Panuccio, M.; Agostini, N.; Mellone, U.; Bogliani, G. Circannual variation in movement patterns of the Black Kite (Milvus migrans migrans): A review. Ethol. Ecol. Evol. 2014, 26, 1–18. [Google Scholar] [CrossRef]
- Mellone, U. Sea crossing as a major determinant for the evolution of migratory strategies in soaring birds. J. Anim. Ecol. 2020, 89, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Kerlinger, P. Water-crossing behavior of raptors during migration. Wilson Bull. 1985, 97, 109–113. [Google Scholar]
- Meyer, S.K.; Spaar, R.; Bruderer, B. Sea crossing behaviour of falcons and harriers at the southern Mediterranean coast of Spain. Avian Sci. 2003, 3, 153–162. [Google Scholar]
- Robinson, W.D.; Bowlin, M.S.; Bisson, I.; Shamoun-Baranes, J.; Thorup, K.; Diehl, R.H.; Kunz, T.H.; Mabey, S.; Winkler, D.W. Integrating concepts and technologies to advance the study of bird migration. Front. Ecol. Environ. 2010, 8, 354–361. [Google Scholar] [CrossRef]
- Newson, S.E.; Moran, N.J.; Musgrove, A.J.; Pearce-Higgins, J.W.; Gillings, S.; Atkinson, P.W.; Miller, R.; Grantham, M.J.; Baillie, S.R. Long-term changes in the migration phenology of UK breeding birds detected by large-scale citizen science recording schemes. Ibis 2016, 158, 481–495. [Google Scholar] [CrossRef]
- Heim, W.; Heim, R.J.; Beermann, I.; Burkovskiy, O.A.; Gerasimov, Y.; Ktitorov, P.; Ozaki, K.; Panov, I.; Sander, M.M.; Sjöberg, S. Using geolocator tracking data and ringing archives to validate citizen-science based seasonal predictions of bird distribution in a data-poor region. Glob. Ecol. Conserv. 2020, 24, e01215. [Google Scholar] [CrossRef]
- Tende, T.; Iniunam, I.A.; Ivande, S.T.; Awoyemi, A.G.; Danmallam, B.A.; Ringim, A.S.; Bako, L.A.; Ramzy, F.J.; Kazeh, N.W.; Izang, A.I. Citizen science mitigates the lack of distributional data on Nigerian birds. Ecol. Evol. 2024, 14, e11280. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, L.J.; Leseberg, N.P.; Callaghan, C.T.; Sanderson, C.; Fuller, R.A.; Watson, J.E. Using citizen science to identify Australia’s least known birds and inform conservation action. Emu-Austral Ornithol. 2024, 124, 199–205. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen science as an ecological research tool: Challenges and benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Devictor, V.; Whittaker, R.J.; Beltrame, C. Beyond scarcity: Citizen science programmes as useful tools for conservation biogeography. Divers. Distrib. 2010, 16, 354–362. [Google Scholar] [CrossRef]
- De la Cruz, A.; Morales, A.; Korneeva, Y.; Castro, M. Analysing citizen science data to address the demographic expansion of the Eurasian Magpie (Pica pica) in southern Spain. J. Ornithol. 2024, 165, 805–813. [Google Scholar] [CrossRef]
- Fink, D.; Hochachka, W.M.; Zuckerberg, B.; Winkler, D.W.; Shaby, B.; Munson, M.A.; Hooker, G.; Riedewald, M.; Sheldon, D.; Kelling, S. Spatiotemporal exploratory models for broad-scale survey data. Ecol. Appl. 2010, 20, 2131–2147. [Google Scholar] [CrossRef]
- Supp, S.; Sorte, F.A.L.; Cormier, T.A.; Lim, M.C.; Powers, D.R.; Wethington, S.M.; Goetz, S.; Graham, C.H. Citizen-science data provides new insight into annual and seasonal variation in migration patterns. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Walker, J.; Taylor, P. Using eBird data to model population change of migratory bird species. Avian Conserv. Ecol. 2017, 12, 4. [Google Scholar] [CrossRef]
- Horns, J.J.; Adler, F.R.; Şekercioğlu, Ç.H. Using opportunistic citizen science data to estimate avian population trends. Biol. Conserv. 2018, 221, 151–159. [Google Scholar] [CrossRef]
- Stuber, E.F.; Robinson, O.J.; Bjerre, E.R.; Otto, M.C.; Millsap, B.A.; Zimmerman, G.S.; Brasher, M.G.; Ringelman, K.M.; Fournier, A.M.; Yetter, A. The potential of semi-structured citizen science data as a supplement for conservation decision-making: Validating the performance of eBird against targeted avian monitoring efforts. Biol. Conserv. 2022, 270, 109556. [Google Scholar] [CrossRef]
- De la Cruz, A.; Numa, C. Habitat availability decline for waterbirds in a sensitive wetland: Climate change impact on the Ebro Delta. Ecol. Model. 2024, 498, 110896. [Google Scholar] [CrossRef]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Strimas-Mackey, M.; Miller, E.; Hochachka, W. R Package Version 0.3. 0, auk: eBird Data Extraction and Processing with AWK; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Johnston, A.; Hochachka, W.M.; Strimas-Mackey, M.E.; Ruiz Gutierrez, V.; Robinson, O.J.; Miller, E.T.; Auer, T.; Kelling, S.T.; Fink, D. Analytical guidelines to increase the value of community science data: An example using eBird data to estimate species distributions. Divers. Distrib. 2021, 27, 1265–1277. [Google Scholar] [CrossRef]
- Hoekstra, B.; Jansen, J.; Engelen, D.; Boer, F.D.; Benjumea, R.; Wehrmann, J.; Cavaillès, S.; Kaasiku, T.; Jansen, D.; Fetting, P. Batumi Raptor Count: From Migration Counts to Conservation in a Raptor Flyway Under Threat. Br. Birds. 2020, 113, 439–460. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Cañizares, J.R.; Edwards, C.B.; Reed, J.M. Quantifying phenological landmarks of migration shows nonuniform use of the Caribbean by shorebirds. Ecol. Evol. 2023, 13, e9954. [Google Scholar] [CrossRef]
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef]
- Panuccio, M.; Agostini, N.; Massa, B. Spring raptor migration over Ustica, southern Italy. Br. Birds 2004, 97, 400–403. [Google Scholar]
- Panuccio, M. Across and around a barrier: Migration ecology of raptors in the Mediterranean basin. Sci. Acta 2011, 5, 27–36. [Google Scholar]
- Panuccio, M.; Agostini, N.; Barboutis, C. Raptor migration in Greece: A review. Avocetta 2013, 37, 1–7. [Google Scholar]
- Premuda, G.; Gustin, M.; Pandolfi, M.; Sonet, L.; Cento, M. Spring raptor migration along the Adriatic coast (Italy): A comparative study over three sites. Avocetta 2008, 32, 13–20. [Google Scholar]
- Handrinos, G.; Akriotis, T. The Birds of Greece; Christopher Helm Ltd.: London, UK, 1997; p. 336. [Google Scholar]
- Meininger, P.L. Birds of the Wetlands in North-East Greece, Spring 1987; Foundation Working Group International Wader and Waterfowl Research: Nijmegen, The Netherlands, 1990. [Google Scholar]
- Ristow, D.; Wink, M. The Diet of Eleonora’s Falcons (Falco eleonorae) during the Autumn Migration of Passerine Birds across the Aegean Sea. Diversity 2024, 16, 538. [Google Scholar] [CrossRef]
- Agostini, N.; Lucia, G.; Mellone, U.; Panuccio, M.; Von Hardenberg, J.; Evangelidis, A.; Kominos, T. Loop migration of adult European Honey Buzzards (Pernis apivorus Linnaeus, 1758) through the central-eastern Mediterranean. Ital. J. Zool. 2012, 79, 280–286. [Google Scholar] [CrossRef]
- Bounas, A.; Sotiropoulos, K. Change of feeding strategy prior to migration: A comparative diet analysis in the Lesser Kestrel (Falco naumanni). Avian Biol. Res. 2017, 10, 27–35. [Google Scholar] [CrossRef]
- Bounas, A.; Tsaparis, D.; Gustin, M.; Mikulic, K.; Sarà, M.; Kotoulas, G.; Sotiropoulos, K. Using genetic markers to unravel the origin of birds converging towards pre-migratory sites. Sci. Rep. 2018, 8, 8326. [Google Scholar] [CrossRef]
- Bounas, A.; Tsiakiris, R.; Vlachopoulos, K.; Bukas, N.; Stara, K.; Sotiropoulos, K. Large Premigratory Roost of Lesser Kestrels (Falco naumanni) in Ioannina City, Greece: Trends, Roost Characteristics, and Implications for Conservation. J. Raptor Res. 2016, 50, 416–421. [Google Scholar] [CrossRef]
- Safriel, U. Bird migration at Elat, Israel. Ibis 1968, 110, 283–320. [Google Scholar] [CrossRef]
- Lucia, G.; Agostini, N.; Panuccio, M.; Mellone, U.; Chiatante, G.; Tarini, D.; Evangelidis, A. Raptor migration at Antikythira, in southern Greece. Br. Birds 2011, 104, 266. [Google Scholar]
- Panuccio, M.; Agostini, N.; Bogliani, G. Mount-Olympus: A new raptor migration bottle-neck in northern Greece. In Proceedings of the XVI Italian Ornithological Congress, Cervia, Italy, 22–25 September 2011. [Google Scholar]
- Bounas, A.; Solanou, M.; Panuccio, M.; Barišić, S.; Bino, T.; Erciyas-Yavuz, K.; Iankov, P.; Ieronymidou, C.; Barboutis, C. Mining citizen science data to explore stopover sites and spatiotemporal variation in migration patterns of the red-footed falcon. Curr. Zool. 2020, 66, 467–475. [Google Scholar] [CrossRef]
- Guetté, A.; Caillault, S.; Pithon, J.; Pain, G.; Daniel, H.; Marchadour, B.; Beaujouan, V. Who and Where Are the Observers behind Biodiversity Citizen Science Data? Effect of Landscape Naturalness on the Spatial Distribution of French Birdwatching Records. Land 2022, 11, 2095. [Google Scholar] [CrossRef]
- Tang, B.; Clark, J.S.; Gelfand, A.E. Modeling spatially biased citizen science effort through the eBird database. Environ. Ecol. Stat. 2021, 28, 609–630. [Google Scholar] [CrossRef]
- Bounas, A.; Panuccio, M.; Evangelidis, A.; Sotiropoulos, K.; Barboutis, C. The Migration of the Lesser Kestrel Falco naumanni in Eastern Europe-A Ringing Recovery and Direct Observation Approach. Acrocephalus 2016, 37, 49–56. [Google Scholar] [CrossRef]
- Shamoun-Baranes, J.; Liechti, F.; Vansteelant, W.M. Atmospheric conditions create freeways, detours and tailbacks for migrating birds. J. Comp. Physiol. A 2017, 203, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Newton, I. The Migration Ecology of Birds; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Merken, R.; Deboelpaep, E.; Teunen, J.; Saura, S.; Koedam, N. Wetland suitability and connectivity for trans-Saharan migratory waterbirds. PLoS ONE 2015, 10, e0135445. [Google Scholar] [CrossRef]
- Barboutis, C.; Bounas, A.; Navarrete, E.; Fransson, T. Stopover Ecology of the European Turtle Dove (Streptopelia turtur), a Threatened Migratory Bird Species, after the Crossing of an Extended Ecological Barrier. Birds 2023, 4, 202–212. [Google Scholar] [CrossRef]
- Barboutis, C.; Navarrete, E.; Karris, G.; Xirouchakis, S.; Fransson, T.; Bounas, A. Arriving depleted after crossing of the Mediterranean: Obligatory stopover patterns underline the importance of Mediterranean islands for migrating birds. Anim. Migr. 2022, 9, 27–36. [Google Scholar]
- Ferretti, A.; Maggini, I.; Fusani, L. How to recover after sea crossing: The importance of small islands for passerines during spring migration. Ethol. Ecol. Evol. 2021, 33, 307–320. [Google Scholar] [CrossRef]
- Runge, C.A.; Martin, T.G.; Possingham, H.P.; Willis, S.G.; Fuller, R.A. Conserving mobile species. Front. Ecol. Environ. 2014, 12, 395–402. [Google Scholar] [CrossRef]
- Oppel, S.; Dobrev, V.; Arkumarev, V.; Saravia, V.; Bounas, A.; Kret, E.; Velevski, M.; Stoychev, S.; Nikolov, S.C. High juvenile mortality during migration in a declining population of a long-distance migratory raptor. Ibis 2015, 157, 545–557. [Google Scholar] [CrossRef]
- Bounas, A.; Georgopoulou, E.; Arkumarev, V.; Liapakis, S.; Samaritakis, N.; Vavylis, D.; Dobrev, D.; Dobrev, V.; Sotiropoulos, K.; Xirouchakis, S. Increased occurrence of Egyptian vultures in Crete: Exploring movement patterns and potential for wintering. Ethol. Ecol. Evol. 2024, 36, 663–678. [Google Scholar] [CrossRef]
- Spyridonidou, S.; Vagiona, D.G.; Loukogeorgaki, E. Strategic planning of offshore wind farms in Greece. Sustainability 2020, 12, 905. [Google Scholar] [CrossRef]
- Kafetzis, A.; Kret, E.; Skartsi, D.; Vasilakis, D.; Christopoulou, I. Wind Farms in areas of high ornithological value—Conflicts, solutions, challenges: The case of Thrace, Greece. In Wind Energy and Wildlife Interactions: Presentations from the CWW2015 Conference, Online, 20 November 2017; Springer: Cham, Switzerland, 2017; pp. 191–205. [Google Scholar]
- Bennun, L.; van Bochove, J.; Ng, C.; Fletcher, C.; Wilson, D.; Phair, N. Mitigating Biodiversity Impacts Associated with Solar and Wind Energy Development: Guidelines for Project Developers; IUCN: Gland, Switzerland, 2021. [Google Scholar]
- Bounas, A.; Vasilakis, D.; Kret, E.; Zakkak, S.; Chatzinikolaou, Y.; Kapsalis, E.; Arkumarev, V.; Dobrev, D.; Stamenov, A.; Stoychev, S. Cumulative collision risk and population-level consequences of industrial wind-power plant development for two vulture species: A quantitative warning. Environ. Impact Assess. Rev. 2025, 110, 107669. [Google Scholar] [CrossRef]

| Common Name | Scientific Name | Group |
|---|---|---|
| Pallid Harrier | Circus macrourus | harriers |
| Montagu’s Harrier | Circus pygargus | harriers |
| Marsh Harrier | Circus aeruginosus | harriers |
| Booted Eagle | Hieraaetus pennatus | eagles |
| Imperial Eagle | Aquila heliaca | eagles |
| Lesser Spotted Eagle | Clanga pomarina | eagles |
| Short-toed Eagle | Circaetus gallicus | eagles |
| Osprey | Pandion haliaetus | eagles |
| Black Kite | Milvus migrans | other raptors |
| Honey Buzzard | Pernis apivorus | other raptors |
| Levant Sparrowhawk | Accipiter brevipes | other raptors |
| Egyptian Vulture | Neophron percnopterus | other raptors |
| Eleonora’s Falcon | Falco eleonorae | falcons |
| Lesser Kestrel | Falco naumanni | falcons |
| Red-footed Falcon | Falco vespertinus | falcons |
| White Stork | Ciconia ciconia | other soaring birds |
| Black Stork | Ciconia nigra | other soaring birds |
| Great White Pelican | Pelecanus onocrotalus | other soaring birds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounas, A.; Tsiopelas, N.; Evangelidis, A.; Barboutis, C. Migration Phenology and Spatial Distribution of Soaring Birds in Greece: From Citizen Science Data to Implications for Monitoring and Conservation Strategies. Birds 2025, 6, 6. https://doi.org/10.3390/birds6010006
Bounas A, Tsiopelas N, Evangelidis A, Barboutis C. Migration Phenology and Spatial Distribution of Soaring Birds in Greece: From Citizen Science Data to Implications for Monitoring and Conservation Strategies. Birds. 2025; 6(1):6. https://doi.org/10.3390/birds6010006
Chicago/Turabian StyleBounas, Anastasios, Nikos Tsiopelas, Angelos Evangelidis, and Christos Barboutis. 2025. "Migration Phenology and Spatial Distribution of Soaring Birds in Greece: From Citizen Science Data to Implications for Monitoring and Conservation Strategies" Birds 6, no. 1: 6. https://doi.org/10.3390/birds6010006
APA StyleBounas, A., Tsiopelas, N., Evangelidis, A., & Barboutis, C. (2025). Migration Phenology and Spatial Distribution of Soaring Birds in Greece: From Citizen Science Data to Implications for Monitoring and Conservation Strategies. Birds, 6(1), 6. https://doi.org/10.3390/birds6010006






