Zebra Finch Females Avoided the Scent of Males with Greater Body Condition
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Species
2.2. Classification of Scent-Donour Males
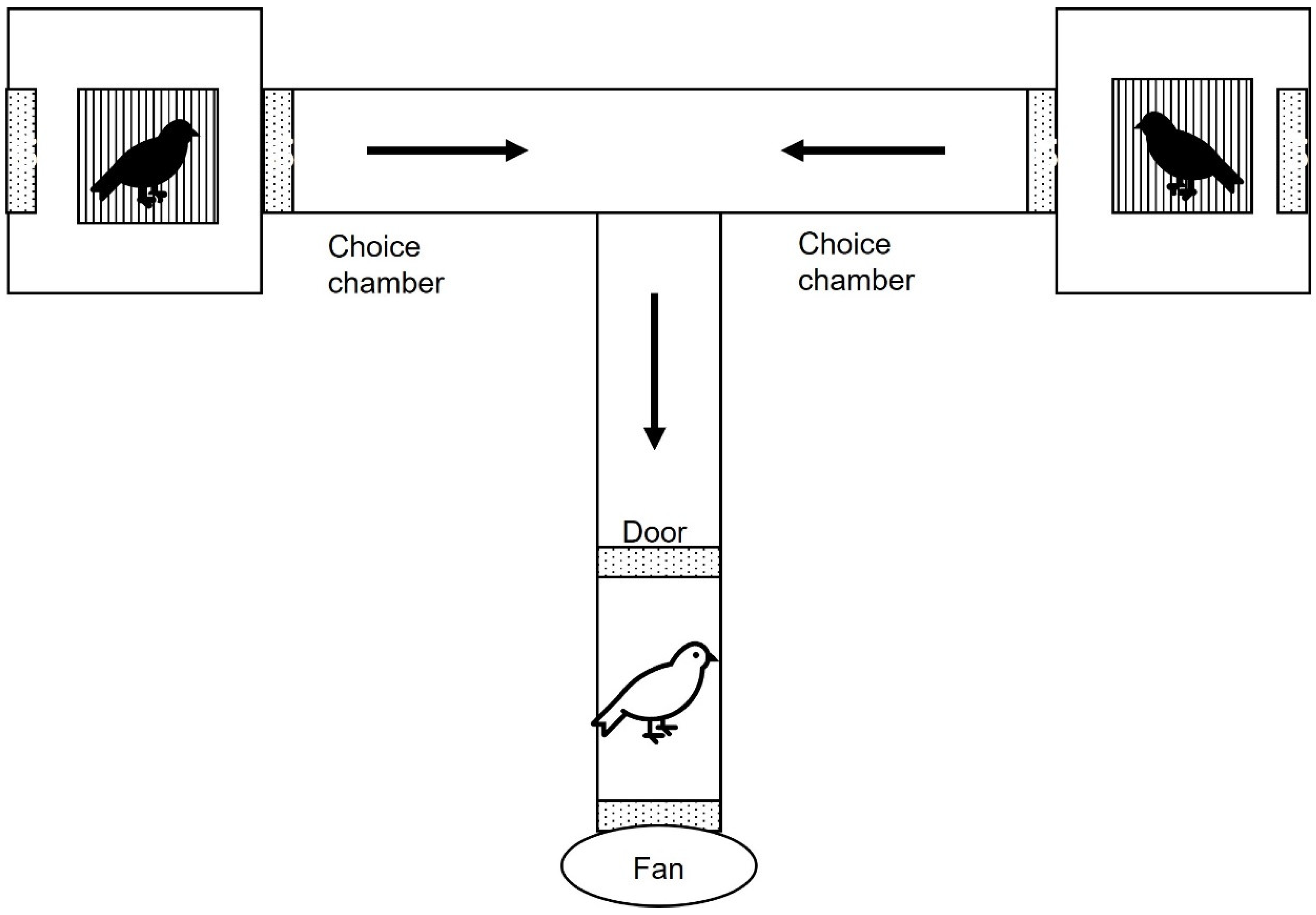
2.3. Behavioural Experiments
2.4. Data Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grafen, A. Biological signals as handicaps. J. Theor. Biol. 1990, 144, 517–546. [Google Scholar] [CrossRef] [PubMed]
- Kokko, H.; Jennions, M.D.; Brooks, R. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 43–66. [Google Scholar] [CrossRef]
- Trivers, R. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Campbell, B., Ed.; Aldine: Chicago, IL, USA, 1972; pp. 136–179. [Google Scholar]
- Fisher, R.A. The Genetical Theory of Natural Selection; Oxford University Press: Oxford, UK, 1930. [Google Scholar]
- Zahavi, A. Mate selection—A selection for a handicap. J. Theor. Biol. 1975, 67, 205–214. [Google Scholar] [CrossRef]
- Ahnesjö, I.; Vincent, A.; Alatalo, R.; Halliday, T.; Sutherland, W.J. The role of females in influencing mating patterns. Behav. Ecol. 1993, 4, 187–189. [Google Scholar] [CrossRef]
- Hamilton, W.D.; Zuk, M. Heritable True Fitness and Bright Birds: A Role for Parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E. A Red Bird in a Brown Bag. In The Function and Evolution of Colourful Plumage in the House Finch; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Mcgraw, K.J.; Nolan, P.M.; Crino, O.L. Carotenoid accumulation strategies for becoming a colourful House Finch: Analyses of plasma and liver pigments in wild moulting birds. Funct. Ecol. 2006, 20, 678–688. [Google Scholar] [CrossRef]
- Laucht, S.; Dale, J. Development of Badges of Status in Captive Male House Sparrows (Passer domesticus) in Relation to the Relative Ornamentation of Flock-Mates. Ethology 2012, 118, 644–653. [Google Scholar] [CrossRef]
- Grieves, L.A.; Kelly, T.R.; Bernards, M.A.; MacDougall-Shackleton, E.A. Malarial infection alters wax ester composition of preen oil in songbirds: Results of an experimental study. Ornithology 2018, 135, 767–776. [Google Scholar] [CrossRef]
- Whittaker, D.J.; Slowinski, S.P.; Greenberg, J.M.; Alian, O.; Winters, A.D.; Ahmad, M.M.; Burrell, M.J.E.; Soini, H.A.; Novotny, M.V.; Ketterson, E.D.; et al. Experimental evidence that symbiotic bacteria produce chemical cues in a songbird. J. Exp. Biol. 2019, 222, jeb202978. [Google Scholar] [CrossRef]
- Jacob, J.; Zisweiler, V. The uropygial gland. In Avian Biology; Farner, D.S., King, J.R., Eds.; Academic Press: New York, NY, USA, 1982; Volume 6, pp. 199–314. [Google Scholar]
- López-Rull, I.; Pagán, I.; Garcia, C.M. Cosmetic enhancement of signal coloration: Experimental evidence in the house finch. Behav. Ecol. 2010, 21, 781–787. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Uropygial gland and bib colouration in the house sparrow. PeerJ 2016, 4, e2102. [Google Scholar] [CrossRef] [PubMed]
- Reneerkens, J.; Piersma, T.; Damsté, J.S.S. Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc. R. Soc. B Biol. Sci. 2002, 269, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Amo, L.; Avilés, J.M.; Parejo, D.; Peña, A.; Rodríguez, J.; Tomás, G. Sex recognition by odour and variation in the uropygial gland secretion in starlings. J. Anim. Ecol. 2012, 81, 605–613. [Google Scholar] [CrossRef]
- Grieves, L.A.; Bernards, M.A.; MacDougall-Shackleton, E.A. Wax ester composition of songbird preen oil varies seasonally and differs between sexes, ages, and populations. J. Chem. Ecol. 2019, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.J.; Richmond, K.M.; Miller, A.K.; Kiley, R.; Burns, C.B.; Atwell, J.W.; Ketterson, E.D. Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav. Ecol. 2011, 22, 1256–1263. [Google Scholar] [CrossRef]
- Sandilands, V.; Powell, K.; Keeling, L.; Savory, C. Preen gland function in layer fowls: Factors affecting preen oil fatty acid composition. Br. Poult. Sci. 2004, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, V.; Savory, J.; Powell, K. Preen gland function in layer fowls: Factors affecting morphology and feather lipid levels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.J.; Levin, T.C.; Owen, J.C.; Garvin, M.C. Short-chain carboxylic acids from gray catbird (Dumetella carolinensis) uropygial secretions vary with testosterone levels and photoperiod. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 156, 183–188. [Google Scholar] [CrossRef]
- Whittaker, D.J.; Rosvall, K.A.; Slowinski, S.P.; Soini, H.A.; Novotny, M.V.; Ketterson, E.D. Songbird chemical signals reflect uropygial gland androgen sensitivity and predict aggression: Implications for the role of the periphery in chemosignaling. J. Comp. Physiol. A 2018, 204, 5–15. [Google Scholar] [CrossRef]
- Pap, P.L.; Vágási, C.I.; Osváth, G.; Mureşan, C.; Barta, Z. Seasonality in the uropygial gland size and feather mite abundance in house sparrows Passer domesticus: Natural covariation and an experiment. J. Avian Biol. 2010, 41, 653–661. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Uropygial gland size correlates with feather holes, body condition and wingbar size in the house sparrow Passer domesticus. J. Avian Biol. 2010, 41, 229–236. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Body-mass-dependent trade-off between immune response and uropygial gland size in house sparrows Passer domesticus. J. Avian Biol. 2015, 46, 40–45. [Google Scholar] [CrossRef]
- Tuttle, E.M.; Sebastian, P.J.; Posto, A.L.; Soini, H.A.; Novotny, M.V.; Gonser, R.A. Variation in preen oil composition pertaining to season, sex, and genotype in the polymorphic white-throated sparrow. J. Chem. Ecol. 2014, 40, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Mardon, J.; Saunders, S.M.; Anderson, M.J.; Couchoux, C.; Bonadonna, F. Species, gender, and identity: Cracking petrels’ sociochemical code. Chem. Senses 2010, 35, 309–321. [Google Scholar] [CrossRef]
- Whittaker, D.J.; Soini, H.A.; Atwell, J.W.; Hollars, C.; Novotny, M.V.; Ketterson, E.D. Songbird chemosignals: Volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav. Ecol. 2010, 21, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Leclaire, S.; Merkling, T.; Raynaud, C.; Giacinti, G.; Bessière, J.-M.; Hatch, S.A.; Danchin, É. An individual and a sex odor signature in kittiwakes? Study of the semiochemical composition of preen secretion and preen down feathers. Sci. Nat. 2011, 98, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Campagna, S.; Mardon, J.; Celerier, A.; Bonadonna, F. Potential semiochemical molecules from birds: A practical and comprehensive compilation of the last 20 years studies. Chem. Senses 2011, 37, 3–25. [Google Scholar] [CrossRef]
- Leclaire, S.; Merkling, T.; Raynaud, C.; Mulard, H.; Bessière, J.-M.; Lhuillier, É.; Hatch, S.A.; Danchin, É. Semiochemical compounds of preen secretion reflect genetic make-up in a seabird species. Proc. R. Soc. B Biol. Sci. 2011, 279, 1185–1193. [Google Scholar] [CrossRef]
- Leclaire, S.; van Dongen, W.F.D.; Voccia, S.; Merkling, T.; Ducamp, C.; Hatch, S.A.; Blanchard, P.; Danchin, É.; Wagner, R.H. Preen secretions encode information on MHC similarity in certain sex-dyads in a monogamous seabird. Sci. Rep. 2014, 4, 6920. [Google Scholar] [CrossRef]
- Strandh, M.; Westerdahl, H.; Pontarp, M.; Canbäck, B.; Dubois, M.-P.; Miquel, C.; Taberlet, P.; Bonadonna, F. Major histocompatibility complex class II compatibility, but not class I, predicts mate choice in a bird with highly developed olfaction. Proc. R. Soc. B Biol. Sci. 2012, 279, 4457–4463. [Google Scholar] [CrossRef]
- Slade, J.W.G.; Watson, M.J.; Kelly, T.R.; Gloor, G.B.; Bernards, M.A.; MacDougall-Shackleton, E.A. Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161966. [Google Scholar] [CrossRef] [PubMed]
- Coffin, H.R.; Watters, J.V.; Mateo, J.M. Odor-Based Recognition of Familiar and Related Conspecifics: A First Test Conducted on Captive Humboldt Penguins (Spheniscus humboldti). PLoS ONE 2011, 6, e25002. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.T.; Krüger, O.; Kohlmeier, P.; Caspers, B.A. Olfactory kin recognition in a songbird. Biol. Lett. 2012, 8, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, F.; Sanz-Aguilar, A. Kin recognition and inbreeding avoidance in wild birds: The first evidence for individual kin-related odour recognition. Anim. Behav. 2012, 84, 509–513. [Google Scholar] [CrossRef]
- Leclaire, S.; Strandh, M.; Mardon, J.; Westerdahl, H.; Bonadonna, F. Odour-based discrimination of similarity at the major histocompatibility complex in birds. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162466. [Google Scholar] [CrossRef] [PubMed]
- Grieves, L.; Gloor, G.; Bernards, M.; MacDougall-Shackleton, E. Songbirds show odour-based discrimination of similarity and diversity at the major histocompatibility complex. Anim. Behav. 2019, 158, 131–138. [Google Scholar] [CrossRef]
- Amo, L.; López-Rull, I.; Pagán, I.; Garcia, C.M. Male quality and conspecific scent preferences in the house finch, Carpodacus mexicanus. Anim. Behav. 2012, 84, 1483–1489. [Google Scholar] [CrossRef]
- Johansson, B.G.; Jones, T.M. The role of chemical communication in mate choice. Biol. Rev. 2007, 82, 265–289. [Google Scholar] [CrossRef]
- Thomas, M.L. Detection of female mating status using chemical signals and cues. Biol. Rev. 2011, 86, 1–13. [Google Scholar] [CrossRef]
- Caspers, B.A.; Hagelin, J.C.; Paul, M.; Bock, S.; Willeke, S.; Krause, E.T. Zebra Finch chicks recognise parental scent, and retain chemosensory knowledge of their genetic mother, even after egg cross-fostering. Sci. Rep. 2017, 7, 12859. [Google Scholar] [CrossRef]
- Caspers, B.A.; Gagliardo, A.; Krause, E.T. Impact of kin odour on reproduction in zebra finches. Behav. Ecol. Sociobiol. 2015, 69, 1827–1833. [Google Scholar] [CrossRef]
- Caspers, B.A.; Hagelin, J.; Bock, S.; Krause, E.T. An Easy Method to Test Odour Recognition in Songbird Hatchlings. Ethology 2015, 121, 882–887. [Google Scholar] [CrossRef]
- Golüke, S.; Bischof, H.-J.; Caspers, B.A. Nestling odour modulates behavioural response in male, but not in female zebra finches. Sci. Rep. 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.T.; Brummel, C.; Kohlwey, S.; Baier, M.C.; Müller, C.; Bonadonna, F.; Caspers, B.A. Differences in olfactory species recognition in the females of two Australian songbird species. Behav. Ecol. Sociobiol. 2014, 68, 1819–1827. [Google Scholar] [CrossRef]
- Krause, E.T.; Bischof, H.J.; Engel, K.; Golüke, S.; Maraci, O.; Mayer, U.; Sauer, J.; Caspers, B.A. Olfaction in the Zebra Finch (Taeniopygia guttata): What Is Known and Further Perspectives. In Advances in the Study of Behavior; Naguib, M., Barrett, L., Healy, S.D., Podos, J., Simmons, L.W., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 37–85. [Google Scholar]
- Krause, E.T.; Paul, M.; Krüger, O.; Caspers, B.A. Olfactory sex preferences in six Estrildid Finch species. Front. Ecol. Evol. 2023, 11, 1000531. [Google Scholar] [CrossRef]
- Bonadonna, F.; Nevitt, G.A. Partner-Specific Odor Recognition in an Antarctic Seabird. Science 2004, 306, 835. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, F.; Caro, S.; Jouventin, P.; Nevitt, G.A. Evidence that blue petrel, Halobaena caerulea, fledglings can detect and orient to dimethyl sulfide. J. Exp. Biol. 2006, 209, 2165–2169. [Google Scholar] [CrossRef] [PubMed]
- Crino, O.L.; van Oorschot, B.K.; Crandell, K.E.; Breuner, C.W.; Tobalske, B.W. Flight performance in the altricial zebra finch: Developmental effects and reproductive consequences. Ecol. Evol. 2017, 7, 2316–2326. [Google Scholar] [CrossRef]
- De Kogel, C.H.; Prijs, H.J. Effects of brood size manipulations on sexual attractiveness of offspring in the zebra finch. Anim. Behav. 1996, 51, 699–708. [Google Scholar] [CrossRef]
- Holveck, M.-J.; Riebel, K. Low-quality females prefer low-quality males when choosing a mate. Proc. R. Soc. B Biol. Sci. 2010, 277, 153–160. [Google Scholar] [CrossRef]
- Holveck, M.-J.; Geberzahn, N.; Riebel, K. An experimental test of condition-dependent male and female mate choice in zebra finches. PLoS ONE 2011, 6, e23974. [Google Scholar] [CrossRef]
- Griffith, S.C.; Ton, R.; Hurley, L.L.; McDiarmid, C.S.; Pacheco-Fuentes, H. The ecology of the zebra finch makes it a great laboratory model but an outlier amongst passerine birds. Birds 2021, 2, 60–76. [Google Scholar] [CrossRef]
- Bolund, E.; Schielzeth, H.; Forstmeier, W. Intrasexual competition in zebra finches, the role of beak colour and body size. Anim. Behav. 2007, 74, 715–724. [Google Scholar] [CrossRef]
- Arakawa, H.; Blanchard, D.C.; Arakawa, K.; Dunlap, C.; Blanchard, R.J. Scent marking behavior as an odorant communication in mice. Neurosci. Biobehav. Rev. 2008, 32, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.T.; Parker, M.R. Social behavior and pheromonal communication in reptiles. J. Comp. Physiol. A 2010, 196, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Maynard Smith, J.; Parker, G.A. The logic of asymmetrical contests. Anim. Behav. 1976, 32, 564–578. [Google Scholar] [CrossRef]
- Mardon, J.; Saunders, S.M.; Bonadonna, F. From preen secretions to plumage: The chemical trajectory of blue petrels’ Halobaena caerulea social scent. J. Avian Biol. 2011, 42, 29–38. [Google Scholar] [CrossRef]
- Golüke, S.; Caspers, B.A. Sex-specific differences in preen gland size of Zebra Finches during the course of breeding. Ornithology 2017, 134, 821–831. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amo, L.; López-Rull, I. Zebra Finch Females Avoided the Scent of Males with Greater Body Condition. Birds 2024, 5, 127-136. https://doi.org/10.3390/birds5010009
Amo L, López-Rull I. Zebra Finch Females Avoided the Scent of Males with Greater Body Condition. Birds. 2024; 5(1):127-136. https://doi.org/10.3390/birds5010009
Chicago/Turabian StyleAmo, Luisa, and Isabel López-Rull. 2024. "Zebra Finch Females Avoided the Scent of Males with Greater Body Condition" Birds 5, no. 1: 127-136. https://doi.org/10.3390/birds5010009
APA StyleAmo, L., & López-Rull, I. (2024). Zebra Finch Females Avoided the Scent of Males with Greater Body Condition. Birds, 5(1), 127-136. https://doi.org/10.3390/birds5010009




