How Safe Is Gadobutrol? Examining the Effect of Gadolinium Deposition on the Nervous System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment Regimen
2.3. Animal Perfusion
2.4. Quantification of Gadolinium Using ICP-MS
2.5. LDH Assay
2.6. Behavioral Tests
2.6.1. Heat Sensitivity Test
2.6.2. Beam Walking Test
2.6.3. Spontaneous Alternation T-Maze Test
2.7. Electrophysiological Assessment
2.8. Statistical Analysis
3. Results
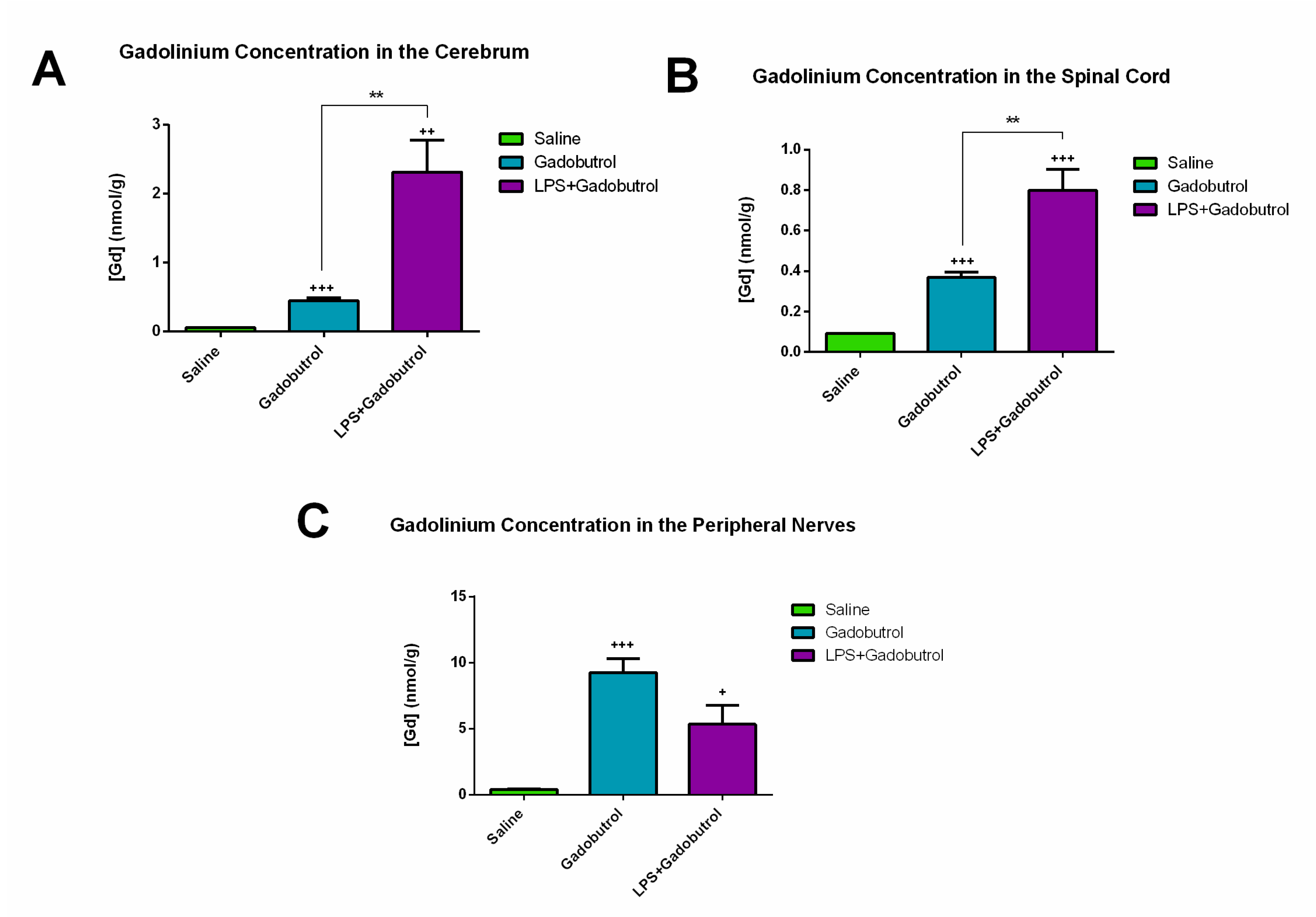
3.1. Quantification of Gadolinium Using ICP-MS
3.2. LDH Assay
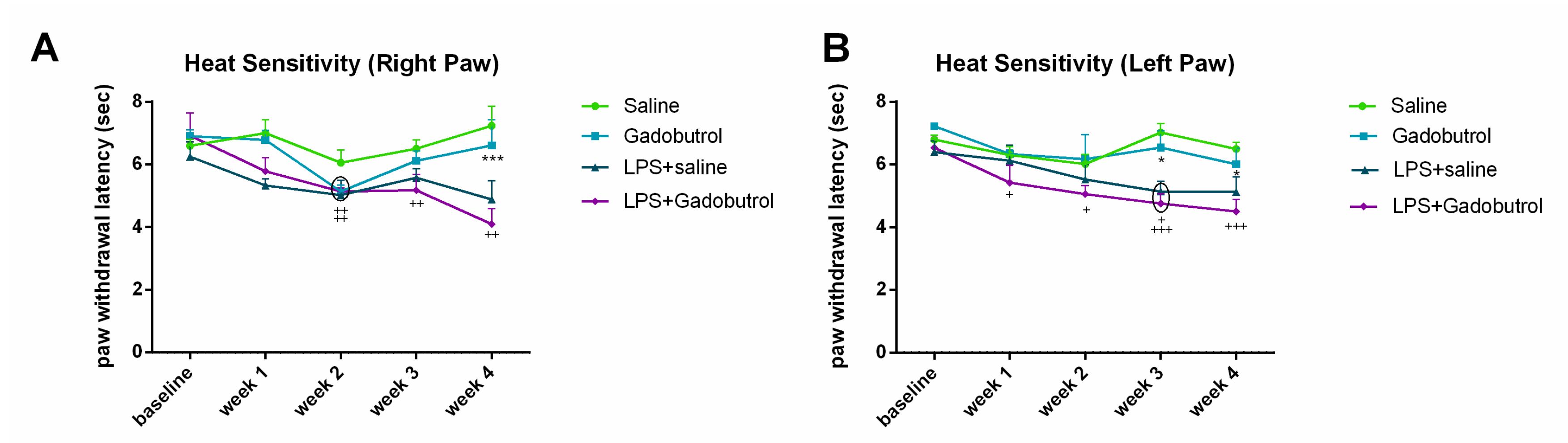
3.3. Heat Sensitivity Test
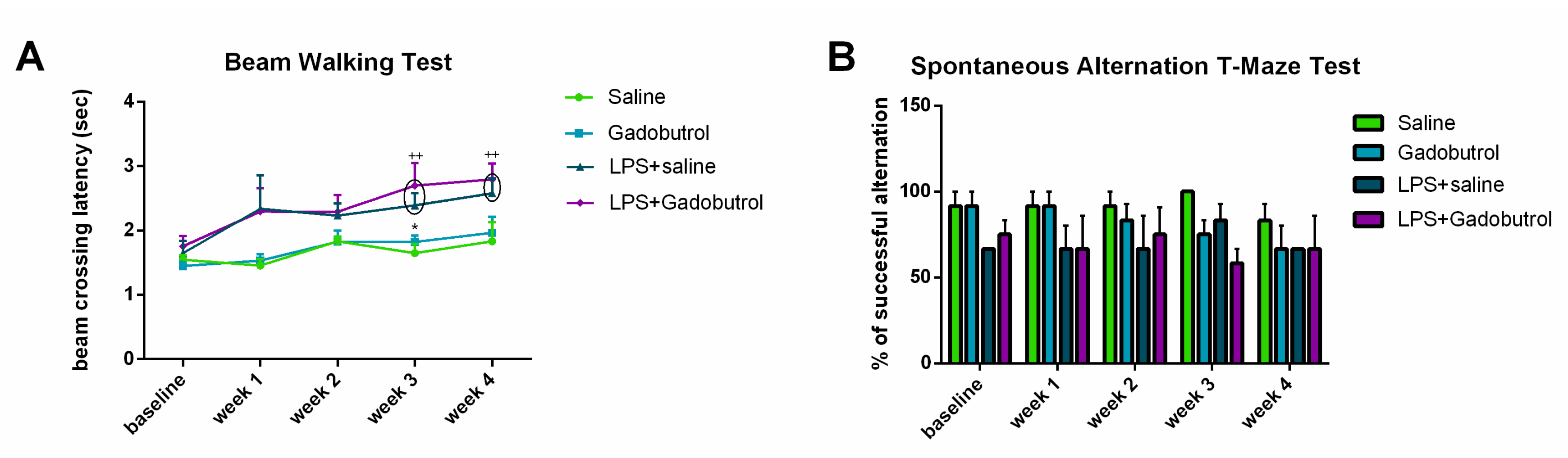
3.4. Beam Walking Test
3.5. Spontaneous Alternation T-Maze Test
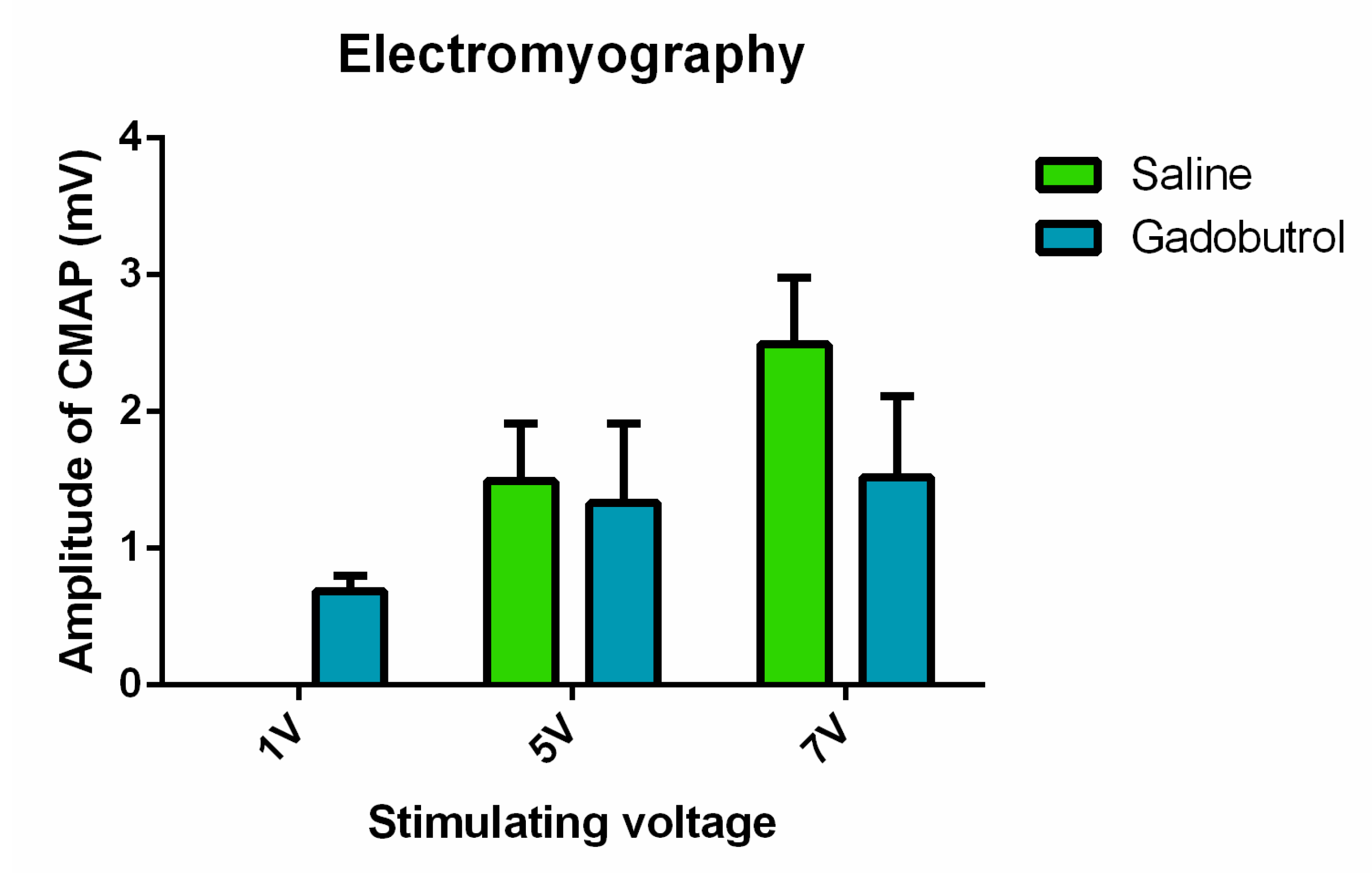
3.6. Electrophysiological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botta, M. Second coordination sphere water molecules and relaxivity of gadolinium (III) complexes: Implications for MRI contrast agents. Eur. J. Inorg. Chem. 2000, 2000, 399–407. [Google Scholar] [CrossRef]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evans, P. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Dai, D.; Schroeder, D.; Jentoft, M.E.; Murray, D.L.; Kadirvel, R.; Eckel, L.J.; Kallmes, D.F. Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 2017, 285, 536–545. [Google Scholar]
- Alkhunizi, S.M.; Fakhoury, M.; Abou-Kheir, W.; Lawand, N. Gadolinium Retention in the Central and Peripheral Nervous System: Implications for Pain, Cognition, and Neurogenesis. Radiology 2020, 297, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Erdene, K.; Nakajima, T.; Kameo, S.; Khairinisa, M.A.; Lamid-Ochir, O.; Tumenjargal, A.; Koibuchi, N.; Koyama, H.; Tsushima, Y. Organ retention of gadolinium in mother and pup mice: Effect of pregnancy and type of gadolinium-based contrast agents. Jpn. J. Radiol. 2017, 35, 568–573. [Google Scholar] [CrossRef]
- Khairinisa, M.A.; Takatsuru, Y.; Amano, I.; Erdene, K.; Nakajima, T.; Kameo, S.; Koyama, H.; Tsushima, Y.; Koibuchi, N. The Effect of Perinatal Gadolinium-Based Contrast Agents on Adult Mice Behavior. Investig. Radiol. 2018, 53, 110–118. [Google Scholar] [CrossRef]
- Saupe, N.; Zanetti, M.; Pfirrmann, C.W.; Wels, T.; Schwenke, C.; Hodler, J. Pain and other side effects after MR arthrography: Prospective evaluation in 1085 patients. Radiology 2009, 250, 830–838. [Google Scholar] [CrossRef]
- Semelka, R.C.; Commander, C.W.; Jay, M.; Burke, L.M.; Ramalho, M. Presumed gadolinium toxicity in subjects with normal renal function: A report of 4 cases. Investig. Radiol. 2016, 51, 661–665. [Google Scholar] [CrossRef]
- Ayers-Ringler, J.; McDonald, J.S.; Connors, M.A.; Fisher, C.R.; Han, S.; Jakaitis, D.R.; Scherer, B.; Tutor, G.; Wininger, K.M.; Dai, D.; et al. Neurologic Effects of Gadolinium Retention in the Brain after Gadolinium-based Contrast Agent Administration. Radiology 2022, 302, 676–683. [Google Scholar] [CrossRef]
- Scott, L.J. Gadobutrol: A Review in Contrast-Enhanced MRI and MRA. Clin. Drug Investig. 2018, 38, 773–784. [Google Scholar] [CrossRef]
- Kaunzner, U.W.; Gauthier, S.A. MRI in the assessment and monitoring of multiple sclerosis: An update on best practice. Ther. Adv. Neurol. Disord. 2017, 10, 247–261. [Google Scholar] [CrossRef] [PubMed]
- FDA. Gadavist (Gadobutrol) Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201277s000lbl.pdf (accessed on 1 August 2021).
- Port, M.; Idée, J.-M.; Medina, C.; Robic, C.; Sabatou, M.; Corot, C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: A critical review. Biometals 2008, 21, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Glutig, K.; Hahn, G.; Kuvvetli, P.; Endrikat, J. Safety of gadobutrol: Results of a non-interventional study of 3710 patients, including 404 children. Acta Radiol. 2019, 60, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Endrikat, J.; Schwenke, C.; Prince, M. Gadobutrol for contrast-enhanced magnetic resonance imaging in elderly patients: Review of the safety profile from clinical trial, post-marketing surveillance, and pharmacovigilance data. Clin. Radiol. 2015, 70, 743–751. [Google Scholar] [CrossRef]
- Ozturk, K.; Nascene, D. Effect of at least 10 serial gadobutrol administrations on brain signal intensity ratios on T1-weighted MRI in children: A matched case-control study. Am. J. Roentgenol. 2021, 217, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Mlinar, B.; Enyeart, J. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J. Physiol. 1993, 469, 639–652. [Google Scholar] [CrossRef]
- Green, C.; Jost, G.; Frenzel, T.; Boyken, J.; Schwenke, C.; Pietsch, H. The Effect of Gadolinium-Based Contrast Agents on Longitudinal Changes of Magnetic Resonance Imaging Signal Intensities and Relaxation Times in the Aging Rat Brain. Investig. Radiol. 2022, 57, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xia, Q.; Yuan, L.; Yang, X.; Wang, K. Impaired mitochondrial function and oxidative stress in rat cortical neurons: Implications for gadolinium-induced neurotoxicity. Neurotoxicology 2010, 31, 391–398. [Google Scholar] [CrossRef]
- Xia, Q.; Feng, X.; Huang, H.; Du, L.; Yang, X.; Wang, K. Gadolinium-induced oxidative stress triggers endoplasmic reticulum stress in rat cortical neurons. J. Neurochem. 2011, 117, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.A.; Apaydin, M.; Armagan, G.; Taskiran, D. Evaluation of toxicity of gadolinium-based contrast agents on neuronal cells. Acta Radiol. 2021, 62, 206–214. [Google Scholar] [CrossRef]
- Wermuth, P.J.; Jimenez, S.A. Induction of a type I interferon signature in normal human monocytes by gadolinium-based contrast agents: Comparison of linear and macrocyclic agents. Clin. Exp. Immunol. 2014, 175, 113–125. [Google Scholar] [CrossRef]
- Schmidt-Lauber, C.; Bossaller, L.; Abujudeh, H.H.; Vladimer, G.I.; Christ, A.; Fitzgerald, K.A.; Latz, E.; Gravallese, E.M.; Marshak-Rothstein, A.; Kay, J. Gadolinium-based compounds induce NLRP3-dependent IL-1β production and peritoneal inflammation. Ann. Rheum. Dis. 2015, 74, 2062–2069. [Google Scholar] [CrossRef]
- Weng, T.-I.; Chen, H.J.; Lu, C.-W.; Ho, Y.-C.; Wu, J.-L.; Liu, S.-H.; Hsiao, J.-K. Exposure of macrophages to low-dose gadolinium-based contrast medium: Impact on oxidative stress and cytokines production. Contrast Media Mol. Imaging 2018, 2018, 3535769. [Google Scholar] [CrossRef] [PubMed]
- Bossù, P.; Cutuli, D.; Palladino, I.; Caporali, P.; Angelucci, F.; Laricchiuta, D.; Gelfo, F.; De Bartolo, P.; Caltagirone, C.; Petrosini, L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J. Neuroinflammation 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.I.; Souza, J.P.; Muller, C.C.; Muller, A.L.H.; Mello, P.A.; Bizzi, C.A. Microwave-assisted wet digestion with H2O2 at high temperature and pressure using single reaction chamber for elemental determination in milk powder by ICP-OES and ICP-MS. Talanta 2016, 156–157, 232–238. [Google Scholar] [CrossRef]
- Deacon, R.M.; Rawlins, J.N.P. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7. [Google Scholar] [CrossRef]
- Aggleton, J.P.; Hunt, P.; Rawlins, J. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav. Brain Res. 1986, 19, 133–146. [Google Scholar] [CrossRef]
- Varatharaj, A.; Carare, R.O.; Weller, R.O.; Gawne-Cain, M.; Galea, I. Gadolinium enhancement of cranial nerves: Implications for interstitial fluid drainage from brainstem into cranial nerves in humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2106331118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hesse, B.; Roman, M.; Stier, D.; Castillo-Michel, H.; Cotte, M.; Suuronen, J.-P.; Lagrange, A.; Radbruch, H.; Paul, F. Increased retention of gadolinium in the inflamed brain after repeated administration of gadopentetate dimeglumine: A proof-of-concept study in mice combining ICP-MS and micro–and Nano–SR-XRF. Investig. Radiol. 2019, 54, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Anderhalten, L.; Silva, R.V.; Morr, A.; Wang, S.; Smorodchenko, A.; Saatz, J.; Traub, H.; Mueller, S.; Boehm-Sturm, P.; Rodriguez-Sillke, Y. Different impact of gadopentetate and gadobutrol on inflammation-promoted retention and toxicity of gadolinium within the mouse brain. Investig. Radiol. 2022, 57, 677–688. [Google Scholar] [CrossRef]
- Fu, H.Q.; Yang, T.; Xiao, W.; Fan, L.; Wu, Y.; Terrando, N.; Wang, T.L. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS ONE 2014, 9, e106331. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrian, O.; Ramos, M.; Bachiller, S.; Lundgaard, I.; Deierborg, T. Acute systemic LPS-exposure impairs perivascular CSF distribution in mice. J. Neuroinflammation 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Montague-Cardoso, K.; Malcangio, M. Changes in blood–spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. Pain Rep. 2021, 6, e879. [Google Scholar] [CrossRef]
- Lauer, M.; Lauer, A.; You, S.J.; Kluge, S.; Hattingen, E.; Harter, P.N.; Senft, C.; Wagner, M.; Voss, M. Neurotoxicity of subarachnoid Gd-based contrast agent accumulation: A potential complication of intraoperative MRI? Neurosurg. Focus 2021, 50, E12. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation 2015, 12, 223. [Google Scholar] [CrossRef]
- Bassi, G.S.; Kanashiro, A.; Santin, F.M.; de Souza, G.E.; Nobre, M.J.; Coimbra, N.C. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin. Pharmacol. Toxicol. 2012, 110, 359–369. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jammoul, M.; Abou-Kheir, W.; Lawand, N. How Safe Is Gadobutrol? Examining the Effect of Gadolinium Deposition on the Nervous System. Radiation 2023, 3, 75-86. https://doi.org/10.3390/radiation3020007
Jammoul M, Abou-Kheir W, Lawand N. How Safe Is Gadobutrol? Examining the Effect of Gadolinium Deposition on the Nervous System. Radiation. 2023; 3(2):75-86. https://doi.org/10.3390/radiation3020007
Chicago/Turabian StyleJammoul, Maya, Wassim Abou-Kheir, and Nada Lawand. 2023. "How Safe Is Gadobutrol? Examining the Effect of Gadolinium Deposition on the Nervous System" Radiation 3, no. 2: 75-86. https://doi.org/10.3390/radiation3020007
APA StyleJammoul, M., Abou-Kheir, W., & Lawand, N. (2023). How Safe Is Gadobutrol? Examining the Effect of Gadolinium Deposition on the Nervous System. Radiation, 3(2), 75-86. https://doi.org/10.3390/radiation3020007






