Potential Effects of Anthropogenic Radiofrequency Radiation on Cetaceans
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Stranding Types and Causes
1.1.1. Strandings with Direct Human Intervention
1.1.2. Strandings with No Direct Human Intervention
1.2. Spatial Distribution and Numerical Trends of Strandings
1.3. Mass Live Strandings without a Known Cause
1.4. Proposed Explanations for Mass Live Strandings
1.5. Aims and Objectives
2. Methods
3. Results
4. Discussion
4.1. Magnetoreception in Cetaceans and Its Involvement in Mass Live Strandings
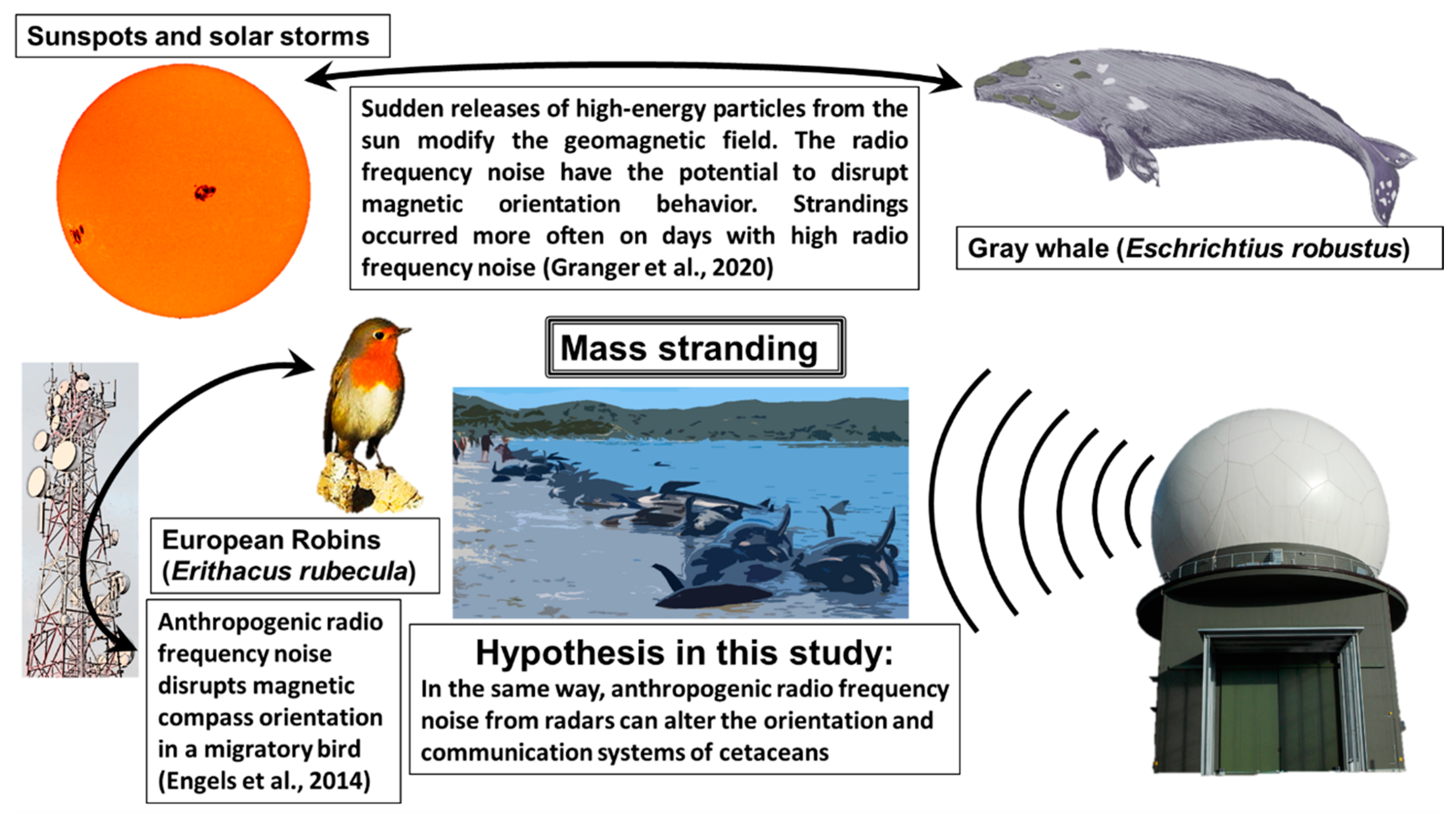
4.2. Anthropogenic RFR May Have the Same Effects
4.3. Plausible Action Mechanisms
4.3.1. The Thermoelastic Expansion Mechanism
4.3.2. Delphinid Vocalizations
4.3.3. Interference with Echolocation Systems
4.3.4. Interference with the Pulse Communication Systems of Odontocetes
4.3.5. Other Possible Mechanisms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bearzi, G.; Pierantonio, N.; Affronte, M.; Holcer, D.; Nicola MA, I.O.; Di Sciara, G.N. Overview of sperm whale Physeter macrocephalus mortality events in the Adriatic Sea, 1555–2009. Mammal Rev. 2011, 41, 276–293. [Google Scholar] [CrossRef]
- Simmonds, M.P. The meaning of cetacean strandings. Bull. Inst. R. Sci. Nat. Belg. Biol. 1997, 67, 29–34. [Google Scholar]
- Arbelo, M.; de los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Fernández, A. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999−2005). Dis. Aquat. Org. 2013, 103, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.; Walkowicz, L.; Fitak, R.; Johnsen, S. Gray whales strand more often on days with increased levels of atmospheric radio-frequency noise. Curr. Biol. 2020, 30, R155–R156. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.J.; Coughran, D.K. Three decades of cetacean strandings in Western Australia: 1981 to 2010. J. R. Soc. West. Aust. 2012, 95, 63. [Google Scholar]
- Cordes, D.O. The causes of whale strandings. N. Z. Vet. J. 1982, 30, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, D.E. Mass strandings of toothed whales (Odontoceti) as a population phenomenon. Sci. Rep. Whales Res. Inst. 1982, 34, 1–47. [Google Scholar]
- Mazzuca, L.; Atkinson, S.; Keating, B.; Nitta, E. Cetacean mass strandings in the Hawaiian Archipelago, 1957–1998. Aquat. Mamm. 1999, 25, 105–114. [Google Scholar]
- Pyenson, N.D. Carcasses on the coastline: Measuring the ecological fidelity of the cetacean stranding record in the eastern North Pacific Ocean. Paleobiology 2010, 36, 453–480. [Google Scholar] [CrossRef]
- Augé, A.A.; Oyley, H.; Rendell, N.; Frans, V.F. Spatial distribution of cetacean strandings in the Falkland Islands to define monitoring opportunities. J. Cetacean Res. Manag. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Obusan MC, M.; Rivera, W.L.; Siringan MA, T.; Aragones, L.V. Stranding events in the Philippines provide evidence for impacts of human interactions on cetaceans. Ocean. Coast. Manag. 2016, 134, 41–51. [Google Scholar] [CrossRef]
- Leeney, R.H.; Amies, R.; Broderick, A.C.; Witt, M.J.; Loveridge, J.; Doyle, J.; Godley, B.J. Spatio-temporal analysis of cetacean strandings and bycatch in a UK fisheries hotspot. Biodivers. Conserv. 2008, 17, 2323. [Google Scholar] [CrossRef]
- Tonay, A.M.; Dede, A.; Öztürk, A.A.; Öztürk, B. Cetacean strandings in the Turkish Western Black Sea coast during 2007–2009. J. Black Sea/Mediterr. Environ. 2012, 18, 246–250. [Google Scholar]
- Karaa, S.; Bradai, M.N.; Jribi, I.; El Hili, H.A.; Bouain, A. Status of cetaceans in Tunisia through analysis of stranding data from 1937 to 2009. Mammalia 2012, 76, 21–29. [Google Scholar] [CrossRef]
- Peltier, H.; Authier, M.; Deaville, R.; Dabin, W.; Jepson, P.D.; van Canneyt, O.; Pierre, D.; Ridoux, V. Small cetacean bycatch as estimated from stranding schemes: The common dolphin case in the northeast Atlantic. Environ. Sci. Policy 2016, 63, 7–18. [Google Scholar] [CrossRef]
- Vianna TD, S.; Loch, C.; Castilho PV, D.; Gaidzinski, M.C.; Cremer, M.J.; Simões-Lopes, P.C. Review of thirty-two years of toothed whale strandings in Santa Catarina, southern Brazil (Cetacea: Odontoceti). Zoologia 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Alava, J.J.; Barragan, M.J.; Castro, C.; Carvajal, R. A note on strandings and entanglements of humpback whales (Megaptera novaeangliae) in Ecuador. J. Cetacean Res. Manag. 2005, 7, 163. [Google Scholar] [CrossRef]
- Meynecke, J.O.; Meager, J.J. Understanding strandings: 25 years of humpback whale (Megaptera novaeangliae) strandings in Queensland, Australia. J. Coast. Res. 2016, 75, 897–901. [Google Scholar] [CrossRef]
- Song, K.J. Cetacean Strandings in Korean Waters 1. Pac. Sci. 2016, 70, 35–44. [Google Scholar] [CrossRef]
- Fernández, A.; Edwards, J.F.; Rodriguez, F.; De Los Monteros, A.E.; Herraez, P.; Castro, P.; Arbelo, M. “Gas and fat embolic syndrome” involving a mass stranding of beaked whales (Family Ziphiidae) exposed to anthropogenic sonar signals. Vet. Pathol. 2005, 42, 446–457. [Google Scholar] [CrossRef]
- Rommel, S.A.; Costidis, A.M.; Fernandez, A.; Jepson, P.D.; Pabst, D.A.; Houser, D.S.; Barros, N.B. Elements of beaked whale anatomy and diving physiology and some hypothetical causes of sonar-related stranding. J. Cetacean Res. Manag. 2006, 7, 189–209. [Google Scholar] [CrossRef]
- Weilgart, L.S. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Parsons EC, M.; Dolman, S.J.; Wright, A.J.; Rose, N.A.; Burns, W.C.G. Navy sonar and cetaceans: Just how much does the gun need to smoke before we act? Mar. Pollut. Bull. 2008, 56, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Gisiner, R.C.; Ketten, D.R.; Hammock, J.A.; Johnson, C.; Tyack, P.L.; Mead, J. Beaked whale strandings and naval exercises. Aquat. Mamm. 2009, 35, 452–472. [Google Scholar] [CrossRef]
- Filadelfo, R.; Mintz, J.; Michlovich, E.; D’Amico, A.; Tyack, P.L.; Ketten, D.R. Correlating military sonar use with beaked whale mass strandings: What do the historical data show? Aquat. Mamm. 2009, 35, 435–444. [Google Scholar] [CrossRef]
- Jepson, P.D.; Arbelo, M.; Deaville, R.; Patterson IA, P.; Castro, P.; Baker, J.R.; Fernández, A. Gas-bubble lesions in stranded cetaceans. Nature 2003, 425, 575–576. [Google Scholar] [CrossRef]
- Evans, P.G.; Hammond, P.S. Monitoring cetaceans in European waters. Mammal Rev. 2004, 34, 131–156. [Google Scholar] [CrossRef]
- Yang, W.C.; Chou, L.S.; Jepson, P.D.; Brownell, R.L.; Cowan, D.; Chang, P.H.; Wang, P.J. Unusual cetacean mortality event in Taiwan, possibly linked to naval activities. Vet. Rec. 2008, 162, 184–186. [Google Scholar] [CrossRef]
- Aragones, L.V.; Roque MA, A.; Flores, M.B.; Encomienda, R.P.; Laule, G.E.; Espinos, B.G.; Braun, R.C. The Philippine marine mammal strandings from 1998 to 2009: Animals in the Philippines in peril? Aquat. Mamm. 2010, 36, 219. [Google Scholar] [CrossRef]
- Engel, M.H.; Marcondes, M.C.; Martins, C.C.; Luna, F.O.; Lima, R.P.; Campos, A. Are seismic surveys responsible for cetacean strandings? An unusual mortality of adult humpback whales in Abrolhos Bank, northeastern coast of Brazil. 2004, (in press). [Google Scholar]
- Walker, R.; Keith, E.; Yankovsky, A.; Odell, D.K. Environmental correlates of cetacean mass stranding sites in Florida. Mar. Mammal Sci. 2005, 21, 327–335. [Google Scholar] [CrossRef]
- Bogomolni, A.L.; Pugliares, K.R.; Sharp, S.M.; Patchett, K.; Harry, C.T.; LaRocque, J.M.; Moore, M. Mortality trends of stranded marine mammals on Cape Cod and southeastern Massachusetts, USA, 2000 to 2006. Dis. Aquat. Org. 2010, 88, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G.; Di Francesco, C.E.; Eleni, C.; Cocumelli, C.; Scholl, F.; Casalone, C.; Leonardi, L. Morbillivirus infection in cetaceans stranded along the Italian coastline: Pathological, immunohistochemical and biomolecular findings. Res. Vet. Sci. 2013, 94, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Guerri, C.; Melero, M.; Esperón, F.; Bellière, E.N.; Arbelo, M.; Crespo, J.L.; Sierra EGarcía-Párraga DSánchez-Vizcaíno, J.M. Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean Sea. BMC Vet. Res. 2013, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Casalone, C.; Mazzariol, S.; Pautasso, A.; Di Guardo, G.; Di Nocera, F.; Lucifora, G.; Cersini, A. Cetacean strandings in Italy: An unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis. Aquat. Org. 2014, 109, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Poje, A.C.; Veit, R.R.; Nganguia, H. Acoustical dead zones and the spatial aggregation of whale strandings. J. Theor. Biol. 2006, 238, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Fire, S.E.; Wang, Z.; Berman, M.; Langlois, G.W.; Morton, S.L.; Sekula-Wood, E.; Benitez-Nelson, C.R. Trophic Transfer of the Harmful Algal Toxin Domoic Acid as a Cause of Death in a Minke Whale (Balaenoptera acutorostrata) Stranding in Southern California. Aquat. Mamm. 2010, 36, 342–350. [Google Scholar] [CrossRef]
- McManus, T.J.; Wapstra, J.E.; Guiler, E.R.; Munday, B.L.; Obendorf, D.L. Cetacean strandings in Tasmania from February 1978 to May 1983. Pap. Proc. R. Soc. Tasman. 1984, 118, 117–135. [Google Scholar] [CrossRef]
- MacLeod, C.D.; Pierce, G.J.; Santos, M.B. Geographic and temporal variations in strandings of beaked whales (Ziphiidae) on the coasts of the UK and the Republic of Ireland from 1800–2002. J. Cetacean Res. Manag. 2004, 6, 79–86. [Google Scholar] [CrossRef]
- Evans, K.; Thresher, R.; Warneke, R.M.; Bradshaw, C.J.; Pook, M.; Thiele, D.; Hindell, M.A. Periodic variability in cetacean strandings: Links to large-scale climate events. Biol. Lett. 2005, 1, 147–150. [Google Scholar] [CrossRef]
- Lloyd, H.B.; Ross, G.A. Long-term trends in cetacean incidents in New South Wales, Australia. Aust. Zool. 2015, 37, 492–500. [Google Scholar] [CrossRef]
- Brabyn, M.W. An Analysis of the New Zealand Whale Stranding Record; Department of Conservation, Head Office: Wellington, New Zealand, 1991. [Google Scholar]
- Liu, M.; Lin, M.; Zhang, P.; Xue, T.; Li, S. An overview of cetacean stranding around Hainan Island in the South China Sea, 1978–2016: Implications for research, conservation and management. Mar. Policy 2019, 101, 147–153. [Google Scholar] [CrossRef]
- Kirschvink, J.L. Geomagnetic sensitivity in cetaceans: An update with live stranding records in the United States. In Sensory Abilities of Cetaceans; Springer: Boston, MA, USA, 1990; pp. 639–649. [Google Scholar]
- Hutchison, Z.L.; Gill, A.B.; Sigray, P.; He, H.; King, J.W. Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci. Rep. 2020, 10, 4219. [Google Scholar] [CrossRef] [PubMed]
- Levitt, B.B.; Lai, H. Biological effects from exposure to electromagnetic radiation emitted by cell tower base stations and other antenna arrays. Environ. Rev. 2010, 18, 369–395. [Google Scholar] [CrossRef]
- Balmori, A. Electrosmog and species conservation. Sci. Total Environ. 2014, 496, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Balmori, A. Anthropogenic radiofrequency electromagnetic fields as an emerging threat to wildlife orientation. Sci. Total Environ. 2015, 518, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Levitt, B.B.; Lai, H.C.; Manville, A.M. Effects of non-ionizing electromagnetic fields on flora and fauna, part 1. Rising ambient EMF levels in the environment. Rev. Environ. Health 2022, 37, 81–122. [Google Scholar] [CrossRef]
- Levitt, B.B.; Lai, H.C.; Manville, A.M. Effects of non-ionizing electromagnetic fields on flora and fauna, Part 2 impacts: How species interact with natural and man-made EMF. Rev. Environ. Health 2022, 37, 327–406. [Google Scholar] [CrossRef]
- Coombs, E.J.; Deaville, R.; Sabin, R.C.; Allan, L.; O’Connell, M.; Berrow, S.; Cooper, N. What can cetacean stranding records tell us? A study of UK and Irish cetacean diversity over the past 100 years. Mar. Mammal Sci. 2019, 35, 1527–1555. [Google Scholar] [CrossRef]
- Berrow, S.D.; Rogan, E. Review of cetaceans stranded on the Irish coast, 1901–1995. Mammal Rev. 1997, 27, 51–75. [Google Scholar] [CrossRef]
- Coughran, D.K.; Gales, N.J.; Smith, H.C. A note on the spike in recorded mortality of humpback whales (Megaptera novaeangliae) in Western Australia. J. Cetacean Res. Manag. 2013, 13, 105–108. [Google Scholar] [CrossRef]
- McGovern, B.; Culloch, R.M.; O’Connell, M.; Berrow, S. Temporal and spatial trends in stranding records of cetaceans on the Irish coast, 2002–2014. J. Mar. Biol. Assoc. UK 2016, 98, 977–989. [Google Scholar] [CrossRef]
- Alvarado-Rybak, M.; Toro, F.; Escobar-Dodero, J.; Kinsley, A.C.; Sepúlveda, M.A.; Capella, J.; Mardones, F.O. 50 Years of Cetacean Strandings Reveal a Concerning Rise in Chilean Patagonia. Sci. Rep. 2020, 10, 9511. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.N.; Asmutis, R.A. Stranding and mortality of humpback whales, Megaptera novaeangliae. Fish. Bull. 1995, 93, 196–205. [Google Scholar]
- Peltier, H.; Baagøe, H.J.; Camphuysen, K.C.; Czeck, R.; Dabin, W.; Daniel, P.; Jepson, P.D. The stranding anomaly as population indicator: The case of harbour porpoise Phocoena phocoena in North-Western Europe. PLoS ONE 2013, 8, e62180. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Santos, M.B.; Pierce, G.J.; González, Á.F.; Valeiras, X.; Guerra, Á. Trends in strandings and by-catch of marine mammals in north-west Spain during the 1990s. J. Mar. Biol. Assoc. UK 2002, 82, 513–521. [Google Scholar] [CrossRef]
- Quiroga, J.M. Primeros desarrollos de tecnología radar en los principales beligerantes de la II Guerra Mundial. Un análisis desde la perspectiva Ciencia, Tecnología y Sociedad. Cienc. Docencia Y Tecnol. 2018, 57, 36–59. [Google Scholar] [CrossRef]
- Pikesley, S.K.; Witt, M.J.; Hardy, T.; Loveridge, J.; Loveridge, J.; Williams, R.; Godley, B.J. Cetacean sightings and strandings: Evidence for spatial and temporal trends? J. Mar. Biol. Assoc. UK 2012, 92, 1809–1820. [Google Scholar] [CrossRef]
- Podesta, M.; DAmico, A.; Pavan, G.; Drougas, A.; Komnenou, A.; Portunato, N. A review of Cuvier’s beaked whale strandings in the Mediterranean Sea. J. Cetacean Res. Manag. 2006, 7, 251. [Google Scholar] [CrossRef]
- Maldini, D.; Mazzuca, L.; Atkinson, S. Odontocete Stranding Patterns in the Main Hawaiian Islands (1937–2002): How Do They Compare with Live Animal Surveys? 1. Pac. Sci. 2005, 59, 55–67. [Google Scholar] [CrossRef]
- Brownell, R.L., Jr.; Yao, C.J.; Lee, C.S.; Wang, M.C. Worldwide Review of Pygmy Killer Whales, Feresa Attenuate, Mass Strandings Reveals Taiwan Hot Spot; U.S. Department of Commerce: Washington, DC, USA, 2009. [Google Scholar]
- Masski, H.; De Stéphanis, R. Cetaceans of the Moroccan coast: Information from a reconstructed strandings database. J. Mar. Biol. Assoc. UK 2018, 98, 1029–1037. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Barreiros, J.P.; Azevedo, J.; Norberto, R. Cetaceans stranded in the Azores during 1992–1996. Arquipélago Ciências Biológicas E Mar. Life Mar. Sci. 1996, 14, 57–65. [Google Scholar]
- Pimper, L.E.; Goodall RN, P.; Gibbons JO RG, E.; Sobral, A.P.; Lockyer, C.H.; Praderi, R. A Review of Strandings of Sperm Whales from the Strait of Magellan to Cape Horn; Paper SC/60/O3 Presented to the International Whaling Commission Scientific Committee; International Whaling Commission Office: Cambridge, UK, 2008; 8p. [Google Scholar]
- Mazzariol, S.; Di Guardo, G.; Petrella, A.; Marsili, L.; Fossi, C.M.; Leonzio, C.; Fernández, A. Sometimes sperm whales (Physeter macrocephalus) cannot find their way back to the high seas: A multidisciplinary study on a mass stranding. PLoS ONE 2011, 6, e19417. [Google Scholar] [CrossRef] [PubMed]
- Borsa, P. Marine mammal strandings in the New Caledonia region, Southwest Pacific. Comptes Rendus Biol. 2006, 329, 277–288. [Google Scholar] [CrossRef]
- Caracappa, S.; Puleio, R.; Loria, G.R.; Gentile, A.; Persichetti, M.F.; Caracappa, G.; Arculeo, M. Stranding patterns of the striped dolphin (Stenella coeruleoalba, Meyen 1833, Delphinidae) along the Sicilian coast (Mediterranean Sea). Mar. Mammal Sci. 2019, 35, 1083–1091. [Google Scholar] [CrossRef]
- Rojo–Nieto, E.; Álvarez–Díaz, P.D.; Morote, E.; Burgos–Martín, M.; Montoto–Martínez, T.; Sáez–Jiménez, J.; Toledano, F. Strandings of cetaceans and sea turtles in the Alboran Sea and Strait of Gibraltar: A long–term glimpse at the north coast (Spain) and the south coast (Morocco). Anim. Biodivers. Conserv. 2011, 34, 151–163. [Google Scholar] [CrossRef]
- Brabyn, M.W.; McLean, I.G. Oceanography and coastal topography of herd-stranding sites for whales in New Zealand. J. Mammal. 1992, 73, 469–476. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; Van Neer, A.; Deaville, R.; Begeman, L.; van de Bildt, M.; van den Brand, J.M.; Brownlow, A.; Czeck, R.; Dabin, W.; ten Doeschate, M.; et al. Beached bachelors: An extensive study on the largest recorded sperm whale Physeter macrocephalus mortality event in the North Sea. PLoS ONE 2018, 13, e0201221. [Google Scholar] [CrossRef]
- Klinowska, M. Cetacean live stranding dates relate to geomagnetic disturbances. Aquat. Mamm. 1986, 11, 109–119. [Google Scholar]
- Klinowska, M. Cetacean live stranding sites relate to geomagnetic topography. Aquat. Mamm. 1985, 1, 27–32. [Google Scholar]
- Walker, M.M.; Kirschvink, J.L.; Ahmed, G.; Dizon, A.E. Evidence that fin whales respond to the geomagnetic field during migration. J. Exp. Biol. 1992, 171, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, K.H.; Jacobsen, S.; Hall, C.; Garthe, S. Solar storms may trigger sperm whale strandings: Explanation approaches for multiple strandings in the North Sea in 2016. Int. J. Astrobiol. 2018, 17, 336–344. [Google Scholar] [CrossRef]
- Burda, H.; Begall, S.; Hart, V.; Malkemper, E.P.; Painter, M.S.; Phillips, J.B. Magnetoreception in Mammals. In The Senses: A Comprehensive Reference; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 421–444. [Google Scholar]
- Vanselow, K.H.; Ricklefs, K. Are solar activity and sperm whale Physeter macrocephalus strandings around the North Sea related? J. Sea Res. 2005, 53, 319–327. [Google Scholar] [CrossRef]
- Ferrari, T.E. Cetacean beachings correlate with geomagnetic disturbances in Earth’s magnetosphere: An example of how astronomical changes impact the future of life. Int. J. Astrobiol. 2017, 16, 163–175. [Google Scholar] [CrossRef]
- Wiltschko, R.; Thalau, P.; Gehring, D.; Nießner, C.; Ritz, T.; Wiltschko, W. Magnetoreception in birds: The effect of radio-frequency fields. J. R. Soc. Interface 2015, 12, 20141103. [Google Scholar] [CrossRef] [PubMed]
- Engels, S.; Schneider, N.L.; Lefeldt, N.; Hein, C.M.; Zapka, M.; Michalik, A.; Elbers, D.; Kittel, A.; Hore, P.J.; Mouritsen, H. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 2014, 509, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Leberecht, B.; Wong, S.Y.; Satish, B.; Döge, S.; Hindman, J.; Venkatraman, L.; Hore, P.J. Upper bound for broadband radiofrequency field disruption of magnetic compass orientation in night-migratory songbirds. Proc. Natl. Acad. Sci. USA 2023, 120, e2301153120. [Google Scholar] [CrossRef]
- Vácha, M.; Půžová, T.; Kvíćalová, M. Radio frequency magnetic fields disrupt magnetoreception in American cockroach. J. Exp. Biol. 2009, 212, 3473–3477. [Google Scholar] [CrossRef]
- Malkemper, P.; Eder, S.H.K.; Begall, S.; Phillips, J.B.; Winklhofer, M.; Hart, V.; Burda, H. Magnetoreception in the wood mouse (Apodemus sylvaticus): Influence of weak frequency-modulated radio frequency fields. Sci. Rep. 2015, 5, 9917. [Google Scholar] [CrossRef]
- Phillips, J.; Painter, M. Small differences in weak electromagnetic fields disrupt magnetic compass orientation of C57 BL/6 mice (Rodentia: Muridae). Lynx Ser. Nova 2022, 53, 219–234. [Google Scholar] [CrossRef]
- Bartos, P.; Netusil, R.; Slaby, P.; Dolezel, D.; Ritz, T.; Vacha, M. Weak radiofrequency fields affect the insect circadian clock. J. R. Soc. Interface 2019, 16, 20190285. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Thalau, P.; Phillips, J.B.; Wiltschko, R.; Wiltschko, W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 2004, 429, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.G.; Mouritsen, H.; Manolopoulos, D.E.; Hore, P.J. Disruption of magnetic compass orientation in migratory birds by radiofrequency electromagnetic fields. Biophys. J. 2017, 113, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, E.; Randolet, J.; DeVault, T.L.; Seamans, T.W.; Blackwell, B.F.; Fernández-Juricic, E. The effects of radar on avian behavior: Implications for wildlife management at airports. Appl. Anim. Behav. Sci. 2015, 171, 241–252. [Google Scholar] [CrossRef]
- Southall, B.L.; Braun, R.C.; Gulland, F.; Heard, A.; Baird, R.W.; Wilkins, S. Hawaiian Melon-Headed Whale (Peponacephala electra) Mass Stranding Event of July 3–4, 2004; NOAA Technical Memorandum NMFS-OPR-31; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2006. [Google Scholar]
- D’Spain, G.L.; D’Amico, A.; Fromm, D.M. Properties of the underwater sound fields during some well documented beaked whale mass stranding events. J. Cetacean Res. Manag. 2006, 7, 223–238. [Google Scholar] [CrossRef]
- Frey, A.H. Auditory system response to radio frequency energy. AeMed 1961, 32, 1140–1142. [Google Scholar]
- Foster, K.R.; Finch, E.D. Microwave hearing: Evidence for thermoacoustic auditory stimulation by pulsed microwaves. Science 1974, 185, 256–258. [Google Scholar] [CrossRef]
- Lin, J.C. Hearing microwaves: The microwave auditory phenomenon. IAPM 2001, 43, 166–168. [Google Scholar] [CrossRef]
- Elder, J.A.; Chou, C.K. Auditory response to pulsed radiofrequency energy. Bioelectromagnetics 2003, 6, 162–173. [Google Scholar] [CrossRef]
- Nicholls, B.; Racey, P.A. Bats avoid radar installations: Could electromagnetic fields deter bats from colliding with wind turbines? PLoS ONE 2007, 2, e297. [Google Scholar] [CrossRef]
- Popper, A.N.; Fay, R.R.; Au, W. Hearing by whales and dolphins. In Springer Handbook of Auditory Research; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000; Volume 12. [Google Scholar]
- Panagopoulos, D.J.; Messini, N.; Karabarbounis, A.; Filippetis, A.L.; Margaritis, L.H. A mechanism for action of oscillating electric fields on cells. Biochem. Biophys. Res. Commun. 2000, 272, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002, 298, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Balmori, A. On the biophysical mechanism of sensing atmospheric discharges by living organisms. Sci. Total Environ. 2017, 599–600, 2026–2034. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Balmori, A.; Chrousos, G.P. On the biophysical mechanism of sensing upcoming earthquakes by animals. Sci. Total Environ. 2020, 717, 136989. [Google Scholar] [CrossRef]
- Balmori, A. Evidence for a health risk by RF on humans living around mobile phone base stations: From radiofrequency sickness to cancer. Environ. Res. 2022, 214, 2. [Google Scholar] [CrossRef]
- Mann, K.; Roschkle, J. Effects of pulsed high-frequency electromagnetic fields on human sleep. Neuropsychobiology 1996, 33, 41–47. [Google Scholar] [CrossRef]
- Beasond, R.C.; Semm, P. Responses of neurons to an amplitude modulated microwave stimulus. Neurosci. Lett. 2002, 33, 175–178. [Google Scholar] [CrossRef]
- Kramarenko, A.V.; Tan, U. Effects of high frequency electromagnetic fields on human EEG: A brain mapping study. Int. J. Neurosci. 2003, 113, 1007–1019. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Juanes, F. The soundscape of the Anthropocene ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Butchart, S.H.; Connor, B.; Culshaw, C.; Dicks, L.V.; Dinsdale, J.; Doran, H.; Entwistle, A.C.; Fleishman, E.; Gibbons, D.W.; et al. A 2018 horizon scan of emerging issues for global conservation and biological diversity. Trends Ecol. Evol. 2018, 33, 47–58. [Google Scholar] [CrossRef] [PubMed]

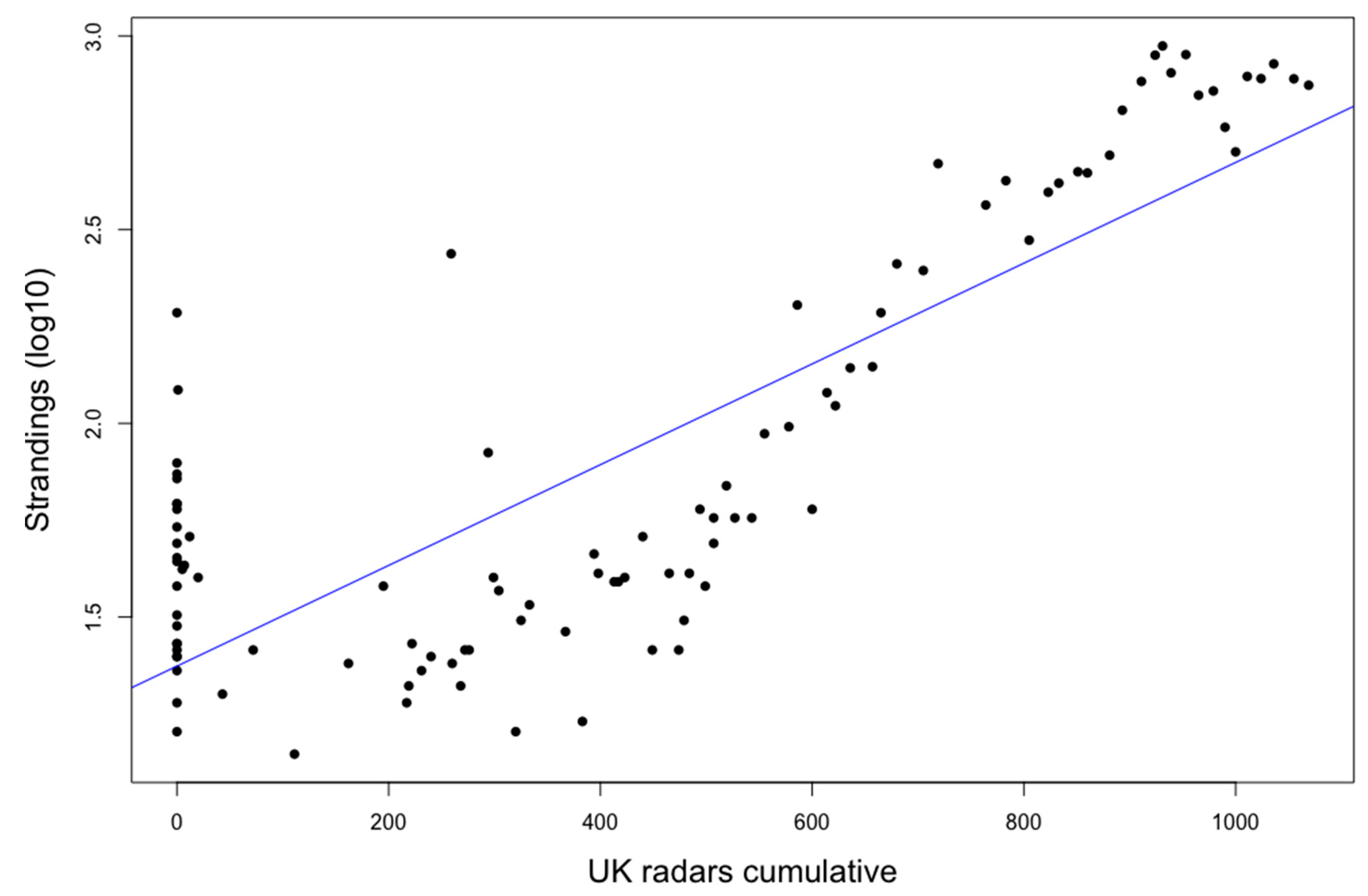
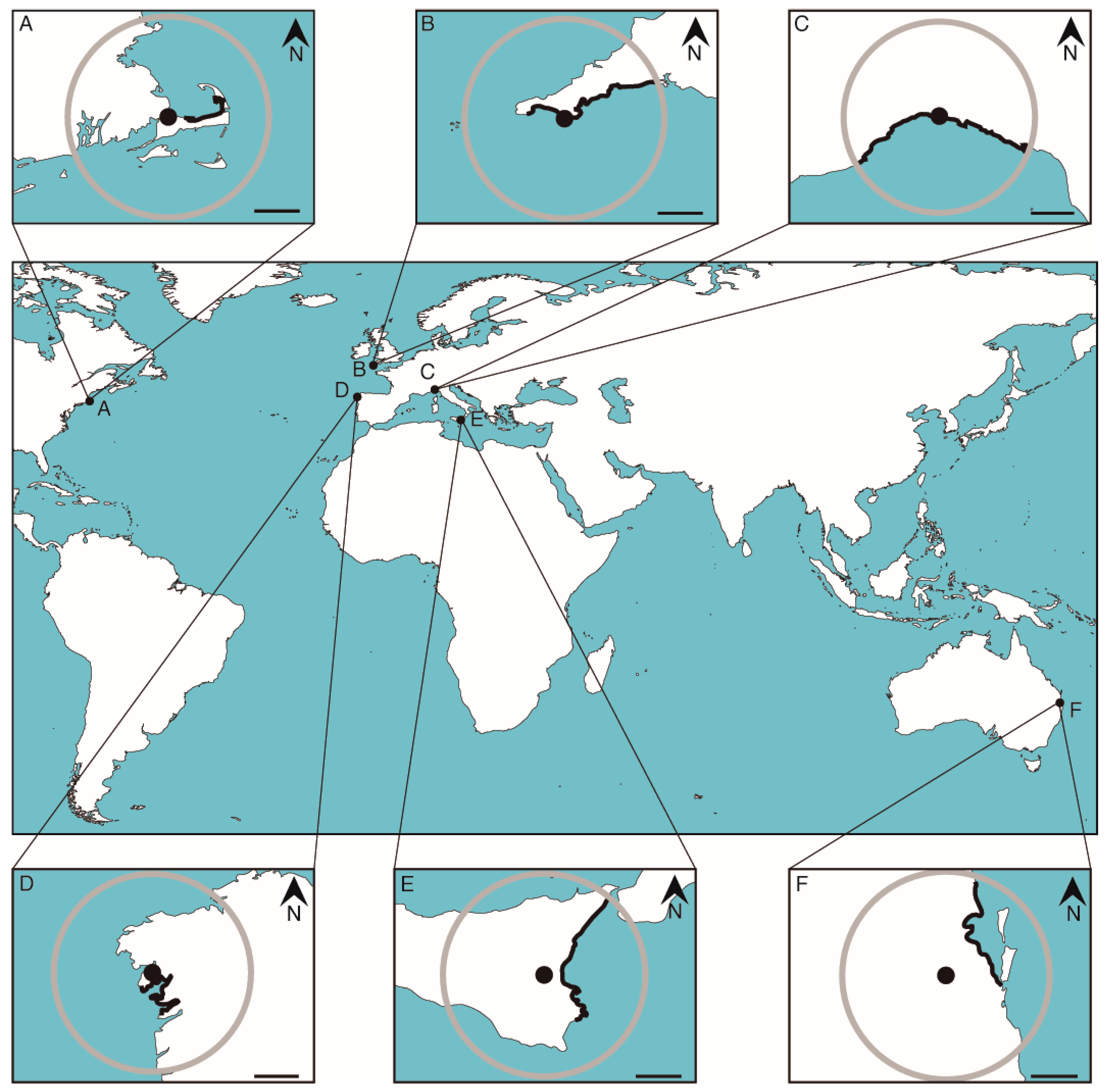
| Reference | Study Species | Time Period | Study Area | Closest Radar(s) Found | Date | Considerations | S | T |
|---|---|---|---|---|---|---|---|---|
| Leeney et al., 2008 [12] | Cetaceans | 1911–2006 | UK (Cornwall and the Isles of Scilly) | Cornwall meteorological radar (Predannack) | 1986 | Yes | Yes | |
| Pikesley et al., 2012 [60] | Cetaceans | 1991–2008 | UK (Cornwall and the Isles of Scilly) | Cornwall meteorological radar (Predannack) | 1986 | Data after radar setup | Yes | |
| Podesta et al., 2006 [61] | Cuvier’s beaked whales | 1803–2003 | Mediterranean Sea | Marconi radar | 1962 | Yes | Yes | |
| Bogomolni et al., 2010 [32] | Marine mammals | 2000–2006 | USA (Massachusetts) | Cape Cod Air Force Station | 1980 | Data after radar setup | Yes | |
| Maldini et al., 2005 [62] | Odontocetes | 1950–2002 | USA (Hawaii, Oahu) | Pearl Harbour base | 1941 | More prospection in that island | ||
| Lloyd and Ross, 2015 [41] | Cetaceans | 1970–2013 | Australia (NSW) | Woolloomooloo base | 1941 | Data after radar setup; scattered stranding hotspots | ||
| D’amico et al., 2009 [24] | Beaked whales | 1874–2015 | Global | Yokosuka naval base | 1951 | Strandings linked to naval sonars | ||
| Brownell et al., 2009 [63] | Pygmy killer whales | 1968–2006 | Taiwan | Tainan and Kaohsiung military bases | - | Without radar running date | ||
| Masski and De Stéphanis 2018 [64] | Cetaceans | 1980–2009 | Morocco | 1: Tarifa radar 2: Casablanca marine base | 1: 2009 2: - | Data before radar setup | ||
| Groom and Coughran 2012 [5] | Cetaceans | 1981–2010 | Australia (WA) | Perth meteorological radar | 2009 | Data before radar setup | ||
| Mcgovern et al., 2016 [54] | Cetaceans | 2002–2014 | Ireland | - | - | Radar not found | ||
| Walker et al., 2005 [31] | Cetaceans | 1977–2001 | USA (Florida) | Key West meteorological radar and naval air station (south Florida) | 2012 | Data before radar setup | ||
| Gonçalves et al., 1996 [65] | Cetaceans | 1992–1996 | Portugal (Azores) | - | - | Radar not found | ||
| Pimper et al., 2008 [66] | Sperm whales | 1977–1981 | Argentina (Tierra del Fuego) | - | - | Radar not found | ||
| Mazzariol et al., 2011 [67] | Sperm whales | 2009 | Italy (Adriatic) | Remote radar squadron and Jacotenente Air Force detachment | 1963 | Only one stranding event after radar setup | ||
| Bearzi et al., 2011 [1] | Sperm whales | 1955–2009 | Italy (Adriatic) | Potenza Picena radar | 1970–1998 | Strandings scattered in time | ||
| Song 2016 [19] | Cetaceans | 1997–2004 | Korea | - | - | Radar not found | ||
| Augé et al., 2018 [10] | Cetaceans | 1980–2015 | UK (Falkland Islands) | Mont Pleasant Air Base | 1986 | Scattered stranding hotspots; reliable only from 2007 | ||
| Aragones et al., 2010 [29] | Marine mammals | 1998–2009 | Philippines | - | - | Radar not found; scattered stranding hotspots | ||
| Borsa 2006 [68] | Marine mammals | 1877–2005 | New Caledonia | - | - | Radar not found | ||
| Caracappa et al., 2018 [69] | Striped dolphins | 2013–2016 | Sicily | Sigonella Naval Air Station | 1959 | Data after radar setup; some morbillivirus effects | Yes | |
| Coughran et al., 2013 [53] | Humpback whales | 1989–2012 | Australia (WA) | - | - | Radar not found | ||
| López et al., 2002 [58] | Marine mammals | 1990–1999 | Spain (Northwest) | Iroite military radar | 1980 | Data after radar setup | Yes | |
| McManus et al., 1984 [38] | Cetacean strandings | 1978–1983 | Tasmania | Hobart Military and Naval Base | - | Without radar running date | ||
| Meynecke & Meager 2016 [18] | Humpback whales | 1989–2014 | Australia (Queensland) | 1: Ipswich Base 2: Marburg meteorological radar 3: Brisbane meteorological radar | 1: 1938 2: 1993 3: 2005 | Difficult to correlate strandings and localities | Yes | |
| Rojo-Nieto et al., 2011 [70] | Cetaceans and sea turtles | 1991–2008 | Spain and Morocco | Tarifa radar | 2009 | Data before radar setup | ||
| Alvarado-Rybak et al., 2020 [55] | Cetaceans | 1968–2020 | Chile | Bío Bío radars | 2016 | Only one stranding event after radar setup | Yes | |
| Brabyn 1991 [42] | Cetaceans | 1840–1989 | New Zealand | - | - | Radar not found | ||
| Brabyn & McLean 1992 [71] | Cetaceans | 1840–1993 | New Zealand | - | - | Radar not found | ||
| Sundaram, 2006 [36] | Whale strandings | 1850–2005 | USA (Massachusetts) and New Zealand | - | - | Radar not found | ||
| IJsseldijk et al., 2018 [72] | White-beaked dolphins | 1991–2017 | North Sea | - | - | Radar not found |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmori-de la Puente, A.; Balmori, A. Potential Effects of Anthropogenic Radiofrequency Radiation on Cetaceans. Radiation 2024, 4, 1-16. https://doi.org/10.3390/radiation4010001
Balmori-de la Puente A, Balmori A. Potential Effects of Anthropogenic Radiofrequency Radiation on Cetaceans. Radiation. 2024; 4(1):1-16. https://doi.org/10.3390/radiation4010001
Chicago/Turabian StyleBalmori-de la Puente, Alfonso, and Alfonso Balmori. 2024. "Potential Effects of Anthropogenic Radiofrequency Radiation on Cetaceans" Radiation 4, no. 1: 1-16. https://doi.org/10.3390/radiation4010001
APA StyleBalmori-de la Puente, A., & Balmori, A. (2024). Potential Effects of Anthropogenic Radiofrequency Radiation on Cetaceans. Radiation, 4(1), 1-16. https://doi.org/10.3390/radiation4010001







