Chick Provisioning in Grey-Faced Petrel (Pterodroma gouldi) under Environmental Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
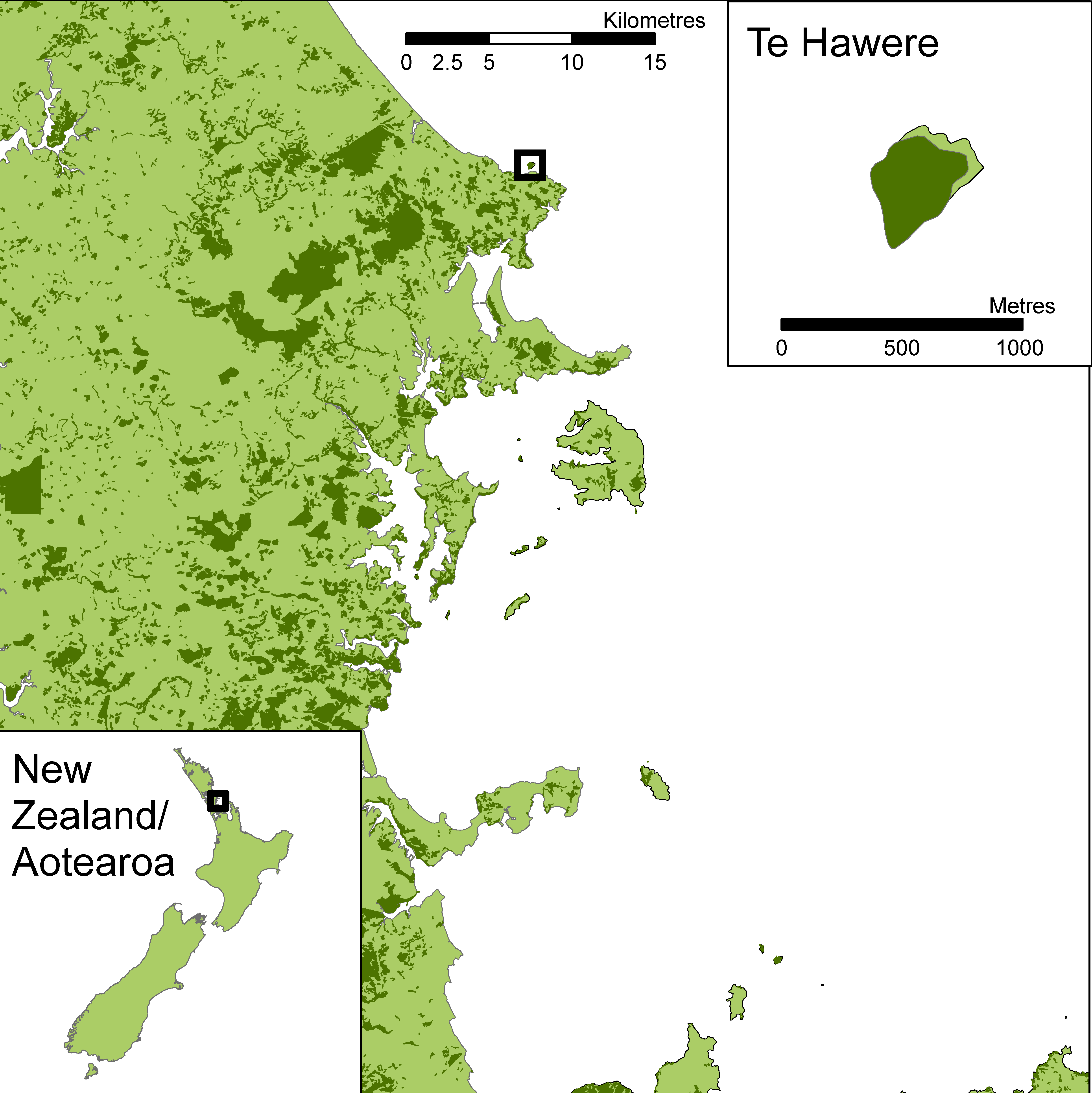
2.1. Study Site
2.2. Field Methods
2.3. Statistical Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghalambor, C.K.; Martin, T.E. Fecundity-survival trade-offs and parental risk-taking in birds. Science 2001, 292, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Wagner, G.F.; Drobniak, S.M.; Ekman, J. Reproductive trade-offs in a long-lived bird species: Condition-dependent reproductive allocation maintains female survival and offspring quality. J. Evol. Biol. 2017, 30, 782–795. [Google Scholar] [CrossRef]
- Erikstad, K.E.; Fauchald, P.; Tveraa, T.; Steen, H. On the cost of reproduction in long-lived birds: The influence of environmental variability. Ecology 1998, 79, 1781–1788. [Google Scholar] [CrossRef]
- Wiley, E.M.; Ridley, A.R. The effects of temperature on offspring provisioning in a cooperative breeder. Anim. Behav. 2016, 117, 187–195. [Google Scholar] [CrossRef]
- Grissot, A.; Araya-Salas, M.; Jakubas, D.; Kidawa, D.; Boehnke, R.; Błachowiak-Samołyk, K.; Wojczulanis-Jakubas, K. Parental coordination of chick provisioning in a planktivorous arctic seabird under divergent conditions on foraging grounds. Front. Ecol. Evol. 2019, 7, 349. [Google Scholar] [CrossRef]
- Regular, P.M.; Hedd, A.; Montevecchi, W.A.; Robertson, G.J.; Storey, A.E.; Walsh, C.J. Why timing is everything: Energetic costs and reproductive consequences of resource mismatch for a chick-rearing seabird. Ecosphere 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Székely, T.; Webb, J.N.; Houston, A.I.; McNamara, J.M. An evolutionary approach to offspring desertion in birds. In Current Ornithology; Springer: Boston, MA, USA, 1996; pp. 271–330. [Google Scholar]
- Weimerskirch, H.; Zimmermann, L.; Prince, P.A. Influence of environmental variability on breeding effort in a long-lived seabird, the yellow-nosed albatross. Behav. Ecol. 2001, 12, 22–30. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Ancel, A.; Caloin, M.; Zahariev, A.; Spagiari, J.; Kersten, M.; Chastel, O. Foraging efficiency and adjustment of energy expenditure in a pelagic seabird provisioning its chick. J. Anim. Ecol. 2003, 72, 500–508. [Google Scholar] [CrossRef]
- Karris, G.; Xirouchakis, S.; Maina, I.; Grivas, K.; Kavadas, S. Home range and foraging habitat preference of Scopoli’s Shearwater Calonectris diomedea during the early chick-rearing phase in the eastern Mediterranean. Wildl. Biol. 2018, 2018, wlb-00388. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Fradet, G.; Cherel, Y. Natural and experimental changes in chick provisioning in a long-lived seabird, the Antarctic prion. J. Avian. Biol. 1999, 30, 165–174. [Google Scholar] [CrossRef]
- Kadin, M.; Olsson, O.; Hentati-Sunberg, J.; Ehrning, E.W.; Blenckner, T. Common guillemot Uria aalge parents adjust provisioning rates to compensate for low food quality. Ibis 2016, 158, 167–178. [Google Scholar] [CrossRef]
- Limmer, B.; Becker, P.H. Improvement in chick provisioning with parental experience in a seabird. Anim. Behav. 2009, 77, 1095–1101. [Google Scholar] [CrossRef]
- Wojczulanis-Jakubas, K.; Araya-Salas, M.; Jakubas, D. Seabird parents provision their chick in a coordinated manner. PLoS ONE 2018, 13, e0189969. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Pterodroma gouldi. 2022. Available online: http://www.birdlife.org (accessed on 21 August 2022).
- Greene, B.S.; Taylor, G.A.; Earl, R. Distribution, population status and trends of grey-faced petrel (Pterodroma macroptera gouldi) in the northern North Island, New Zealand. Notornis 2015, 62, 143–161. [Google Scholar]
- Miskelly, C.M.; Gilad, D.; Taylor, G.A.; Tennyson, A.J.; Waugh, S.M. A review of the distribution and size of gadfly petrel (Pterodroma spp.) colonies throughout New Zealand. Tuhinga 2019, 30, 93–173. [Google Scholar]
- Russell, J.C.; Welch, J.R.; Bourgeois, K.; Dromzée, S.; Dunn, R.; Friesen, M.R.; Rayner, M.J. Climatic effects on Grey-faced Petrel (Pterodroma gouldi) chick growth and survival. Birds 2022, 3, 138–148. [Google Scholar] [CrossRef]
- Mullan, A.B. On the linearity and stability of Southern Oscillation-climate relationships for New Zealand. Int. J. Climat. 1995, 15, 1365–1386. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Meal sizes and feeding rates of Christmas shearwaters and Phoenix petrels on Christmas Island, Central Pacific Ocean. Ornis Scand. 1984, 15, 16–22. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 7 February 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-152. 2021. Available online: https://CRAN.R-project.org/package=nlme (accessed on 7 February 2022).
- Warham, J. The Petrels: Their Ecology and Breeding Systems; Academic Press: London, UK, 1990. [Google Scholar]
- Schreiber, E.A. Experimental manipulation of feeding in Red-tailed Tropicbird chicks. Col. Waterbirds 1996, 1, 45–55. [Google Scholar] [CrossRef]
- Schultz, M.A.; Klomp, N.I. Chick-provisioning behaviour of two shearwaters breeding in south-eastern Australia. Austral. Ecol. 2000, 25, 319–326. [Google Scholar] [CrossRef]
- Ramos, J.A.; Pacheco, C. Chick growth and provisioning of surviving and nonsurviving White-tailed tropicbirds (Phaethon lepturus). Wilson Bull. 2003, 115, 414–422. [Google Scholar] [CrossRef]
- Croxall, J.; Reid, K.; Prince, P. Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 1999, 177, 115–131. [Google Scholar] [CrossRef]
- Burke, C.M.; Montevecchi, W.A. The foraging decisions of a central place foraging seabird in response to fluctuations in local prey conditions. J. Zool. 2009, 278, 354–361. [Google Scholar] [CrossRef]
- Ramos, J.A.; Rodrigues, I.; Melo, T.; Geraldes, P.; Paiva, V.H. Variation in ocean conditions affects chick growth, trophic ecology, and foraging range in Cape Verde Shearwater. Condor 2018, 120, 283–290. [Google Scholar] [CrossRef]
- Cerveira, L.R.; Ramos, J.A.; Rodrigues, I.; Almeida, N.; Araújo, P.M.; dos Santos, I.; Vieira, C.; Pereira, J.M.; Ceia, F.R.; Geraldes, P.; et al. Inter-annual changes in oceanic conditions drives spatial and trophic consistency of a tropical marine predator. Mar. Environ. Res. 2020, 162, 105165. [Google Scholar] [CrossRef] [PubMed]
- Wanless, S.; Harris, M.P.; Redman, P.; Speakman, J.R. Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar. Ecol. Prog. Ser. 2005, 294, 1–8. [Google Scholar] [CrossRef]
- Kowalczyk, N.D.; Chiaradia, A.; Preston, T.J.; Reina, R.D. Linking dietary shifts and reproductive failure in seabirds: A stable isotope approach. Funct. Ecol. 2014, 28, 755–765. [Google Scholar] [CrossRef]
- Scopel, L.; Diamond, A.; Kress, S.; Shannon, P. Varied breeding responses of seabirds to a regime shift in prey base in the Gulf of Maine. Mar. Ecol. Prog. Ser. 2019, 626, 177–196. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Mougey, T.; Hindermeyer, X. Foraging and provisioning strategies of black-browed albatrosses in relation to the requirements of the chick: Natural variation and experimental study. Behav. Ecol. 1997, 8, 635–643. [Google Scholar] [CrossRef]
- Schrimpf, M.B.; Parrish, J.K.; Pearson, S.F. Trade-offs in prey quality and quantity revealed through the behavioural compensation of breeding seabirds. Mar. Ecol. Prog. Ser. 2012, 460, 247–259. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Chastel, O.; Ackermann, L.; Chaurand, T.; Cuenotchaillet, F.; Hindermeyer, X.; Judas, J. Alternate long and short foraging trips in pelagic seabird parents. Anim. Behav. 1994, 47, 472–476. [Google Scholar] [CrossRef]
- Shoji, A.; Aris-Brosou, S.; Fayet, A.; Padget, O.; Perrins, C.; Guilford, T. Dual foraging and pair coordination during chick provisioning by Manx shearwaters: Empirical evidence supported by a simple model. J. Exp. Biol. 2015, 218, 2116–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steen, H.; Vogedes, D.; Broms, F.; Falk-Petersen, S.; Berge, J. Little auks (Alle alle) breeding in a High Arctic fjord system: Bimodal foraging strategies as a response to poor food quality? Polar Res. 2007, 26, 118–125. [Google Scholar] [CrossRef]
- Phillips, R.A.; Hammer, K.C. Growth and provisioning strategies of Northern Fulmars Fulmarus glacialis. Ibis 2000, 142, 435–445. [Google Scholar] [CrossRef]
- Carey, M.J. The effects of investigator disturbance on procellariiform seabirds: A review. N. Z. J. Zool. 2009, 36, 367–377. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, J.C.; Welch, J.R.; Dunn, R.; Bourgeois, K. Chick Provisioning in Grey-Faced Petrel (Pterodroma gouldi) under Environmental Stress. Birds 2022, 3, 285-292. https://doi.org/10.3390/birds3030019
Russell JC, Welch JR, Dunn R, Bourgeois K. Chick Provisioning in Grey-Faced Petrel (Pterodroma gouldi) under Environmental Stress. Birds. 2022; 3(3):285-292. https://doi.org/10.3390/birds3030019
Chicago/Turabian StyleRussell, James C., Jemma R. Welch, Rob Dunn, and Karen Bourgeois. 2022. "Chick Provisioning in Grey-Faced Petrel (Pterodroma gouldi) under Environmental Stress" Birds 3, no. 3: 285-292. https://doi.org/10.3390/birds3030019




