The Effects of Disturbance Intensity on Tropical Forest Bird Communities and Vegetation Structure after Two Decades of Recovery
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
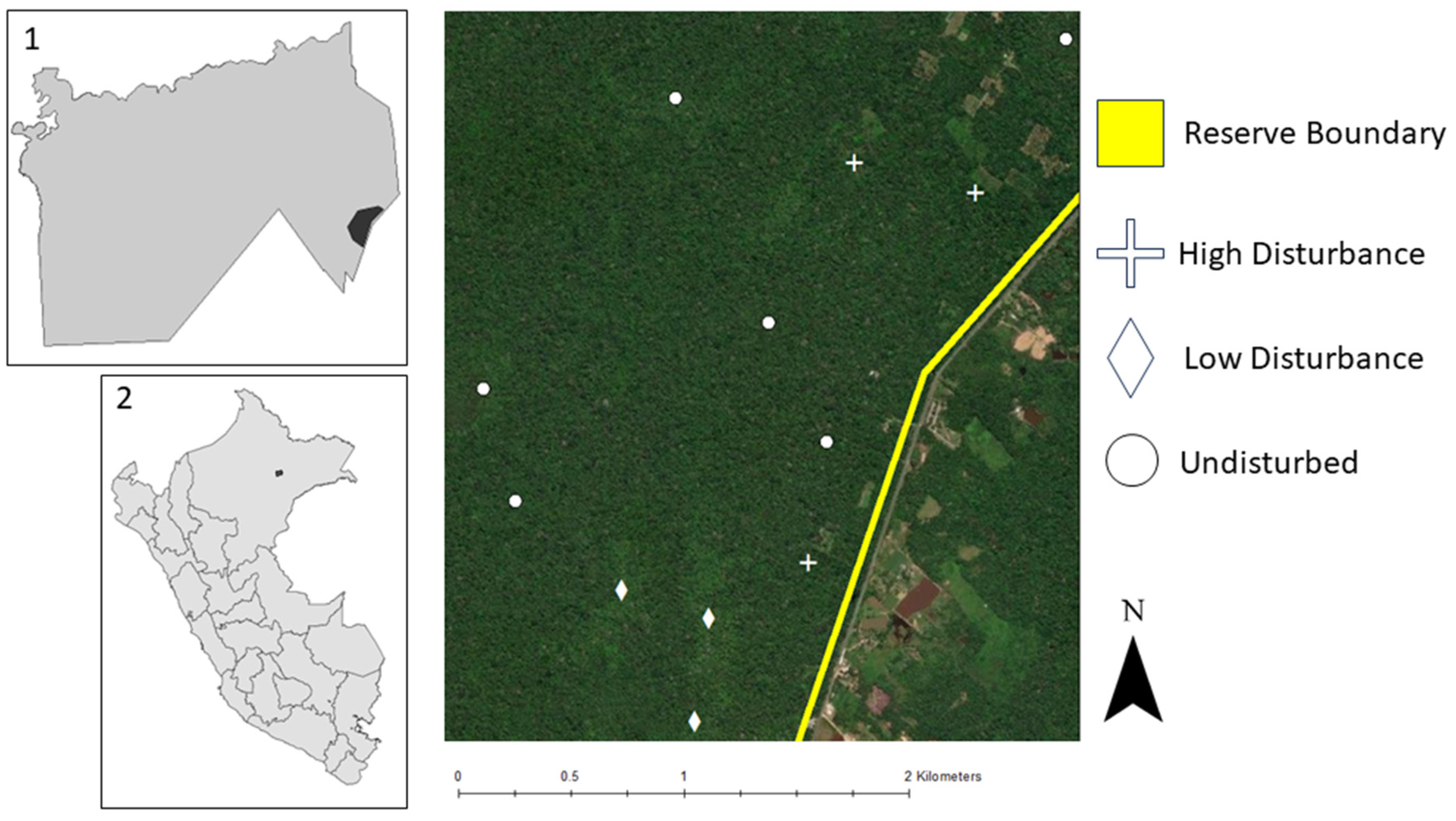
2.1. Study Area and Study Design
2.2. Bird Sampling
2.3. Vegetation Sampling
2.4. Statistical Analyses
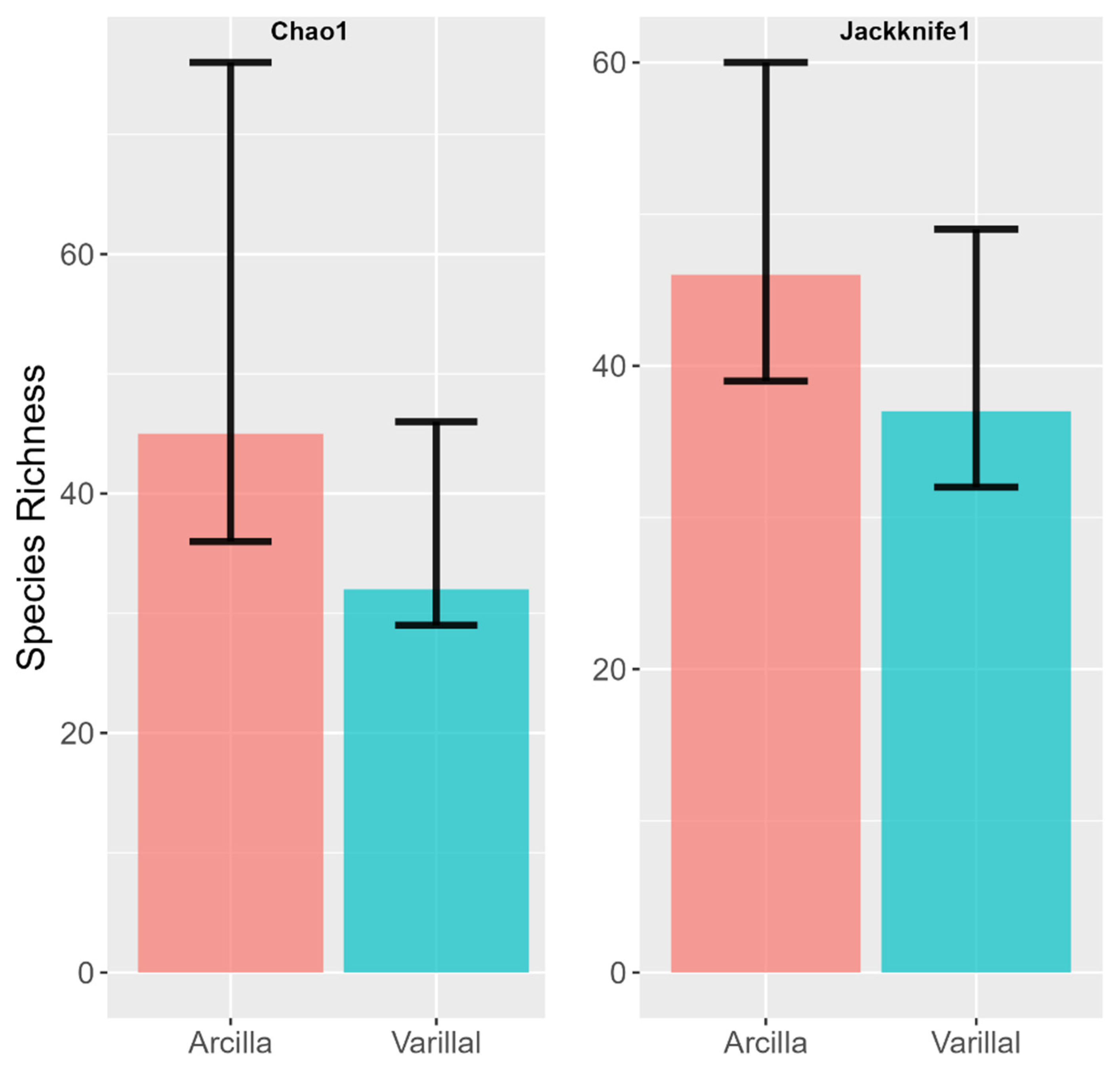
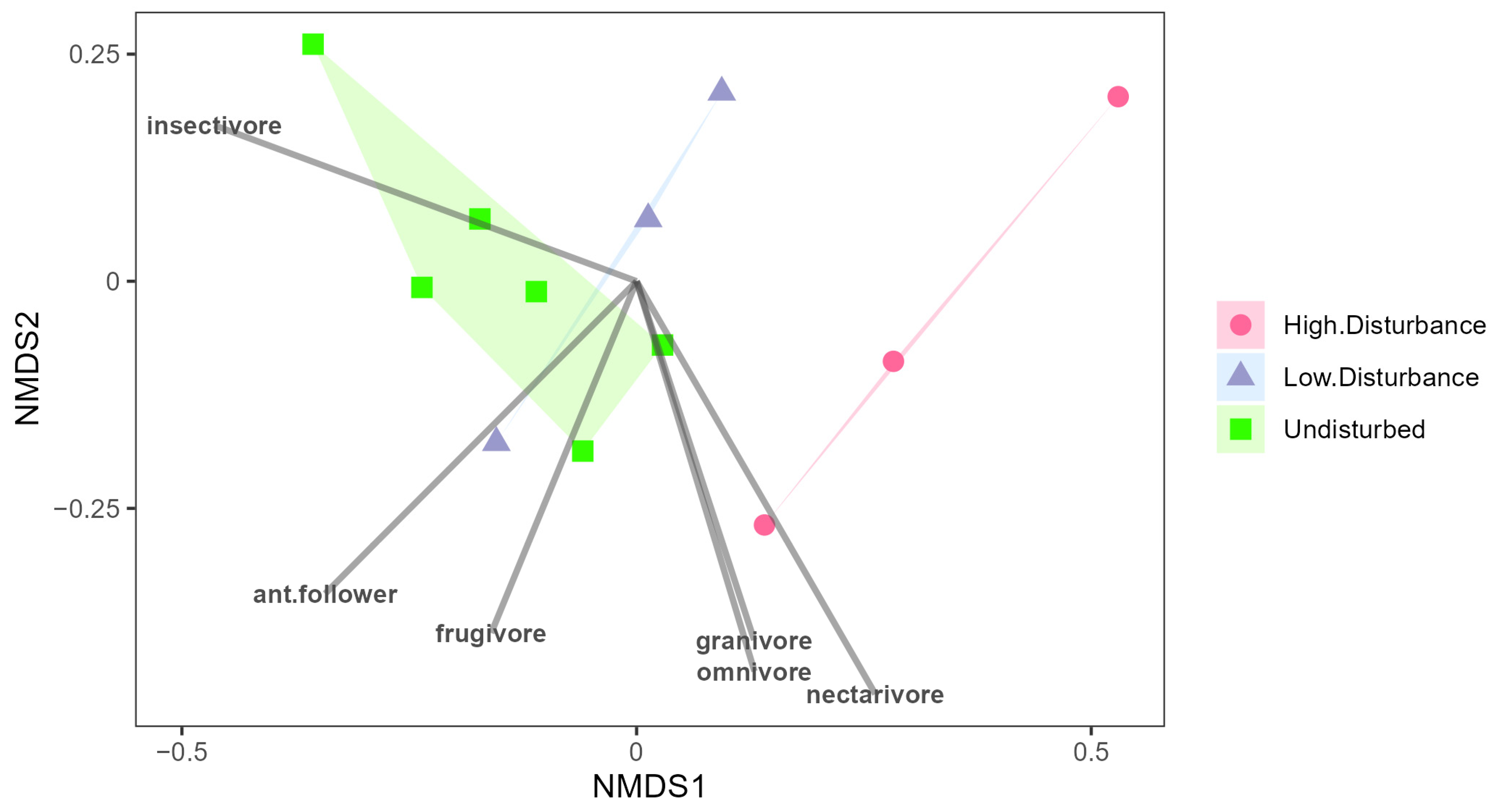
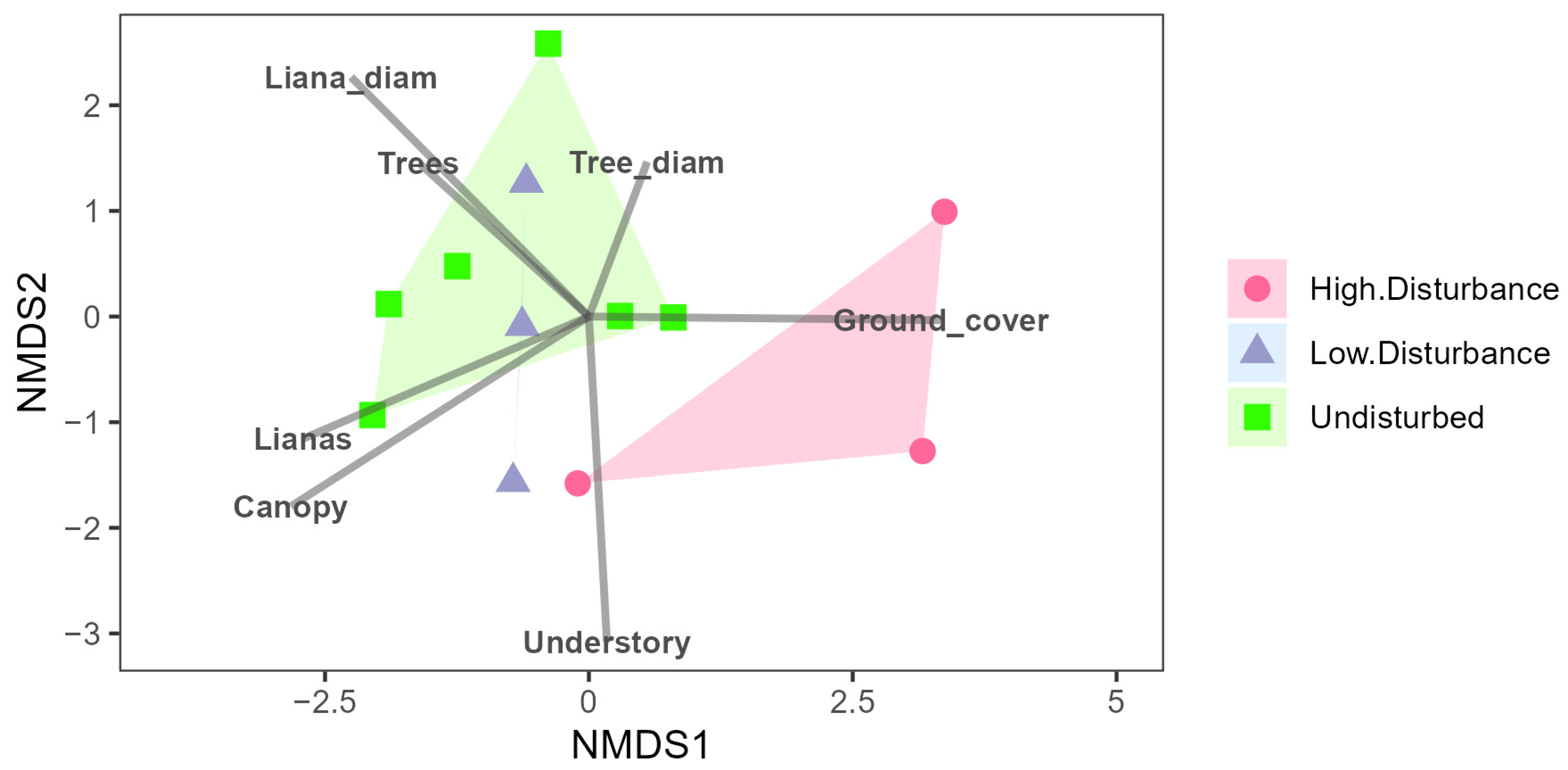
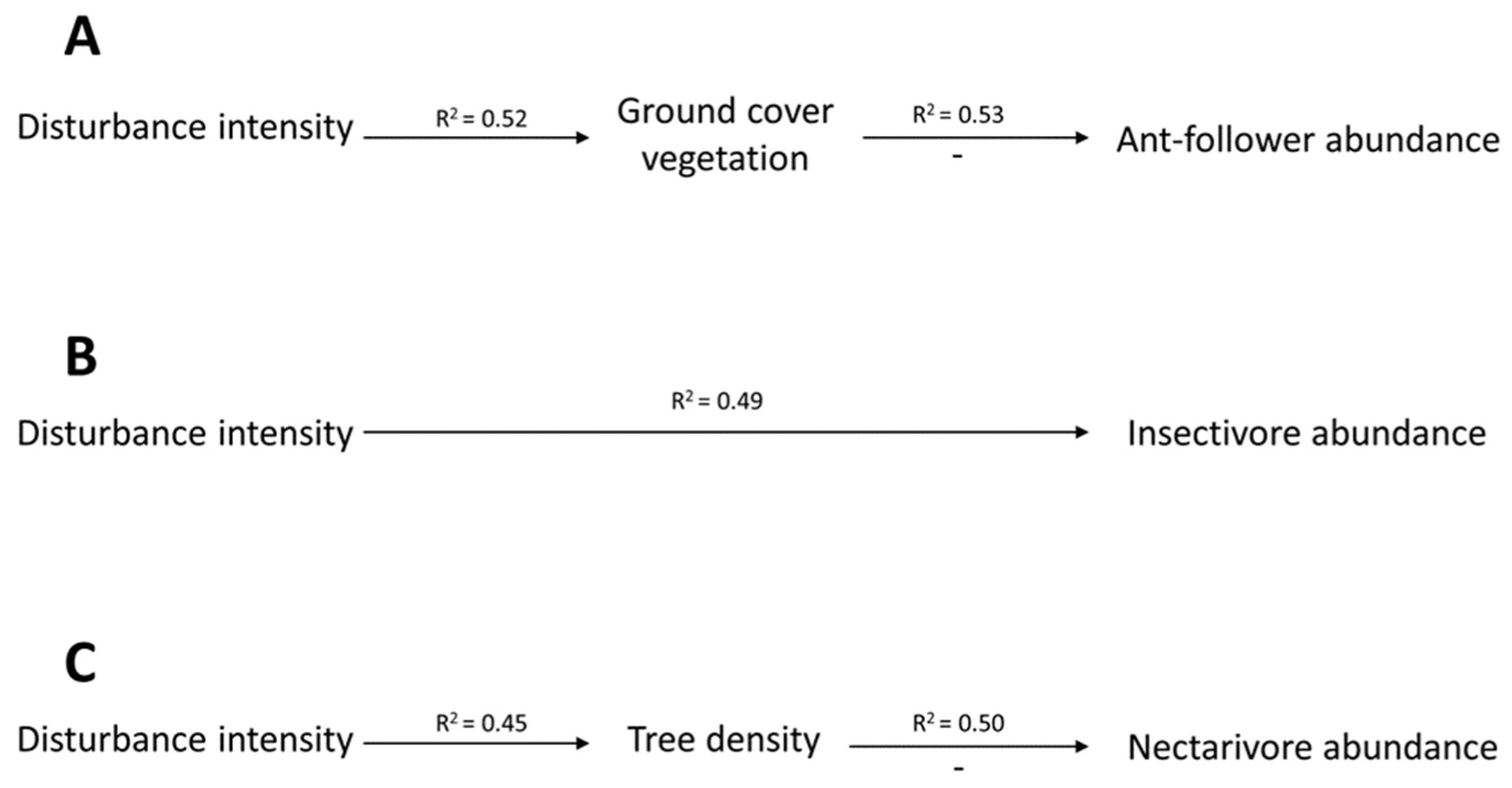
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Species | Scientific Name | Guild | Count |
|---|---|---|---|
| Amazonian Grosbeak | Cyanoloxia rothschildii | Granivore | 3 |
| American Pygmy Kingfisher | Chloroceryle aenea | Omnivore | 2 |
| Ash-throated Gnateater | Conopophaga peruviana | Insectivore | 1 |
| Black and White Tody-flycatcher | Poecilotriccus capitalis | Insectivore | 1 |
| Black-faced Antbird | Myrmoborus myotherinus | Insectivore | 17 |
| Blue-capped Manakin | Lepidothrix coronata | Frugivore | 19 |
| Chestnut Woodpecker | Celeus elegans | Omnivore | 1 |
| Cinereous Antshrike | Thamnomanes caesius | Insectivore | 4 |
| Collared Puffbird | Bucco capensis | Omnivore | 2 |
| Common Scale-backed Antbird | Willisornis poecilinotus | Insectivore | 11 |
| Double-banded Pygmy-tyrant | Lophotriccus vitiosus | Insectivore | 2 |
| Dusky-throated Antshrike | Thamnomanes ardesiacus | Insectivore | 5 |
| Elegant Woodcreeper | Xiphorhynchus elegans | Insectivore | 4 |
| Fork-tailed Woodnymph | Thalurania furcata | Nectarivore | 1 |
| Golden-headed Manakin | Ceratopipra erythrocephala | Frugivore | 3 |
| Gould’s Jewelfront | Heliodoxa aurescens | Nectarivore | 1 |
| Gray Antwren | Myrmotherula menetriesii | Insectivore | 1 |
| Great-billed Hermit | Phaethornis malaris | Nectarivore | 38 |
| Ivory-billed Aracari | Pteroglossus azara | Frugivore | 1 |
| Lunulated Antbird | Oneillornis lunulatus | Insectivore | 1 |
| Ocellated Woodcreeper | Xiphorhynchus ocellatus | Insectivore | 3 |
| Ochre-bellied Flycatcher | Mionectes oleagineus | Omnivore | 13 |
| Orange-bellied Euphonia | Euphonia xanthogaster | Frugivore | 1 |
| Pale-tailed Barbthroat | Threnetes leucurus | Nectarivore | 1 |
| Pearly Antshrike | Megastictus margaritatus | Insectivore | 10 |
| Peruvian Warbling-antbird | Hypocnemis peruviana | Insectivore | 2 |
| Plain Xenops | Xenops minutus | Insectivore | 8 |
| Plain-brown Woodcreeper | Dendrocincla fuliginosa | Insectivore | 6 |
| Plain-throated Antwren | Isleria hauxwelli | Insectivore | 14 |
| Purple Honeycreeper | Cyanerpes caeruleus | Omnivore | 1 |
| Royal Flycatcher | Onychorhynchus coronatus | Insectivore | 2 |
| Ruddy Foliage-gleaner | Clibanornis rubiginosus | Omnivore | 1 |
| Ruddy Quail-dove | Geotrygon montana | Omnivore | 3 |
| Ruddy Spinetail | Synallaxis rutilans | Insectivore | 4 |
| Ruddy-tailed Flycatcher | Terenotriccus erythrurus | Insectivore | 5 |
| Rufous-backed Stipplethroat | Epinecrophylla haematonota | Insectivore | 5 |
| Rufous-breasted Hermit | Glaucis hirsutus | Nectarivore | 4 |
| Scaly-breasted Wren | Microcerculus marginatus | Insectivore | 3 |
| Slate-colored Grosbeak | Saltator grossus | Omnivore | 1 |
| Slaty-backed Forest-falcon | Micrastur mirandollei | Carnivore | 1 |
| Slender-footed Tyrannulet | Zimmerius gracilipes | Insectivore | 1 |
| Sooty Antbird | Hafferia fortis | Insectivore | 1 |
| Spot-winged Antbird | Myrmelastes leucostigma | Insectivore | 3 |
| Straight-billed Hermit | Phaethornis bourcieri | Nectarivore | 13 |
| Swainson’s Thrush | Catharus ustulatus | Omnivore | 1 |
| Undulated Antshrike | Frederickena unduliger | Insectivore | 1 |
| Wedge-billed Woodcreeper | Glyphorynchus spirurus | Insectivore | 49 |
| White-bearded Hermit | Phaethornis hispidus | Nectarivore | 4 |
| White-bearded Manakin | Manacus manacus | Omnivore | 11 |
| White-cheeked Antbird | Gymnopithys leucaspis | Insectivore | 12 |
| White-crowned Manakin | Pseudopipra pipra | Frugivore | 9 |
| White-flanked Antwren | Myrmotherula axillaris | Insectivore | 6 |
| White-necked Thrush | Turdus albicollis | Omnivore | 1 |
| White-plumed Antbird | Pithys albifrons | Insectivore | 9 |
| Yellow-margined Flycatcher | Tolmomyias assimilis | Insectivore | 2 |
Appendix C
References
- Gibson, L.; Lee, T.M.; Pin Koh, L.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef]
- Barlow, J.; Franca, F.; Gardner, T.A.; Hicks, C.C.; Lennox, G.D.; Berenguer, E.; Castello, L.; Economo, E.P.; Ferriera, J.; Guenard, B.; et al. The future of hyperdiverse tropical ecosystems. Nature 2018, 559, 517–526. [Google Scholar] [CrossRef]
- Gardner, T. Monitoring Forest Biodiversity: Improving Conservation through Ecologically-Responsible Management, 1st ed.; Earthscan: Washington, DC, USA; London, UK, 2010. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Barreto, P.; Souza, C., Jr.; Noguerón, R.; Anderson, A.; Salomão, R. Human Pressure on the Brazilian Amazon Forests; World Resources Institute: Washington, DC, USA, 2006. [Google Scholar]
- Bax, V.; Francesconi, W.; Quintero, M. Spatial modeling of deforestation processes in the Central Peruvian Amazon. J. Nat. Conserv. 2016, 29, 79–88. [Google Scholar] [CrossRef]
- Dent, D.H.; Wright, S.J. The future of tropical species in secondary forests: A quantitative review. Biol. Conserv. 2009, 142, 2833–2843. [Google Scholar] [CrossRef]
- Edwards, D.P.; Tobias, J.A.; Sheil, D.; Meijaard, E.; Laurance, W.F. Maintaining ecosystem function and services in logged tropical forests. Trends Ecol. Evol. 2014, 29, 511–520. [Google Scholar] [CrossRef]
- Lennox, G.D.; Gardner, T.A.; Thomson, J.R.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Mac Nally, R.; Aragão, L.E.; Ferraz, S.F.; Louzada, J.; et al. Second rate or a second chance? Assessing biomass and biodiversity recovery in regenerating Amazonian forests. Glob. Chang. Biol. 2018, 24, 5680–5694. [Google Scholar] [CrossRef]
- Arcilla, N.; Strazds, M. Ten principles for bird-friendly forestry: Conservation approaches in natural forests used for timber production. Birds 2023, 4, 245–261. [Google Scholar] [CrossRef]
- Dunn, R.R. Recovery of faunal communities during tropical forest regeneration. Conserv. Biol. 2004, 18, 302–309. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A.; Henriques, L.M.P.; Stouffer, P.C.; Wunderle, J.M. The responses of understorey birds to forest fragmentation, logging and wildfires: An Amazonian synthesis. Biol. Conserv. 2006, 128, 182–192. [Google Scholar] [CrossRef]
- Burivalova, Z.; Lee, T.M.; Giam, X.; Şekercioğlu, Ç.H.; Wilcove, D.S.; Koh, L.P. Avian responses to selective logging shaped by species traits and logging practices. Proc. R. Soc. B 2015, 282, 20150164. [Google Scholar] [CrossRef]
- Tobias, J.A. Hidden impacts of logging. Nature 2015, 523, 163–164. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Miller, S.E. The potential for species conservation in tropical secondary forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef]
- Whitworth, A.; Downie, R.; von May, R.; Villacampa, J.; MacLeod, R. How much potential biodiversity and conservation value can a regenerating rainforest provide? A ‘best-case scenario’ approach from the Peruvian Amazon. Trop. Conserv. Sci. 2016, 9, 224–245. [Google Scholar] [CrossRef]
- Whitworth, A.; Pillco-Huarcaya, R.; Downie, R.; Villacampa, J.; Braunholtz, L.D.; MacLeod, R. Long lasting impressions: After decades of regeneration rainforest biodiversity remains differentially affected following selective logging and clearance for agriculture. Glob. Ecol. Conserv. 2018, 13, e00375. [Google Scholar] [CrossRef]
- Terborgh, J. Habitat selection in Amazonian birds. In Habitat Selection in Birds; Cody, M.L., Ed.; Academic Press Inc.: New York, NY, USA, 1985; pp. 311–338. [Google Scholar]
- Barlow, J.; Gardner, T.A.; Araújo, I.S.; Ávila-Pire, T.C.; Bonaldo, A.B.; Costa, J.E.; Espósito, M.C.; Ferreira, L.V.; Hawes, J.; Hernández, M.I.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forest. Proc. Natl. Acad. Sci. USA 2007, 105, 18555–18560. [Google Scholar] [CrossRef]
- Castaño-Villa, G.J.; Ramos-Valencia, S.A.; Fontúrbel, F.E. Fine-scale habitat structure complexity determines insectivorous bird diversity in a tropical forest. Acta Oecol. 2014, 61, 19–23. [Google Scholar] [CrossRef]
- Atikah, S.N.; Yahya, M.S.; Norhisham, A.R.; Kamarudin, N.; Sanusi, R.; Azhar, B. Effects of vegetation structure on avian biodiversity in a selectively logged hill dipterocarp forest. Glob. Ecol. Conserv. 2021, 28, e01660. [Google Scholar] [CrossRef]
- Corlett, R.; Primack, R. Tropical Rain Forests: An Ecological and Biogeographical Comparison, 2nd ed.; Wiley-Blackwell: West Sussex, UK, 2011. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Sekercioglu, C.H.; Barlow, J.; Robinson, S.K. Conservation of Tropical Birds, 1st ed.; Wiley-Blackwell: West Sussex, UK, 2011. [Google Scholar]
- Venier, L.A.; Pearce, J.L. Boreal forest landbirds in relation to forest composition, structure, and landscape: Implications for forest management. Can. J. For. Res. 2007, 37, 1214–1226. [Google Scholar] [CrossRef]
- Jokimäki, J.; Solonen, T. Habitat associations of old forest bird species in managed boreal forests characterized by forest inventory data. Ornis Fenn. 2011, 88, 57–70. [Google Scholar] [CrossRef]
- Moura, N.G.; Lees, A.C.; Andretti, C.B.; Davis, B.J.; Solar, R.R.; Aleixo, A.; Barlow, J.; Ferreira, J.; Gardner, T.A. Avian biodiversity in multiple-use landscapes of the Brazilian Amazon. Biol. Conserv. 2013, 167, 339–348. [Google Scholar] [CrossRef]
- Tobias, J.A.; Şekercioğlu, Ç.H.; Vargas, F.H. Bird conservation in tropical ecosystems: Challenges and opportunities. Key Top. Conserv. Biol. 2013, 2, 258–276. [Google Scholar] [CrossRef]
- Durães, R.; Carrasco, L.; Smith, T.B.; Karubian, J. Effects of forest disturbance and habitat loss on avian communities in a Neotropical biodiversity hotspot. Biol. Conserv. 2013, 166, 203–211. [Google Scholar] [CrossRef]
- Álvarez Alonso, J.; Whitney, B.M. New distributional records of birds from white-sand forests of the northern Peruvian Amazon, with Implications for biogeography of northern South America. Ornithol. Appl. 2003, 105, 552–566. [Google Scholar] [CrossRef]
- Shany, N.; Díaz Alván, J.; Álvarez Alonso, J. Finding white-sand forest specialists in Allpahuayo-Mishana Reserve, Peru. Neotrop. Bird. 2007, 2, 60–68. [Google Scholar]
- Álvarez Alonso, J.; Díaz Alván, J.; Shany, N. Avifauna de la Reserva Nacional Allpahuayo Mishana, Loreto, Perú. Cotinga 2012, 34, 132–152. [Google Scholar]
- Edwards, D.P.; Woodcock, P.; Newton, R.J.; Edwards, F.A.; Andrews, D.J.; Docherty, T.D.; Mitchell, S.L.; Ota, T.; Benedick, S.; Bottrell, S.H.; et al. Trophic flexibility and the persistence of understory birds in intensively logged rainforest. Conserv. Biol. 2013, 27, 1079–1086. [Google Scholar] [CrossRef]
- Arcilla, N.; Holbech, L.H.; O’Donnell, S. Severe declines of understory birds follow illegal logging in Upper Guinea forests of Ghana, West Africa. Biol. Conserv. 2015, 188, 41–49. [Google Scholar] [CrossRef]
- Powell, L.L.; Cordeiro, N.J.; Stratford, J.A. Ecology and conservation of avian insectivores of the rainforest understory: A pantropical perspective. Biol. Conserv. 2015, 188, 1–10. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Sanford, R.L. Nutrient cycling in moist tropical forest. Annu. Rev. Ecol. Evol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Garcia-Villacorta, R.; Ahuite-Reátegui, M.; Olortegui-Zumaeta, M. Clasificación de bosques sobre arena blanca de la Zona Reservada Allpahuayo-Mishana. Folia Amazón. 2003, 14, 17–33. [Google Scholar] [CrossRef]
- Adeney, J.M.; Christensen, N.L.; Vicentini, A.; Cohn-Haft, M. White-sand ecosystems in Amazonia. Biotropica 2016, 48, 7–23. [Google Scholar] [CrossRef]
- Salo, M.; Pyhälä, A. Exploring the gap between conservation science and protected area establishment in the Allpahuayo-Mishana National Reserve (Peruvian Amazonia). Environ. Conserv. 2007, 34, 23–32. [Google Scholar] [CrossRef]
- Schulenberg, T.S.; Stotz, D.F.; Lane, D.F.; O’Niell, J.P.; Parker, T.A., III. Birds of Peru, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 20 June 2023).
- Mattsson, B.J.; Zipkin, E.F.; Gardner, B.; Blank, P.J.; Sauer, J.R.; Royle, J.A. Explaining local-scale species distributions: Relative contributions of spatial autocorrelation and landscape heterogeneity for an avian assemblage. PLoS ONE 2013, 8, e55097. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Staudhammer, C.L.; Escobedo, F.J.; Blood, A. Assessing methods for comparing species diversity from disparate data sources: The case of urban and peri-urban forests. Ecosphere 2018, 9, e02450. [Google Scholar] [CrossRef]
- Horn, H.S. Measurement of “overlap” in comparative ecological studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C. SpadeR: Species-Richness Prediction and Diversity Estimation with R. R Package Version 0.1.1. 2016. Available online: https://cran.r-project.org/web/packages/SpadeR/index.html (accessed on 20 June 2023).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. R Package Version 3.0.0. 2022. Available online: https://cran.r-project.org/web/packages/iNEXT/index.html (accessed on 20 June 2023).
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. (Eds.) Birds of the World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2022; Available online: https://birdsoftheworld.org/bow/home (accessed on 15 June 2023).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 June 2023).
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Grace, J.B.; Schoolmaster, D.R., Jr.; Guntenspergen, G.R.; Little, A.M.; Mitchell, B.R.; Miller, K.M.; Schweiger, E.W. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 2012, 3, 73. [Google Scholar] [CrossRef]
- Mestre, L.A.M.; Cosset, C.C.P.; Nienow, S.S.; Krul, R.; Rechetelo, J.; Festti, L.; Edwards, D.P. Impacts of selective logging on avian phylogenetic and functional diversity in the Amazon. Anim. Conserv. 2020, 23, 725–740. [Google Scholar] [CrossRef]
- Chazdon, R.L. Chance and determinism in tropical forest succession. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Blackwell: Malden, MA, USA, 2008; pp. 384–408. [Google Scholar]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.; Sloan, S.P.; Plumptre, A. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef]
- Acevedo-Charry, O.; Aide, T.M. Recovery of amphibian, reptile, bird and mammal diversity during secondary forest succession in the tropics. Oikos 2019, 128, 1065–1078. [Google Scholar] [CrossRef]
- Brotons, L.; Pons, P.; Herrando, S. Colonization of dynamic Mediterranean landscapes: Where do birds come from after fire? J. Biogeogr. 2005, 32, 789–798. [Google Scholar] [CrossRef]
- Markl, J.S.; Schleuning, M.; Forget, P.M.; Jordano, P.; Lambert, J.E.; Traveset, A.; Wright, S.J.; Bohning-Gaese, K. Meta-analysis of the effects of human disturbance on seed dispersal by animals. Conserv. Biol. 2012, 26, 1072–1081. [Google Scholar] [CrossRef]
- Van Bael, S.A.; Brawn, J.D.; Robinson, S.K. Birds defend trees from herbivores in a Neotropical forest canopy. Proc. Natl. Acad. Sci. USA 2003, 100, 8304–8307. [Google Scholar] [CrossRef]
- Hamer, K.C.; Newton, R.J.; Edwards, F.A.; Denedick, S.; Bottrell, S.H.; Edwards, D.P. Impacts of selective logging on insectivorous birds in Borneo: The importance of trophic position, body size and foraging height. Biol. Conserv. 2015, 188, 82–88. [Google Scholar] [CrossRef]
- Cleary, D.F.; Boyle, T.J.; Setyawati, T.; Anggraeni, C.D.; Loon, E.E.V.; Menken, S.B. Bird species and traits associated with logged and unlogged forest in Borneo. Ecol. Appl. 2007, 17, 1184–1197. [Google Scholar] [CrossRef]
- Marques, J.T.; Ramos Periera, M.J.; Marques, T.A.; Santos, C.D.; Santana, J.; Beja, P.; Palmeirim, J.M. Optimizing sampling design to deal with mist-net avoidance in Amazonian birds and bats. PLoS ONE 2013, 8, e74505. [Google Scholar] [CrossRef]
- Wang, Y.; Finch, D.M. Consistency of mist netting and point counts in assessing landbird species richness and relative abundance during migration. Ornithol. Appl. 2002, 104, 59–72. [Google Scholar] [CrossRef]
- Martin, T.E.; Blackburn, G.A.; Simcox, W. An assessment of the effectiveness of two methods in describing a neotropical cloud forest bird community. Ornitol. Neotrop. 2010, 21, 131–147. [Google Scholar]
- Dunn, E.H.; Ralph, C.J. Use of mist nets as a tool for bird population monitoring. Stud. Avian Biol. 2004, 29, 1–6. [Google Scholar]
- Pérez-Granados, C.; Traba, J. Estimating bird density using passive acoustic monitoring: A review of methods and suggestions for further research. IBIS 2021, 163, 765–783. [Google Scholar] [CrossRef]
- Tito, R.; Salinas, N.; Cosio, E.G.; Boza Espinoza, T.E.; Muñiz, J.G.; Aragón, S.; Nina, A.; Roman-Cuesta, R. Secondary forests in Peru: Differential provision of ecosystem services compared to other post-deforestation forest transitions. Ecol. Soc. 2022, 27, 12. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glass, A.; Arcilla, N. The Effects of Disturbance Intensity on Tropical Forest Bird Communities and Vegetation Structure after Two Decades of Recovery. Birds 2024, 5, 388-403. https://doi.org/10.3390/birds5030026
Glass A, Arcilla N. The Effects of Disturbance Intensity on Tropical Forest Bird Communities and Vegetation Structure after Two Decades of Recovery. Birds. 2024; 5(3):388-403. https://doi.org/10.3390/birds5030026
Chicago/Turabian StyleGlass, Alex, and Nico Arcilla. 2024. "The Effects of Disturbance Intensity on Tropical Forest Bird Communities and Vegetation Structure after Two Decades of Recovery" Birds 5, no. 3: 388-403. https://doi.org/10.3390/birds5030026
APA StyleGlass, A., & Arcilla, N. (2024). The Effects of Disturbance Intensity on Tropical Forest Bird Communities and Vegetation Structure after Two Decades of Recovery. Birds, 5(3), 388-403. https://doi.org/10.3390/birds5030026





