Simple Summary
Radiotherapy is widely used to treat cancer by directing high-energy X-rays at tumors while limiting harm to healthy tissue. However, radiation can also influence the immune system in ways that are not fully understood. In this study, we investigated how different radiation doses affect the survival and function of various immune cell types. We found that some immune cells, such as cytotoxic T cells, are highly sensitive to radiation, whereas others, like natural killer cells, are more resistant. Radiation also increased certain immune signals, such as interleukin-12, which can boost immune activity. These changes occurred rapidly after exposure, suggesting that the timing of treatment may be crucial when combining radiotherapy with immune-based therapies. Our findings may help guide future strategies to improve cancer treatment by integrating radiotherapy with immunotherapy.
Abstract
Radiotherapy employs high-energy X-rays to precisely target tumor tissues while minimizing damage to the surrounding healthy structures. Although its clinical efficacy is well established, the immunomodulatory effects of ionizing radiation remain complex and context-dependent. This study investigated the biological effects of radiotherapeutic doses on immune cells by evaluating lymphocyte viability, phenotypic profiles, and cytokine expression levels. Peripheral blood mononuclear cells (PBMCs) were isolated from six healthy donors and irradiated with 0, 2, or 6 Gy using a 6 MV linear accelerator (LINAC). Dose validation with an ionization chamber demonstrated strong agreement between estimated and measured values (intraclass correlation coefficient = 1, 95% CI). Immune subsets, including T cells (CD3+), helper T cells (CD3+CD4+), cytotoxic T cells (CD3+CD8+), regulatory T cells (CD3+CD4+Foxp3+), and natural killer (CD3-CD56+) cells, along with intracellular cytokines interleukin-12 (IL-12) and interferon-gamma (IFN-γ), were analyzed via flow cytometry at multiple time points. The results showed a significant, dose-dependent decline in overall lymphocyte viability (p < 0.01) compared to control. Cytotoxic T cells were the most radiosensitive, followed by helper and regulatory T cells, while NK cells were the most radioresistant. IL-12 expression initially increased post-irradiation, while IFN-γ levels remained variable. These findings demonstrate that radiation induces distinct alterations in immune phenotypes and cytokine profiles, which may shape the immune response. Immune profiling following irradiation may therefore provide valuable insights for optimizing combination strategies that integrate radiotherapy and immunotherapy in cancer treatment.
1. Introduction
Radiotherapy (RT) is used in more than 60% of cancer treatments. Its goal is to deliver maximum radiation to tumors while minimizing exposure to surrounding healthy tissues, thereby optimizing the therapeutic ratio [1]. The effectiveness of RT relies on the higher sensitivity of cancer cells to radiation-induced DNA damage compared with normal cells [2,3]. Radiation causes DNA breaks and generates free radicals, ultimately leading to cell death via failed repair mechanisms. Advanced techniques such as IMRT, VMAT, and SBRT allow precise tumor targeting and dose escalation [4,5,6], while emerging technologies, including FLASH radiotherapy, proton therapy, and carbon ion therapy, further enhance treatment precision [7]. Despite these advancements, individual responses to RT vary [8], and side effects caused by damage to nearby normal tissues remain a challenge.
Beyond its cytotoxic effects, RT can significantly affect the immune system, exerting both suppressive or stimulatory effects [9]. High doses of total body irradiation (TBI) can suppress immunity by reducing circulating lymphocytes [10], including T cells, B cells, and natural killer (NK) cells, which are essential for immune defense [11]. This damage may result in leukopenia and impaired production and function of white blood cells [12,13,14]. Prosser initially reported that the survival of T and B lymphocytes following X-ray irradiation (assayed with trypan blue) declined rapidly after relatively low doses [15]. Subsequently, Kwon et al. showed that B lymphocytes were slightly more radiosensitive than T lymphocytes [16], and Han et al. confirmed that B lymphocytes are more sensitive to low-dose radiation-induced cell lethality in vitro than T lymphocytes [17]. Each group consistently reported B cells to be more sensitive to low radiation doses than T cells. Nakamura et al. investigated the subsets of CD4+ and CD8+ lymphocytes in peripheral blood following irradiation. They concluded that the doses required to reduce the surviving fractions of cytotoxic T cells and helper T cells were similar [18]. In contrast, Seki et al. investigated cytokine-mediated protection against apoptosis in lymphocyte subpopulations. Their data showed that NK cells were the most sensitive, while T and B cells showed weaker susceptibility, and helper T cells were relatively radioresistant compared to T cytotoxic cells [19]. These findings highlight the complexity of radiation-induced changes in lymphocyte quantity and function, which depend on various factors, including radiation dose and type, exposure duration, and the specific components of the immune system under consideration [8,20,21,22].
Radiation-induced cellular damage also triggers inflammatory responses, leading to the release of cytokines and chemokines. These inflammatory responses can enhance the antigen-presenting function of dendritic cells [23] and promote the activation and proliferation of T and NK cells [24,25,26], which are critical for adaptive immune responses against pathogens and cancer cells. T and NK cells are types of immune cells known for their ability to recognize and kill cancer cells [27], and radiotherapy has been shown to moderate their activity. Radiation induces these immune responses by stimulating the expression of anti-tumor and related molecules [28].
Overall, the effects of radiation on immune responses are complex and context-dependent. High doses of RT can suppress immune function and increase susceptibility to inflammation, which may stimulate and potentially enhance the anti-tumor immune responses of cancer therapy. However, the balance between these opposing effects and their clinical implications requires careful consideration. In this study, we aimed to investigate the immune response to conventional radiotherapy in an in vitro model. Specifically, we assessed the radiobiological effects by quantifying cell viability loss, characterizing changes in lymphocyte populations, and evaluating functional responses through cytokine production.
2. Materials and Methods
2.1. Sample Preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from six healthy adult donors (three males and three females), aged 25–35 years. All donors were non-smokers and free of chronic illness. Prior to sample collection, each participant provided written informed consent in accordance with institutional guidelines. Ethical approval was obtained from Mahidol University Central Institutional Review Board (MU-CIRB; Reference no. 2019/119.1904). For each donor, 20 mL of blood was drawn by venipuncture and collected into sodium heparin tubes (BD Biosciences, Franklin Lakes, NJ, USA). The samples were gradually overlaid onto the LymphoprepTM solution at a 1:1 volume-to-volume (v/v) ratio, followed by PBMCs separation via gradient centrifugation at 900× g and 20 °C for 30 min. After centrifugation, plasma and red blood cells (RBCs) were collected for storage at −20 °C. PBMCs were harvested and washed twice with Roswell Park Memorial Institute-1640 (RPMI-1640) medium (Gibco, Grand Island, NY, USA). Cell viability was determined using the trypan blue exclusion method.
2.2. Cultivation and Expansion of PBMCs
To activate lymphocytes for clonal expansion, PBMCs were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, USA), 1% penicillin–streptomycin (10,000 U/mL), and 2 µg/mL phytohemagglutinin-M (Merck, Darmstadt, Germany) for 24 h prior to irradiation. Cells were incubated at 37 °C with 5% CO2 in a humidified atmosphere. Cell viability was assessed using the trypan blue exclusion method.
2.3. Irradiation
For each condition, 5 × 106 PBMCs were irradiated using a 6 MV TrueBeam LINAC (Varian Medical Systems, Inc., Palo Alto, CA, USA) at Siriraj Hospital, Bangkok, Thailand. A 30 cm × 30 cm × 30 cm water phantom was used, with samples placed at a source-to-axis distance (SAD) of 100 cm and 10 cm below the water surface. The exposure technique used for our clinical setup followed the protocol provided by the Department of Radiology at the Faculty of Medicine, Siriraj Hospital. Radiation doses of 2 and 6 Gy were delivered at a dose rate of 4.014 Gy/min with a field size of 10 × 10 cm2. Before irradiation, the treatment plans were validated by measuring exposure within the phantom using an ionization chamber connected to an electrometer. This comprehensive setup ensured precise and controlled irradiation of the PBMC samples in accordance with established clinical protocols and standards.
2.4. Determination of Radiation-Induced Changes in Lymphocytes
Following irradiation, PBMCs were cultured in 24-well plates and harvested for analysis from Day 1 to Day 7.
- Cell viability analysis via flow cytometry
A total of 1 × 106 irradiated cells were washed with 1 mL PBS buffer, diluted in 100 µL of Zombie Green™ solution (Cat. No. 423111) and incubated at room temperature for 15 min in the dark. Then, cells were washed with 1 mL of FACS buffer containing 0.1% bovine serum albumin (BSA) and resuspended in 500 µL of FACS buffer for analysis.
- Lymphocyte phenotype analysis
PBMCs were analyzed via flow cytometry to determine the phenotype of the lymphocyte subpopulations. Cells were stained with fluorescent dye-labeled monoclonal antibodies to determine the cell populations, which included CD3-PE (clone OKT3, Cat# 317308), CD4-PE/Dazzle™ 594 (clone OKT4, Cat# 317448), CD8-PE/Cyanine7 (clone SK1, Cat# 344712), FoxP3-Alexa Fluor® 647 (clone 206D, Cat# 320114), and CD56-Alexa Fluor® 700 (clone HCD56, Cat# 318316) from BioLegend, San Diego, CA, USA. Data were analyzed on a FACS Canto II flow cytometer (BD Biosciences, USA) using both histogram and quadrant analyses.
- Intracellular cytokine staining
To measure cytokine production, PBMCs were collected from culture at various time points from Day 0 (4 h after irradiation) to Day 7. The cells were fixed with 4% paraformaldehyde (Sigma, Lee County, VA, USA) and treated with a permeabilization wash buffer (0.1% Triton X-100). The cells were then stained with fluorescent dye-labeled monoclonal antibodies specific to the cytokines interleukin 12; IL-12-APC (clone C11.5, Cat# 554576) and interferon-gamma; and IFN-γ-APC/Cyanine7 (clone B27, Cat# 506524). Results were acquired and analyzed using a FACS Canto II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
2.5. Statistical Analysis
Dose response results are presented as the mean ± standard error of the mean (SEM). The experiments were performed using biological replicates in 3 males and 3 females from independent donors. Data were analyzed using the FlowJo v10 software and GraphPad Prism version v9.5.1 (GraphPad Software, CA, USA). For all statistical analyses, p-values ≤ 0.05 were considered significant. The data were assessed for normality and homogeneity of variance using Shapiro–Wilk and Levene’s tests, respectively. Where assumptions were met, radiation effects on the immune responses on different days were compared using a one-way ANOVA test with LSD post hoc tests, while dose comparisons were carried out using Student’s t-test. Nonparametric data were analyzed using the Mann–Whitney U test for two groups or the Kruskal–Wallis test for more than two groups. Statistical analyses were performed using SPSS v18.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Intraclass Correlation Coefficients of Irradiation
Radiation exposure at the central irradiated field, based on our treatment plan, is summarized and illustrated in Figure 1a,b. The exposure output was measured using an ionization chamber in three independent sessions prior to sample irradiation, with two samples measured per session. The measured outputs for the 2 and 6 Gy dose plans are presented in Figure 1a as monitor units (MU), which were used to calculate delivered doses. For the 2 Gy dose, the delivered doses were measured as 200.025, 199.974, and 200.035 cGy. Similarly, for the 6 Gy dose, the delivered doses were 599.995, 600.002, and 600.025 cGy. A strong positive correlation was observed between the measured delivered doses obtained from the three independent irradiations, with an intraclass correlation coefficient of 1.0 (95% CI) across the estimated dose range of 2–6 Gy, as shown in Figure 1c.
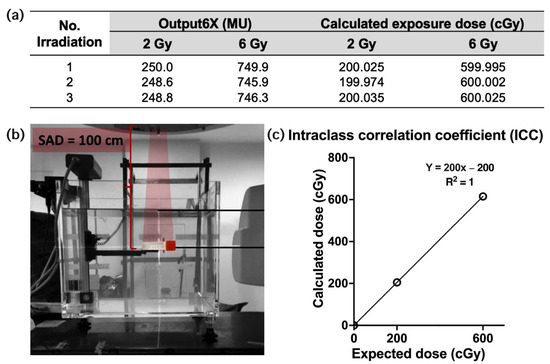
Figure 1.
Setup of sample positioning and intraclass correlation coefficient (ICC) of radiation exposures. (a) A table showing the output (MU) of the 2 and 6 Gy doses measured in the ionization chamber, as well as the calculated exposure dose. (b) A water phantom containing blood samples in cryovial tubes was positioned at SAD 100 cm (6 MV TrueBeam LINAC). (c) The graph of the ICC of irradiation, calculated and expected exposures.
3.2. Viability of Irradiated Lymphocytes
PBMCs from six healthy donors were collected and exposed to radiation doses of 2 and 6 Gy. Irradiation reduced total cell counts and altered the proportions of various immune subpopulations. The average dose response of lymphocytes to radiation was determined, with error bars representing the SEMs. This analysis assessed cell viability as an indicator of cytotoxicity by utilizing Zombie Green fixable viability dye in flow cytometry. This dye irreversibly binds to cellular amines upon loss of membrane integrity across multiple time points up to one week post-irradiation.
Figure 2 illustrates the percentage of irradiated and non-irradiated lymphocytes viable on Day 0 through Day 7. Both the 2 and 6 Gy irradiation doses resulted in significant decrease in cell viability, with the decrease being dose dependent. At Day 7, cell viability percentages showed a mean difference of 15.29% (2 Gy = 59.86%) and 46.18% (6 Gy = 28.97%) compared to controls (75.15%), with p = 0.02, Cohen’s d = 1.61, 95% CI [2.01 to 28.62] at Day 5 and p < 0.01 (Cohen’s d = 2.92, 95% CI [32.88 to 59.49] at Day 7, respectively. However, a partial recovery in cell viability was observed as 55.72% after Day 3 at the lower dose (2 Gy), with viability reaching ~59.86% at Day 7, which remains significantly lower than non-irradiated controls.
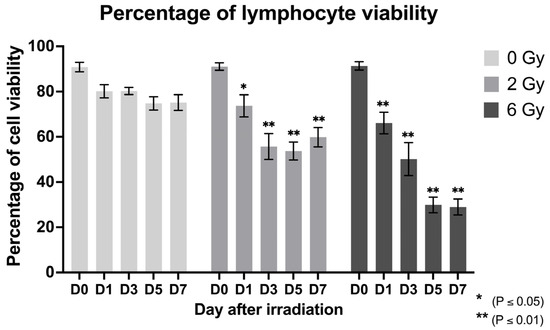
Figure 2.
Cell viability percentage of irradiated (2 and 6 Gy) lymphocytes at different time points (Days 0, 1, 3, 5, and 7 post-irradiation); mean (SEM).
3.3. Radiation Effects on Lymphocyte Subpopulations
Flow cytometric analysis revealed differences in the irradiation susceptibility of lymphocyte subpopulations cultured post-irradiation as shown in Supplementary Materials Figure S1. Figure 3 shows the mean relative counts of lymphocyte subsets (CD3+, CD4+, CD8+, CD4+/CD8+, Foxp3+, and NK) over time, with non-irradiated cells serving as controls. The relative proportions of T lymphocytes (CD3+) and cytotoxic T (CD3+CD8+) cells gradually decreased following irradiation in a dose-dependent manner. Conversely, T-cells (CD3+CD4+Foxp3+) and NK cells (CD3-CD56+) exhibited an increasing trend following irradiation (Figure 3). There were similar proportions of helper T (CD3+CD4+) cells observed in the irradiated and non-irradiated samples over time. The immunoregulatory index, which was determined by the CD4+-to-CD8+ ratio, indicated that cytotoxic T cells were more radiosensitive than helper T cells, while NK cells demonstrated the highest degree of radioresistance.
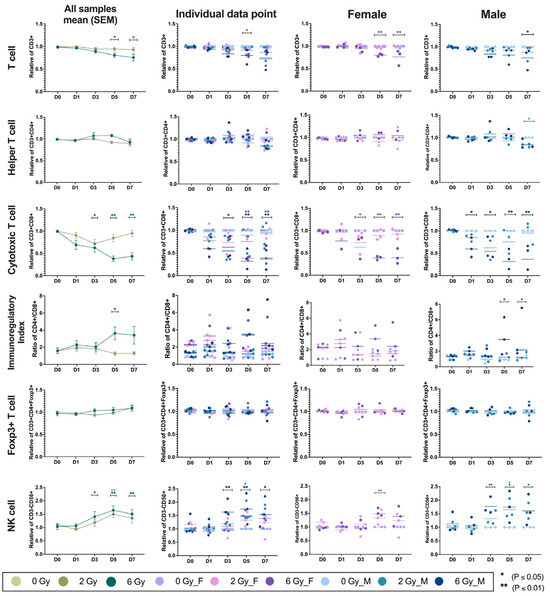
Figure 3.
This figure illustrates the temporal dynamics of lymphocyte subpopulations following exposure to 2 or 6 Gy of ionizing radiation. Immune cell subsets are shown across five time points (Days 0, 1, 3, 5, and 7): CD3+ T cells (row 1), CD3+CD4+ T cells (row 2), CD3+CD8+ T cells (row 3), CD4+/CD8+ ratio as an immunoregulatory index (row 4), CD3+CD4+Foxp3+ T cells (row 5), and CD3-CD56+ NK cells (row 6). Data are presented in mean ± SEM across all samples (6 donors), individual data points (3 males and 3 females), female-specific responses (n = 3), and male-specific responses (n = 3). Color-coded groups represent radiation dose and sex. This layout enables comparison of dose-dependent and sex-specific immune responses over time.
At a radiation dose of 6 Gy, there was a decrease in the relative counts of CD3+ cells, with mean differences of 0.19 (p = 0.02, Cohen’s d = 1.58, 95% CI [0.09 to 0.30]) on Day 5 and 0.24 on Day 7 (p = 0.04, Cohen’s d = 1.78, 95% CI [0.14 to 0.35]). CD3+CD8+ cells demonstrated marked reductions with mean differences of 0.38 (p = 0.03, Cohen’s d = 0.89, 95% CI [0.17 to 0.58]) on Day 3, 0.62 (p < 0.01, Cohen’s d = 2.39, 95% CI [0.42 to 0.82]) on Day 5, and 0.56 (p < 0.01, Cohen’s d = 1.87, 95% CI [0.36 to 0.77]) on Day 7. In contrast, irradiated lymphocytes showed an increase in the relative proportions of CD3+CD4+Foxp3+ and CD3-CD56+ cells, particularly at higher radiation doses. Foxp3+ regulatory T cells gradually increased at later time points (Day 3, 5, and 7), particularly following 6 Gy irradiation, with a smaller rise also observed at Day 7 in the 2 Gy group. This regulatory feedback mechanism corresponded to a relative deficiency in CD3+CD8+ cells, resulting in a significantly elevated CD4+/CD8+ ratio, most pronounced after 6 Gy exposure with a mean difference of 2.51 (p = 0.04, Cohen’s d = 1.93, 95% CI [4.19 to 0.84]) on Day 5. Higher levels of CD3-CD56+ cells were observed after irradiation with 6 Gy, with significant mean differences of 0.40, (p = 0.02, Cohen’s d = 1.27, 95% CI [0.78 to 0.032]) on Day 3, 0.64 (p < 0.01, Cohen’s d = 2.09, 95% CI [1.01 to 0.27]) on Day 5, and 0.50 (p < 0.01, Cohen’s d = 1.48, 95% CI [0.87 to 0.13]) on Day 7. Additionally, on Day 5, there was an increase in the relative number of NK cells, with a mean difference of 0.50 (p = 0.01, Cohen’s d = 1.98, and 95% CI [0.87 to 0.13]) following exposure to 2 Gy.
Radiation exposure thus induced dynamic, dose-dependent alterations in immune cell populations, with additional sex-dependent effects. Following 6 Gy irradiation, CD3+ T cells were significantly reduced in females on Day 5 (p = 0.03, Cohen’s d = 1.8, 95% CI [0.06 to 0.34]). CD3+CD8+ cells showed marked decreases in both sexes, with reductions in males at Day 3 (p = 0.02, Cohen’s d = 1.54, 95% CI [0.04 to 0.80]), Day 5 (p = 0.01, Cohen’s d = 2.49, 95% CI [0.25 to 1.01]), and Day 7 (p = 0.01, Cohen’s d = 1.93, 95% CI [0.17 to 0.93]), and in females at Day 5 (p = 0.01, Cohen’s d = 2.78, 95% CI [0.23 to 0.99]) and Day 7 (p = 0.01, Cohen’s d = 2.36, 95% CI [0.20 to 0.96]). CD3+CD4+ and CD3+CD4+Foxp3+ cell levels remained relatively stable, and the pronounced decline in CD3+CD8+ cells led to an elevated CD4+/CD8+ ratio across irradiated groups. Statistically significant increases in CD3-CD56+ were observed in males at Day 3 (p = 0.01, Cohen’s d = 2.32, 95% CI [1.38 to 0.16]), Day 5 (p = 0.01, Cohen’s d = 1.68, 95% CI [1.36 to 0.14]), and Day 7 (p = 0.05, Cohen’s d = 1.39, 95% CI [1.22 to 0.01]). Additionally, 2 Gy irradiation on Day 5 significantly modulated immune subsets (p = 0.03, Cohen’s d = 1.91, 95% CI [1.25 to 0.03]). Notably, CD3-CD56+ cell proportions differed significantly between sexes, with males exhibiting greater sensitivity to radiation than females p = 0.01, Cohen’s d = 0.46, 95% CI [1.34 to 0.12].
In specific groups, lymphocyte subsets exhibited similar trends in phenotypic shifts, with notable sex-dependent differences. CD3+ and CD3+CD8+ T cells progressively declined in both sexes in a dose-dependent manner, whereas CD3+CD4+ T cells and CD3+CD4+Foxp3+ T cells remained relatively stable. This imbalance resulted in a significant increase in the CD4+/CD8+ ratio, especially in males (Day 5: p = 0.01, Cohen’s d = 1.69, 95% CI [5.12 to 0.59]; Day 7: p = 0.03, Cohen’s d = 1.11, 95% CI [4.81 to 0.28]). CD3-CD56+ cells showed increased proportions post-irradiation, with males demonstrating greater sensitivity and variability across time points. These findings underscore the differential radiosensitivity of lymphocyte subsets and highlight sex-specific immune modulation following radiation exposure.
3.4. The Radiation Effect on Cytokine Production
The effect of radiation on immune function was evaluated via cytokine production levels. The mean relative fluorescent intensities (MFIs) of interleukin-12 (IL-12) and interferon-gamma (IFN-γ) were determined using flow cytometry across six donors following irradiation with various doses (0, 2 and 6 Gy) at five timepoints: Days 0, 1, 3, 5, and 7 after irradiation (Figure 4). The MFI of IL-12 increased immediately after exposure to radiation (from 1967 to 2509) and then declined significantly on Day 1 compared to Day 0 at both 2 and 6 Gy. From Day 1 to Day 7, IL-12 level remained relatively stable. IFN-γ showed fewer differences, although a significant reduction was observed on Day 1 after 6 Gy (MFI = 577 vs. 720 at Day 0; p < 0.05), followed by a rebound increase on subsequent days.
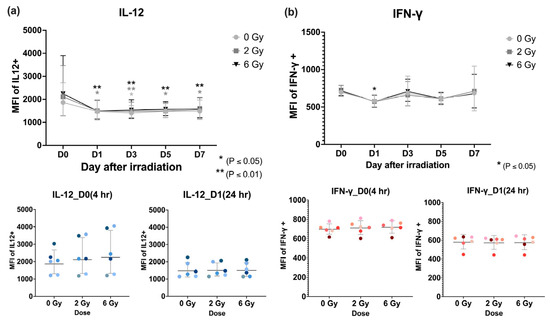
Figure 4.
Flow cytometric analysis of the mean fluorescent intensities (MFIs) and mean (SEM) averaged over six donors of intracellular cytokines, (a) interleukin-12, and (b) interferon-gamma after lymphocyte irradiation (0, 2, or 6 Gy).
4. Discussion
The radiosensitivity of lymphocytes has been studied extensively using various approaches, including assays of survival, mortality, and immune function. The available published data come from pre-clinical and human studies using in vitro and in vivo assays [29]. In our study, PBMCs irradiated with 0, 2, and 6 Gy were analyzed for T cells (CD4+, CD8+, and Foxp3+) and NK cells across five timepoints (Days 0, 1, 3, 5, and 7). We observed an upregulation of NK cells and Foxp3+ T cells, a relatively stable level of CD4+ T cells, and significant decline in CD8+ T cells. Moreover, the immunoregulatory index (CD4+/CD8+ ratio) was approximately two after 2 and 6 Gy irradiation. On Day 5, 6 Gy irradiation induced a significant increase in the immune regulatory index, while 2 Gy irradiation showed a reduction in the CD4+/CD8+ ratio. These results indicated that subpopulations of lymphocytes differed in radiosensitivity. After irradiation with doses of up to 6 Gy, the ranking of radiosensitivity was T (CD3+CD8+) > T (CD3+CD4+) > T (CD3+CD4+Foxp3+) > NK (CD3-CD56+), which is consistent with published in vitro studies [30,31,32,33]. Our ranking also aligns with in vivo studies based on lymphocyte depletion, suggesting that B cells are the most radiosensitive, followed by T cells and NK cells [34,35,36]. However, interpreting lymphocyte radiosensitivity is complex due to biological and spatial variability. While in vitro studies (e.g., Phillipp et al. [31]) suggest that helper T cells are more radiosensitive than cytotoxic T cells, in vivo conditions introduce further challenges. Circulating lymphocytes may be only partially irradiated depending on their location during treatment [37], and their dose exposure differs from whole blood due to varying recirculation and transit times among subsets [38]. Moreover, circulating lymphocytes are generally more radiosensitive than tissue-resident ones [29,39]. These factors highlight the difficulty in directly translating experimental findings to clinical settings.
Our data also suggests that reduced levels of cytokines are linked to a reduction in T lymphocytes, especially cytotoxic T cells, due to radiation-induced immunogenic cell death. This process triggers the development of danger-associated molecular patterns (DAMPs) and the release of pro-inflammatory cytokines such as IL-12 and IFN-γ [40]. Reductions in cytotoxic T cells may weaken immune defenses, hinder the elimination of pathogens and tumor cells, and affect the development of CD8+ memory cells [41]. CD4+ T cells consist of Th1 and Th2 subpopulations, with Th1 cells being the main producers of pro-inflammatory cytokines [42]. A slight decline in the proportion of irradiated CD4+ T cells observed in our study may have contributed to reductions in IL-12 and IFN-γ levels, as also reported by Vanbuskirk et al. [43]. Regulatory T cells (Tregs), marked by Foxp3+ expression, regulate immune homeostasis by suppressing excessive activation [44,45]. In our study, the reduction in CD8+ T cells after irradiation may correlate with a relative increase in Tregs, potentially dampening anti-tumor immunity. This suggests that combining RT with immunotherapies targeting Tregs, such as checkpoint inhibitors or depletion strategies, could enhance efficacy. Interestingly, NK cell levels greatly increased post-irradiation. Prior studies reported that NK cell activation during RT correlates with better outcomes, due to their ability to target cancer cells [46]. Radiation may enhance NK cell recruitment and activity by upregulating chemokines like CXCL6 [47] and modifying the tumor microenvironment [48]. In breast cancer patients, RT has been shown to increase tumor-infiltrating NK cells and promote IFN-γ and TNF-α production [49,50]. These findings support the potential of RT-induced NK cell activation as part of a synergistic cancer treatment strategy.
Radiotherapy has been shown to modulate immune responses through distinct cytokine profiles [51]. In our study, IL-12 levels increased rapidly within hours of radiation exposure, reflecting early activation of antigen-presenting cells. This early surge in IL-12 is known to promote Th1 polarization and prime both NK and CD8+ T cells [52,53], which subsequently contribute to IFN-γ production via STAT4 signaling [54]. Interestingly, despite a reduction in CD8+ T cell numbers, IFN-γ levels remained stable, suggesting a compensatory response by NK cells, which are known to be potent producers of IFN-γ [55]. The observed rise in IFN-γ at later time points aligns with downstream signaling cascade. IFN-γ plays a central role in anti-tumor immunity by sustaining cytotoxic activity post-irradiation [56], enhancing NK cell-mediated cytotoxicity, promoting antigen presentation, and shaping adaptive immune responses. Collectively, the sequential elevation of IL-12 followed by IFN-γ suggests a coordinated immune response to radiation-induced stress, wherein early innate activation drives functional modulation of lymphocyte subsets [57,58]. In addition to IL-12 and IFN-γ, radiation exposure modulates several key cytokines that shape the immune response [59]. IL-6 and TNF-α typically rise early, reflecting acute inflammation and innate immune activation [60,61], while IL-2 supports proliferation of effector and regulatory T cells, though its levels may fluctuate due to lymphocyte depletion [62]. IL-10 increases later, acting as a counter-regulatory signal to suppress excessive inflammation and restore immune balance [60,61]. These coordinated shifts contribute to a dynamic immune landscape that transitions from activation to resolution, consistent with previous reports in both cancer patients and irradiated lymphocyte models. Our study captured immune alterations within the first 7 Days post-irradiation, consistent with previous reports showing dynamic shifts in CD4-associated cytokines such as IL-6, IL-4, IL-1β, IL-10, IL-13, IL-17, IFN-γ, and TNF-α during this period [63]. Recovery profiles of T-cell subsets suggest gradual reconstitution beyond the first week, with distinct kinetics across naïve, memory, and regulatory populations [64]. Additionally, caspase-1 p10 and pyroptosis markers peak at Day 7 and normalize by Day 14, indicating transient innate immune activation [65].
Although the present study provides reproducible trends in immune cell dynamics following irradiation within an in vitro PBMC model, the limited donor pool may not fully capture inter-individual variability. These findings demonstrate a temporal shift in immune cell dynamics following irradiation within an in vitro PBMC model. CD8+ T cells exhibited high radiosensitivity, undergoing apoptosis shortly after exposure, likely due to radiation-induced DNA damage and limited repair capacity. In contrast, NK cells displayed greater resistance, consistent with their innate immune characteristics and non-proliferative nature. While these changes in cytotoxic T cell and NK cell proportions may serve as potential biomarkers for radiation-induced immune modulation, it is important to acknowledge that in vitro systems cannot fully replicate the complexity of the tumor microenvironment, including stromal interactions, hypoxia, and tumor–immune cell crosstalk. Nonetheless, the controlled conditions of this model enabled precise quantification of dose-dependent effects and minimized confounding variables inherent to in vivo systems. The observed increase in IL-12 levels alongside sustained IFN-γ expression suggests that radiation influences not only direct cytotoxicity but also immune signaling pathways. These mechanistic insights provide a foundation for integrating radiotherapy with immunotherapeutic strategies, such as IL-12-based therapies or NK cell-targeted approaches. Future studies involving larger and more diverse donor cohorts will be important to validate and extend the current findings. Incorporating multiplex cytokine analysis could offer deeper insight into the regulatory and proliferative dynamics of immune cells following irradiation, particularly in the context of immune recovery and compensatory mechanisms that may shape long-term immune remodeling. In addition, validation using tumor-bearing models and patient-derived samples will be essential for optimizing the timing and therapeutic efficacy of radiation-based combination strategies.
5. Conclusions
Radiation alters lymphocyte viability, subset distribution, and cytokine production in a dose- and time-dependent manner. CD8+ T cells were most radiosensitive, while NK cells exhibited the most radioresistance. Elevated CD4+/CD8+ ratios and sequential IL-12/IFN-γ responses reflect coordinated immune modulation. These findings show a potential in supporting the integration of radiotherapy with immunotherapeutic strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/radiation5040029/s1, Figure S1: Gating strategy applied for flow cytometry analysis of immune cell subsets. Initial gating was performed to identify lymphocytes based on FSC and SSC parameters. Non-lymphoid events were excluded from further analysis. T cells (CD3+), helper T cells (CD3+CD4+), cytotoxic T cells (CD3+CD8+), regulatory T cells (CD3+CD4+foxP3+), and NK cells (CD3-CD56+) were identified using sequential gating. All gates were defined using fluorescence minus one (FMO) controls to ensure specificity.
Author Contributions
Conceptualization, K.J. and R.W.; methodology, P.Y. and W.P.; validation, P.Y. and R.W.; formal analysis, R.W.; investigation, P.Y.; resources, P.D.; software, W.P.; writing—original draft preparation, P.Y.; writing—review and editing, K.J. and R.W.; visualization, R.W. and P.Y.; supervision, K.J., D.P., P.D. and A.E.; funding acquisition, K.J., D.P., R.W. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Thailand Graduate Institute of Science and Technology (TGIST) Scholarship [TG-55-14-60-012D] from the National Science and Technology Development Agency (NSTDA), and Thailand Science Research and Innovation (TSRI), Ministry of Higher Education, Science, Research and Innovation, project no. 197246. This project was partially supported by the Franco-Thai Mobility Program 2018–2019. The funding sources were not involved in the study design, data collection, analysis, interpretation, or writing of this manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Mahidol University Central Institutional Review Board (MU-CIRB), which gave approval (Reference no. 1191904).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request (corresponding author: kulachart.jan@mahidol.edu).
Acknowledgments
The authors thank Wisawa Phongprapun and the Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, for providing radiation equipment and assisting with dose measurements and assessments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| DNA | Deoxyribonucleic Acid |
| IMRT | Intensity Modulated Radiation Therapy |
| VMAT | Volumetric Modulated Arc Therapy |
| SBRT | Stereotactic Body Radiation Therapy |
| TBI | Total Body Irradiation |
| CD | Cluster of differentiation |
| PBMCs | Peripheral Blood Mononuclear Cells |
| RPMI-1640 | Roswell Park Memorial Institute-1640 |
| BSA | Bovine Serum Albumin |
| FACS | Fluorescence-Activated Cell Sorting |
| LINAC | Linear Accelerator |
| SAD | Source-to-Axis Distance |
| Gy | Gray |
| MU | Monitor Unit |
| IL-12 | Interleukin-12 |
| IFN-γ | Interferon-Gamma |
| SEM | Standard Error of The Mean |
| DAMPs | Danger-Associated Molecular Patterns |
| Th1 | T Helper 1 |
| Treg | T Regulatory Cell |
| STAT4 | Signal Transducer and Activator of Transcription 4 |
References
- Kirthi Koushik, A.S.; Harish, K.; Avinash, H.U. Principles of radiation oncology: A beams eye view for a surgeon. Indian J. Surg. Oncol. 2013, 4, 255–262. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, F.; Liu, H. Radiation-induced Cell Death and Its Mechanisms. Health Phys. 2022, 123, 376–386. [Google Scholar] [CrossRef]
- Ghaderi, N.; Jung, J.; Brüningk, S.C.; Subramanian, A.; Nassour, L.; Peacock, J. A Century of Fractionated Radiotherapy: How Mathematical Oncology Can Break the Rules. Int. J. Mol. Sci. 2022, 23, 1316. [Google Scholar] [CrossRef]
- Toma-Dasu, I.; Moiseenko, V.; Purdie, T.G.; Carlson, D.J. Recent Developments in the Prediction of Clinical Outcomes Data in Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Loughery, B.; Knill, C.; Silverstein, E.; Zakjevskii, V.; Masi, K.; Covington, E.; Snyder, K.; Song, K.; Snyder, M. Multi-institutional evaluation of end-to-end protocol for IMRT/VMAT treatment chains utilizing conventional linacs. Med. Dosim. 2019, 44, 61–66. [Google Scholar] [CrossRef]
- Higginson, D.S.; Morris, D.E.; Jones, E.L.; Clarke-Pearson, D.; Varia, M.A. Stereotactic body radiotherapy (SBRT): Technological innovation and application in gynecologic oncology. Gynecol. Oncol. 2011, 120, 404–412. [Google Scholar] [CrossRef]
- Fiorino, C.; Guckemberger, M.; Schwarz, M.; van der Heide, U.A.; Heijmen, B. Technology-driven research for radiotherapy innovation. Mol. Oncol. 2020, 14, 1500–1513. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Liu, C.; Chikina, M.; Deshpande, R.; Menk, A.V.; Wang, T.; Tabib, T.; Brunazzi, E.A.; Vignali, K.M.; Sun, M.; Stolz, D.B.; et al. Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8+ T Cell-Derived Interferon-γ. Immunity 2019, 51, 381–397.e6. [Google Scholar] [CrossRef]
- Ellsworth, S.G.; Yalamanchali, A.; Lautenschlaeger, T.; Grossman, S.A.; Grassberger, C.; Lin, S.H.; Mohan, R. Lymphocyte Depletion Rate as a Biomarker of Radiation Dose to Circulating Lymphocytes During Fractionated Partial-Body Radiation Therapy. Adv. Radiat. Oncol. 2022, 7, 100959. [Google Scholar] [CrossRef]
- Terrones-Campos, C.; Ledergerber, B.; Forbes, N.; Smith, A.G.; Petersen, J.; Helleberg, M.; Lundgren, J.; Specht, L.; Vogelius, I.R. Prediction of Radiation-induced Lymphopenia following Exposure of the Thoracic Region and Associated Risk of Infections and Mortality. Clin. Oncol. 2023, 35, e434–e444. [Google Scholar] [CrossRef]
- Yoon, C.I.; Hwang, J.; Kim, D.; Ji, J.H.; Lee, J.; Bae, S.J.; Jeong, J.; Chang, J.-S.; Cho, Y.; Lee, H.S.; et al. Prognostic impact of radiotherapy-induced-lymphopenia in patients treated with breast-conservative surgery. Sci. Rep. 2023, 13, 14372. [Google Scholar] [CrossRef]
- Chen, F.; Jin, J.Y.; Hui, T.S.K.; Jing, H.; Zhang, H.; Nong, Y.; Han, Y.; Wang, W.; Ma, L.; Yi, F.; et al. Radiation Induced Lymphopenia Is Associated with the Effective Dose to the Circulating Immune Cells in Breast Cancer. Front. Oncol. 2022, 12, 768956. [Google Scholar] [CrossRef]
- Prosser, J.S. Survival of Human T and B Lymphocytes after X-irradiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 30, 459–465. [Google Scholar] [CrossRef]
- Kwan, D.K.; Norman, A. Radiosensitivity of Human Lymphocytes and Thymocytes. Radiat. Res. 1977, 69, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Pauly, J.L.; Minowada, J. In vitro preferential effect of irradiation on cultured T lymphoid cell line. Clin. Exp. Immunol. 1974, 17, 455–462. [Google Scholar] [PubMed]
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 Positive Human T-Lymphocytes by an In Vitro Colony Formation Assay. Radiat. Res. 1990, 123, 224–227. [Google Scholar] [CrossRef]
- Seki, H.; Iwai, K.; Kanegane, H.; Konno, A.; Ohta, K.; Ohta, K.; Yachie, A.; Taniguchi, N.; Miyawaki, T. Differential Protective Action of Cytokines on Radiation-Induced Apoptosis of Peripheral Lymphocyte Subpopulations. Cell. Immunol. 1995, 163, 30–36. [Google Scholar] [CrossRef]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef]
- Boopathi, E.; Den, R.B.; Thangavel, C. Innate Immune System in the Context of Radiation Therapy for Cancer. Cancers 2023, 15, 3972. [Google Scholar] [CrossRef]
- Sologuren, I.; Rodríguez-Gallego, C.; Lara, P.C. Immune effects of high dose radiation treatment: Implications of ionizing radiation on the development of bystander and abscopal effects. Transl. Cancer Res. 2014, 3, 18–31. [Google Scholar]
- Roses, R.E.; Datta, J.; Czerniecki, B.J. Radiation as immunomodulator: Implications for dendritic cell-based immunotherapy. Radiat. Res. 2014, 182, 211–218. [Google Scholar] [CrossRef]
- Canter, R.J.; Grossenbacher, S.K.; Foltz, J.A.; Sturgill, I.R.; Park, J.S.; Luna, J.I.; Kent, M.S.; Culp, W.T.N.; Chen, M.; Modiano, J.F.; et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunother. Cancer 2017, 5, 98. [Google Scholar] [CrossRef]
- Chen, F.; Yu, H.; Zhang, H.; Nong, Y.; Wang, Q.; Jing, H.; Han, Y.; Wu, J.; Zhou, Z.; Yang, L.; et al. Risk factors for radiation induced lymphopenia in patients with breast cancer receiving adjuvant radiotherapy. Ann. Transl. Med. 2021, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Bernard, B.; Rajamanickam, V.; Dubay, C.; Piening, B.; Alonso, E.; Jutric, Z.; Tang, E.; Newell, P.; Hansen, P.; Medler, T.; et al. Transcriptional and immunohistological assessment of immune infiltration in pancreatic cancer. PLoS ONE 2020, 15, e0238380. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zeng, Z.; Li, J.; Luo, Y.; Sun, W.; Gong, Y.; Zhang, J.; Wu, Q.; Xie, C. Immunomodulation of NK Cells by Ionizing Radiation. Front. Oncol. 2020, 10, 874. [Google Scholar] [CrossRef]
- Gallo, P.M.; Gallucci, S. The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Front. Immunol. 2013, 4, 138. [Google Scholar] [CrossRef]
- Paganetti, H. A review on lymphocyte radiosensitivity and its impact on radiotherapy. Front. Oncol. 2023, 13, 1201500. [Google Scholar] [CrossRef] [PubMed]
- Heylmann, D.; Ponath, V.; Kindler, T.; Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021, 11, 2478. [Google Scholar] [CrossRef] [PubMed]
- Philippé, J.; Louagie, H.; Thierens, H.; Vral, A.; Cornelissen, M.; De Ridder, L. Quantification of apoptosis in lymphocyte subsets and effect of apoptosis on apparent expression of membrane antigens. Cytometry 1997, 29, 242–249. [Google Scholar] [CrossRef]
- Wilkins, R.C.; Kutzner, B.C.; Truong, M.; McLean, J.R.N. The effect of the ratio of CD4 + to CD8 + T-cells on radiation-induced apoptosis in human lymphocyte subpopulations. Int. J. Radiat. Biol. 2002, 78, 681–688. [Google Scholar] [CrossRef]
- Horn, S.; Barnard, S.; Brady, D.; Prise, K.M.; Rothkamm, K. Combined analysis of gamma-H2AX/53BP1 foci and caspase activation in lymphocyte subsets detects recent and more remote radiation exposures. Radiat. Res. 2013, 180, 603–609. [Google Scholar] [CrossRef]
- Chambers, K.A.; Harrington, N.P.; Ross, W.M.; Filion, L.G. Relative alterations in blood mononuclear cell populations reflect radiation injury in mice. Cytometry 1998, 31, 45–52. [Google Scholar] [CrossRef]
- Harrington, N.P.; Chambers, K.A.; Ross, W.M.; Filion, L.G. Radiation damage and immune suppression in splenic mononuclear cell populations. Clin. Exp. Immunol. 1997, 107, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Bogdándi, E.N.; Balogh, A.; Felgyinszki, N.; Szatmári, T.; Persa, E.; Hildebrandt, G.; Sáfrány, G.; Lumniczky, K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat. Res. 2010, 174, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Bornschlegl, S.; Park, S.S.; Gastineau, D.A.; Roberts, L.R.; Dietz, A.B.; Hallemeier, C.L. Comprehensive assessment of circulating immune cell populations in response to stereotactic body radiation therapy in patients with liver cancer. Adv. Radiat. Oncol. 2017, 2, 540–547. [Google Scholar] [CrossRef]
- Eckert, D.; Rapp, F.; Tsedeke, A.T.; Molendowska, J.; Lehn, R.; Langhans, M.; Fournier, C.; Rödel, F.; Hehlgans, S. ROS- and Radiation Source-Dependent Modulation of Leukocyte Adhesion to Primary Microvascular Endothelial Cells. Cells 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.L.; Sukhina, A.S.; Seed, T.M.; Manley, N.R.; Sempowski, G.D.; van den Brink, M.R.; Smithey, M.J.; Nikolich-Žugich, J. Histone deacetylation critically determines T cell subset radiosensitivity. J. Immunol. 2014, 193, 1451–1458. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Zhang, J.; Xue, K.; Ma, X.; Yu, B.; Jin, X. Pyroptotic cell death: An emerging therapeutic opportunity for radiotherapy. Cell Death Discov. 2024, 10, 32. [Google Scholar] [CrossRef]
- Uhl, L.F.K.; Cai, H.; Oram, S.L.; Mahale, J.N.; MacLean, A.J.; Mazet, J.M.; Piccirilli, T.; He, A.J.; Lau, D.; Elliott, T.; et al. Interferon-γ couples CD8+ T cell avidity and differentiation during infection. Nat. Commun. 2023, 14, 6727. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. How diverse--CD4 effector T cells and their functions. J. Mol. Cell Biol. 2009, 1, 20–36. [Google Scholar] [CrossRef]
- Vanbuskirk, A.; Oberyszyn, T.M.; Kusewitt, D.F. Depletion of CD8+ or CD4+ lymphocytes enhances susceptibility to transplantable ultraviolet radiation-induced skin tumours. Anticancer. Res. 2005, 25, 1963–1967. [Google Scholar]
- Simon, S. Dynamic Regulation of CD4+ Regulatory T Cells by Radiation Treatment. Ph.D. Thesis, Georgia State University, Atlanta, GA, USA, December 2019. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yin, C.F.; Qin, Q.H.; Dong, N.; Zhu, X.M.; Sheng, Z.Y.; Zhang, Q.H.; Yao, Y.M. Effect of Regulatory T Cells on Promoting Apoptosis of T Lymphocyte and Its Regulatory Mechanism in Sepsis. J. Interferon Cytokine Res. 2015, 35, 969–980. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Yoon, M.S.; Pham, C.T.; Phan, M.T.; Shin, D.J.; Jang, Y.Y.; Park, M.H.; Kim, S.K.; Kim, S.; Cho, D. Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy 2016, 18, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef]
- Muraro, E.; Furlan, C.; Avanzo, M.; Martorelli, D.; Comaro, E.; Rizzo, A.; Fae, D.A.; Berretta, M.; Militello, L.; Del Conte, A.; et al. Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer. Front. Immunol. 2017, 8, 1476. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Tang, J.; Cao, M. Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment. Front. Immunol. 2022, 13, 1074477. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Ornelles, D.A.; Mitchell, L.M.; Brzoza-Lewis, K.L.; Hiltbold, E.M. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J. Immunol. 2008, 181, 8576–8584. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, X.; Tong, Y. Interleukin-12 in multimodal tumor therapies for induction of anti-tumor immunity. Discov. Oncol. 2024, 15, 170. [Google Scholar] [CrossRef]
- Thierfelder, W.E.; van Deursen, J.M.; Yamamoto, K.; Tripp, R.A.; Sarawar, S.R.; Carson, R.T.; Sangster, M.Y.; Vignali, D.A.; Doherty, P.C.; Grosveld, G.C.; et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 1996, 382, 171–174. [Google Scholar] [CrossRef]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8+ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Seong, J.; Oh, H.J.; Koom, W.S.; Choi, K.J.; Yun, C.O. A novel combination treatment of armed oncolytic adenovirus expressing IL-12 and GM-CSF with radiotherapy in murine hepatocarcinoma. J. Radiat. Res. 2011, 52, 646–654. [Google Scholar] [CrossRef]
- Wu, C.J.; Tsai, Y.T.; Lee, I.J.; Wu, P.Y.; Lu, L.S.; Tsao, W.S.; Huang, Y.J.; Chang, C.C.; Ka, S.M.; Tao, M.H. Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology 2018, 7, e1477459. [Google Scholar] [CrossRef] [PubMed]
- Gkika, E.; Adebahr, S.; Brenner, A.; Schimek-Jasch, T.; Radicioni, G.; Exner, J.-P.; Rühle, A.; Spohn, S.K.B.; Popp, I.; Zamboglou, C.; et al. Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies. Cancers 2021, 13, 5725. [Google Scholar] [CrossRef] [PubMed]
- Meirovitz, A.; Gross, M.; Cohen, S.; Popovtzer, A.; Barak, V. Effect of irradiation on cytokine production in cancer patients. Int. J. Biol. Markers 2022, 37, 360–367. [Google Scholar] [CrossRef]
- Li, B.; Chen, S.; Lu, H.; Tan, Y. Predictive values of TNF-[alpha], IL-6, IL-10 for radiation pneumonitis. Int. J. Radiat. Res. 2016, 14, 173. [Google Scholar]
- Zakeri, F.; Hirobe, T.; Akbari Noghabi, K. Biological effects of low-dose ionizing radiation exposure on interventional cardiologists. Occup. Med. 2010, 60, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Paun, A.; Kunwar, A.; Haston, C.K. Acute adaptive immune response correlates with late radiation-induced pulmonary fibrosis in mice. Radiat. Oncol. 2015, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Guo, Y.; Wang, L.; Zhang, H.; Wang, S.; Wang, L.; An, L.; Zhou, X.; Li, X.; Yao, C. Recovery Profiles of T-Cell Subsets Following Low-Dose Total Body Irradiation and Improvement with Cinnamon. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1118–1126. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).