Simple Summary
Through the examination of several blood metabolites in two migratory warblers at two stopover sites in Portugal, Sedge warblers showed higher values of triglycerides (TRIG) + glycerol (GLY) than Eurasian Reed warblers, both during post-flight fasting and when stopping naturally. The higher values of TRIG + GLY in circulation should reflect a higher fat reserve mobilization by Sedge warblers, attributed to the fewer possibilities to refuel, relying more on fat reserves during their migratory journeys than those of Eurasian Reed warblers. Sedge warblers use more lipids or more efficiently the reserves in each migratory step, due to longer fasting periods causing lower glycaemia levels. Our study revealed differences in the level of unsaturation subcutaneous fatty acids, mainly the higher levels of polyunsaturated fatty acids in Sedge warbler. Differences in preying habits may trigger a series of adaptations that results in different migratory strategies between Sedge and Eurasian Reed warblers.
Abstract
The overall speed of bird migration is limited by the amount of fuel stores acquired during the initial phases of migration. The ability to mobilize fat is crucial for migratory birds that can exhibit different migratory strategies. Birds mobilize triglycerides during nocturnal flight thus increasing circulating fatty acids and glycerol to meet the metabolic demands of flight. Eurasian Reed (Acrocephalus scirpaceus) and Sedge (Acrocephalus schoenobaenus) Warblers were captured at Portuguese stopover sites during spring and autumn migration. These species were selected based on their different migration strategies and dietary preferences during migration. Blood metabolites and fat composition were analyzed to determine their nutritional states. Sedge Warblers had higher blood triglyceride and glycerol levels during post-flight fasting than in non-fasting periods. Furthermore, Sedge Warblers had higher triglyceride and glycerol levels than Eurasian Reed Warblers in both post-flight fasting and non-fasting condition. The differences found may reflect distinct approaches in re-feeding activity (e.g., feeding intensely) associated with the number of stopovers during migratory cycle. Dietary preferences affect the fat composition available for oxidation during long-term exercise in migratory flight. Nuclear magnetic resonance analysis of subcutaneous fat composition revealed that Sedge Warblers presented higher levels of polyunsaturated fatty acid levels than Eurasian Reed Warblers. The distinct lipidic profiles observed and differences in feeding ecology may explain the different migration strategies of these species. Overall and despite their ecological similarity, our study species showed pronounced differences in blood metabolites levels and subcutaneous fatty acids composition, likely attributed to the migratory strategy and foraging preferences during their migratory cycle.
1. Introduction
Migratory birds have developed different strategies to deal with long-distance movements, with most species using stopover sites to refuel along their journeys [1]. The frequency and duration of these stopovers determine migration speed and, therefore, migrants face trade-offs between refuelling and carrying heavier fuel stores. During fasting or high energy demand, such as migratory flight, triglycerides (TRIG) are mobilized and hydrolyzed, releasing free fatty acids (FFA) and glycerol (GLY) into the circulation, increasing their bloodstream concentration [2]. To meet the energetic demands of flight, migrating birds can feed at stopover sites, leading to the synthesis of TRIG in the liver that is stored in adipose tissue for later use.
Researchers commonly use plasma metabolites as indicators of the migratory state of songbirds, particularly TRIG, but also glucose (GLUC), GLY, cholesterol, FFA, β-hydroxybutyrate, and uric acid [3,4,5]. TRIG and GLY can be used to infer metabolic condition, although levels in the blood have been found to vary among species. For example, Jenni-Eiermann and Jenni found lower TRIG levels in flying than resting Red Knots (Calidris canutus) [6]. However, Jenni-Eiermann and Jenni [7,8] showed that small migratory passerines had high levels of TRIG in their blood plasma during flight. The difference among several species in TRIG levels during stopover can be attributed to metabolic differences between passerines and non-passerines, or their fasting duration due to flight distance [6]. Given these differences, Jenni-Eiermann and Jenni suggested an additional pathway involving TRIG and FFA to flight muscles [6]. Therefore, levels of TRIG in flying birds should vary according to their migratory state.
Blood GLUC concentration is the most commonly measured physiological variable in fasting animals [9], but levels of GLUC in the blood of birds are usually higher than in other vertebrates with similar body mass [10,11]. GLUC is involved in several metabolic pathways and is especially important in energy production and FA synthesis [10]. However, the mechanisms behind how birds maintain such high blood GLUC levels have not yet been clarified. Carbohydrates are the primary fuel for flight [12,13], but with the increasing flight duration fatty acid oxidation becomes an increasingly important source of fuel [13,14]. Overall, high blood GLUC concentrations are maintained at the expense of FA oxidation to fuel long distance migrations [10,15]. After depletion of glycogen and fat reserves, proteins are also catabolized, resulting in muscle loss and increasing levels of uric acid in the blood [13,15,16].
FA composition is influenced mainly by diet and can affect the migratory performance of birds, which may be characterized by the FA profile [17,18]. Price stated that both FA chain length and the degree of unsaturation influenced the rate of mobilization, transport, and oxidation of lipid reserves and affect overall flight endurance, such as shorter or with more double bonds FA are likely to increase flight performance due to their higher transport rates en route to oxidation [19]. Birds may alter FA composition either through endogenous mechanisms or though their diet [19]. Given that birds are incapable of de novo synthesis of some FA, such as polyunsaturated fatty acids (PUFAs), these must be obtained from dietary sources [19,20]. Therefore, diet composition, therefore, influences the FA composition of fuel stores in birds [21], as well as seasonal and pre-migratory changes in the FA composition of adipose tissue [22,23,24].
Eurasian Reed (Acrocephalus scirpaceus) and Sedge (Acrocephalus schoenobaenus) Warblers are nocturnal long-distance migratory Palearctic passerines, similar in body size and morphology, that winter in tropical Africa [25]. Despite their similarity, these two species differ in feeding ecology, diet, and stopover behavior [26,27,28,29], and can present differences in their metabolic profiles [30]. Some Eurasian Reed Warblers breed in Portuguese reedbeds, but Sedge Warblers only breed in central and northern Europe. During spring migration, Sedge Warblers leave their wintering areas with sufficient fat reserves to fly at least for two days without refuelling, whereas Eurasian Reed Warblers leave wintering areas with low fat stores [31]. Therefore, Sedge Warblers should accumulate greater fat stores than Eurasian Reed Warblers. During autumn migration, Sedge Warblers accomplish this by feeding more on reed aphids (Hyalopterus pruni), a lipid-rich diet [29,32,33], and their fattening process results from a surplus of calories made available by hyperphagia. Eurasian Reed Warblers accumulate fat stores by feeding primarily on other insects during stopovers [34]. These differences in feeding behaviour could lead to physiological differences in how these species accumulate and use fuel stores during migration. Furthermore, a high fat diet might facilitate fattening and enhance lipid oxidation, but whether this is the case for birds is still unclear [35,36,37].
There are no detailed studies on migratory passerines regarding FA composition during migration obtained from fat reserves of lived animals. To better understand these issues, in this study, individuals were induced to stop during nocturnal flight using tape lured calls (henceforth “post-flight fasting birds”), and blood and subcutaneous fat samples were collected. Given the diurnal dietary habits of these species, these samples represented the metabolism occurring in the fasting periods of flight. Birds that stopped naturally at dawn at our study site were also sampled. These samples represent the non-fasting/re-feeding birds (henceforth ‘non-fasting birds’). Our objective was to determine both if blood metabolites (TRIG, GLY, and GLUC) and FA composition varies in Eurasian Reed and Sedge Warblers that exhibit differential migratory stopover strategies and nutritional states over Europe during migration. As part of this study, we used portable instruments together with nuclear magnetic resonance (NMR), and the innovative non-lethal fat sampling procedure that we have optimized [38] to unravel the potential differences in migratory stopover strategies of two similar migratory passerines species, the Eurasian Reed and Sedge Warbler. We expected that post-flight fasting birds would exhibit higher levels of TRIG + GLY due to higher TRIG mobilization from adipose tissue into GLY. Furthermore, Sedge Warblers should present, also higher TRIG + GLY levels than Eurasian Reed Warblers during post-flight fasting period, due to TRIG hydrolysis into GLY and FFA due to their differing migratory strategy. According to Scanes and Braun, migratory birds primarily use fat reserves to fuel migratory flights [11], and therefore we expected that GLUC concentrations in blood circulation stay constant during, or as result of a long-distance flight [22]. Non-fasting birds that stopped naturally at our stopover sites should present higher GLUC levels due to their diet preferences at our study site and their likely recent feeding activity. Finally, we expected that Sedge Warblers should present higher PUFAs and n-3 FA, given that do not refuel along their migratory route benefiting of their feeding habits on reed aphids at the Northern stopover sites [30]. On the other hand, Eurasian Reed warblers would present highest proportion of SFA, because birds store and mobilize preferentially shorter and more saturated FAs in their adipose tissues, mainly species whose major fattening occurs later in the migration route [30].
2. Methods
2.1. Study Site
Eurasian Reed and Sedge Warblers were captured at two stopover sites in central Portugal: the reedbeds of Paul do Taipal (40°11′ N 8°41′ W—233 ha) and Paul da Madriz (40°7′ N 8°38′ W—89 ha). Paul do Taipal is a densely vegetated marsh resulting from the permanent flooding of long- abandoned rice fields. It supports a wide variety of breeding water birds and is of national importance for wintering ducks, as well as an important stop-over site for migrating passerines. It is composed of Common Reed (Phragmites australis) and Bulrush (Scirpus lacustres) and is surrounded by a hedge of Willow (Salix alba) and Iberian Alder (Alnus lusitanica) on the north margin, which is starting to encroach the area of Common Reed. By the other side Paul da Madriz is located circa12km to the south of Taipal and is a mixed riparian forest/wetland with typical aquatic species such as the Common Reed, the Bulrush, the European White Water-lily (Nymphaea alba), and trees species as Iberian alder (Alnus lusitanica) and Grey Willow (Salix atrocinerea). The habitat was surrounded by mixed Pinus spp., Quercus spp. and agricultural fields. At both sites, the dominant vegetation near water consists of Reed and Bulrush. Both sites were designated Ramsar sites.
2.2. Study Species
Eurasian Reed and Sedge warblers are closely related insectivores passerines of similar morphology. In Europe they are present in high numbers during spring and autumn migrations [26,27], as in our study sites. Eurasian Reed warblers from Western Europe (including Iberia Peninsula) overwinter in Western Africa, mostly Senegambia, similarly to Sedge warblers, that includes the Western Sahelian wetlands [39,40]. Both species breed in Eurasia and winter in Central Africa, follow the same migration routes and use similar stopover sites. Eurasian Reed warblers do not fly directly from Central Europe to their wintering areas; they accumulate at each stopover site the amount of energy which is needed to fly safely to the next site. On the other hand, Sedge warblers in autumnal migration tend to fuel up extensively as soon as they encounter a site with super-abundant food (aphids or other aggregated prey), and then fly directly to wintering grounds without refuelling. Furthermore, both Eurasian Reed and Sedge warblers present parallel migration patterns, which give rise to high connectivity between breeding sites and wintering quarters in Africa [39,40].
2.3. Mist-Netting
At each site, mist-nets were erected before dawn (between 00:00 and 6:00) and operated for a maximum of 2 h after twilight to capture birds on two non-consecutive days per week during spring (15 April–30 May) and autumn (1 August–30 September) of 2011 and 2012 to determine the metabolite profiles of non-fasting birds. Tape lures were used, at both sites, for 5 days every other week in 2011/2012 and during autumn migration in 2014 to evaluate differences in metabolite profiles of birds with differentiated migration status. Both species are nocturnal migrants that use calls of conspecifics to select stopover sites [41], thus allowing induced stops at specific sites by playing vocalizations at night [42]. During tape-lure sessions, mist-nets were opened at 01:00 and operated until 05:00, i.e., before dawn. The tape lure consisted of two Sony Creative Zen Mosaic EZ300 player loudspeakers, oriented northward, with songs of Eurasian Reed and Sedge Warblers played simultaneously and continuously. All captured birds were ringed, morphological measurements were taken (tarsus length, wing length, bill length, tail length, and body mass), and fat scores were assessed on a scale from 0 to 8 (where 0 is no fat and 8 is maximum fat [43]).
2.4. Blood and Fat Sampling
In 2011 and 2012, a blood sample (~75 μL) was obtained from the brachial veins of the first five individuals captured of each species (Table 1) using heparinized micro-hematocrit capillary tubes. Measurements of TRIG and GLUC concentrations were immediately taken using portable instruments (TRIG, Roche Accutrend GCT®, range = 50–600 mg/dL, ±6 mg/dL, 0.80–6.86 mmol/L; GLUC: Roche Accu-Chek Advantage®. Range = 30–500 mg/dL, ±5 mg/dL). The method for chemical detection of TRIG used by the Roche Accutrend GCT® is the same used by others commercially available TRIG meters. TRIG are hydrolysed into GLY and FFA and two subsequent reactions convert GLY into hydroacetone phosphate and hydrogen peroxide. Hydrogen peroxide, in the presence of peroxidase, converts an indicator to a dye by oxidation. Concentration of the dye is then measured by reflectance photometry to give the TRIG concentration [44]. Furthermore, the Roche Accutrend GCT® do not differentiate between TRIG and GLY.

Table 1.
Sample size of birds captured during spring (15 March 2011–15 October 2012) and autumn migratory seasons (1 August–15 October of 2011, 2012 and 2014) and the number of birds captured with and without playback (Tape lure = Post-flight fasting birds, non-tape lure = Non-fasting birds).
Mist-nets were monitored constantly, and birds were sampled immediately after extracted from mist-nets (<5 min between extraction and sampling). To validate the values of the portable TRIG and GLUC instruments during autumn migration (season with higher number of birds captured) of 2014, a blood sample (~150 μL) was taken immediately from the brachial vein into two heparinized micro-hematocrit capillary tubes. One sample was stored at −20 °C for later laboratorial analysis and the other was used to immediately measure TRIG concentrations using portable instruments (as above). At the same time, a small amount of subcutaneous fat was collected through a small incision in the furcular zone, after a local anaesthetic (Tetracaine hydrochloride 7.5 mg/g, B. BRAUN®, for further details see the procedure described by Rocha et al. 2016 [38]), directly into amber glass flasks with chloroform (N = 10 by species). Fat samples were taken only during autumn migration because birds captured during spring migration had low fat scores, and the number of the captures was low, mainly for Sedge Warblers. These samples were stored at −80 °C for posterior nuclear magnetic resonance (NMR) analyses.
2.5. Blood Sample Analyses
Blood samples were centrifuged (3200 rpm) for 10 min and the plasma was separated within 3 h after sampling. Before analysis, plasma was diluted three-fold with 0.9% NaCl. Metabolites were measured via colorimetric enzymatic endpoint assays in 200-μL flat-bottomed microplates using a microplate spectrophotometer (BioTek Powerwave X340, Agilent, Coimbra, Portugal). We measured TRIG and GLY as described by Guglielmo et al. [45].
2.6. Fat Sample Analyses
Lipids were extracted by rinsing the freeze-dried fat samples in 450 µL of chloroform. Pyrazine was added as standard. Samples were analysed by 1H NMR spectroscopy using a Bruker Avance III HD, 11.7 T magnet (1H operating frequency 500 MHz) equipped with a 5-mm 2H-selective probe with 19F lock and 1H-decoupling coil. Spectra were acquired using a 90° pulse, 8-sec relaxation delay, and 3-sec acquisition time for a total of 16 scans run at 25 °C and were processed by applying 0.5 Hz of exponential line broadening. Spectra were analysed using the line-fitting routine supplied with the ACDLabs 1D NMR processor software. The obtained spectra revealed hydrogens corresponding to different lipid species (peaks were assigned based on literature and databases) from which fatty acid composition and average molecular structure were estimated following the work of Duarte et al. (2014) [46]. Briefly, considering a fatty acid molecule as a polymer, i.e., OOC-(CH2)x-(HC=CH)y-CH3, 1H NMR techniques can distinguish and quantify the signal area from hydrogens present in the methyl (terminal CH3), methylenes (CH2), and olefinic (HC=CH) functional groups. Given that each fatty acid possesses a terminal methyl group, an average composition of methylenes and olefinic groups per fatty acid molecule can be calculated. From here, relative proportions of lipid classes (SFAs, UFAs (MUFAs, and PUFAs) and 3-omega) can be determined [17].
2.7. Data Analysis
Pearson correlations were used to evaluate the relationship in Eurasian Reed and Sedge Warblers during ‘post-flight fasting’ between TRIG + GLY values measured in the field with TRIG + GLY values, GLY and TRIG values measured in the lab. Because there was no difference in TRIG + GLY and GLUC values between years (t-test, P > 0.100), we pooled data for both 2011 and 2012. To evaluate possible differences in metabolites levels of Eurasian Reed and Sedge Warblers during post-flight fasting and non-fasting in each migratory season we used a Linear Mixed Model (GLMM) with Species (Sedge Warblers Vs. Eurasian Reed Warblers), Season (spring Vs autumn) and Condition (post flying fasting Vs. non-fasting) in fixed effects structure using the metabolites levels (TRIG + GLY and GLUC) as sampling units. To evaluate if: (a) laboratorial and field TRIG + GLY varies of ’post-flight fasting’ Sedge and Eurasian Reed Warblers during autumnal migration we used a LMM: with TRIG + GLY as response variable, and species and laboratorial or field analyses as fixed effects; (b) field TRIG + GLY varies of post-flight fasting’ Sedge and Eurasian Reed Warblers during both migratory periods we used a LMM: with TRIG + GLY as response variable, and species and season as fixed effects; (c) field TRIG + GLY varies of ‘non-fasting’ Sedge and Eurasian Reed Warblers during both migratory periods we used a LM: with TRIG + GLY as response variable, and species and season as fixed effects; (d) field TRIG + GLY varies of ‘post-flight fasting’ and non-fasting’ Sedge and Eurasian Reed Warblers during both migratory periods we used a LM: with TRIG + GLY as response variable, and species, season and condition as fixed effects. To evaluate if: (e) field GLUC levels varies of post-flight fasting Sedge and Eurasian Reed Warblers during both migratory periods we used a LM: with GLUC as response variable, and species and season as fixed effects; (f) field GLUC varies of non-fasting Sedge and Eurasian Reed Warblers during both migratory periods we used a LM: with GLUC as response variable, and species and season as fixed effects (g) field GLUC varies of post-flight fasting and non-fasting Sedge and Eurasian Reed Warblers during both migratory periods we used a LMM: with GLUC as response variable, and species, season and condition as fixed effects. All the LMM were runed with Gaussian distribution and identity link function. All the dependent variables were tested for normality and homogeneity of variances. We compared the proportion of residual data left unexplained by the model, and visualized residual plots to validate the models.
Differences in FA composition between species were analysed with Student’s two-tailed unpaired t-test. Computations were carried out using several functions within different R packages, (e.g., lmerTest, lme4, psych, doBy, plyr, and MASS) and using Graphpad Prism (Version 8). Values are presented as means ± SE, and differences were considered statistically significant at P < 0.05.
3. Results
3.1. Blood Metabolites and Body Mass of ‘Non-Fasting’ Sedge and Eurasian Reed Warblers
We found no correlation between TRIG + GLY values measured in the field and body mass of Eurasian Reed Warblers during both spring and autumn migration (r = 0.17, P = 0.24; r = −0.04, P = 0.75 respectively). We also found no correlation between TRIG + GLY values measured in the field and body mass of Sedge Warblers during spring and autumn migration (r = 0.08, P = 0.80; r = 0.04, P = 0.81, respectively).
3.2. Blood Metabolites and Body Mass of ‘Fasting’ Sedge and Eurasian Reed Warblers
We found no significant correlation between TRIG + GLY values measured in the field and body mass for Eurasian Reed Warblers during both spring and autumn migration (r = −0.26, P = 0.54; r = −0.01, P = 0.95 respectively). Moreover, no correlation was found between TRIG + GLY values measured in the field and body mass for Sedge warblers during spring and autumn migration (r = −0.40, P = 0.75; r = 0.11, P = 0.67, respectively).
3.3. Blood Metabolites of ‘Post-Flight Fasting’ Sedge and Eurasian Reed Warblers during Autumnal Migration
A positive and significant correlation was found between TRIG + GLY values measured in the field and TRIG + GLY concentration in the lab, for both Sedge and Eurasian Reed Warblers (r = 0.82, P = 0.001, r = 0.70, P = 0.001; respectively—Figure 1A). TRIG + GLY values measured in the field and TRIG concentration measured in the lab showed a positive and significant correlation for both Sedge and Eurasian Reed Warblers (r = 0.80, P = 0.002, r = 0.62, P = 0.006, respectively; Figure 1B). TRIG + GLY values from the field and GLY concentration measured in the lab showed the “same” positive and significant correlation for Sedge and Eurasian Reed Warblers (r = 0.79, P = 0.002 and r = 0.64, P = 0.004, respectively; Figure 1C). Finally, we found a positive and significant correlation between TRIG and GLY concentration both measured in the lab, for Sedge and Eurasian Reed Warblers (r = 0.78, P = 0.005, r = 0.48, P = 0.037; respectively—Figure 1D).
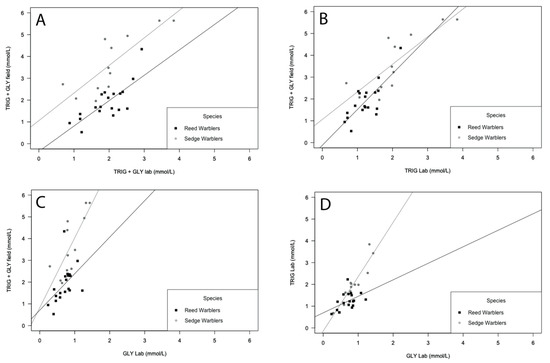
Figure 1.
Spearman correlation for Sedge (grey circles) and Eurasian Reed Warblers (Reed Warblers—black squares) between: (A) TRIG + GLY (mmol/L) concentrations measured in the field in the laboratory; (B) Triglycerides (TRIG) + Glycerol (GLY) (mmol/L) concentrations measured in the field and TRIG (mmol/L) concentrations measured in the laboratory; (C) TRIG + GLY (mmol/L) concentrations measured in the field and GLY (mmol/L) concentrations measured in the laboratory; (D) TRIG (mmol/L) concentrations measured in the laboratory and GLY (mmol/L) concentrations measured in the laboratory. Field results were converted from mg/dL to mmol/L (1 mM—88.825 mg/dL of TRIG; 1 mM—88.652 mg/dL of GLY).
Furthermore, during autumn migration 2014, post-flight fasting Sedge Warblers had significantly higher plasma TRIG + GLY concentrations measured in the lab (E = −118.29, SE = 21.14; T value = −5.596, P < 0.001; Figure 2) than post-flight fasting Eurasian Reed Warblers. These results agreed with our field results, with Sedge Warblers having higher TRIG levels than Eurasian Reed Warblers (E = −123.32, SE = 13.96, T value = −9.195, P< 0.001; Figure 2).
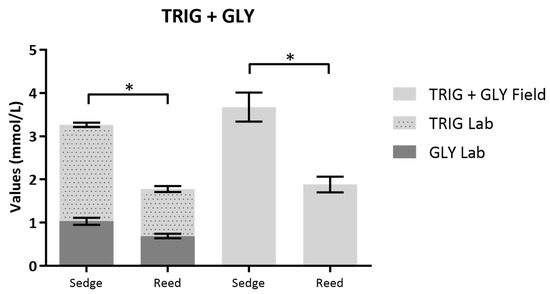
Figure 2.
Triglycerides (TRIG) + Glycerol (GLY) (mmol/L) concentration (mean ± SE) for laboratorial analyses and TRIG + GLY (mmol/L) for field results of Sedge and Eurasian Reed Warblers captured during post-flight fasting (with tape lure). Field results were converted from mg/dL to mmol/L (1 mM—88.825 mg/dL of TRIG; 1 mM—88.652 mg/dL of GLY). Significance codes: *** = P < 0.001; ** P < 0.01; * P < 0.05.
3.4. Seasonal Variation in Fat Scores and Blood Metabolites of Non-Fasting Sedge and Eurasian Reed Warblers
Non-fasting Sedge warblers had higher fat scores than non-fasting Eurasian Reed Warblers during autumn migration (4.0 ± 2.2 vs. 2.6 ± 2.0; t = 2.3, P < 0.001), but fat scores for the two species were similar during spring migration (2.7 ± 0.8 vs. 2.1 ± 1.4; t = 0.3, P = 0.76). TRIG + GLY levels measured with portable machines in 2011/2012 (pooled data) showed that Sedge Warblers had significantly higher TRIG + GLY levels (E = −0.691, SE = 0.057, T value = −12.03, P < 0.001) than Eurasian Reed Warblers (Figure 3A), but there was no effect of season (E = 0.106, SE = 0.060, T value = 1.760, P = 0.079; Figure 3A).
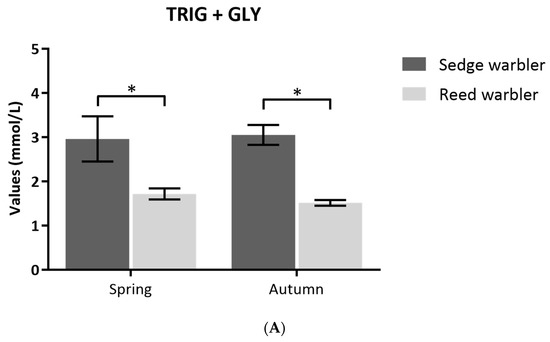
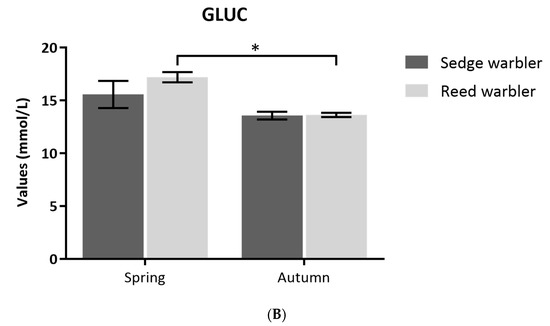
Figure 3.
(A) Comparison of Triglycerides (TRIG) + Glycerol (GLY) and (B) Glucose (GLUC) (mmol/L) values (mean ± SE) measured at field of non-fasting Sedge and Eurasian Reed Warblers (Reed Warbler) captured after stopping naturally during spring and autumn migration. Significance codes: *** = P < 0.001; ** P < 0.01; * P < 0.05.
Both species did not differ significantly in blood GLUC levels (E = 6.581, SE = 7.05, T value = 0.934, P = 0.351; Figure 3B). However, significant differences were found between seasons (E = 44.62, SE = 6.84, T value = 6.53, P < 0.001).
3.5. Circulating Blood Metabolites of Sedge and Eurasian Reed Warblers between Post-Flight Fasting and Non-Fasting Birds Using Portable Field Equipment
Sedge Warblers had significantly higher TRIG + GLY levels than Eurasian Reed Warblers in both nutritional states (non-fasting E = −128.32, SE = 13.96, T value = −9.195, P< 0.001; post-flying fasting E = 196.54, SE = 16.56, T value = −11.87, P < 0.001; Figure 4A). Sedge Warblers captured in post-flight fasting had significantly higher TRIG + GLY levels than those that stopped naturally (E = −54.94, SE = 28.04, T value = −1.959, P = 0.0439), but there were no differences between Eurasian Reed Warblers caught in post-flight fasting and those that stopped naturally (E = 0.000, SE = 0.001, T value = 0.00, P = 1).
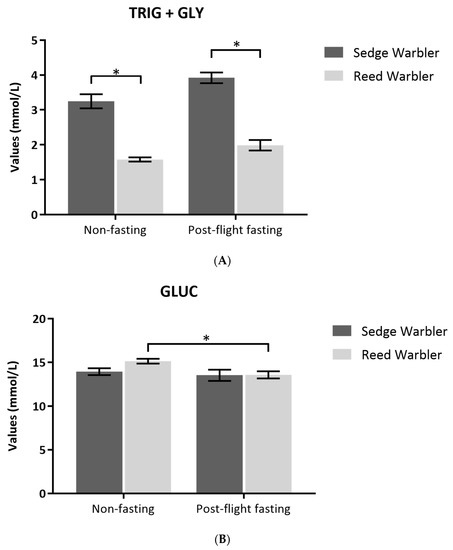
Figure 4.
(A) Comparison of Triglycerides (TRIG) + Glycerol (GLY) (mmol/L) and (B) Glucose (GLUC) (mmol/L) values (mean ± SE) measure at field between non-fasting (stopped naturally) and post-flight fasting birds (tape lured) of Sedge and Eurasian Reed Warblers (Reed Warbler). Significance codes: *** = P < 0.001; ** P < 0.01; * P < 0.05.
For GLUC analyses, we found no difference between species (E = 6.581, SE = 7.05, T value = 0.934, P = 0.351; Figure 4B). However, non-fasting birds had higher GLUC levels than post-flight fasting birds (E = 22.75, SE = 7.67, T value = 2.967, P = 0.003; Figure 4B). Non-fasting Eurasian Reed Warblers that stopped naturally had higher GLUC levels than post-flight fasting birds (E = 34.926, SE = 9.677, T value = 3.609, P < 0.001).
3.6. Comparing Fatty Acid Composition and Average Molecular Structure between Sedge and Eurasian Reed Warblers during Post-Flight Fasting
Analysis of subcutaneous fat of both post-flight fasting Eurasian Reed and Sedge Warblers by 1H NMR spectroscopy revealed different molecular profiles of FA between the two species (Table 2). Sedge Warblers had increased relative levels of unsaturated fatty acids (UFA) due to a higher PUFA fraction. This increase in PUFAs from dietary sources was accompanied by a reduced relative proportion of SFAs whereas MUFAs and ω-3 levels remained similar relatively to Eurasian Reed Warblers.

Table 2.
Subcutaneous fatty acid composition (%) and molecular structure for Eurasian Reed and Sedge Warblers obtained by 1H Nuclear Magnetic Ressonance (RMN). Values are means ± SE (N = 10). Significant differences between species are indicated by an asterisk (t-test, * P < 0.05). FA: fatty acids; SFA: saturated fatty acids; UFA: unsaturated fatty acids; PUFA: polyunsaturated fatty acids; MUFA: monounsaturated fatty acid; n-3 FA: omega-3 fatty acids.
4. Discussion
Two migratory warblers in central Portugal stopover sites had different plasma levels of TRIG + GLY, during both post-flight fasting and non-fasting periods, and this was consistent with differences found in FA composition. According to Jenni-Eirmann (1992) [8], plasma TRIG increases during long-distance migratory flights. However, TRIG, GLY, and FFA all increase in flying birds when compared with inactive overnight fasted birds or birds feeding at stopover sites [7]. The TRIG and GLY levels in circulation (observed both in field and in laboratory) should reflect mobilization of larger fat stores by Sedge Warblers, attributed to the fewer possibilities to refuel, therefore relying more on fat reserves during migratory flight than Eurasian Reed Warblers [29]. Despite our results indicated higher TRIG and GLY levels in circulation in Sedge Warblers using portable equipment, we cannot ensure that both TRIG and GLY levels increased at the same level. High levels of PUFA in the subcutaneous fat profile of Sedge Warblers may indicate a better capacity for endurance flight [47] when compared with Eurasian Reed Warblers. Analysing the circulating substrates and adipose tissue profile during fasting, we can infer which reserves are mobilized and correlated with physiological states during migratory flights.
Non-fasting Eurasian Reed Warblers that stopped naturally had higher blood GLUC levels than post-flight fasting Eurasian Reed Warblers, suggesting they were capable to re-feed at our study site. Despite the importance of GLUC for burst flight (e.g., escape from predators) and in early migratory flights while FA mobilization is being triggered [12], the contribution of GLUC is too small to meaningfully contribute to multi-hour or multi-day flights [48,49,50]. GLUC concentrations in plasma stay constant during long-distance migratory journeys [15], mainly because migratory birds primarily use FA reserves to fuel migratory flights [11]. Eurasian Reed Warblers are not a long-term fasting species given their high number of short stopovers, accumulating the required fuel load at each one to reach the next, before taking longer stopovers, such as in north Africa to accumulate the necessary fuel load just prior to crossing the Sahara [33,51].
High TRIG levels in the blood of post-flight fasting birds can be explained by the fact that they were using their fat stores, given the high energy requirements of flight [52]. However, non-fasting birds captured during natural stopover had significantly lower TRIG + GLY levels, presumably because they were not as dependent on fat stores at the time of capture, and their GLUC reserves were higher, probably because they were able to re-feed. Another possible explanation is that our portable machine did not distinguish between TRIG and GLY, and the decrease on TRIG levels could be due to a decrease in GLY and not TRIG. By feeding more frequently, Eurasian Reed Warblers can maintain GLUC levels at the expense of catabolically faster substrates, namely dietary carbohydrates and glycogen reserves, reducing their dependence on fat reserves.
Levels of blood metabolites such as GLUC depend on the composition of energy substrates, exogenous diet sources, and on muscle energetic needs during migratory flight [53]. Sedge Warblers captured during post-flight fasting and when stopping naturally had similarly high TRIG + GLY values, probably because both fattened in Northern and Western Europe and should be more dependent on fat stores than Eurasian Reed Warblers during migration. The highly abundant and nutritious food source in Northern and Western Europe would amplify this differential feeding behaviour to a point that would lead to differential accumulation of fat reserves, with consequent implications on migratory strategies [30]. Presumably, Sedge Warblers are more efficient at preying on reed aphids in Northern Europe than Eurasian Reed Warblers [54]. Reed aphids are scarce in the Iberian Peninsula during the peak of warbler migration [29,55], making stopovers for Sedge Warblers less profitable at Portuguese reedbeds. Because the probability of replenishing their fat reserves preying on reed aphids is lower, Sedge Warblers are likely more reliant on stored fat reserves and stop fewer times and for less time per stopover.
The n-3 long-chain PUFA are mainly synthesized from microalgae or bacteria and transferred up the food web [56], and only birds that consume marine invertebrates have higher proportions of this specific PUFA in their cell membranes and lipid stores, such as shorebirds [22,57,58]. In the case of insectivores, granivores or frugivores passerines, they tend to have diets richer in the n-6 PUFA. According to natural doping theory, birds that feed on n-3 PUFA-rich diets fly longer with fewer stopovers [22]. On the other hand, species that rely more on non n-3 PUFA-rich diets, should stop more often and for longer periods. The hypothesis of natural doping proposed by Maillet and Weber (2006, 2007) [22,59] posted that Semipalmated Sandpipers (Calidris pusilla) increased flight endurance due to their rich diet in n-3 long chain PUFAs on marine invertebrates during the stopover. However, migratory White-throated Sparrows (Zonotrichia albicollis) supplemented with n-3 and n-6 PUFA diets showed no effects on the upregulation of aerobic capacity [60].
There is still controversy on the effect of PUFAs enhanced the endurance flight performance in migratory songbirds (Dick and Guglielmo, 2019). However, differences in the level of unsaturation in subcutaneous FA, and the elevated levels of PUFA observed in Sedge Warblers agrees with Price and Guglielmo (2009) [61] findings. Diets enriched in n-6 PUFAs could lead to a better exercise performance increasing the metabolic rate and potentiate the migration endurance [60,62]. The higher percentage of PUFAs on Sedge Warblers can be attributed to their diet or to a preferential mobilization of shorter chain FAs increasing its percentage in the adipose tissue. Mustonem et al. (2005) reported that minks were capable to use fat reserves to overcome seasonal variations in food availability and evolved better adaptations to overcome food scarcity [63]. This particular pattern described for minks can potentially indicate that also birds can use a preferential mobilization of particular FA to overcome seasonal variations in food availability, and for this reason have evolved better adaptations to cope with their non-stop flights.
During spring migration, both post-flight fasting Sedge and Eurasian Reed Warblers had higher GLUC levels than during autumn migration, suggesting that birds had already made a stopover before we captured them at our study site, where they probably re-fed, increasing their GLUC levels. In conclusion, differences in foraging habits may trigger a series of adaptations that results in different migratory strategies between Sedge and Eurasian Reed Warblers. Presumably, by feeding intensely on aphids near the breeding grounds, Sedge Warblers present higher fat scores, and accumulate more unsaturated FA, which can be supplied to working muscles by increasing blood FFA and GLY levels.
In technical terms, using portable equipment to measure blood metabolites in the field provides a faster and easier way to assess GLUC and TRIG + GLY values in migratory songbirds and other animals than more conventional approaches that use stored blood samples to measure metabolites in the lab. By sampling captured birds immediately, we minimized this issue. However, the part of signal is GLY and we cannot tease apart what is happening in the different metabolic states.
Author Contributions
P.M.A. and J.A.R. conceived and designed the experiment; P.M.A. organized fieldwork. P.M.A., I.V. and L.C.T. performed the lab work; P.M.A., L.P.D.S. and P.B.L. performed the field captures; P.M.A. led the writing of the manuscript with substantial inputs from all other authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All sampling procedures and/or experimental manipulations were reviewed and specifically approved as part of obtaining the field license. All work was approved by the relevant authorities (Instituto da Conservação da Natureza e da Biodiversidade, research permits 107/2006, 116/2007, 333/2007/CAPT).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in [FigShare] at [https://doi.org/10.6084/m9.figshare.21280515.v1].
Acknowledgments
PMA and LPS acknowledges the individual research contracts given by “Fundação para a Ciência e Tecnologia” (Portugal, 2020.01494.CEECIND and CEECIND/02064/2017, respectively). We thank the ‘national conservation and forests institution’ (ICNF) for the legal permits. This paper benefited from the comments of Ana Claudia Norte, José Alves, Vitor Hugo Paiva and Christopher G. Guglielmo. This study had the support of FCT through the strategic project UIDB/04292/2020 awarded to MARE and through project LA/P/0069/2020 granted to the Associate Laboratory ARNET.
Conflicts of Interest
The authors declare no competing or financial interests.
References
- Alerstam, T.; Hedenström, A. The development of bird migration theory. J. Avian Biol. 1998, 29, 343–369. [Google Scholar] [CrossRef]
- Price, E.R.; Krokfors, A.; Guglielmo, C.G. Selective mobilization of fatty acids from adipose tissue in migratory birds. J. Exp. Biol. 2007, 211, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jenni-Eiermann, S.; Jenni, L. Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the Garden Warbler. Auk 1994, 111, 888–899. [Google Scholar] [CrossRef]
- Guglielmo, C.G.; Cesarale, D.J.; Eldermire, C. A field validation of plasma metabolite profiling to assess refueling performance of migratory birds. Physiol. Biochem. Zool. 2005, 78, 116–125. [Google Scholar] [CrossRef]
- Cesarale, D.J.; Guglielmo, C.G. An integrative assessment of the effects of Tamarisk on stopover ecology of a long-distance migrant along the San Pedro River, Arizona. Auk 2010, 127, 636–646. [Google Scholar] [CrossRef]
- Jenni-Eeiermann, S.; Jenni, L. Fuel deposition rates in migrating birds: Causes, constraints and consequences. In Avian Migration; Berthold, P., Gwinner, E., Sonnenschein, Eds.; Springer: New York, NY, USA, 2003; pp. 293–306. [Google Scholar]
- Jenni-Eiermann, S.; Jenni, L. Metabolic responses to flight and fasting in night-migrating passerines. J. Comp. Physiol. B 2003, 161, 465–474. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Jenni, L. High plasma triglyceride levels in small birds during migratory flight: A new pathway for fuel supply during endurance locomotion at very high mass-specific metabolic rates? Physiol Zool. 1992, 65, 112–123. [Google Scholar] [CrossRef]
- Mccue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Phys. A 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. Phys. B 2008, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G.; Braun, E. Avian metabolism: Its control and evolution. Front. Biol. 2013, 8, 134–159. [Google Scholar] [CrossRef]
- Gerson, A.R.; Guglielmo, C.G. Energetics and metabolite profiles during early flight in American Robins (Turdus Migratorius). Comp. Biochem. Phys. B 2013, 183, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, C.G.; Gerson, A.R.; Price, E.R.; Hays, Q.R. The effects of dietary macronutrients on flight ability, energetics, and fuel metabolism of Yellow-rumped Warblers Setophaga coronata. J. Avian Biol. 2017, 48, 133–148. [Google Scholar] [CrossRef]
- Klaassen, M.; Kvist, A. Flight costs and fuel composition of a bird migrating in a wind tunnel. Condor 2000, 102, 444–451. [Google Scholar] [CrossRef]
- Landys, M.M.; Piersma, T.; Guglielmo, C.G.; Jukema, J.; Ramenofsky, M.; Wingfield, J.C. Metabolic profile of long-distance migratory flight and stopover in a shorebird. P. Roy. Soc. B 2005, 272, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bauchinger, U.; Wohlmann, A.; Biebach, H. Flexible remodeling of organ size during spring migration of the Garden Warbler (Sylvia borin). Zoology 2005, 108, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Viegas, I.; Araújo, P.M.; Rocha, A.D.; Villegas, A.; Jones, J.G.; Ramos, J.A.; Masero, J.A.; Alves, J.A. Metabolic plasticity for subcutaneous fat accumulation in a long-distance migratory bird traced by 2H2O. J. Exp. Biol. 2017, 220, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.M.; Viegas, I.; Rocha, A.D.; Villegas, A.; Jones, J.G.; Ramos, J.A.; Masero, J.A.; Alves, J.A. Does fasting enhance lipogenesis during migration re-fuelling? A test between traditional and novel diets in a long-distance migratory bird. Sci. Rep. 2019, 9, 10065. [Google Scholar] [CrossRef] [PubMed]
- Price, E.R. Dietary lipid composition and avian migratory flight performance: Development of a theoretical. Comp. Biochem. Phy. A 2010, 157, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Mcwilliams, S.R.; Guglielmo, C.G.; Pierce, B.J.; Klaassen, M. Flying, fasting, and feeding in birds during migration: A nutritional and physiological ecology perspective. J. Avian Biol. 2004, 35, 377–393. [Google Scholar] [CrossRef]
- Nagahuedi, S.; Popesku, J.T.; Trudeau, V.L.; Weber, J.M. Mimicking the natural doping of migrant sandpipers in sedentary quails: Effects of dietary n-3 fatty acids on muscle membranes and PPAR expression. J. Exp. Biol. 2009, 212, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Maillet, D.; Weber, J.M. Performance-enhancing role of dietary fatty acids in a long-distance migrant shorebird: The Semipalmated Sandpiper. J. Exp. Biol. 2006, 209, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Klaiman, J.M.; Price, E.R.; Guglielmo, C.G. Fatty acid composition of pectoralis muscle membrane, intramuscular fat stores and adipose tissue of migrant and wintering White-throated Sparrows (Zonotrichia albicollis). J. Exp. Biol. 2009, 212, 3865–3872. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.N.; Wang, H.L.; Nord, A.; Salmón, P.; Isaksson, C. Composition of physiologically important fatty acids in Great Tits differs between urban and rural populations on a seasonal basis. Front. Ecol. Evol. 2015, 3, 522. [Google Scholar] [CrossRef]
- Moreau, R.E. The Palaearctic-African Bird Migration Systems; Academic Press: London, UK, 1972. [Google Scholar]
- Green, R.E.; Davies, N.B. Feeding ecology of Reed and Sedge warblers. Wicken Fen Group Rep. 1972, 4, 8–14. [Google Scholar]
- Green, R.E.; Bibby, C.J. Sedge Warblers and aphids. Wicken Fen Group Rep. 1973, 5, 7–11. [Google Scholar]
- Green, R.E. Adult survival rates for Reed and Sedge warblers. Wicken Fen Group Rep. 1976, 8, 23–26. [Google Scholar]
- Bibby, C.; Green, R. Autumn migration strategies of Reed and Sedge warblers. Ornis Scand. 1981, 12, 1–12. [Google Scholar] [CrossRef]
- Lee, M.; Viegas, I.; Norte, A.C.; Ramos, J.A.; Araújo, P.M. Assessing the fatty acid profile of migratory birds with different fuelling strategies. Ibis 2022, in press. [Google Scholar] [CrossRef]
- Bolshakov, C.V.; Bulyuk, V.N.; Mukhin, A.; Chernetsov, N. Body mass and fat reserves of Sedge Warblers during vernal nocturnal migration: Departure versus arrival. J. Field Ornithol. 2003, 74, 81–89. [Google Scholar] [CrossRef]
- Koskimies, P.; Saurola, P. Autumn migration strategy of the Sedge Warbler Acrocephalus schoenobaenus in Finland: A preliminary report. Ornis Fenn. 1985, 62, 145–152. [Google Scholar]
- Schaub, M.; Jenni, L. Variation of fuelling rates among sites, days and individuals in migrating passerine birds. Funct. Ecol. 2001, 15, 584–594. [Google Scholar] [CrossRef]
- Araújo, P.M.; Lopes, P.B.; da Silva, L.P.; Ramos, J.A. The importance of reedbeds and riparian areas for Cetti’s Warbler Cettia cetti along its annual cycle. Wetlands 2016, 36, 875–887. [Google Scholar] [CrossRef]
- Gannes, L.Z. Comparative fuel use of migrating passerines: Effects of fat stores, migration distance, and diet. Auk 2001, 118, 665–677. [Google Scholar] [CrossRef]
- Volek, J.S.; Noakes, T.; Phinney, S.D. Rethinking fat as a fuel for endurance exercise. Eur. J. Sport Sci. 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jenni-Eiermann, S. Energy metabolism during endurance flight and the post-flight recovery phase. J. Comp. Physiol. A 2017, 203, 431–438. [Google Scholar] [CrossRef]
- Rocha, A.; Araújo, P.M.; Martinho, F.; Ramos, J.A.; Masero, J.A. A non-lethal biopsy technique for sampling subcutaneous adipose tissue of small and medium-sized birds. J. Field Ornithol. 2016, 87, 213–221. [Google Scholar] [CrossRef]
- Arizaga, J. Reed and Sedge warblers. In The Eurasian African Bird Migration Atlas, 1st ed.; Spina, F., Baillie, S.R., Bairlein, F., Fiedler, W., Thorup, K., Eds.; 2022; EURING/CMS; Available online: https://migrationatlas.org (accessed on 14 June 2022).
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvaňová, A.; Kalyakin, M.V. European Breeding Bird Atlas 2: Distribution, Abundance and Change; European Bird Census Council: Beek, The Netherlands; Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Thomson, R.L.; Forsman, J.T.; Mönkkönen, M. Positive interactions between migrant and resident birds: Testing the heterospecific attraction hypothesis. Oecologia 2003, 134, 431–438. [Google Scholar] [CrossRef]
- Mukhin, A.; Chernetsov, A.; Kishkinev, D. Acoustic information as a distant cue for habitat recognition by nocturnally migrating passerines during landfall. Behav. Ecol. 2008, 19, 716–723. [Google Scholar] [CrossRef]
- Kaiser, A. A new multi-category classification of subcutaneous fat deposits of songbirds. J. Field Ornithol. 1993, 64, 246–255. [Google Scholar]
- Irvine, K.L.; Mans, C.; Friedrichs, K.R. Validation of 2 point-of-care meters for measuring triglycerides in chickens using whole blood and plasma. J. Vet. Diagnost. Investig. 2018, 30, 197–204. [Google Scholar] [CrossRef]
- Guglielmo, C.G.; Williams, T.D.; Zwingelstein, G.; Brichon, G.; Weber, J.M. Plasma and muscle phospholipids are involved in the metabolic response to long-distance migration in a shorebird. J. Comp. Physiol. B 2002, 172, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.A.; Carvalho, G.F.; Pearson, M.; Horton, J.D.; Browning, J.D.; Jones, J.G.; Burgess, S.C. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J. Lipid Res. 2014, 55, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.J.; Mcwilliams, S.R. Seasonal changes in composition of lipids stores in migratory birds: Causes and consequences. Condor 2005, 107, 269–279. [Google Scholar] [CrossRef]
- Blem, C.R. Avian energy storage. Curr. Ornithol. 1990, 7, 59–113. [Google Scholar]
- Driedzic, W.R.; Crowe, K.L.; Hicklin, P.W.; Sephton, D.H. Adaptations in pectoralis muscle, heart mass, and energy metabolism during premigratory fattening in Semipalmated Sandpipers (Calidris pusilla). Can. J. Zool. 1993, 71, 1602–1608. [Google Scholar] [CrossRef]
- Banerjee, S.; Chaturvedi, C.M. Migratory preparation associated alterations in pectoralis muscle biochemistry and proteome in Palearctic–Indian emberizid migratory finch, Red-headed Bunting, Emberiza bruniceps. J. Comp. Physiol. D 2016, 17, 9–25. [Google Scholar] [CrossRef]
- Yohannes, E.; Biebach, H.; Nikolaus, G.; Pearson, D.J. Passerine migration strategies and body mass variation along geographic sectors across East Africa, the Middle East and the Arabian Peninsula. J. Ornithol. 2009, 150, 369. [Google Scholar] [CrossRef][Green Version]
- Bairlein, F.; Fritz, J.; Scope, A.; Schwendenwein, I.; Stanclova, G.; van Dijk, G.; Meijer, H.A.J.; Verhulst, S.; Dittam, J. Energy expenditure and metabolic changes of free-flying migrating Northern Bald Ibis. PLoS ONE 2015, 10, e0134433. [Google Scholar] [CrossRef]
- Jenni, L.; Jenni-Eiermann, S. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol. 1998, 29, 521–528. [Google Scholar] [CrossRef]
- Bayly, N. Extreme fattening by Sedge Warblers, Acrocephalus schoenobaenus, is not triggered by food availability alone. Ani. Behav 2007, 74, 471–479. [Google Scholar] [CrossRef]
- Villarán, A. Cettia cetti. Rev. Catalanes 2000, 17, 1–9. [Google Scholar]
- Nichols, D.S. Prokaryotes and the input of polyunsaturated fatty acids to the marine food web. FEMS Microbiol. Lett. 2003, 219, 1–7. [Google Scholar] [CrossRef]
- Wang, S.W.; Iverson, S.J.; Springer, A.M.; Hatch, S.A. Fatty acid signatures of stomach oil and adipose tissue of Northern Fulmars (Fulmarus glacialis) in Alaska: Implications for diet analysis of Procellariiform birds. J. Comp. Physiol. B 2007, 177, 893–903. [Google Scholar] [CrossRef]
- Weber, J.M. The physiology of long-distance migration: Extending the limits of endurance metabolism. J. Exp. Biol. 2009, 212, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Maillet, D.; Weber, J.M. Relationship between n-3 PUFA content and energy metabolism in the flight muscles of a migrating shorebird: Evidence for natural doping. J. Exp. Biol. 2007, 21, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Price, E.R.; Guglielmo, C.G. The effect of muscle phospholipid fatty acid composition on exercise performance: A direct test in the migratory White- throated Sparrow (Zonotrichia albicollis). Am. J. Physiol. 2009, 297, R775–R782. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.F.; Guglielmo, C.G. Dietary polyunsaturated fatty acids influence flight muscle oxidative capacity but not endurance flight performance in a migratory songbird. Am. J. Physiol. 2019, 316, R362–R375. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.J.; McWilliams, S.R. The fat of the matter: How dietary fatty acids can affect exercise performance. Integr. Comp. Biol. 2014, 54, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Mustonem, A.M.; Pyykonen, T.; Paakkonen, T.; Ryokkynen, A.; Asikainen, J.; Aho, J.; Mononen, J.; Nieminen, P. Adaptations to fasting in the American mink (Mustela vison): Carbohydrate and lipid metabolism. J. Comp. Physiol. A 2005, 140, 195–202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).