Diversity of Culturable Yeasts in the Feces of Mew Gulls Breeding in Natural and Urban Habitats, with Insights into the Antifungal Susceptibility of the Observed Pathogens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Collection and Processing
2.3. Molecular Identification of Pure Cultures
2.4. Antifungal Susceptibility Testing
2.5. Statistical Data Analyses
3. Results
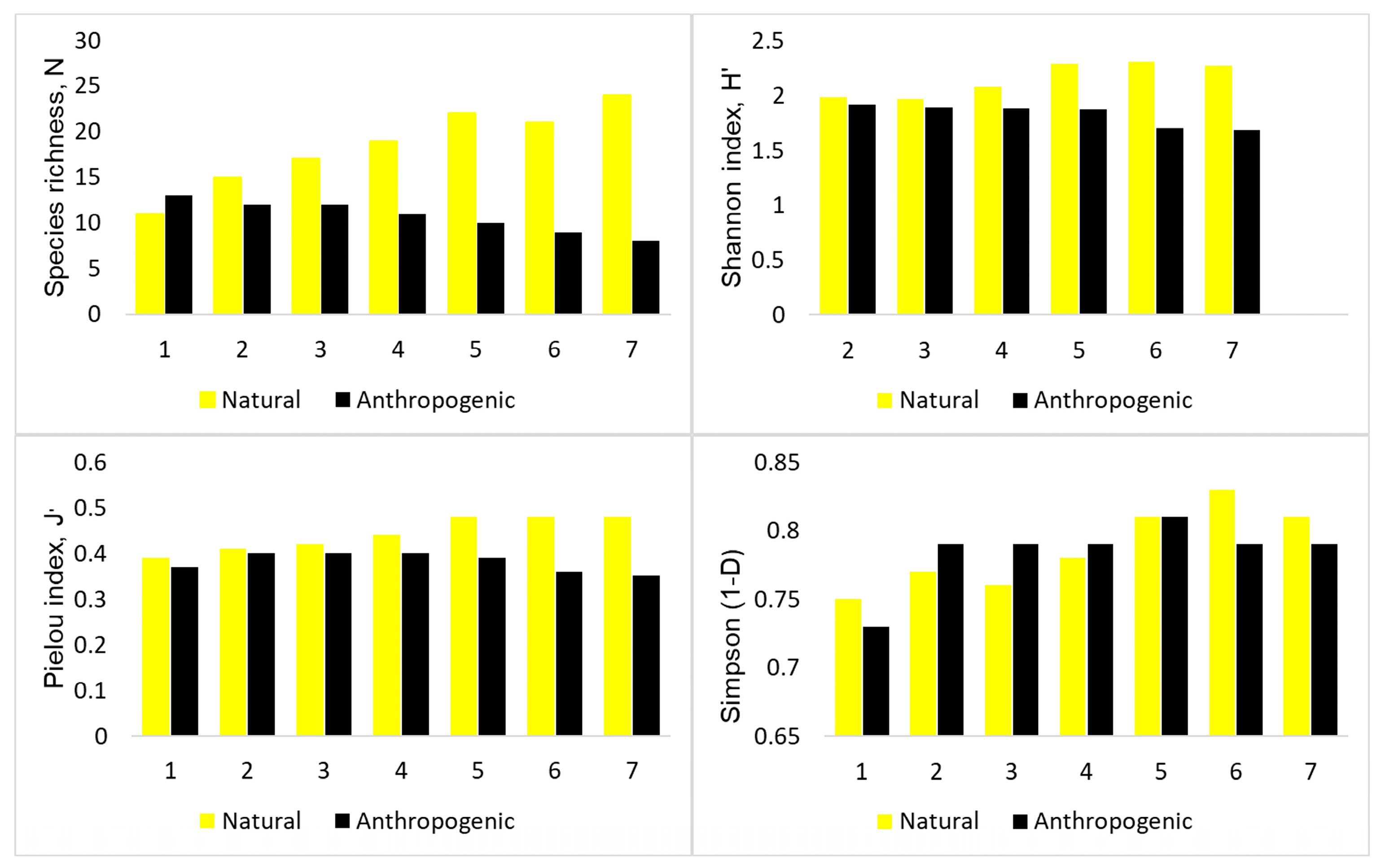
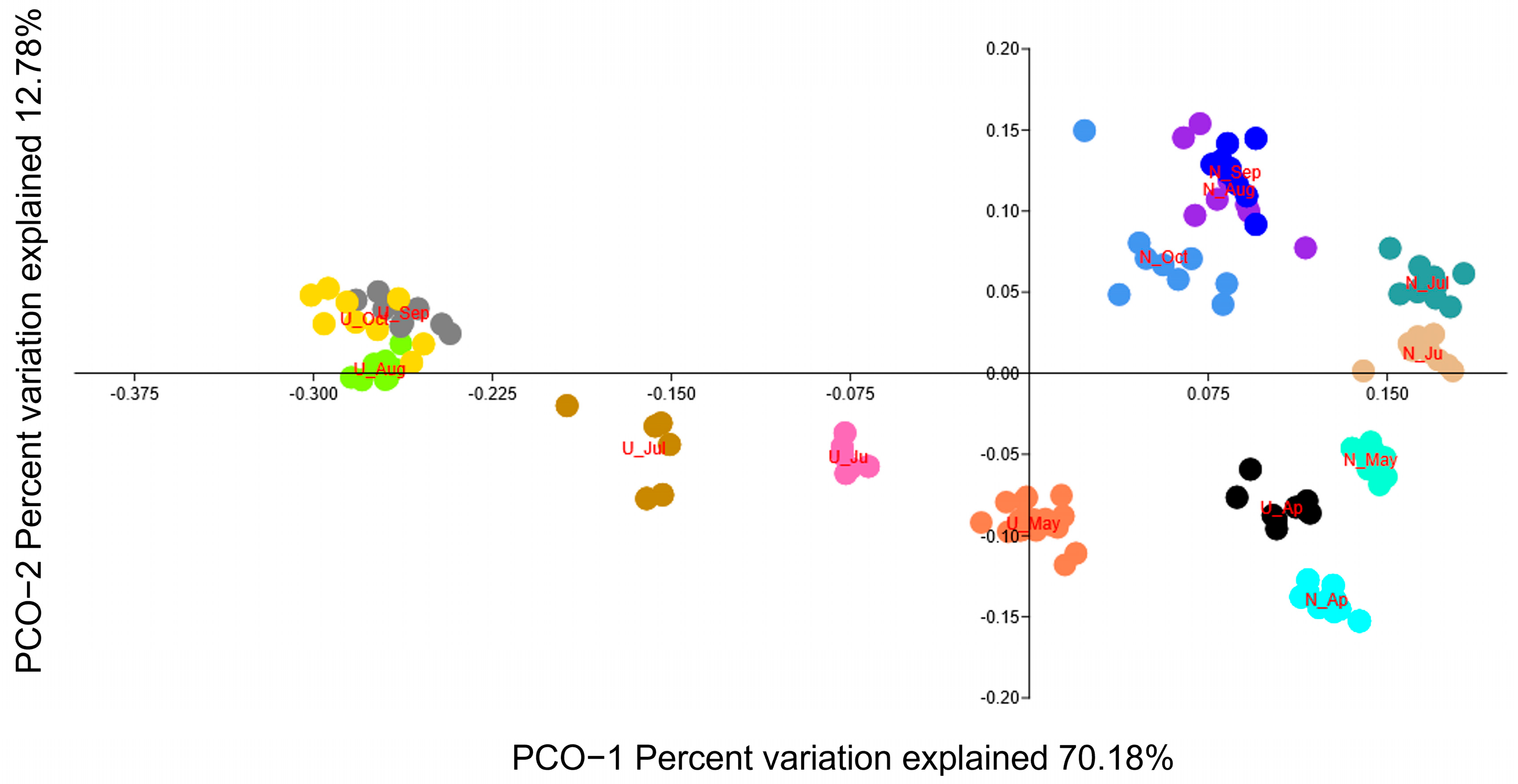
3.1. Main Features of Observed Yeast Diversity
3.2. Susceptibility of Rh. mucilaginosa, C. parapsilosis and C. tropicalis to Conventional Antifungal Agents
- Rh. mucilaginosa
- C. parapsilosis
- C. tropicalis
4. Discussion
4.1. Main Features of Yeast Communities in the Feces of Mew Gulls from Natural and Urban Colonies
4.2. Susceptibility Profiles of Potentially Pathogenic Yeast Species Observed in Feces
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partecke, J.; Schwabl, I.; Gwinner, E. Stress and the city: Urbanization and its effects on the stress physiology in European blackbirds. Ecology 2006, 87, 1945–1952. [Google Scholar] [CrossRef]
- Lopes, C.S. The Physiology and Health Condition of Urban Dweller Gulls in Increasingly Urbanized Areas. Ph.D. Dissertation, Universidade de Coimbra, Coimbra, Portugal, 2022. Available online: https://hdl.handle.net/10316/100394 (accessed on 13 January 2024).
- Marzluff, J.M. Worldwide urbanization and its effects on birds. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer: Boston, MA, USA, 2001; pp. 19–47. [Google Scholar] [CrossRef]
- Luniak, M. Fauna of the Big City—Estimating Species Richness and Abundance in Warsaw Poland. In Urban Ecology; Marzluff, J.M., Endlicher, W., Bradley, G., Simon, U., Shulenberger, E., Alberti, M., Ryan, C., ZumBrunnen, C., Eds.; Springer: Boston, MA, USA, 2008; pp. 349–354. [Google Scholar] [CrossRef]
- Reusch, K. Foraging Ecology of Kelp Gulls in Natural and Anthropogenically Modified Environments. Ph.D. Dissertation, School of Environmental Sciences, Faculty of Sciences, Nelson Mandela University, Gqeberha, South Africa, 2021. Available online: http://hdl.handle.net/10948/54106 (accessed on 13 January 2024).
- Belant, J.L.; Ickes, S.K.; Seamans, T.W. Importance of landfills to urban-nesting herring and ring-billed gulls. Landsc. Urban Plan. 1998, 43, 11–19. [Google Scholar] [CrossRef]
- De Faria, J.P.; Vaz, P.T.; Lopes, C.S.; Calado, J.G.; Pereira, J.M.; Veríssimo, S.N.; Paiva, V.H.; Gonçalves, A.M.M.; Ramos, J.A. The importance of marine resources in the diet of urban gulls. Mar. Ecol. Prog. Ser. 2021, 660, 189–201. [Google Scholar] [CrossRef]
- Spelt, A.; Soutar, O.; Williamson, C.; Memmott, J.; Shamoun-Baranes, J.; Rock, P.; Windsor, S. Urban gulls adapt foraging schedule to human-activity patterns. Ibis 2021, 163, 274–282. [Google Scholar] [CrossRef]
- Pais de Faria, J.; Lopes, C.S.; Kroc, E.; Blight, L.K.; Nager, R.G. Urban gulls living with humans. In Seabird Biodiversity and Human Activities; Ramos, J.A., Pereira, L., Eds.; Aquatic Sciences; CRC Press: Boca Raton, FL, USA, 2022; Volume 1, pp. 90–105. [Google Scholar]
- Van Toor, M.L.; Arriero, E.; Holland, R.A.; Huttunen, M.J.; Juvaste, R.; Müller, I.; Thorup, K.; Wikelski, M.; Safi, K. Flexibility of habitat use in novel environments: Insights from a translocation experiment with lesser black-backed gulls. R. Soc. Open Sci. 2017, 4, 160164. [Google Scholar] [CrossRef]
- Mendes, R.F.; Ramos, J.A.; Paiva, V.H.; Calado, J.G.; Matos, D.M.; Ceia, F.R. Foraging strategies of a generalist seabird species, the yellow-legged gull, from GPS tracking and stable isotope analyses. Mar. Biol. 2018, 165, 168. [Google Scholar] [CrossRef]
- Oro, D.; Genovart, M.; Tavecchia, G.; Fowler, M.S.; Martínez-Abraín, A. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 2013, 16, 1501–1514. [Google Scholar] [CrossRef]
- Ramos, R.; Ramírez, F.; Sanpera, C.; Jover, L.; Ruiz, X. Diet of Yellow-legged Gull (Larus michahellis) chicks along the Spanish Western Mediterranean coast: The relevance of refuse dumps. J. Ornithol. 2009, 150, 265–272. [Google Scholar] [CrossRef]
- Lopes, C.S.; Laranjeiro, M.I.; Lavers, J.L.; Finger, A.; Provencher, J. Seabirds as indicators of metal and plastic pollution. In Seabird Biodiversity and Human Activities; Ramos, J.A., Pereira, L., Eds.; Aquatic Sciences; CRC Press: Boca Raton, FL, USA, 2022; Volume 1, pp. 169–188. [Google Scholar] [CrossRef]
- De la Casa-Resino, I.; Hernández-Moreno, D.; Castellano, A.; Pérez-López, M.; Soler, F. Breeding near a landfill may influence blood metals (Cd, Pb, Hg, Fe, Zn) and metalloids (Se, As) in white stork (Ciconia ciconia) nestlings. Ecotoxicology 2014, 23, 1377–1386. [Google Scholar] [CrossRef]
- Pierotti, R.; Annett, C.A. Diet and reproductive output in seabirds. BioScience 1990, 40, 568–574. [Google Scholar] [CrossRef]
- Pierotti, R.; Annett, C.A. Diet choice in the herring gull: Constraints imposed by reproductive and ecological factors. Ecology 1991, 72, 319–328. [Google Scholar] [CrossRef]
- Murray, M.H.; Sánchez, C.A.; Becker, D.J.; Byers, K.A.; Worsley-Tonks, K.E.; Craft, M.E. City sicker? A meta-analysis of wildlife health and urbanization. Front. Ecol. Environ. 2019, 17, 575–583. [Google Scholar] [CrossRef]
- Hubálek, Z. Pathogenic microorganisms associated with gulls and terns (Laridae). J. Vertebr. Biol. 2021, 70, 21009-1. [Google Scholar] [CrossRef]
- Cafarchia, C.; Romito, D.; Coccioli, C.; Camarda, A.; Otranto, D. Phospholipase activity of yeasts from wild birds and possible implications for human disease. Sabouraudia 2008, 46, 429–434. [Google Scholar] [CrossRef]
- Francesca, N.; Canale, D.E.; Settanni, L.; Moschetti, G. Dissemination of wine-related yeasts by migratory birds. Environ. Microbiol. Rep. 2012, 4, 105–112. [Google Scholar] [CrossRef]
- Francesca, N.; Carvalho, C.; Guerreiro, M.A.; Alfonzo, A.; Gaglio, R.; Settanni, L.; Sampaio, J.P.; Moschetti, G. The migratory birds: Novel ecological niche of fungal diversity? In Yeasts Biodiversity and Biotechnology in the Twenty-First Century; University of Perugia: Perugia, Italy, 2015; Available online: https://iris.unipa.it/handle/10447/200656 (accessed on 15 January 2024).
- Moschetti, G.; Alfonzo, A.; Francesca, N. Yeasts in birds. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 435–454. [Google Scholar] [CrossRef]
- Kawakita, S.; van Uden, N. Occurrence and population densities of yeast species in the digestive tracts of gulls and terns. Microbiology 1965, 39, 125–129. [Google Scholar] [CrossRef]
- Buck, J.D. Occurrence of Candida albicans in fresh gull feces in temperate and subtropical areas. Microb. Ecol. 1983, 9, 171–176. [Google Scholar] [CrossRef]
- Buck, J.D. A note on the experimental uptake and clearance of Candida albicans in a young captive gull (Larus sp.). Mycopathologia 1986, 94, 59–61. [Google Scholar] [CrossRef]
- Buck, J.D. Isolation of Candida albicans and halophilic Vibrio spp. from aquatic birds in Connecticut and Florida. Appl. Environ. Microbiol. 1990, 56, 826–828. [Google Scholar] [CrossRef]
- Al-Yasiri, M.H.; Normand, A.C.; L’ollivier, C.; Lachaud, L.; Bourgeois, N.; Rebaudet, S.; Piarroux, R.; Mauffrey, J.-F.; Ranque, S. Opportunistic fungal pathogen Candida glabrata circulates between humans and yellow-legged gulls. Sci. Rep. 2016, 6, 36157. [Google Scholar] [CrossRef]
- Al-Yasiri, M.H.; Normand, A.C.; Piarroux, R.; Ranque, S.; Mauffrey, J.F. Gut yeast communities in Larus michahellis from various breeding colonies. Med. Mycol. 2017, 55, 436–444. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 11 January 2024).
- Van Asbeck, E.C.; Huang, Y.C.; Markham, A.N.; Clemons, K.V.; Stevens, D.A. Candida parapsilosis fungemia in neonates: Genotyping results suggest healthcare workers hands as source, and review of published studies. Mycopathologia 2007, 164, 287–293. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY antifungal surveillance program: Results for Candida species from 1997–2016. Open Forum Infect. Dis. 2019, 6, 79–94. [Google Scholar] [CrossRef]
- Branco, J.; Miranda, I.M.; Rodrigues, A.G. Candida parapsilosis virulence and antifungal resistance mechanisms: A comprehensive review of key determinants. J. Fungi 2023, 9, 80. [Google Scholar] [CrossRef]
- Prigitano, A.; Blasi, E.; Calabrò, M.; Cavanna, C.; Cornetta, M.; Farina, C.; Grancini, A.; Innocenti, P.; Lo Cascio, G.; Nicola, L.; et al. Yeast bloodstream infections in the COVID-19 patient: A multicenter Italian study (FiCoV Study). J. Fungi 2023, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Demirci-Duarte, S.; Arikan-Akdagli, S.; Gülmez, D. Species distribution, azole resistance and related molecular mechanisms in invasive Candida parapsilosis complex isolates: Increase in fluconazole resistance in 21 years. Mycoses 2021, 64, 823–830. [Google Scholar] [CrossRef]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiao, M.; Liao, K.; Kudinha, T.; Wang, H.; Zhang, L.; Hou, X.; Kong, F.; Xu, Y.-C. Notable increasing Trend in Azole Non-susceptible Candida tropicalis Causing Invasive Candidiasis in China (August 2009 to July 2014): Molecular epidemiology and clinical azole consumption. Front. Microbiol. 2017, 8, 464. [Google Scholar] [CrossRef]
- Glushakova, A.; Kachalkin, A. Wild and partially synanthropic bird yeast diversity, in vitro virulence, and antifungal susceptibility of Candida parapsilosis and Candida tropicalis strains isolated from feces. Int. Microbiol. 2023, 27, 883–897. [Google Scholar] [CrossRef]
- Kulesza, K.; Biedunkiewicz, A.; Nowacka, K.; Dynowska, M.; Urbaniak, M.; Stępień, Ł. Dishwashers as an extreme environment of potentially pathogenic yeast species. Pathogens 2021, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Brizzio, S.; van Broock, M. Rhodotorula mucilaginosa, a carotenoid producing yeast strain from a patagonian high-altitude lake. Folia Microbiol. 2004, 49, 19–25. [Google Scholar] [CrossRef]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An emerging pathogen. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 465717. [Google Scholar] [CrossRef]
- De Sousa, J.R.; Goncalves, V.N.; de Holanda, R.A.; Santos, D.A.; Bueloni, C.F.; Costa, A.O.; Petry, M.V.; Rosa, C.A.; Rosa, L.H. Pathogenic potential of environmental resident fungi from ornithogenic soils of Antarctica. Fungal Biol. 2017, 121, 991–1000. [Google Scholar] [CrossRef]
- Kemler, M.; Witfeld, F.; Begerow, D.; Yurkov, A. Phylloplane Yeasts in Temperate Climates. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 171–197. [Google Scholar] [CrossRef]
- Ioannou, P.; Vamvoukaki, R.; Samonis, G. Rhodotorula species infections in humans: A systematic review. Mycoses 2018, 62, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, P. Evaluation of Selected Virulence Factors of Rhodotorula Fungi. Ph.D. Thesis, Wydział Lekarski, Krakow, Poland, 2010. [Google Scholar]
- Jarros, I.C.; Veiga, F.F.; Corrêa, J.L.; Barros, I.L.E.; Gadelha, M.C.; Voidaleski, M.F.; Pieralisi, N.; Pedroso, R.B.; Vicente, V.A.; Negri, M.; et al. Microbiological and virulence aspects of Rhodotorula mucilaginosa. EXCLI J. 2020, 19, 687–704. [Google Scholar] [PubMed] [PubMed Central]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Robert, V.A.; Casadevall, A. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 2009, 200, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Jokimäki, J.; Skorka, P.; Tryjanowski, P. Loss of migration and urbanization in birds: A case study of the blackbird (Turdus merula). Oecologia 2014, 175, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Stedt, J.; Bonnedahl, J.; Hernandez, J.; McMahon, B.J.; Hasan, B.; Olsen, B.; Drobni, M.; Waldenström, J. Antibiotic resistance patterns in Escherichia coli from gulls in nine European countries. Infect. Ecol. Epidemiol. 2014, 4, 21565. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M.; Fournier, C.; Lopes, E.; de Lencastre, H.; Nordmann, P.; Poirel, L. High colonization rate and heterogeneity of ESBL-and carbapenemase-producing Enterobacteriaceae isolated from gull feces in Lisbon, Portugal. Microorganisms 2020, 8, 1487. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.P.; Pace, A.; Varriale, L.; Borrelli, L.; Gargiulo, A.; Pompameo, M.; Fioretti, A.; Dipineto, L. Prevalence and antimicrobial resistance of enteropathogenic bacteria in yellow-legged gulls (Larus michahellis) in Southern Italy. Animals 2021, 11, 275. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; Navarro, J.; Figuerola, J.; Aymí, R.; Sabaté, S.; Planell, R.; Vila, J.; Montalvo, T. A spatial analysis of urban gulls contribution to the potential spread of zoonotic and antibiotic-resistant bacteria. Sci. Total Environ. 2024, 912, 168762. [Google Scholar] [CrossRef] [PubMed]
- Hoff, G.L.; Bigler, W.J. The role of bats in the propagation and spread of histoplasmosis: A review. J. Wildl. Dis. 1981, 17, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, M.P. Mycetes and urban areas. Parassitologia 2006, 48, 121–124. [Google Scholar] [PubMed]
- Irga, P.J.; Armstrong, B.; King, W.L.; Burchett, M.; Torpy, F.R. Correspondence between urban bird roosts and the presence of aerosolised fungal pathogens. Mycopathologia 2016, 181, 689–699. [Google Scholar] [CrossRef]
- Kavitha, N.; Amtuz, Z. Fungal diversity in droppings of spot-billed pelican, Pelecanus philippensis. Int. J. Zool. Investig. 2021, 7, 259–265. [Google Scholar] [CrossRef]
- Tsiodras, S.; Kelesidis, T.; Kelesidis, I.; Bauchinger, U.; Falagas, M.E. Human infections associated with wild birds. J. Infect. 2008, 56, 83–98. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; de Vries, M.; Verkleij, G.J.M.; Crous, P.W.; Boekhout, T.; et al. DNA barcoding analysis of more than 9 000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. [Google Scholar] [CrossRef]
- Glushakova, A.M.; Kachalkin, A.V. Endophytic yeasts in Malus domestica and Pyrus communis fruits under anthropogenic impact. Microbiology 2017, 86, 128–135. [Google Scholar] [CrossRef]
- Kachalkin, A.V.; Glushakova, A.M.; Venzhik, A.S. Presence of clinically significant endophytic yeasts in agricultural crops: Monitoring and ecological safety assessment. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 042005. [Google Scholar] [CrossRef]
- CLSI. Epidemiological Cut-Off Values for Antifungal Susceptibility Testing, M59, 2nd ed.; Clinical Laboratory Standards Institute: Wayne, IL, USA, 2018. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI Supplement M60, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, IL, USA, 2020. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI Supplement M27M44S, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, IL, USA, 2022. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. ACM SIGMOBILE Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Klaps, J.; Lievens, B.; Álvarez-Pérez, S. Towards a better understanding of the role of nectar-inhabiting yeasts in plant–animal interactions. Fungal Biol. Biotechnol. 2020, 7, 1. [Google Scholar] [CrossRef]
- Skotniczny, M.; Satora, P.; Pańczyszyn, K.; Cioch-Skoneczny, M. Growth dynamics and diversity of yeasts during spontaneous plum mash fermentation of different varieties. Foods 2020, 9, 1054. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R.; Flores-Félix, J.D. Diversity of Culture Microorganisms from Portuguese Sweet Cherries. Life 2023, 13, 2323. [Google Scholar] [CrossRef]
- Agarbati, A.; Gattucci, S.; Canonico, L.; Ciani, M.; Comitini, F. Yeast communities related to honeybees: Occurrence and dis-tribution in flowers, gut mycobiota, and bee products. Appl. Microbiol. Biotechnol. 2024, 108, 175. [Google Scholar] [CrossRef]
- Gouliamova, D.E.; Dimitrov, R.A.; Smith, M.T.; Groenewald, M.; Stoilova-Disheva, M.M.; Gueorguiev, B.V.; Boekhout, T. DNA barcoding revealed Nematodospora valgi gen. nov., sp. nov. and Candida cetoniae sp. nov. in the Lodderomyces clade. Fungal Biol. 2016, 120, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Leroux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.J.; Heykoop, M.; et al. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, S.Y.; Lee, H.B.; Lee, J.S. Isolation of wild yeasts from soils of reed fields in Seocheon-gun county, Chungcheongnam-do, south Korea, and characterization of unrecorded yeasts. Korean J. Mycol. 2017, 45, 234–240. [Google Scholar] [CrossRef]
- Prista, C.; Michán, C.; Miranda, I.M.; Ramos, J. The halotolerant Debaryomyces hansenii, the Cinderella of non-conventional yeasts. Yeast 2016, 33, 523–533. [Google Scholar] [CrossRef]
- Navarrete, C.; Martínez, J.L. Non-conventional yeasts as superior production platforms for sustainable fermentation based bio-manufacturing processes. AIMS Bioeng. 2020, 7, 289–305. [Google Scholar] [CrossRef]
- Campana, R.; Fanelli, F.; Sisti, M. Role of melanin in the black yeast fungi Aureobasidium pullulans and Zalaria obscura in promoting tolerance to environmental stresses and to antimicrobial compounds. Fungal Biol. 2022, 126, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Á.; Inácio, J. Phylloplane Yeasts. In Biodiversity and Ecophysiology of Yeasts. The Yeast Handbook, 1st ed.; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 263–301. [Google Scholar] [CrossRef]
- Gouka, L.; Raaijmakers, J.M.; Cordovez, V. Ecology and functional potential of phyllosphere yeasts. Trends Plant Sci. 2022, 27, 1109–1123. [Google Scholar] [CrossRef]
- Chernov, I.Y. Yeast in Nature; Association of Scientific Publications of KMC: Moscow, Russia, 2013. (In Russian) [Google Scholar]
- Mannazzu, I.; Landolfo, S.; da Silva, T.L.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Jiang, L.; Qiu, J.; Gao, Y.; Gu, T.; Li, Z. Responses of Rhodotorula mucilaginosa under Pb (II) stress: Carotenoid production and budding. Environ. Microbiol. 2021, 24, 678–688. [Google Scholar] [CrossRef]
- Segal-Kischinevzky, C.; Romero-Aguilar, L.; Alcaraz, L.D.; López-Ortiz, G.; Martínez-Castillo, B.; Torres-Ramírez, N.; Sandoval, G.; González, J. Yeasts inhabiting extreme environments and their biotechnological applications. Microorganisms 2022, 10, 794. [Google Scholar] [CrossRef]
- Lord, A.T.; Mohandas, K.; Somanath, S.; Ambu, S. Multidrug resistant yeasts in synanthropic wild birds. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 11. [Google Scholar] [CrossRef]
- Reis, E.J.; Buscariolo, F.; Siqueira, J.P.; Castilho, E.M.; Almeida, M.T. Agapornis sp. pet birds: Source of dissemination of azole-resistant yeasts. Med. Mycol. 2019, 57, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, W.; Aneke, C.I.; Annoscia, G.; Camarda, A.; Mosca, A.; Cantacessi, C.; Otranto, D.; Cafarchia, C. Virulence and in vitro antifungal susceptibility of Candida albicans and Candida catenulata from laying hens. Int. Microbiol. 2021, 24, 57–63. [Google Scholar] [CrossRef]
- Silva, F.A.D.; Medeiros, S.M.D.F.R.D.S.; Costa-Junior, S.D.D.; Roberto, A.E.M.; Palácio, S.B.; Lima-Neto, R.G.D.; Cavalcanti, I.M.F. Antimicrobial resistance profile and biofilm production of microorganisms isolated from oropharynx of Rupornis magnirostris (Gmelin, 1788) and Caracara plancus (Miller, 1777). Vet. Med. Int. 2020, 2020, 8888618. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]


| Month | Temperature, Day (°C) | Temperature, Night (°C) | Wind Speed, (m/s) | Air Humidity, (%) |
|---|---|---|---|---|
| April | 13/14 | 4/6 | 2.1/1.0 | 62/62 |
| May | 17/16 | 8/8 | 1.7/0.9 | 59/63 |
| June | 19/21 | 9/12 | 1.8/1.0 | 59/61 |
| July | 21/21 | 14/15 | 2.3/0.9 | 75/74 |
| August | 24/23 | 15/16 | 1.5/0.7 | 71/74 |
| September | 19/20 | 10/12 | 1.7/0.7 | 74/72 |
| October | 5/7 | 3/5 | 3.3/0.6 | 83/81 |
| Mew Gulls (Natural Habitat) | Mew Gulls (Anthropogenic Habitat Habitat) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeast Species | GenBank Accession No. | 1 * | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Aureobasidium pullulans | PP481694 | 1.2 | 1.1 | 1.4 | 0.9 | 3.1 | 3.5 | 3.6 | 1.5 | 4.2 | 10.8 | 15.8 | 20.6 | 20.8 | 20.5 |
| Candida boleticola | PP481695 | 0 | 0 | 5.1 | 4.4 | 4.2 | 3.1 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida cetoniae | PP481696 | 0 | 5.7 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Candida parapsilosis | PP481697 | 0 | 0 | 0 | 0 | 1,1 | 0 | 0 | 0 | 1.1 | 2.4 | 3.2 | 2.1 | 2.5 | 2.4 |
| Candida santamariae | PP481698 | 5.2 | 1.4 | 2.2 | 2.1 | 1.9 | 2.4 | 2.1 | 6.1 | 4.2 | 2.1 | 2.1 | 1.8 | 1.6 | 1.9 |
| Candida tropicalis | PP481699 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 | 2.2 | 3.3 | 2.1 | 2.1 |
| Candida zeylanoides | PP481700 | 20.8 | 21.4 | 25.7 | 22.3 | 20.1 | 18.5 | 19.6 | 22.8 | 20 | 21.7 | 19.8 | 18.1 | 19.5 | 18.4 |
| Cyberlindnera misumaensis | PP481701 | 0 | 0 | 1.7 | 1.1 | 1.2 | 0.8 | 1.5 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Debaryomyces hansenii | PP481702 | 42.4 | 40.4 | 40.2 | 40.6 | 36.4 | 35.3 | 37.8 | 44.2 | 39 | 37.1 | 35.2 | 28.2 | 30.1 | 29.1 |
| Hanseniaspora uvarum | PP481703 | 7.2 | 5.6 | 3.1 | 2.5 | 2.1 | 2.4 | 2.6 | 10.2 | 5.6 | 2.4 | 1.2 | 0 | 0 | 0 |
| Metschnikowia colchici | PP481704 | 0 | 1.2 | 0 | 0 | 0.9 | 1.1 | 1.1 | 0 | 5.2 | 5.1 | 5.1 | 4.2 | 2.1 | 0 |
| Metschnikowia pulcherrima | PP481705 | 3.2 | 3.1 | 1.1 | 1.5 | 2.2 | 2.1 | 1.8 | 1.2 | 6.8 | 3.8 | 5.1 | 3.5 | 3.2 | 3.2 |
| Meyerozyma guilliermondii | PP481706 | 5.2 | 4.8 | 3.4 | 2.8 | 3.2 | 3.5 | 2.8 | 2.1 | 4.2 | 4.1 | 1.2 | 1.5 | 0 | 0 |
| Priceomyces vitoshaensis | PP481707 | 0 | 0 | 0 | 0 | 1.2 | 1.4 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yarrowia alimentaria | PP481708 | 0 | 0 | 0 | 2.4 | 2.1 | 0.9 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yarrowia lipolytica | PP481709 | 4.5 | 3.2 | 3.1 | 2.6 | 2.4 | 2 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yarrowia sp. | OR582608 | 0 | 0 | 0 | 2.2 | 2 | 2.1 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wickerhamia sp. | OR582606 | 0 | 0 | 0 | 0 | 0 | 0 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wickerhamomyces sp. | OR582609 | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cutaneotrichosporon moniliforme | PP481710 | 0 | 2.2 | 1.8 | 1.5 | 1 | 1.1 | 1.5 | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Goffeauzyma gastrica | PP481711 | 3.7 | 1.5 | 1.2 | 2.3 | 2.4 | 2.2 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Naganishia adeliensis | PP481712 | 0 | 1.1 | 1.2 | 1.1 | 2.1 | 2.4 | 2.1 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhodotorula mucilaginosa | PP481713 | 4.4 | 3.2 | 3.5 | 3.3 | 5.6 | 6.2 | 8.1 | 5.6 | 8.8 | 9.1 | 9.1 | 16.7 | 20.1 | 22.4 |
| Tausonia pullulans | PP481714 | 2.2 | 4.1 | 3.5 | 4.3 | 3.2 | 3.5 | 1.1 | 1.5 | 1.1 | 0.6 | 0 | 0 | 0 | 0 |
| Vanrija albida | PP481715 | 0 | 0 | 1.1 | 2.1 | 1.6 | 5.5 | 0.5 | 1.1 | 0.5 | 0 | 0 | 0 | 0 | 0 |
| Tausonia sp. | PP481716 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glushakova, A.; Kachalkin, A. Diversity of Culturable Yeasts in the Feces of Mew Gulls Breeding in Natural and Urban Habitats, with Insights into the Antifungal Susceptibility of the Observed Pathogens. Birds 2024, 5, 543-557. https://doi.org/10.3390/birds5030036
Glushakova A, Kachalkin A. Diversity of Culturable Yeasts in the Feces of Mew Gulls Breeding in Natural and Urban Habitats, with Insights into the Antifungal Susceptibility of the Observed Pathogens. Birds. 2024; 5(3):543-557. https://doi.org/10.3390/birds5030036
Chicago/Turabian StyleGlushakova, Anna, and Aleksey Kachalkin. 2024. "Diversity of Culturable Yeasts in the Feces of Mew Gulls Breeding in Natural and Urban Habitats, with Insights into the Antifungal Susceptibility of the Observed Pathogens" Birds 5, no. 3: 543-557. https://doi.org/10.3390/birds5030036
APA StyleGlushakova, A., & Kachalkin, A. (2024). Diversity of Culturable Yeasts in the Feces of Mew Gulls Breeding in Natural and Urban Habitats, with Insights into the Antifungal Susceptibility of the Observed Pathogens. Birds, 5(3), 543-557. https://doi.org/10.3390/birds5030036






