Combining Vascular Targeting Agents with Radiation: An Effective Anti-Tumor Treatment but Associated with Radiation-Induced Systemic Toxicity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Tumor Model
2.2. Drug Preparation
2.3. Radiation Treatment
2.4. Treatment Response
2.5. Cytokine Assays
2.6. Data and Statistical Analysis
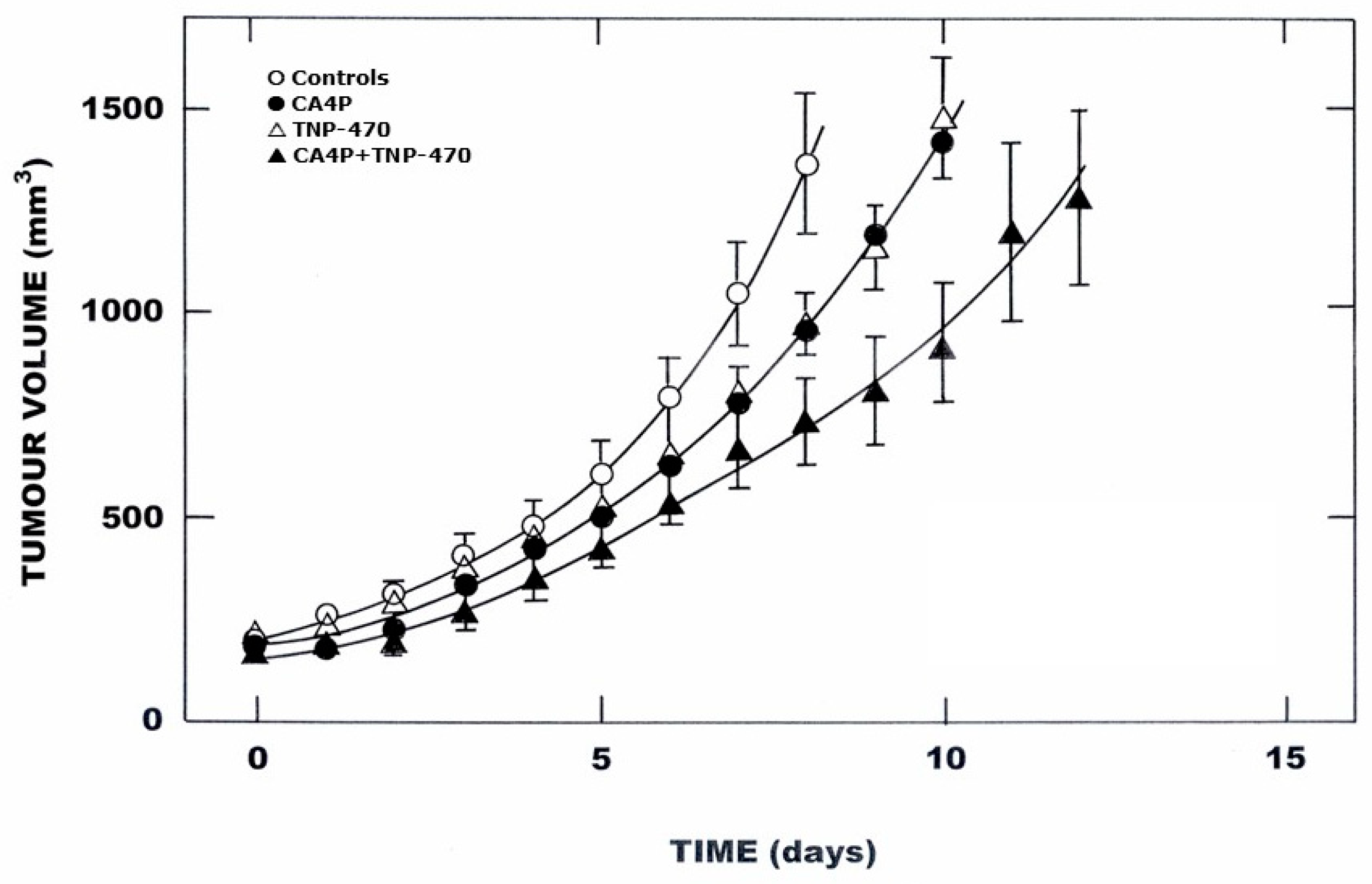
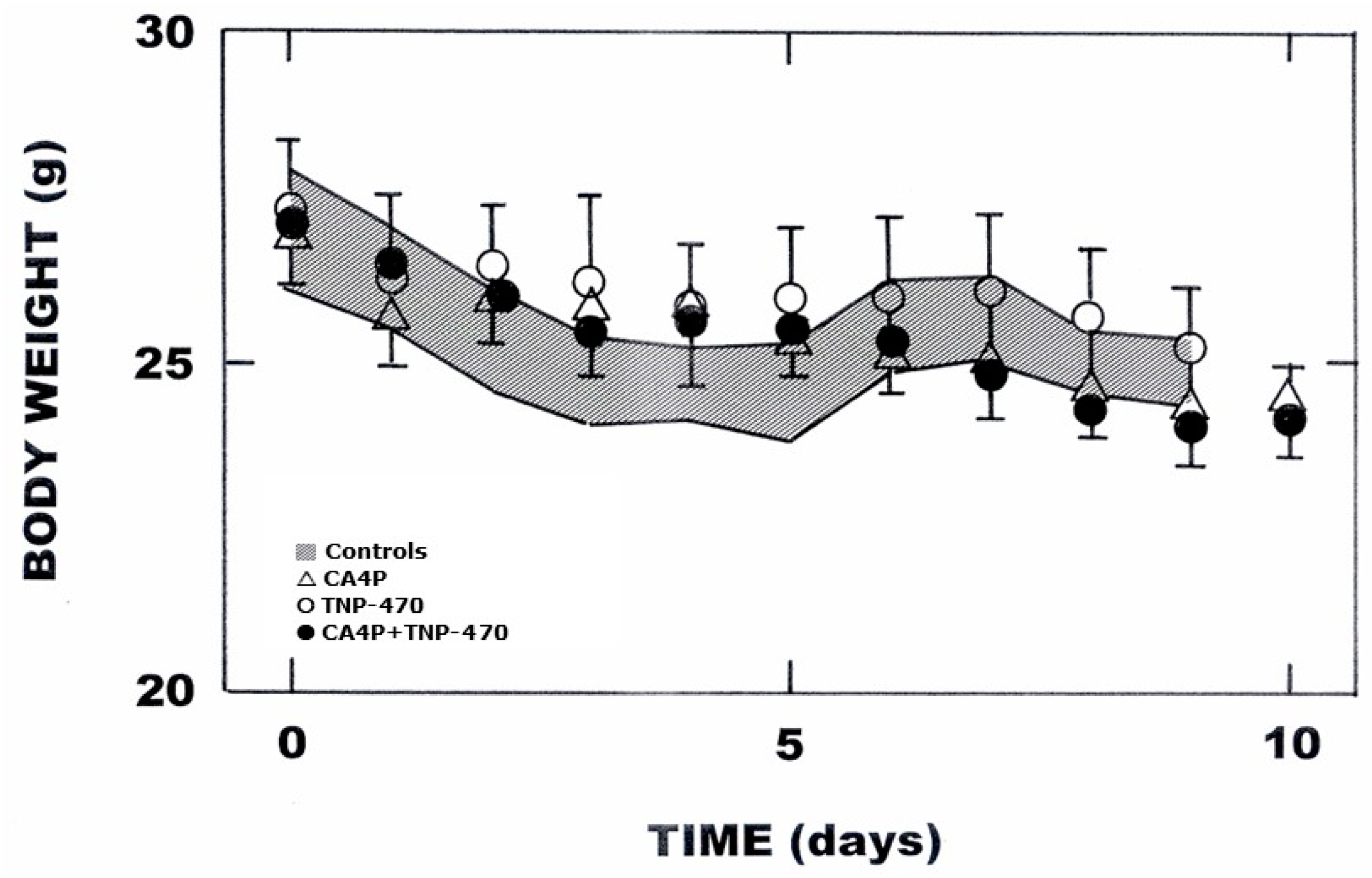
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brem, S.; Brem, H.; Folkman, J.; Finkelstein, D.; Patz, A. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res. 1976, 36, 2807–2812. [Google Scholar] [PubMed]
- Folkman, J. How is blood vessel growth regulated in normal and neoplastic tissue? Cancer Res. 1986, 46, 467–473. [Google Scholar] [PubMed]
- Siemann, D.W.; Bibby, M.C.; Dark, G.; Dicker, A.; Eskens, F.A.L.M.; Horsman, M.R.; Marmé, D.; LoRusso, P.M. Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res. 2005, 11, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Chaplin, D.J.; Horsman, M.R. Realizing the potential of vascular targeted therapy: The rationale for combining vascular disrupting agents and anti-angiogenic agents to treat cancer. Cancer Investig. 2017, 35, 519–534. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Marmé, D. The impact of anti-angiogenic agents on cancer therapy. J. Cancer Res. Clin. Oncol. 2003, 129, 607–620. [Google Scholar] [CrossRef]
- Eskens, F.A.L.M. Angiogenesis inhibitors in clinical development; where are we now and where are we going? Br. J. Cancer 2004, 90, 1–7. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef]
- Abe, J.; Zhou, W.; Takuwa, N.; Taguchi, J.; Kurokawa, K.; Kumada, M.; Takuwa, Y. A fumagillin derivative angiogenesis inhibitor, AGM-1470, inhibits activation of cyclin-dependent kinases and phosphorylation of retinoblastoma gene product but not protein tyrosyl phosphorylation or protooncogene expression in vascular endothelial cells. Cancer Res. 1994, 54, 3407–3412. [Google Scholar] [PubMed]
- Satchi-Fainaro, R.; Mamluk, R.; Wang, L.; Short, S.M.; Nagy, J.A.; Feng, D.; Dvorak, A.M.; Dvorak, H.F.; Puder, M.; Mukhopadhyay, D.; et al. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, carplostatin. Cancer Cell 2005, 7, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Collen, D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. N. Y. Acad. Sci. 2000, 902, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Claesson-Welsh, L. VEGF receptor signal transduction. Sci. STKE 2001, 2001, re21. [Google Scholar] [CrossRef]
- Horsman, M.R.; Siemann, D.W. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006, 66, 11520–11539. [Google Scholar] [CrossRef]
- Denekamp, J.; Hill, S. Angiogenic attack as a therapeutic strategy for cancer. Radiother. Oncol. 1991, 20, 103–112. [Google Scholar] [CrossRef]
- Thorpe, P.E. Vascular targeting agents as cancer therapeutics. Clin. Cancer Res. 2004, 10, 415–427. [Google Scholar] [CrossRef]
- Siemann, D.W.; Chaplin, D.J.; Walicke, P.A. A review and update of the current status of the vascular-disabling agent combretastatin-A4 phosphate (CA4P). Exp. Opin. Investig. Drugs 2009, 18, 189–197. [Google Scholar] [CrossRef]
- Siemann, D.W.; Horsman, M.R. Vascular targeted therapies in oncology. Cell Tissue Res. 2009, 335, 241–248. [Google Scholar] [CrossRef]
- Vens, C.; Koritzinsky, M.; Wouters, B.G. Irradiation-induced damage and the DNA damage response. In Basic Clinical Radiobiology, 5th ed.; Joiner, M.C., van der Kogel, A.J., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 9–20. [Google Scholar]
- Zeman, E.M. The biological basis of radiation oncology. In Clinical Radiation Oncology, 5th ed.; Tepper, J.E., Foote, R.L., Michalski, J.M., Eds.; Elsevier: Philadelphia, PA, USA, 2021; pp. 2–38. [Google Scholar]
- Horsman, M.R.; Murata, R. Combination of vascular targeting agents with thermal and radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1518–1523. [Google Scholar] [CrossRef]
- Wittenborn, T.R.; Horsman, M.R. Targeting tumour hypoxia to improve outcome of stereotactic radiotherapy. Acta Oncol. 2015, 54, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Breidahl, T.; Nielsen, F.U.; Stodkilde-Jorgensen, H.; Maxwell, R.J.; Horsman, M.R. The effects of the vascular disrupting agents combretastatin A-4 disodium phosphate, 5,6-dimethylxanthenone-4-acetic acid and ZD6126 in a murine tumour: A comparative assessment using MRI and MRS. Acta Oncol. 2006, 45, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Nishimura, Y.; Hiraoka, M. An antiangiogenic agent (TNP-470) inhibited reoxygenation during fractionated radiotherapy of murine mammray carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 1107–1113. [Google Scholar] [CrossRef]
- Horsman, M.R.; Chaplin, D.J.; Overgaard, J. Combination of nicotinamide and hyperthermia to eliminate radio-resistant chronically and acutely hypoxic tumour cells. Cancer Res. 1990, 50, 7430–7436. [Google Scholar]
- Tozer, G.M.; Kanthou, C.; Baguley, B.C. Disrupting tumour blood vessels. Nat. Rev. Cancer 2005, 5, 423–435. [Google Scholar] [CrossRef]
- Ching, L.M.; Xu, Z.F.; Gummer, B.H.; Palmer, B.D.; Joseph, W.R.; Baguley, B.C. Effect of thalidomide on tumour necrosis factor production and anti-tumour activity induced by 5,6-dimethylxanthenone-4-acetic acid. Br. J. Cancer 1995, 72, 339–343. [Google Scholar] [CrossRef]
- Mysliwski, A.; Bigda, J.; Koszalka, P.; Szmit, E. Synergistic effect of the angiogenesis inhibitor TNP-470 and tumor necrosis factor (TNF) on Bomirski Ab melanoma in hamsters. Anticancer. Res. 2000, 20, 4643–4647. [Google Scholar]
- Siemann, D.W.; Shi, W. Efficacy of combined antiangiogenic and vascular disrupting agents in treatment of solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1233–1240. [Google Scholar] [CrossRef]
- Siemann, D.W.; Shi, W. Dual targeting of tumor vasculature: Combining avastin and vascular disrupting agents (CA4P or Oxi4503). Anticancer. Res. 2008, 28, 2027–2031. [Google Scholar]
- Landuyt, W.; Ahmed, B.; Nuyts, S.; Theys, J.; Op De Beeck, M.; Runders, A.; Anné, J.; van Oosterom, A.; Van Der Bogaert, W.; Lambin, P. In vivo antitumor effect of vascular targeting combined with either ionizing radiation or anti-angiogenesis treatment. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.B.; Busk, M.; Horsman, M.R. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol. 2013, 52, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.B.; Busk, M.; Bertelsen, L.B.; Laustsen, C.; Munk, O.L.; Nielsen, T.; Wittenborn, T.R.; Bussink, J.; Lok, J.; Stødkilde-Jørgensen, H.; et al. The potential of hyperpolarized 13C magnetic resonance spectroscopy to monitor the effect of combretastatin based vascular disrupting agents. Acta Oncol. 2017, 56, 1626–1633. [Google Scholar] [CrossRef]
- Dark, G.D.; Hill, S.A.; Prise, V.E.; Tozer, G.M.; Pettit, G.R.; Chaplin, D.J. Combretastatin A-4, an agent that displays potent and selective toxicity towrads tumor vasculature. Cancer Res. 1997, 57, 1829–1834. [Google Scholar] [PubMed]
- Lash, C.J.; Li, A.E.; Rutland, M.; Baguley, B.C.; Zwi, L.J.; Wilson, W.R. Enhancement of the anti-tumour effects of the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) by the combination with 5-hydroxytryptamine and bioreductive drugs. Br. J. Cancer 1998, 78, 439–445. [Google Scholar] [CrossRef]
- Li, L.; Rojiani, A.; Siemann, D.W. Targeting the tumor vasculature with combretastatin A-4 disodium phosphate: Effects on radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 899–903. [Google Scholar] [CrossRef]
- Siemann, D.W.; Rojiani, A.M. Enhancement of radiation therapy by the novel vascular targeting agent ZD6126. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 164–171. [Google Scholar] [CrossRef]
- Teicher, B.A.; Holden, S.A.; Ara, G.; Alvarez Sotomayor, E.; Huang, Z.D.; Chen, Y.-N.; Brem, H. Potentiation of cytotoxic cancer therapies by TNP-470 alone and with other anti-angiogenic agents. Int. J. Cancer 1994, 57, 920–925. [Google Scholar] [CrossRef]
- Lund, E.L.; Bastholm, L.; Kristjansen, P.E.G. Therapeutic synergy of TNP-470 and ionizing radiation: Effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin. Cancer Res. 2000, 6, 971–978. [Google Scholar]
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Winkler, F.; Kozin, S.V.; Tong, R.; Chae, S.-S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Dai, Y.; Horsman, M.R. Hypoxia, metastasis, and antiangiogenic therapies. In Hypoxia and Cancer: Biological Implications and Therapeutic Opportunities; Melillo, G., Ed.; Springer: New York, NY, USA, 2014; pp. 205–227. [Google Scholar]
- Teicher, B.A.; Holden, S.A.; Ara, G.; Dupuis, N.P.; Liu, F.; Yuan, J.; Ikebe, M.; Kakeji, Y. Influence of an anti-angiogenic treatment on 9L gliosarcoma: Oxygenation and response to cytotoxic therapy. Int. J. Cancer 1995, 61, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.D.; Adams, G.E.; Hewitt, H.B.; Jones, W.B.; Watts, M.E. A post effect of oxygen in irradiated bacteria: A submillisecond fast mixing study. Radiat. Res. 1973, 54, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Zips, D.; Eicheler, W.; Geyer, P.; Hessel, F.; Dörfler, A.; Thames, H.D.; Haberey, M.; Baumann, M. Enhanced susceptibility of irradiated tumor vessels to vascular endothelial growth factor receptor tyrosine kinase inhibition. Cancer Res. 2005, 65, 5374–5379. [Google Scholar] [CrossRef]
- Eskens, F.A.L.M.; Verweij, J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur. J. Cancer 2006, 42, 3127–3139. [Google Scholar] [CrossRef]
- Subbiah, I.M.; Lenihan, D.J.; Tsimberidou, A.M. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist 2011, 16, 1120–1130. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, B.-Y.; Yang, R.; Xia, L.-Y.; Fan, A.-L.; Chu, Y.-C.; Wang, L.-J.; Wang, Z.-C.; Jiang, A.-Q.; Zhu, H.-L. A class of novel tubulin polymerization inhibitors exert effective anti-tumor activity via mitotic catastrophe. Eur. J. Med. Chem. 2019, 163, 896–910. [Google Scholar] [CrossRef]
- Tang, Z.; Xiong, D.; Song, J.; Ye, M.; Liu, J.; Wang, Z.; Zhang, L.; Xiao, X. Antitumor drug combretastatin-A4 phosphate aggravates the symptoms of dextran sulfate sodium-induced ulcerative colitis in mice. Front. Pharmacol. 2020, 11, 339. [Google Scholar] [CrossRef]
- Drevs, J.; Hofmann, I.; Hugenschmidt, H.; Wittig, C.; Majar, H.; Müller, M.; Wood, J.; Martiny-Baron, G.; Unger, C.; Marmé, D. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000, 60, 4819–4824. [Google Scholar]
- Cernaianu, G.; Frank, S.; Erbstösser, K.; Leonhardt, S.; Cross, M.; McIvor, Z.; Scholz, G.; Dansranjavin, T.; Celik, I.; Tannapfel, A.; et al. TNP-470 fails to block the onset of angiogenesis and early tumor establishment in an intravital minimal disease model. Int. J. Color. Dis. 2006, 21, 143–154. [Google Scholar] [CrossRef]
- Emoto, M.; Ishiguro, M.; Iwasaki, H.; Kikuchi, M.; Kawarabayashi, T. Effect of angiogenesis inhibitor TNP-470 on the growth, blood flow, and microvessel density in xenografts of human uterine carcinosarcoma in nude mice. Gynecol. Oncol. 2003, 89, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Brach, M.A.; Gruss, H.J.; Kaisho, T.; Asano, Y.; Hirano, T.; Herrmann, F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa B. J. Biol. Chem. 1993, 268, 8466–8472. [Google Scholar] [CrossRef] [PubMed]
- Beetz, A.; Messer, G.; Oppel, T.; van Beuningen, D.; Peter, R.U.; Kind, P. Induction of interleukin 6 by ionizing radiation in a human epithelial cell line: Control by corticosteroids. Int. J. Radiat. Biol. 1997, 72, 33–43. [Google Scholar] [CrossRef]
- Hong, A.; Zhang, M.; Leigh, B.; Stevens, G. Induction of interleukin-6 and oncostatin M by radiation in Kaposi’s sarcoma cell lines. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 533–540. [Google Scholar] [CrossRef]
- Baselet, B.; Belmans, N.; Coninx, E.; Lowe, D.; Janssen, A.; Michaux, A.; Tabury, K.; Raj, K.; Quintens, R.; Benotmane, M.A.; et al. Functional gene analysis reveals cell cycle changes and infammation in endothelial cells irradiated with a single X-ray dose. Front. Pharmacol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Brøndum, L.; Sørensen, B.S.; Eriksen, J.G.; Mortensen, L.S.; Lønbro, S.; Overgaard, J.; Alsner, A. An evaluation of multiplex bead-based analysis of cytokines and soluble proteins in archived lithium heparin plasma, EDTA plasma and serum samples. Scand. J. Clin. Lab. Investig. 2016, 76, 601–611. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Links between innate immunity and normal tissue radiobiology. Radiat. Res. 2010, 173, 406–417. [Google Scholar] [CrossRef]
- Rubin, P.; Johnston, C.J.; Williams, J.P.; McDonald, S.; Finkelstein, J.N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 99–109. [Google Scholar] [CrossRef]


| IL-1b | IL-2 | IL-6 | IL-10 | IL-12 p40 | IL-12 p70 | IL-13 | IFN-γ | GM-CSF | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | 1.00 ** (0.25) | 1.00 (0.22) | 1.00 (0.09) | 1.00 (0.41) | 1.00 (0.05) | 1.00 (0.22) | 1.00 (0.17) | 1.00 (0.60) | 1.00 (0.08) | 1.00 (0.15) |
| 1 h after RT | 0.58 (0.24) | 0.57 (0.20) | 1.50 † (0.14) | 2.27 (1.43) | 0.83 † (0.06) | 0.55 (0.08) | 0.74 (0.07) | 0.45 (0.18) | 0.79 (0.07) | 0.60 (0.12) |
| 3 h after RT | 0.40 † (0.11) | 0.86 (0.51) | 0.76 † (0.05) | 1.41 (1.11) | 1.09 (0.05) | 0.47 (0.14) | 0.88 (0.08) | 1.44 (1.40) | 0.83 (0.10) | 0.77 (0.13) |
| 6 h after RT | 0.60 (0.10) | 0.48 (0.13) | 0.90 (0.10) | 1.13 (0.36) | 0.98 (0.05) | 0.71 (0.21) | 0.89 (0.15) | 0.63 (0.49) | 0.92 (0.08) | 0.82 (0.12) |
| 24 h after RT | 1.40 (0.65) | 0.76 (0.34) | 0.73 (0.10) | 0.87 (0.44) | 0.83 † (0.06) | 0.56 (0.12) | 0.76 (0.10) | 0.67 (0.32) | 1.01 (0.10) | 0.97 (0.14) |
| 72 h after RT | 1.38 (0.72) | 0.48 (0.15) | 2.29 (1.68) | 0.52 (0.18) | 0.79 † (0.06) | 1.14 (0.32) | 0.75 (0.10) | 0.67 (0.44) | 0.98 (0.10) | 1.05 (0.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, M.; Murata, R.; Brøndum, L.; Ehrnrooth, E.; Sørensen, B.S.; Horsman, M.R. Combining Vascular Targeting Agents with Radiation: An Effective Anti-Tumor Treatment but Associated with Radiation-Induced Systemic Toxicity. Radiation 2024, 4, 325-335. https://doi.org/10.3390/radiation4040024
Nomura M, Murata R, Brøndum L, Ehrnrooth E, Sørensen BS, Horsman MR. Combining Vascular Targeting Agents with Radiation: An Effective Anti-Tumor Treatment but Associated with Radiation-Induced Systemic Toxicity. Radiation. 2024; 4(4):325-335. https://doi.org/10.3390/radiation4040024
Chicago/Turabian StyleNomura, Miwako, Rumi Murata, Line Brøndum, Eva Ehrnrooth, Brita S. Sørensen, and Michael R. Horsman. 2024. "Combining Vascular Targeting Agents with Radiation: An Effective Anti-Tumor Treatment but Associated with Radiation-Induced Systemic Toxicity" Radiation 4, no. 4: 325-335. https://doi.org/10.3390/radiation4040024
APA StyleNomura, M., Murata, R., Brøndum, L., Ehrnrooth, E., Sørensen, B. S., & Horsman, M. R. (2024). Combining Vascular Targeting Agents with Radiation: An Effective Anti-Tumor Treatment but Associated with Radiation-Induced Systemic Toxicity. Radiation, 4(4), 325-335. https://doi.org/10.3390/radiation4040024






