Simple Summary
Survival for birds can be challenging during the cold winter months in Northern Europe. Humans often provide food to wintering birds, thus, improving their winter survival. This study shows that excess underskin fat reserves of Great Tits wintering near permanent feeders can make them slow at take-off, which increases their exposure to predators. We suggest that caution should be taken when choosing a proper place to position bird feeders to prevent making birds at feeders easy prey for predators.
Abstract
The optimal body mass hypothesis posits that the body reserves of wintering birds are balanced between the risk of starvation and predation. In this study, we tested whether the body mass of wintering Great Tits (Parus major) was higher under conditions of less predictable food resources. We compared body mass, body mass index, the speed at take-off, and apparent survival of Great Tit adult males wintering in small urban areas either near feeders providing permanent access to food for months or near feeders providing irregular access to food. Body mass and body mass index were greater, while take-off speed and apparent survival were lower, in birds wintering near permanent feeders than birds wintering near irregular feeders. Thus, urban birds, with their predictable access to high energy food, did not follow the fattening strategy predicted by the optimal body mass hypothesis. This study shows that regular excess amounts of high-energy food may affect urban birds’ physiological and behavioral strategies in a non-adaptive way. We recommend irregular feeding of wintering birds and the placing of feeders in places that are safe against attacking predators.
1. Introduction
Photoperiod and low ambient temperatures directly affect body mass and energy reserves in wintering birds [1]. Resident birds often respond to winter conditions in temperate climates by increasing their subcutaneous fat reserves [2]. Small passerine birds typically gain up to c. 10% extra body mass before a winter night [3,4]. However, during the coldest and longest winter nights in northern Europe, Great Tits (Parus major) lose up to a quarter of their evening body mass, which needs to be replenished the next day [5,6]. While energy reserves are essential to avoid starvation, wintering birds usually maintain their body reserves below their physiological capacity, suggesting costs associated with excessive body energy reserves [7,8]. It has been suggested that predation risk represents a cost of being fat because higher body mass may make birds escaping prey slower to take flight and to escape [9]. Birds were found to reduce their subcutaneous fat reserves and body mass with high levels of perceived predator risk [10,11,12]. However, only a few studies have shown a negative impact of body reserves on take-off speed and maneuverability [13,14,15,16,17]. However, most studies showed no extra body mass effect on the prey’s escape ability [18,19,20,21]. This discrepancy might arise because gaining excess body reserves is a time-consuming activity, increasing exposure to predators.
Bird feeding is the activity of humans supplying food to wild birds in both urban and rural areas, often using specifically designed bird feeders [22,23,24,25]. This activity has a long history and originated centuries ago [26]. It has become the second most popular hobby and a serious part of the economy of some countries where people buy bird food, bird feeders, bird houses, and other equipment for feeding and observing birds [27]. Although wintering birds at feeders that are almost always stocked have unlimited access to food to build up their energy reserves and enhance overwinter survival [28,29,30] and breeding success [31], evidence suggests multiple adverse ecological and physiological effects of unrestricted feeding [32]. The costs of birds feeding are mainly linked to an increased risk of predation, as bird feeders attract many prey individuals and their predators, such as hawks and domestic cats. Bird feeding may foster bird dependency, increase competition, cause malnutrition, and facilitate the spread of infectious diseases [33,34,35]. These costs are less significant for bird species that attend feeders irregularly, as they require a more diverse diet than the feeders can provide. However, bird species that critically depend on supplementary food, especially under harsh climate conditions, may take the opportunity to consume extra amounts of potentially unlimited food supplied by humans at bird feeders.
The regularity with which food is present in feeders should also influence birds’ energy regulation and fat reserves. Dynamic optimization models of winter food-related behavior in both caching and non-caching songbird species predicted that greater variability in access to food should lead to greater body fat reserves than when there is consistent access to food [36]. Experimental tests in captive settings revealed that for both subordinate and dominant Carolina chickadees (Poecile carolinensis) individuals increased fat storage under conditions of irregular feeding during the winter months [37]. Thus, we might expect greater fat reserves in individuals facing unpredictable/irregular food supplies in comparison to individuals facing consistent ad-lib food supplies.
In this study, we compared evening body mass, the speed at takeoff, and overwinter survival of resident adult male Great Tits supplied with permanent feeders and feeders with non-permanent and irregular food resources. The first group of birds was provided with unlimited amounts of sunflower seeds for the whole duration of the non-reproductive season, while under the conditions of non-permanent feeding, birds were provided the food 2–3 times each week for periods shorter than 2 h a day. We predicted higher body mass and lower speed at take-off in Great Tits wintering near permanent feeders, which may affect their winter survival.
2. Materials and Methods
2.1. Study Sites and Procedures
This study was conducted near the town of Krāslava (55°53′ N 27°11′ E) in south-eastern Latvia (55°53′ N 27°11′ E) during five winters (2011/2012, 2012/2013, 2015/2016, 2018/2019, and 2020/2021) [38]. The experimental part of the study was carried out in December, which was a relatively warm month. The average air temperature in December in the study area ranged from 0 to −5 °C. The field study was carried out in two independent areas of holiday cottages separated by 5 km. Both areas of summer cottages are enclosed by a rich mature forest of Norway spruce (Picea abies), Scotch pine (Pinus sylvestris), with an under-story of rowan-tree (Sorbus aucuparia), common juniper (Juniperus communis), common buckthorn (Rhamnus catharticus), and young pines and spruces growing on mesic soil. The proportion of spruce by basal area at the study sites ranged from 3.3% to 91.7% (mean 32.8%). The mean tree height of pine and spruce has been reported to be 26.6 m (range 9.9–38.1 m) and 21.1 m (range 11.2–35.3), respectively [39]. A significant increase in clear-cut areas has occurred in our study site since 2006. Old monoculture spruce stands have been lacking at the studied sites since 2012, substantially affecting old forest patch connectivity [40]. Silver birch (Betula pendula) was the third most common tree species in both study areas [38]. Although other deciduous tree species also occurred, only aspen (Populus tremula) and alders (Alnus incana and A. glutinosa) were common. Other trees, such as common oak (Quercus robur), mountain ash (Fraxinus excelsior), bird cherry (Prunus padus), and European hazel (Corylus avellana), occurred marginally in both study areas. These forests are highly fragmented because of the many clear-cut areas, small semi-open bogs, and areas of pine and spruce saplings [41].
In winter, cottage owners did not feed wintering birds since none of them lived permanently in the cottage areas.
2.2. Feeders and Feeding Regimes
We installed 3–4 bird feeders in each area each winter. The feeders mainly attracted Great Tits, Blue Tits (Cyanistes caeruleus), Nuthatches (Sitta europaea), and Greenfinches (Carduelis chloris). In one cottage area, we provided food from mid-July until the following April. We baited the feeders with black oil sunflower seeds with shells, and the food was available throughout the day and the study season under constant feeding regime (permanent feeding). In another cottage area, we supplied the feeders twice a week with limited food amounts available for c. 2 h (irregular feeding regime).
2.3. Study Species
Several male Great Tits that bred in the area in a previous season preferred to overwinter near their breeding territories without leaving the area. All the males were caught during the breeding season and at time of capture we weighed them and measured their wing lengths. We also caught each of them during the following winter season. The birds were caught between three and five times between the end of November and December (median = 5, Q1–Q3: 4–5). At each capture, we measured the birds’ wing length and weight using Pesola spring scales (PESOLA Präzisionswaagen AG, Schindellegi, Switzerland; precision of 0.1 g). Each bird was banded with metal bands and individually recognizable plastic bands in the preceding summer or previous winter. Birds did not lose any of these bands during the study. Wing (maximum chord) was measured to within 0.5 mm error. We studied 28 adult male Great Tits in the area of permanent feeding and 25 males in the area of irregular feeding. In this study, we considered only adult males because they dominate over females and juvenile males in their social flocks, and their physiological quality and survival do not depend on the social structure of their groups [5,6,42,43].
2.4. Data Collection
To test whether Great Tit body mass impaired their escape speed, we caught the birds during the final 30 min of their daily activities, and after the weighing procedure, each Great Tit was placed in a holding bag (10 × 10 × 10 cm) for 30 s. After this alarming procedure, we opened the holding box and released the bird. The escape flights were video-recorded using JVC Everio GZ-MS215SE, JVCKENWOOD USA Corporation, Long Beach CA, USA. The birds could escape by flying the first meter horizontally because they had to pass through a cardboard box. We measured the time taken for each bird to fly horizontally up to a line marked 40 cm from the head of the bird at the moment of escape. Time taken to complete this distance was then converted into velocity [17]. Only one trial was done for each Great Tit in a week, and each bird was tested three to five times. Birds at both study sites were always caught, and their take-off speed was measured on consecutive days to avoid the possible effects of season and date.
To control the role of body mass for the size of each bird, we calculated a body mass index (BMI) by dividing body mass by the third power of wing length (body mass/(wing length × 103)) [41,42,44]. Extra body mass increases wing load, which negatively affects maneuverability and the speed of escape behavior [17]. Therefore, wing length is of biological significance for energy reserves and, ultimately, predation risk [13,45,46,47]. Ambient temperatures were between 0 and 3 °C during take-off speed measurements.
2.5. Survival
Each year, take-off speed, body mass, and other procedures were completed at the end of December. Bird overwinter survival was checked again at the beginning of the next breeding season, as we used local adult male Great Tits. Thus, we used two time points to estimate survival: the date of the final take-off measurement (December) and the presence of each experimental male in its breeding territory next spring (April–May). Since we could not fully control for reasons for male disappearance from their winter territories, we defined the survival observed in this study as apparent survival.
We visited study sites several times every week to check the feeders and observe bird behavior and their predators. We spent between one to two hours at each feeder each time. In the study areas, Great Tits were often attacked by Eurasian Sparrowhawks (Accipiter nisus) and Pygmy Owls (Glaucidium passerinum). Sparrowhawk attacks were observed 2–3 times a week, and some banded Great Tits were found in Pygmy Owl caches in mid-winter [48].
2.6. Statistical Analyses
We used simple logistic regression to predict the effects of feeding on the overwinter survival of the adult Great Tits. All relevant assumptions (binary dependent variable, independence of the observations and sufficient sample size) for the model were satisfied. t-tests were used to compare average evening body mass, BMI, and average speed at the take-off in two locations with different feeding. We calculated Pearson correlations between body mass and BMI to the average speed at the take-off. We also compared average evening body mass during the summer and winter in two feeding locations using paired t-tests. All tests were two-tailed, where p < 0.05 was considered statistically significant, and results are shown as mean ± SE. Any possible metal or plastic band losses were not included in the analysis because we did not observe either metal or plastic band losses during the study. We also did not estimate any possible emigration of male Great Tits from the study area because: (i) males that owned a breeding territory in a previous breeding season and spent half of the following winter season in or near their breeding territories are not expected to emigrate only a couple of months ahead of the next breeding season; (ii) we have no reason to consider males from the two study groups to be different in their emigration propensity. Therefore, late winter emigration was not considered in the analysis. All statistical analyses were performed using IBM SPSS Statistics 19 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Body Mass, Body Mass Index, and Survival
The simple regression model was statistically significant (χ2 = 6.263, df = 1, p < 0.012), explaining 17.0% of the adult male apparent survival (Nagelkerke R2), with the feeding regime being a significant predictor (Wald χ2 = 4.92, df = 1, p = 0.027). Adult male Great Tits survived better in the irregular feeding group (92%, 23 from 25) than in the regular feeding group (64%, 18 from 28). Thus, the relative risk of not surviving the winter was 4.46 (odds ratio = 6.39).
In winter, adult male Great Tits from the location with constant feeding were significantly heavier (20.4 ± 0.06 g, mean ± SE) than the birds with irregular feeding (18.8 ± 0.04 g: t-test, t = 20.98, df = 45.9, p < 0.0001) and had significantly higher body mass index (42.64 ± 0.2 g compared to 39.54 ± 0.18 g, mean ± SE, respectively, t-test, t = 11.52, df = 51, p < 0.0001). The average speed at take-off was greater in the irregular feeding group, 3.81 ± 0.02 m/s (mean ± SE), than in the constantly feeding group, 3.48 ± 0.023 m/s (mean ± SE), (t-test, t = 10.64, df = 51, p < 0.0001).
There was a significant difference in the average body mass measured in the summer and in the winter in the constant feeding group (paired t-test, t = −22.74, p < 0.0001), and there was a significant difference in this measure between the seasons in the irregular feeding group (paired t-test, t = −17.36, p < 0.0001). In both feeding groups, the birds were heavier in winter (Figure 1A). Additionally, there were significant differences in BMI measured in the summer and in the winter in both constant feeding and irregular feeding groups (paired t-tests, t = −29.76, p < 0.0001 and t = −6.3, p < 0.0001, respectively) (Figure 1B).
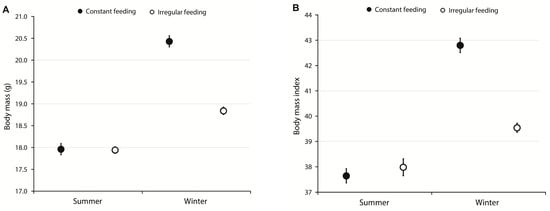
Figure 1.
(A) Average (±2SE) body mass and (B) body mass index (BMI) of male Great Tits from constant (black symbols) and irregular (open symbols) feeding locations in summer and winter.
There was a strong negative correlation between average body mass (winter) and speed at take-off (r = −0.91, p < 0.0001) (Figure 2A) and a strong negative correlation between BMI and speed at take-off (r = −0.80, p < 0.0001) (Figure 2B). Simple linear regression models indicated that increasing the body mass (g) and BMI by a value of one decreased the average speed of male Great Tits at take-off in winter by 0.22 m/s and 0.09 m/s (mean ± SE), respectively (both, p < 0.0001) (Figure 2B).
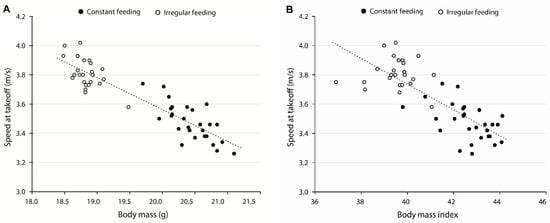
Figure 2.
Relationships between (A) the average body mass and the speed at take-off, and (B) the body mass index (BMI) and the speed at take-off of male Great Tits from both constant (black symbols) and irregular feeding (open symbols) locations in winter.
3.2. Prey Behavior
We observed 17 attacks of Sparrowhawks on Great Tits near feeders. One successful and ten unsuccessful attacks were observed near feeders with irregular food access, and six successful and seven unsuccessful attacks were recorded near permanent feeders. The number of successful attacks was higher at permanent feeders than at irregular feeders (χ2 = 3.96, p = 0.047). Two out of four of the killed Great Tits did not attempt to escape in the vicinity of permanent feeders, and they were caught by Sparrowhawks while pecking seeds. These birds did not utter any alarm call either. All the attacked Great Tits near non-permanent feeders gave alarm and distress calls while escaping the predators. Only two of the four Great Tits gave alarm calls while escaping predators near permanent feeders.
4. Discussion
The results of this study show that body mass and body mass index of adult males was greater in individuals wintering near permanent feeders than near irregular feeders. In contrast, take-off speed and apparent survival were higher in male Great Tits wintering near irregular feeders. This shows that wintering birds do not always follow the fattening strategies predicted by the optimal body mass hypothesis and that there is a link between body mass, take-off speed, and survival [5].
Most experimental studies have not found a significant effect of daily body mass gain of small birds on their escape speed and maneuverability [49,50]. Passerine birds usually gain up to 10% extra body mass, consisting of energy reserves, before a winter night [3,4,51]. It is believed that an increase in body mass of this scale insufficiently affects the speed at take-off and survival. However, the results of this study show a substantial rise in body mass and body mass index, a decrease in take-off speed, and lower apparent survival in Great Tits wintering in areas with food provided ad libitum.
It has generally been assumed that reduced winter fat reserves in small birds are symptomatic of environmental constraints on their ability to fatten further [52]. This study was done during stable winter temperatures across all study winters. Therefore, we cannot attribute the results obtained to the level of winter harshness. We also cannot add anything to the role of social dominance because all individuals in this study were adult males, owners of territories, and dominants within their home ranges [41,53]. The results of this study are relevant only to consider the importance of energy reserves as insurance against the starvation risk. Birds facing a greater starvation risk often carry more body reserves than individuals who enjoy greater predictability of food resources [5,6,44,54,55]. Surprisingly, adult Great Tits were heavier under conditions of more predictable access to food than under irregular feeding, despite similar ambient temperatures and seemingly the same risk of being predated by Eurasian Sparrowhawks and Pygmy Owls.
The likely reason for the greater body mass of male Great Tits wintering in the area of permanently supplied feeders is competition between members of basic flocks. Great Tits do not hoard their food; they do not remove sunflower seeds from feeders to cache them to eat later [56]. Instead, Great Tits tend to consume all food items as soon as possible. Otherwise, the food will be consumed by other members of the basic flock [57]. Although this kind of overeating due to competition can be explained within the time scale of hours and days, building excessive levels of daily body reserves under the conditions of highly predictable food availability and accessibility for months cannot be explained by the optimal body mass hypothesis [8]. This hypothesis suggests that the optimal solution between starvation and predation is the mass that minimizes joint risk [6,45,54]. Importantly, excessive body reserves were gained and carried at the expense of lower overwinter survival, which suggests that more predictable food and its availability ad libitum negatively influenced birds’ fitness, as survival until the next reproductive season is a significant part of an individual’s fitness.
Some studies have shown that birds from urban (with extra food provided by humans) populations may be heavier than individuals from non-urban (no extra food available) habitats [58,59], probably because of the higher energy content in their food in human-populated areas. Sunflower seeds are one of the most popular foods to supply bird feeders. The seeds contain at least 40–50% fat, and this food is termed a high-energy diet for birds [60]. Birds have a fast metabolism, meaning that they can burn calories quickly. Wintering Great Tits typically cover much larger distances while traveling with other members of their compound groups (aggregation of several basic flocks) to find food [61,62]. We often waited for 0.5–1.5 h until the first bird arrived at the feeders with non-permanent access to food, while almost all local male Great Tits stayed 50–150 m away from their permanent feeders. This suggests that Great Tits wintering near permanent feeders might be physically less active while consuming a high-energy diet compared to birds wintering near feeders supplied with sunflower seeds irregularly.
High body fat levels or obesity is well-defined in humans and some domestic mammals [63,64]. However, it is still unclear how to define obesity in birds [65,66]. One definition is that a bird can be obese when its body mass is c. 20% higher than usual [67]. In this study, the evening body mass of birds wintering in the vicinity of permanent feeders was c. 7.7% and BMI was 7.2% higher than birds wintering near the irregular feeders. Our results suggest that most birds around permanent feeders can be considered overweight rather than obese.
Despite the difficulties with the definitions, overweight and obese individuals across different animal taxa can be characterized as those consuming energy-dense foods and following a largely sedentary lifestyle [68]. In humans and domestic birds, obesity is defined as a medical condition in which excess fat has accumulated to such an extent that it may negatively affect health [60,69]. While excess body mass is a major cause of type 2 diabetes, cardiovascular disease, certain types of cancer, and osteoarthritis in humans, in birds, high-calorie diets (all seeds and nuts) and a lack of exercise often cause severe problems like cardiomyopathy, sudden cardiac failure, circulatory collapse, atherosclerosis, and fatty liver syndrome. These and other diseases make birds weak and slow and induce illness responses known as sickness behavior [67], a set of primarily adaptive behavioral changes that develop in response to reorganizing the organism’s priorities to cope with the disease [70]. Sickness behavior is often caused by inflammation in the central nervous system (CNS) [71]. It includes responses such as lethargy, depression, sleepiness, reduction in grooming, and failure to attend to salient stimuli in the environment [72]. In this study, we did not test the birds for possible diseases in the area with permanent feeders. However, the observations of attacks by Eurasian Sparrowhawks suggest that birds were slower to escape and less prone to give alarm calls to warn other group members in the group of birds wintering in the vicinity of permanent feeders. Some previous work shows that high levels of fat reserves may bring costs to migrating birds. For example, the constitutive immune function of Red Knots (Calidris canutus) was stronger in individuals storing fat than in Red Knots recovering proteins at stopover sites [73]. It was shown that migratory endurance flight may weaken the immune function of birds [74,75]. However, it is still unclear whether migratory flight or fat stores are responsible for the deterioration of constitutive immune function in migratory birds [76]. To sum up, this study and studies done on migratory birds suggest that carrying high levels of underskin fat reserves may bring physiological costs to migratory birds and individuals wintering at high latitudes. This topic deserves more research in the future.
Because of the passivity of Great Tits near the permanent feeders avoiding attacking predators, we cannot exclude metabolic diseases due to overeating and being overweight in wild birds. Therefore, birds at permanent feeders need to be further investigated and tested for overweight- and obesity-associated diseases, oxidative stress, and inflammation of their CNS. Overall, this study shows that body reserves of wintering birds are not limited only by an interplay between the perceived predator risk and starvation risk. In urban/suburban areas, birds with unlimited and predictable access to food sources provided by humans can consume much more food than predicted by the optimal body mass hypothesis [5,8,77].
5. Conclusions
The results of this study suggest that competition for food, the energy content of food, and possible overweight- and obesity-associated health issues as drivers of foraging behavior need to be considered and studied in more detail to better understand winter fattening and energy management in birds. The results also show the role of safety against predators when choosing a proper place to position bird feeders to prevent making feeders the sites of ecological traps [78].
Author Contributions
T.K.: conceptualization, funding acquisition, project administration, supervision, formal analysis, investigation, visualization, writing—original draft. R.K.: investigation, methodology, writing—review and editing. S.P.: conceptualization, investigation, writing—review and editing; G.T.: formal analysis, visualization, writing—original draft. M.J.R.: funding acquisition, formal analysis, writing. T.M.F.: writing—original draft, writing—review and editing. I.A.K.: conceptualization, formal analysis, funding acquisition, investigation, methodology, supervision, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Latvian Council of Science (grants lzp-2018/2-00057, lzp-2020/2-0271, lzp-2021/1-0277, and lzp-2022/1-0348), and the Estonian Research Council (grant PUT1223).
Institutional Review Board Statement
Ethical permission #87 was issued by the Food and Veterinary Service of the Republic of Latvia. Birds were caught and banded under bird banding permit #40 issued to Indrikis A. Krams by the Latvian Ringing Centre.
Data Availability Statement
The data that support the findings of this study are available from the Zenodo repository (doi: 10.5281/zenodo.7775391).
Acknowledgments
We would like to thank Kristers Raivo Krams and Jurijs Ivanovs for their help at different stages of the study. TMF acknowledges the support of a Fulbright Teaching Award to work at Daugavpils University, Latvia, during spring 2012.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Houston, A.I.; McNamara, J.M. A Theoretical Investigation of the Fat Reserves and Mortality Levels of Small Birds in Winter. Ornis Scand. 1993, 24, 205. [Google Scholar] [CrossRef]
- Brodin, A. Why Do Hoarding Birds Gain Fat in Winter in the Wrong Way? Suggestions from a Dynamic Model. Behav. Ecol. 2000, 11, 27–39. [Google Scholar] [CrossRef]
- Lehikoinen, E. Seasonality of the Daily Weight Cycle in Wintering Passerines and Its Consequences. Ornis Scand. 1987, 18, 216. [Google Scholar] [CrossRef]
- Haftorn, S. Seasonal and Diurnal Body Weight Variations in Titmice, Based on Analyses of Individual Birds. Wilson Bull. 1989, 101, 217–235. [Google Scholar]
- Krams, I.; Cirule, D.; Suraka, V.; Krama, T.; Rantala, M.J.; Ramey, G. Fattening Strategies of Wintering Great Tits Support the Optimal Body Mass Hypothesis under Conditions of Extremely Low Ambient Temperature. Funct. Ecol. 2010, 24, 172–177. [Google Scholar] [CrossRef]
- Krams, I.; Cīrule, D.; Vrublevska, J.; Nord, A.; Rantala, M.J.; Krama, T. Nocturnal Loss of Body Reserves Reveals High Survival Risk for Subordinate Great Tits Wintering at Extremely Low Ambient Temperatures. Oecologia 2013, 172, 339–346. [Google Scholar] [CrossRef]
- Wood, K.A.; Stillman, R.A.; Newth, J.L.; Nuijten, R.J.M.; Hilton, G.M.; Nolet, B.A.; Rees, E.C. Predicting Avian Herbivore Responses to Changing Food Availability and Competition. Ecol. Model. 2021, 441, 109421. [Google Scholar] [CrossRef]
- Lima, S.L. Predation Risk and Unpredictable Feeding Conditions: Determinants of Body Mass in Birds. Ecology 1986, 67, 377–385. [Google Scholar] [CrossRef]
- Zimmer, C.; Boos, M.; Poulin, N.; Gosler, A.; Petit, O.; Robin, J.-P. Evidence of the Trade-Off between Starvation and Predation Risks in Ducks. PLoS ONE 2011, 6, e22352. [Google Scholar] [CrossRef]
- Lilliendahl, K. The Effect of Predator Presence on Body Mass in Captive Greenfinches. Anim. Behav. 1997, 53, 75–81. [Google Scholar] [CrossRef]
- Gentle, L.K.; Gosler, A.G. Fat Reserves and Perceived Predation Risk in the Great Tit, Parus major. Proc. R. Soc. Lond. B 2001, 268, 487–491. [Google Scholar] [CrossRef]
- Gosler, A.G.; Greenwood, J.J.D.; Perrins, C. Predation Risk and the Cost of Being Fat. Nature 1995, 377, 621–623. [Google Scholar] [CrossRef]
- Witter, M.S.; Cuthill, I.C.; Bonser, R.H.C. Experimental Investigations of Mass-Dependent Predation Risk in the European Starling, Sturnus vulgaris. Anim. Behav. 1994, 48, 201–222. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Ure, S.E. Diurnal Variation in Flight Performance and Hence Potential Predation Risk in Small Birds. Proc. R. Soc. B Biol. Sci. 1995, 261, 395–400. [Google Scholar] [CrossRef]
- Gosler, A.G. Environmental and Social Determinants of Winter Fat Storage in the Great Tit Parus major. J. Anim. Ecol. 1996, 65, 1–17. [Google Scholar] [CrossRef]
- Hake, M. Fattening Strategies in Dominance-Structured Greenfinch (Carduelis chloris) Flocks in Winter. Behav. Ecol. Sociobiol. 1996, 39, 71–76. [Google Scholar] [CrossRef]
- Krams, I. Mass-Dependent Take-off Ability in Wintering Great Tits (Parus major): Comparison of Top-Ranked Adult Males and Subordinate Juvenile Females. Behav. Ecol. Sociobiol. 2002, 51, 345–349. [Google Scholar] [CrossRef]
- van der Veen, I.T.; Lindström, K.M. Escape Flights of Yellowhammers and Greenfinches: More than Just Physics. Anim. Behav. 2000, 59, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.W.; Piersma, T.; Hedenström, A.; Brugge, M. Intraspecific Variation in Avian Pectoral Muscle Mass: Constraints on Maintaining Manoeuvrability with Increasing Body Mass. Funct Ecol. 2007, 21, 317–326. [Google Scholar] [CrossRef]
- van den Hout, P.J.; Mathot, K.J.; Maas, L.R.M.; Piersma, T. Predator Escape Tactics in Birds: Linking Ecology and Aerodynamics. Behav. Ecol. 2010, 21, 16–25. [Google Scholar] [CrossRef]
- Walters, B.T.; Cheng, T.N.N.; Doyle, J.; Guglielmo, C.G.; Clinchy, M.; Zanette, L.Y. Too Important to Tamper with: Predation Risk Affects Body Mass and Escape Behaviour but Not Escape Ability. Funct Ecol 2017, 31, 1405–1417. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Skórka, P.; Sparks, T.H.; Biaduń, W.; Brauze, T.; Hetmański, T.; Martyka, R.; Indykiewicz, P.; Myczko, Ł.; Kunysz, P.; et al. Urban and Rural Habitats Differ in Number and Type of Bird Feeders and in Bird Species Consuming Supplementary Food. Environ. Sci. Pollut. Res. 2015, 22, 15097–15103. [Google Scholar] [CrossRef] [PubMed]
- Tryjanowski, P.; Møller, A.P.; Morelli, F.; Indykiewicz, P.; Zduniak, P.; Myczko, Ł. Food Preferences by Birds Using Bird-Feeders in Winter: A Large-Scale Experiment. Avian. Res. 2018, 9, 16. [Google Scholar] [CrossRef]
- Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L. Residential Areas Support Overwintering Possibilities of Most Bird Species. Annales Zoologici Fennici 2012, 49, 240–256. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J. Distribution and Habitat Selection of Wintering Birds in Urban Environments. Landsc. Urban Plan. 1998, 39, 253–263. [Google Scholar] [CrossRef]
- Jones, W.; Kulma, K.; Bensch, S.; Cichoń, M.; Kerimov, A.; Krist, M.; Laaksonen, T.; Moreno, J.; Munclinger, P.; Slater, F.M.; et al. Interspecific Transfer of Parasites Following a Range-Shift in Ficedula Flycatchers. Ecol. Evol. 2018, 8, 12183–12192. [Google Scholar] [CrossRef]
- Orros, M.E.; Fellowes, M.D.E. Wild Bird Feeding in an Urban Area: Intensity, Economics and Numbers of Individuals Supported. Acta Ornithol. 2015, 50, 43–58. [Google Scholar] [CrossRef]
- Jones, D.N.; James Reynolds, S. Feeding Birds in Our Towns and Cities: A Global Research Opportunity. J. Avian Biol. 2008, 39, 265–271. [Google Scholar] [CrossRef]
- Cox, D.T.C.; Gaston, K.J. Human–Nature Interactions and the Consequences and Drivers of Provisioning Wildlife. Phil. Trans. R. Soc. B 2018, 373, 20170092. [Google Scholar] [CrossRef]
- Shutt, J.D.; Trivedi, U.H.; Nicholls, J.A. Faecal Metabarcoding Reveals Pervasive Long-Distance Impacts of Garden Bird Feeding. Proc. R. Soc. B. 2021, 288, 20210480. [Google Scholar] [CrossRef]
- Broggi, J.; Watson, H.; Nilsson, J.; Nilsson, J. Carry-over Effects on Reproduction in Food-supplemented Wintering Great Tits. J. Avian Biol. 2022, 2022, e02969. [Google Scholar] [CrossRef]
- Broggi, J.; Hohtola, E.; Koivula, K. Winter Feeding Influences the Cost of Living in Boreal Passerines. Ibis 2021, 163, 260–267. [Google Scholar] [CrossRef]
- Malpass, J.S.; Rodewald, A.D.; Matthews, S.N. Species-Dependent Effects of Bird Feeders on Nest Predators and Nest Survival of Urban American Robins and Northern Cardinals. Condor Ornithol. Appl. 2017, 119, 1–16. [Google Scholar] [CrossRef]
- Lawson, B.; Robinson, R.A.; Toms, M.P.; Risely, K.; MacDonald, S.; Cunningham, A.A. Health Hazards to Wild Birds and Risk Factors Associated with Anthropogenic Food Provisioning. Phil. Trans. R. Soc. B 2018, 373, 20170091. [Google Scholar] [CrossRef]
- Shutt, J.D.; Lees, A.C. Killing with Kindness: Does Widespread Generalised Provisioning of Wildlife Help or Hinder Biodiversity Conservation Efforts? Biol. Conserv. 2021, 261, 109295. [Google Scholar] [CrossRef]
- Pravosudov, V.V. A Dynamic Model of Short-Term Energy Management in Small Food-Caching and Non-Caching Birds. Behav. Ecol. 2001, 12, 207–218. [Google Scholar] [CrossRef]
- Pravosudov, V.V.; Lucas, J.R. The Effect of Social Dominance on Fattening and Food-Caching Behaviour in Carolina Chickadees, Poecile carolinensis. Anim. Behav. 2000, 60, 483–493. [Google Scholar] [CrossRef]
- Rytkönen, S.; Krams, I. Does Foraging Behaviour Explain the Poor Breeding Success of Great Tits Parus major in Northern Europe? J. Avian Biol. 2003, 34, 288–297. [Google Scholar] [CrossRef]
- Brūmelis, G.; Dauškane, I.; Elferts, D.; Strode, L.; Krama, T.; Krams, I. Estimates of Tree Canopy Closure and Basal Area as Proxies for Tree Crown Volume at a Stand Scale. Forests 2020, 11, 1180. [Google Scholar] [CrossRef]
- Rendenieks, Z.; Nikodemus, O.; Brūmelis, G. Dynamics in Forest Patterns during Times of Forest Policy Changes in Latvia. Eur. J. For. Res. 2015, 134, 819–832. [Google Scholar] [CrossRef]
- Krams, I. Dominance-Specific Vigilance in the Great Tit. J. Avian Biol. 1998, 29, 55. [Google Scholar] [CrossRef]
- Krams, I. Rank-Dependent Fattening Strategies of Willow Tit Parus montanus and Crested Tit P. cristatus Mixed Flock Members. Ornis Fenn. 1998, 75, 19–26. [Google Scholar]
- Krams, I.; Cīrule, D.; Krama, T.; Vrublevska, J. Extremely Low Ambient Temperature Affects Haematological Parameters and Body Condition in Wintering Great Tits (Parus major). J. Ornithol. 2011, 152, 889–895. [Google Scholar] [CrossRef]
- Ekman, J.B.; Lilliendahl, K. Using Priority to Food Access: Fattening Strategies in Dominance-Structured Willow Tit (Parus montanus) Flocks. Behav. Ecol. 1993, 4, 232–238. [Google Scholar] [CrossRef]
- McNamara, J.M.; Houston, A.I. The Value of Fat Reserves and the Tradeoff between Starvation and Predation: There’s a Special Providence in the Fall of a Sparrow Hamlet Act V Sc Ii. Acta Biotheor 1990, 38, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Pravosudov, V.V.; Grubb, T.C.; Doherty, P.F.; Bronson, C.L.; Pravosudova, E.V.; Dolby, A.S. Social Dominance and Energy Reserves in Wintering Woodland Birds. Condor 1999, 101, 880–884. [Google Scholar] [CrossRef]
- Krams, I.A.; Luoto, S.; Krama, T.; Krams, R.; Sieving, K.; Trakimas, G.; Elferts, D.; Rantala, M.J.; Goodale, E. Egalitarian Mixed-Species Bird Groups Enhance Winter Survival of Subordinate Group Members but Only in High-Quality Forests. Sci. Rep. 2020, 10, 4005. [Google Scholar] [CrossRef] [PubMed]
- Krama, T.; Krams, R.; Cīrule, D.; Moore, F.R.; Rantala, M.J.; Krams, I.A. Intensity of Haemosporidian Infection of Parids Positively Correlates with Proximity to Water Bodies, but Negatively with Host Survival. J. Ornithol. 2015, 156, 1075–1084. [Google Scholar] [CrossRef]
- Kullberg, C.; Fransson, T.; Jakobsson, S. Impaired Predator Evasion in Fat Blackcaps (Sylvia atricapilla). Proc. R. Soc. B Biol. Sci. 1996, 263, 1671–1675. [Google Scholar]
- Veasey, J.S.; Metcalfe, N.B.; Houston, D.C. A Reassessment of the Effect of Body Mass upon Flight Speed and Predation Risk in Birds. Anim. Behav. 1998, 56, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Sargisson, R.J.; McLean, I.G.; Brown, G.S.; White, K.G. Seasonal Variation in Pigeon Body Weight and Delayed Matching-to-Sample Performance. J. Exp. Anal. Behav. 2007, 88, 395–404. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.M.; Ekman, J.; Houston, A.J. The Effect of Thermoregulatory Substitution on Optimal Energy Reserves of Small Birds in Winter. Oikos 2004, 105, 192–196. [Google Scholar] [CrossRef]
- Sandell, M.; Smith, H.G. Dominance, Prior Occupancy, and Winter Residency in the Great Tit (Parus major). Behav. Ecol. Sociobiol. 1991, 29, 147–152. [Google Scholar] [CrossRef]
- Krams, I. Length of Feeding Day and Body Weight of Great Tits in a Single- and a Two-Predator Environment. Behav. Ecol. Sociobiol. 2000, 48, 147–153. [Google Scholar] [CrossRef]
- Gosler, A.G. Strategy and Constraint in the Winter Fattening Response to Temperature in the Great Tit Parus major. J. Anim. Ecol. 2002, 71, 771–779. [Google Scholar] [CrossRef]
- Ekman, J. Ecology of Non-Breeding Social Systems of Parus. Wilson Bull. 1989, 101, 263–288. [Google Scholar]
- Jokimäki, J.; Suhonen, J.; Kaisanlahti-Jokimäki, M.-L. Differential Long-Term Population Responses of Two Closely Related Human-Associated Sparrow Species with Respect to Urbanization. Birds 2021, 2, 230–249. [Google Scholar] [CrossRef]
- Auman, H.J.; Meathrel, C.E.; Richardson, A. Supersize Me: Does Anthropogenic Food Change the Body Condition of Silver Gulls? A Comparison Between Urbanized and Remote, Non-Urbanized Areas. Waterbirds 2008, 31, 122–126. [Google Scholar] [CrossRef]
- Risi, T.C.; Sumasgutner, P.; Cunningham, S.J. Anthropogenic Food Availability and Body Mass Maintenance in Urban Red-Winged Starlings Onychognathus morio. Ostrich 2021, 92, 16–25. [Google Scholar] [CrossRef]
- Hosseinian, S.A.; Hasanzadeh, F. Impact of High Dietary Energy on Obesity and Oxidative Stress in Domestic Pigeons. Vet. Med. Sci. 2021, 7, 1391–1399. [Google Scholar] [CrossRef]
- Hinde, R.A. The Behavior of the Great Tit (Parus major), and Some Other Related Species; E. J. Brill: Leiden, The Netherlands, 1952. [Google Scholar]
- Grabowska-Zhang, A.M.; Sheldon, B.C.; Hinde, C.A. Long-Term Familiarity Promotes Joining in Neighbour Nest Defence. Biol. Lett. 2012, 8, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Giles, E.D.; Jackman, M.R.; MacLean, P.S. Modeling Diet-Induced Obesity with Obesity-Prone Rats: Implications for Studies in Females. Front. Nutr. 2016, 3, 50. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Cherian, G. Metabolic and Cardiovascular Diseases in Poultry: Role of Dietary Lipids. Poult. Sci. 2007, 86, 1012–1016. [Google Scholar] [CrossRef]
- Coogan, S.C.P.; Raubenheimer, D.; Zantis, S.P.; Machovsky-Capuska, G.E. Multidimensional Nutritional Ecology and Urban Birds. Ecosphere 2018, 9. [Google Scholar] [CrossRef]
- Bandyopadhyay, S. Systemic Clinical and Metabolic Diseases. In Pet Bird Diseases and Care; Springer: Singapore, 2017; pp. 167–252. ISBN 978-981-10-3673-6. [Google Scholar]
- Harakeh, S.M.; Khan, I.; Kumosani, T.; Barbour, E.; Almasaudi, S.B.; Bahijri, S.M.; Alfadul, S.M.; Ajabnoor, G.M.A.; Azhar, E.I. Gut Microbiota: A Contributing Factor to Obesity. Front. Cell. Infect. Microbiol. 2016, 6, 95. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Kelley, K.W. Twenty Years of Research on Cytokine-Induced Sickness Behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Bipolar Disorder: An Evolutionary Psychoneuroimmunological Approach. Neurosci. Biobehav. Rev. 2021, 122, 28–37. [Google Scholar] [CrossRef]
- Kelley, K.W.; Bluthé, R.-M.; Dantzer, R.; Zhou, J.-H.; Shen, W.-H.; Johnson, R.W.; Broussard, S.R. Cytokine-Induced Sickness Behavior. Brain Behav. Immun. 2003, 17, 112–118. [Google Scholar] [CrossRef]
- Buehler, D.M.; Tieleman, B.I.; Piersma, T. Indices of Immune Function Are Lower in Red Knots (Calidris canutus) Recovering Protein Than in Those Storing Fat during Stopover in Delaware Bay. Auk 2010, 127, 394–401. [Google Scholar] [CrossRef]
- Matson, K.D.; Horrocks, N.P.C.; Tieleman, B.I.; Haase, E. Intense Flight and Endotoxin Injection Elicit Similar Effects on Leukocyte Distributions but Dissimilar Effects on Plasma-Based Immunological Indices in Pigeons. J. Exp. Biol. 2012, 215 Pt 21, 3734–3741. [Google Scholar] [CrossRef]
- Eikenaar, C.; Hegemann, A.; Packmor, F.; Kleudgen, I.; Isaksson, C. Not Just Fuel: Energy Stores Are Correlated with Immune Function and Oxidative Damage in a Long-Distance Migrant. Curr. Zool. 2020, 66, 21–28. [Google Scholar] [CrossRef]
- Eikenaar, C.; Hessler, S.; Hegemann, A. Migrating Birds Rapidly Increase Constitutive Immune Function during Stopover. R. Soc. Open Sci. 2020, 7, 192031. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.L. Stress and Decision Making under the Risk of Predation: Recent Developments from Behavioral, Reproductive, and Ecological Perspectives. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1998; Volume 27, pp. 215–290. ISBN 978-0-12-004527-3. [Google Scholar]
- Krams, R.; Krama, T.; Brūmelis, G.; Elferts, D.; Strode, L.; Dauškane, I.; Luoto, S.; Šmits, A.; Krams, I.A. Ecological Traps: Evidence of a Fitness Cost in a Cavity-Nesting Bird. Oecologia 2021, 196, 735–745. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).